Molecular Imprinting Technology for Advanced Delivery of Essential Oils
Abstract
1. Introduction
2. Essential Oils’ Active Substances as Part of MIP
3. Opportunities to Apply Molecular Imprinting Technologies for the Delivery of Essential Oils
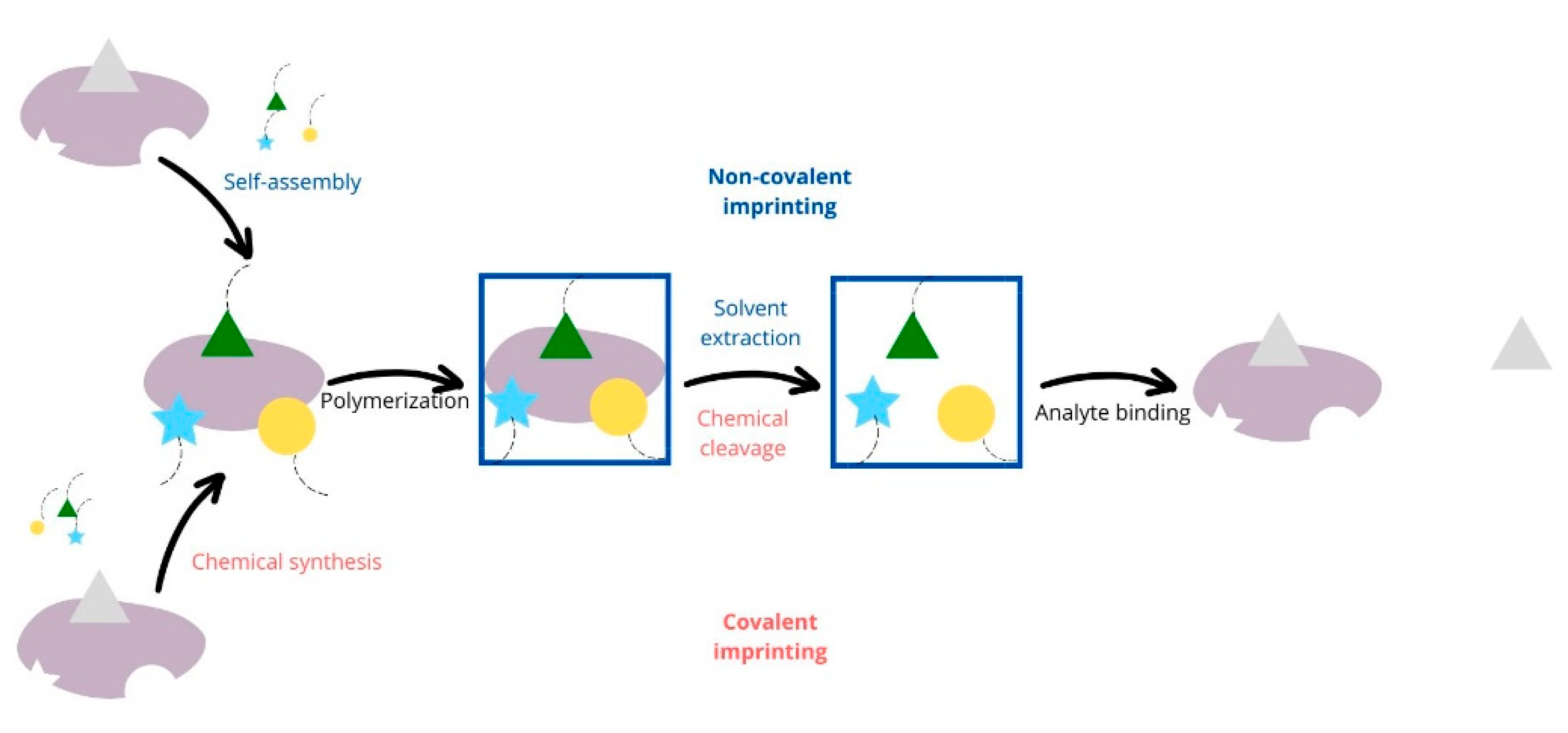
3.1. The Most Promising Types of Molecular Imprinting Technology
3.2. Kinetics’ Role in EO Integration in MIP
3.3. Molecularly Imprinted Polymers Applied to the Development of Biosensors for the Detection of EOs
3.4. Nanotechnological Approaches for the Preparation of Molecularly Imprinted Polymers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dornic, N.; Ficheux, A.S.; Roudot, A.C.; Saboureau, D.; Ezzedine, K. Usage patterns of aromatherapy among the French general population: A descriptive study focusing on dermal exposure. Regul. Toxicol. Pharmacol. 2016, 76, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Nakai, S. Usage patterns of aromatherapy essential oil among Chinese consumers. PLoS ONE 2022, 17, e0272031. [Google Scholar] [CrossRef]
- De Sousa Barros, A.; de Morais, S.M.; Ferreira, P.A.T.; Vieira, Í.G.P.; Craveiro, A.A.; dos Santos Fontenelle, R.O.; de Menezes, J.E.S.A.; da Silva, F.W.F.; de Sousa, H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Pinto, G.B.; dos Reis Corrêa, A.; da Silva, G.N.C.; da Costa, J.S.; Figueiredo, P.L.B. Drug Development from Essential Oils: New Discoveries and Perspectives. In Drug Discovery and Design Using Natural Products; Cruz, J.N., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, G.; Mattiasson, B. Molecular Imprinting Techniques Used for the Preparation of Biosensors. Sensors 2017, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Urriza-Arsuaga, I.; Guadaño-Sánchez, M.; Urraca, J.L. Current Trends in Molecular Imprinting: Strategies, Applications and Determination of Target Molecules in Spain. Int. J. Mol. Sci. 2023, 24, 1915. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, C.; Hasterok, S.; Gjörloff Wingren, A.; Tassidis, H. Recent Advances in Molecularly Imprinted Polymers and Their Disease-Related Applications. Polymers 2023, 15, 4199. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Samukaite-Bubniene, U.; Ratautaite, V.; Bechelany, M.; Ramanavicius, A. Electrochemical molecularly imprinted polymer based sensors for pharmaceutical and biomedical applications (review). J. Pharm. Biomed. Anal. 2022, 215, 114739. [Google Scholar] [CrossRef]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Samukaite-Bubniene, U.; Plausinaitis, D.; Ramanaviciene, A.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polymers for the recognition of biomarkers of certain neurodegenerative diseases. J. Pharm. Biomed. Anal. 2023, 228, 115343. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef]
- Ratautaite, V.; Brazys, E.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical sensors based on l-tryptophan molecularly imprinted polypyrrole and polyaniline. J. Electroanal. Chem. 2022, 917, 116389. [Google Scholar] [CrossRef]
