The Effect of Polymer–Solvent Interaction on the Swelling of Polymer Matrix Tablets: A Magnetic Resonance Microscopy Study Complemented by Bond Fluctuation Model Simulations
Abstract
1. Introduction
2. Materials and Methods