- Brazys, E.; Ratautaite, V.; Brasiunas, B.; Ramanaviciene, A.; Rodríguez, L.; Pinto, A.; Milea, D.; Prentice, U.; Ramanavicius, A. Molecularly imprinted polypyrrole-based electrochemical melamine sensors. Microchem. J. 2024, 199, 109890. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Plausinaitis, D.; Ramanavicius, S.; Samukaite-Bubniene, U.; Bechelany, M.; Ramanavicius, A. Evaluation of the interaction between SARS-CoV-2 spike glycoproteins and the molecularly imprinted polypyrrole. Talanta 2023, 253, 123981. [Google Scholar] [CrossRef]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, S.; Chen, C.-F.; Viter, R.; Ramanavicius, A. Molecularly Imprinted Polymer-Based Electrochemical Sensors for the Diagnosis of Infectious Diseases. Biosensors 2023, 13, 620. [Google Scholar] [CrossRef]
- Kaspute, G.; Arunagiri, B.D.; Alexander, R.; Ramanavicius, A.; Samukaite-Bubniene, U. Development of Essential Oil Delivery Systems by ‘Click Chemistry’ Methods: Possible Ways to Manage Duchenne Muscular Dystrophy. Materials 2023, 16, 6537. [Google Scholar] [CrossRef]
- Elaine, A.A.; Krisyanto, S.I.; Hasanah, A.N. Dual-Functional Monomer MIPs and Their Comparison to Mono-Functional Monomer MIPs for SPE and as Sensors. Polymers 2022, 14, 3498. [Google Scholar] [CrossRef]
- Ramstroem, O.; Andersson, L.I.; Mosbach, K. Recognition sites incorporating both pyridinyl and carboxy functionalities prepared by molecular imprinting. J. Org. Chem. 1993, 58, 7562–7564. [Google Scholar] [CrossRef]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One Hundred Faces of Geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef] [PubMed]
- Karadağ, A.E.; Biltekin, S.N.; Demirci, B.; Demirci, F.; Ghani, U. Comparative In Vitro and In Silico Enzyme Inhibitory Screening of Rosa x damascena and Pelargonium graveolens Essential Oils and Geraniol. Plants 2023, 12, 3296. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Liu, J.; Li, J.; Wang, X.; Lv, M.; Cui, R.; Chen, L. Dual-template molecularly imprinted polymers for dispersive solid-phase extraction of fluoroquinolones in water samples coupled with high performance liquid chromatography. Analyst 2019, 144, 1292–1302. [Google Scholar] [CrossRef]
- Plikusiene, I.; Balevicius, Z.; Ramanaviciene, A.; Talbot, J.; Mickiene, G.; Balevicius, S.; Stirke, A.; Tereshchenko, A.; Tamosaitis, L.; Zvirblis, G.; et al. Evaluation of affinity sensor response kinetics towards dimeric ligands linked with spacers of different rigidity: Immobilized recombinant granulocyte colony-stimulating factor based synthetic receptor binding with genetically engineered dimeric analyte derivatives. Biosens. Bioelectron. 2020, 156, 112112. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Noroozian, E.; Jassbi, A.R. Molecularly imprinted polymer solid-phase extraction for the analysis of 1,8-cineole in thyme and sagebrush distillates. J. Iran. Chem. Soc. 2020, 17, 1153–1161. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Sedaghat, S.; Larijani, K.; Khossravi, M.; Masoudi, S. Composition of the Essential Oil of Ferulago angulata (Schlecht.) Boiss. from Iran. J. Essent. Oil Res. 2002, 14, 447–448. [Google Scholar] [CrossRef]
- Bendimerad, A.; Bendiab, S.A.T.; Breme, K.; Fernandez, X. Essential Oil Composition of Aerial Parts of Sinapis arvensis L. from Algeria. J. Essent. Oil Res. 2007, 19, 206–208. [Google Scholar] [CrossRef]
- Pino, J.; Marbot, R.; Vazquez, C. Volatile constituents of genipap (Genipa americana L.) fruit from Cuba. Flavour Fragr. J. 2005, 20, 583–586. [Google Scholar] [CrossRef]
- Mohsenzadeh, E.; Ratautaite, V.; Brazys, E.; Ramanavicius, S.; Zukauskas, S.; Plausinaitis, D.; Ramanavicius, A. Design of molecularly imprinted polymers (MIP) using computational methods: A review of strategies and approaches. WIREs Comput. Mol. Sci. 2024, 14, e1713. [Google Scholar] [CrossRef]
- Sarmast, E.; Shankar, S.; Salmieri, S.; Rahmouni, S.A.; Mahmud, J.; Lacroix, M. Cross-linked gelatin–riboflavin-based film incorporated with essential oils and silver nanoparticle by gamma-irradiation: A novel approach for extending the shelf life of meat. Food Hydrocoll. 2024, 147, 109330. [Google Scholar] [CrossRef]
- Rojas-Moreno, S.; Osorio-Revilla, G.; Gallardo-Velázquez, T.; Cárdenas-Bailón, F.; Meza-Márquez, G. Effect of the cross-linking agent and drying method on encapsulation efficiency of orange essential oil by complex coacervation using whey protein isolate with different polysaccharides. J. Microencapsul. 2018, 35, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Xu, S.; Zhou, Q.; Wang, S.; Xu, Z.; Liu, Z. Advances in Molecular Imprinting Technology for the Extraction and Detection of Quercetin in Plants. Polymers 2023, 15, 2107. [Google Scholar] [CrossRef] [PubMed]
- Kasiri, E.; Haddadi, H.; Javadian, H.; Asfaram, A. Highly effective pre-concentration of thymol and carvacrol using nano-sized magnetic molecularly imprinted polymer based on experimental design optimization and their trace determination in summer savoury, Origanum majorana and Origanum vulgare extracts. J. Chromatogr. B 2021, 1182, 122941. [Google Scholar] [CrossRef]
- Liu, R.; Poma, A. Advances in Molecularly Imprinted Polymers as Drug Delivery Systems. Molecules 2021, 26, 3589. [Google Scholar] [CrossRef]
- Lusina, A.; Cegłowski, M. Molecularly Imprinted Polymers as State-of-the-Art Drug Carriers in Hydrogel Transdermal Drug Delivery Applications. Polymers 2022, 14, 640. [Google Scholar] [CrossRef]
- Lawai, V.; Ngaini, Z.; Farooq, S.; Wahi, R.; Bhawani, S.A. Current advances in molecularly imprinted polymers and their release mechanisms in drug delivery systems. Polym. Adv. Technol. 2024, 35, e6317. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Leu, Y.-L.; Hwang, T.-L.; Cheng, H.-C.; Hung, C.-F. Development of sesquiterpenes from Alpinia oxyphylla as novel skin permeation enhancers. Eur. J. Pharm. Sci. 2003, 19, 253–262. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Kumar, M.S.; Raju, V.; Lakshmi, M.; Rama, B. Penetration-Enhancing Effect of Ethanolic Solution of Menthol on Transdermal Permeation of Ondansetron Hydrochloride Across Rat Epidermis. Drug Deliv. 2008, 15, 227–234. [Google Scholar] [CrossRef]
- Esmaeili, F.; Rajabnejhad, S.; Partoazar, A.R.; Mehr, S.E.; Faridi-Majidi, R.; Sahebgharani, M.; Syedmoradi, L.; Rajabnejhad, M.R.; Amani, A. Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharm. Dev. Technol. 2016, 21, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Development of molecularly imprinted polymer based phase boundaries for sensors design (review). Adv. Colloid Interface Sci. 2022, 305, 102693. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Topkaya, S.N.; Mikoliunaite, L.; Ozsoz, M.; Oztekin, Y.; Ramanaviciene, A.; Ramanavicius, A. Molecularly Imprinted Polypyrrole for DNA Determination. Electroanalysis 2013, 25, 1169–1177. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Pulsed amperometric detection of DNA with an ssDNA/polypyrrole-modified electrode. Anal. Bioanal. Chem. 2004, 379, 287–293. [Google Scholar] [CrossRef]
- Liustrovaite, V.; Pogorielov, M.; Boguzaite, R.; Ratautaite, V.; Ramanaviciene, A.; Pilvenyte, G.; Holubnycha, V.; Korniienko, V.; Diedkova, K.; Viter, R.; et al. Towards Electrochemical Sensor Based on Molecularly Imprinted Polypyrrole for the Detection of Bacteria—Listeria monocytogenes. Polymers 2023, 15, 1597. [Google Scholar] [CrossRef] [PubMed]
- Boguzaite, R.; Pilvenyte, G.; Ratautaite, V.; Brazys, E.; Ramanaviciene, A.; Ramanavicius, A. Towards Molecularly Imprinted Polypyrrole-Based Sensor for the Detection of Methylene Blue. Chemosensors 2023, 11, 549. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Molecularly imprinted polypyrrole-based synthetic receptor for direct detection of bovine leukemia virus glycoproteins. Biosens. Bioelectron. 2004, 20, 1076–1082. [Google Scholar] [CrossRef]
- Balciunas, D.; Plausinaitis, D.; Ratautaite, V.; Ramanaviciene, A.; Ramanavicius, A. Towards electrochemical surface plasmon resonance sensor based on the molecularly imprinted polypyrrole for glyphosate sensing. Talanta 2022, 241, 123252. [Google Scholar] [CrossRef]
- Viter, R.; Kunene, K.; Genys, P.; Jevdokimovs, D.; Erts, D.; Sutka, A.; Bisetty, K.; Viksna, A.; Ramanaviciene, A.; Ramanavicius, A. Photoelectrochemical Bisphenol S Sensor Based on ZnO-Nanoroads Modified by Molecularly Imprinted Polypyrrole. Macromol. Chem. Phys. 2020, 221, 1900232. [Google Scholar] [CrossRef]
- Ratautaite, V.; Janssens, S.D.; Haenen, K.; Nesládek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Molecularly Imprinted Polypyrrole Based Impedimentric Sensor for Theophylline Determination. Electrochim. Acta 2014, 130, 361–367. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef]
- Karazan, Z.M.; Roushani, M. Synthesis Methods and Strategies for MIPs. In Molecularly Imprinted Polymers as Artificial Antibodies for the Environmental Health: A Step Towards Achieving the Sustainable Development Goals; Patra, S., Sillanpaa, M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 31–52. [Google Scholar] [CrossRef]
- Bakhtiar, S.; Bhawani, S.A.; Shafqat, S.R. Synthesis and characterization of molecular imprinting polymer for the removal of 2-phenylphenol from spiked blood serum and river water. Chem. Biol. Technol. Agric. 2019, 6, 15. [Google Scholar] [CrossRef]
- Hashim, S.N.N.S.; Boysen, R.I.; Schwarz, L.J.; Danylec, B.; Hearn, M.T.W. A comparison of covalent and non-covalent imprinting strategies for the synthesis of stigmasterol imprinted polymers. J. Chromatogr. A 2014, 1359, 35–43. [Google Scholar] [CrossRef]
- Wulff, G. Molecular Imprinting in Cross-Linked Materials with the Aid of Molecular Templates—A Way towards Artificial Antibodies. Angew. Chem. Int. Ed. Engl. 1995, 34, 1812–1832. [Google Scholar] [CrossRef]
- Şenocak, A.; Basova, T.; Demirbas, E.; Durmuş, M. Direct and Fast Electrochemical Determination of Catechin in Tea Extracts using SWCNT-Subphthalocyanine Hybrid Material. Electroanalysis 2019, 31, 1697–1707. [Google Scholar] [CrossRef]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Rodriguez, M.E.; Villar, P.; Vulfson, E.N. A New Method for the Introduction of Recognition Site Functionality into Polymers Prepared by Molecular Imprinting: Synthesis and Characterization of Polymeric Receptors for Cholesterol. J. Am. Chem. Soc. 1995, 117, 7105–7111. [Google Scholar] [CrossRef]
- Qi, P.; Wang, J.; Wang, L.; Li, Y.; Jin, J.; Su, F.; Tian, Y.; Chen, J. Molecularly imprinted polymers synthesized via semi-covalent imprinting with sacrificial spacer for imprinting phenols. Polymer 2010, 51, 5417–5423. [Google Scholar] [CrossRef]
- Soosani, Z.; Rezaei, B.; Heydari-Bafrooei, E.; Ensafi, A.A. Chemical Sensors Based on Molecularly Imprinted Polymers Can Determine Drug Release Kinetics from Nanocarriers without Filtration, Centrifugation, and Dialysis Steps. ACS Sens. 2023, 8, 1891–1900. [Google Scholar] [CrossRef]
- Kim, H.; Kaczmarski, K.; Guiochon, G. Mass transfer kinetics on the heterogeneous binding sites of molecularly imprinted polymers. Chem. Eng. Sci. 2005, 60, 5425–5444. [Google Scholar] [CrossRef]
- Zuo, J.; Ma, P.; Li, Z.; Zhang, Y.; Xiao, D.; Wu, H.; Dong, A. Application of Molecularly Imprinted Polymers in Plant Natural Products: Current Progress and Future Perspectives. Macromol. Mater. Eng. 2023, 308, 2200499. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Recent advances and future trends in molecularly imprinted polymers-based sample preparation. J. Sep. Sci. 2023, 46, 2300157. [Google Scholar] [CrossRef]
- Önal Acet, B.; İnanan, T.; Salieva, K.; Borkoev, B.; Odabaşı, M.; Acet, Ö. Molecular imprinted polymers: Important advances in biochemistry, biomedical and biotechnology. Polym. Bull. 2024, 81, 10439–10459. [Google Scholar] [CrossRef]
- Cengiz, N.; Guclu, G.; Kelebek, H.; Capanoglu, E.; Selli, S. Application of Molecularly Imprinted Polymers for the Detection of Volatile and Off-Odor Compounds in Food Matrices. ACS Omega 2022, 7, 15258–15266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, X. Preparation of molecularly imprinted polymer for vanillin via seed swelling and suspension polymerization. Polym. Sci. Ser. B 2014, 56, 538–545. [Google Scholar] [CrossRef]
- Cao, J.; Shen, C.; Wang, X.; Zhu, Y.; Bao, S.; Wu, X.; Fu, Y. A porous cellulose-based molecular imprinted polymer for specific recognition and enrichment of resveratrol. Carbohydr. Polym. 2021, 251, 117026. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Li, H.; Zhao, L.; Ma, Y.; Zhang, Y.; Liu, J.; Wei, Y. Rigorous recognition mode analysis of molecularly imprinted polymers—Rational design, challenges, and opportunities. Prog. Polym. Sci. 2024, 150, 101790. [Google Scholar] [CrossRef]
- Cagliero, C.; Bicchi, C.; Marengo, A.; Rubiolo, P.; Sgorbini, B. Gas chromatography of essential oil: State-of-the-art, recent advances, and perspectives. J. Sep. Sci. 2022, 45, 94–112. [Google Scholar] [CrossRef]
- Suwanwong, Y.; Boonpangrak, S. Molecularly imprinted polymers for the extraction and determination of water-soluble vitamins: A review from 2001 to 2020. Eur. Polym. J. 2021, 161, 110835. [Google Scholar] [CrossRef]
- Zhi, K.; Duan, J.; Zhang, J.; Huang, L.; Guo, L.; Wang, L. Progress and Prospect of Ion Imprinting Technology in Targeted Extraction of Lithium. Polymers 2024, 16, 833. [Google Scholar] [CrossRef] [PubMed]
- Kavuran, E.; Yurttaş, A. The Effect of Aromatherapy with Lavender Essential Oil on the Sleep and Fatigue Level of Patients with Multiple Sclerosis in Turkey: A Randomized Controlled Trial. Niger. J. Clin. Pract. 2024, 27, 635–642. [Google Scholar] [CrossRef]
- Wakui, N.; Togawa, C.; Ichikawa, K.; Matsuoka, R.; Watanabe, M.; Okami, A.; Shirozu, S.; Yamamura, M.; Machida, Y. Relieving psychological stress and improving sleep quality by bergamot essential oil use before bedtime and upon awakening: A randomized crossover trial. Complement. Ther. Med. 2023, 77, 102976. [Google Scholar] [CrossRef] [PubMed]
- Lucena, L.; Santos-Junior, J.G.; Tufik, S.; Hachul, H. Effect of a lavender essential oil and sleep hygiene protocol on insomnia in postmenopausal women: A pilot randomized clinical trial. EXPLORE 2024, 20, 116–125. [Google Scholar] [CrossRef]
- Pourshaikhian, M.; Moghadamnia, M.T.; Kazemnezhad Leyli, E.; Shafiei Kisomi, Z. Effects of aromatherapy with Matricaria chamomile essential oil on anxiety and hemodynamic indices in patients with acute coronary syndrome, 2021: A randomized controlled trial. BMC Complement. Med. Ther. 2024, 24, 17. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, F.; Hanifi, N.; Mardani, A. Comparison of the Effects of Aromatherapy with Damask Rose and Chamomile Essential Oil on Preoperative Pain and Anxiety in Emergency Orthopedic Surgery: A Randomized Controlled Trial. J. Perianesthesia Nurs. 2024, 39, 583–588. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Progress in dental materials: Application of natural ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds. Molecules 2022, 27, 1716. [Google Scholar] [CrossRef]
- Lanzerstorfer, P.; Sandner, G.; Pitsch, J.; Mascher, B.; Aumiller, T.; Weghuber, J. Acute, reproductive, and developmental toxicity of essential oils assessed with alternative in vitro and in vivo systems. Arch. Toxicol. 2021, 95, 673–691. [Google Scholar] [CrossRef]
- Vostinaru, O.; Heghes, S.C.; Filip, L.; Vostinaru, O.; Heghes, S.C.; Filip, L. Safety Profile of Essential Oils. In Essential Oils—Bioactive Compounds, New Perspectives and Applications; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Ali, S.; Ekbbal, R.; Salar, S.; Yasheshwar; Ali, S.A.; Jaiswal, A.K.; Singh, M.; Yadav, D.K.; Kumar, S.; Gaurav. Quality Standards and Pharmacological Interventions of Natural Oils: Current Scenario and Future Perspectives. ACS Omega 2023, 8, 39945–39963. [Google Scholar] [CrossRef]
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of Essential Oils: A Multitask Issue for Quality Control. Three Case Studies: Lavandula angustifolia Mill., Citrus limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Kong, I.; Irfan, U.; Solihin, M.I.; Pui, L.P. Edible Oils Adulteration: A Review on Regulatory Compliance and Its Detection Technologies. J. Oleo Sci. 2021, 70, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Mishra, M.; Ansari, K.M.; Chaudhari, B.P.; Khanna, R.; Das, M. Edible oil adulterants, argemone oil and butter yellow, as aetiological factors for gall bladder cancer. Eur. J. Cancer 2012, 48, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Viciano-Tudela, S.; Parra, L.; Navarro-Garcia, P.; Sendra, S.; Lloret, J. Proposal of a New System for Essential Oil Classification Based on Low-Cost Gas Sensor and Machine Learning Techniques. Sensors 2023, 23, 5812. [Google Scholar] [CrossRef]
- Karimi Baker, Z.; Sardari, S. Molecularly Imprinted Polymer (MIP) Applications in Natural Product Studies Based on Medicinal Plant and Secondary Metabolite Analysis. Iran. Biomed. J. 2021, 25, 68–77. [Google Scholar] [CrossRef]
- Mazzotta, E.; Di Giulio, T.; Malitesta, C. Electrochemical sensing of macromolecules based on molecularly imprinted polymers: Challenges, successful strategies, and opportunities. Anal. Bioanal. Chem. 2022, 414, 5165–5200. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, D.; Saputri, F.A. Molecularly imprinted polymer nanoparticles (MIP-NPS) applications in electrochemical sensors. Int. J. App. Pharm. 2019, 11, 1–6. [Google Scholar] [CrossRef]
- Ramanavičius, S.; Morkvėnaitė-Vilkončienė, I.; Samukaitė-Bubnienė, U.; Ratautaitė, V.; Plikusienė, I.; Viter, R.; Ramanavičius, A. Electrochemically Deposited Molecularly Imprinted Polymer-Based Sensors. Sensors 2022, 22, 1282. [Google Scholar] [CrossRef]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, A.; Viter, R.; Ramanavicius, S. Molecularly Imprinted Polymers for the Determination of Cancer Biomarkers. Int. J. Mol. Sci. 2023, 24, 4105. [Google Scholar] [CrossRef]
- Mohsenzadeh, E.; Ratautaite, V.; Brazys, E.; Ramanavicius, S.; Zukauskas, S.; Plausinaitis, D.; Ramanavicius, A. Application of computational methods in the design of molecularly imprinted polymers (review). TrAC Trends Anal. Chem. 2024, 171, 117480. [Google Scholar] [CrossRef]
- Zelikovich, D.; Dery, L.; Sagi-Cohen, H.; Mandler, D. Imprinting of nanoparticles in thin films: Quo Vadis? Chem. Sci. 2023, 14, 9630–9650. [Google Scholar] [CrossRef] [PubMed]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly Imprinted Polymer Micro- and Nano-Particles: A Review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef]
- Seifi, M.; Hassanpour Moghadam, M.; Hadizadeh, F.; Ali-Asgari, S.; Aboli, J.; Mohajeri, S.A. Preparation and study of tramadol imprinted micro-and nanoparticles by precipitation polymerization: Microwave irradiation and conventional heating method. Int. J. Pharm. 2014, 471, 37–44. [Google Scholar] [CrossRef]
- Ebrahimzadeh, H.; Dehghani, Z.; Asgharinezhad, A.A.; Shekari, N.; Molaei, K. Determination of haloperidol in biological samples using molecular imprinted polymer nanoparticles followed by HPLC-DAD detection. Int. J. Pharm. 2013, 453, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, Y.; Xiang, Y.; Qiu, F.; Fu, G. Controlled synthesis of PEGylated surface protein-imprinted nanoparticles. Analyst 2019, 144, 5439–5448. [Google Scholar] [CrossRef]
- Pan, S.-D.; Chen, X.-H.; Li, X.-P.; Cai, M.-Q.; Shen, H.-Y.; Zhao, Y.-G.; Jin, M.-C. Double-sided magnetic molecularly imprinted polymer modified graphene oxide for highly efficient enrichment and fast detection of trace-level microcystins from large-volume water samples combined with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1422, 1–12. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, F. Electrochemical sensor for dimetridazole based on novel gold nanoparticles@molecularly imprinted polymer. Sens. Actuators B Chem. 2015, 220, 1017–1022. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Y.; Teng, W.; Tan, J.; Liang, Y.; Tang, Y. Preparation of core-shell magnetic molecular imprinted polymer with binary monomer for the fast and selective extraction of bisphenol A from milk. J. Chromatogr. A 2016, 1462, 2–7. [Google Scholar] [CrossRef]

| Structures Used for the Formation of Nanoparticles (NPs) | Description of Major Achievements |
|---|---|
| Polymeric | Widely used for the extraction and pre-concentration of both small and macromolecules from complex samples, e.g., tramadol and haloperidol [97,98]. |
| Silica | A novel synthesis strategy using silica particles and controlled PEG addition which significantly reduced non-specific binding sites and improved MIP selectivity for lysozyme, increasing the imprinting factor from 2.1 to 9.1. This approach, which employed hydrophilic silica nanocores and click chemistry for AFCTP, was also applied to imprinted bovine hemoglobin, demonstrating its general applicability [99]. |
| Carbon | Graphene oxide was used to synthesize a double-sided magnetic(M) MIP for the selective recognition of microcystins, incorporating Fe3O4 NPs coated with diphenylethene and acrylamide MIP, anchored to both sides of the GO sheets. This material enabled a magnetic solid-phase extraction procedure with an enrichment factor of 2000, limiting the quantification from 0.1 to 2.0 ng·L−1, and with recoveries of 84% to 98%, showing superior analytical performance and the potential for environmental microcystin removal [100]. |
| Gold | The MIPs with AuNPs were developed for the selective detection of dimetridazole. The AuNPs were synthesized by reducing HAuCl4 and were coated with 3-propyl-1-vinylimidazolium bromide, an ionic liquid used as a monomer for MIP synthesis. These imprinted AuNPs were then applied to modified glassy carbon electrodes, resulting in a sensor with a detection limit of 5.0 × 10−10 mol·L−1 for dimetridazole, successfully tested on food samples [101]. |
| Magnetic | A novel strategy for extracting and pre-concentrating bisphenol A from milk using a MIP with MNPs’ core synthesized through reversible addition-fragmentation chain transfer polymerization was reported. The MIP, containing β-cyclodextrin and 4-vinyl pyridine as functional monomers and bisphenol A as the template, achieved highly selective cavities, superior selectivity, a detection limit of 3.7 µg·L−1, and high recovery rates of 97% to 99% [102]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaspute, G.; Ramanavicius, A.; Prentice, U. Molecular Imprinting Technology for Advanced Delivery of Essential Oils. Polymers 2024, 16, 2441. https://doi.org/10.3390/polym16172441
Kaspute G, Ramanavicius A, Prentice U. Molecular Imprinting Technology for Advanced Delivery of Essential Oils. Polymers. 2024; 16(17):2441. https://doi.org/10.3390/polym16172441
Chicago/Turabian StyleKaspute, Greta, Arunas Ramanavicius, and Urte Prentice. 2024. "Molecular Imprinting Technology for Advanced Delivery of Essential Oils" Polymers 16, no. 17: 2441. https://doi.org/10.3390/polym16172441
APA StyleKaspute, G., Ramanavicius, A., & Prentice, U. (2024). Molecular Imprinting Technology for Advanced Delivery of Essential Oils. Polymers, 16(17), 2441. https://doi.org/10.3390/polym16172441








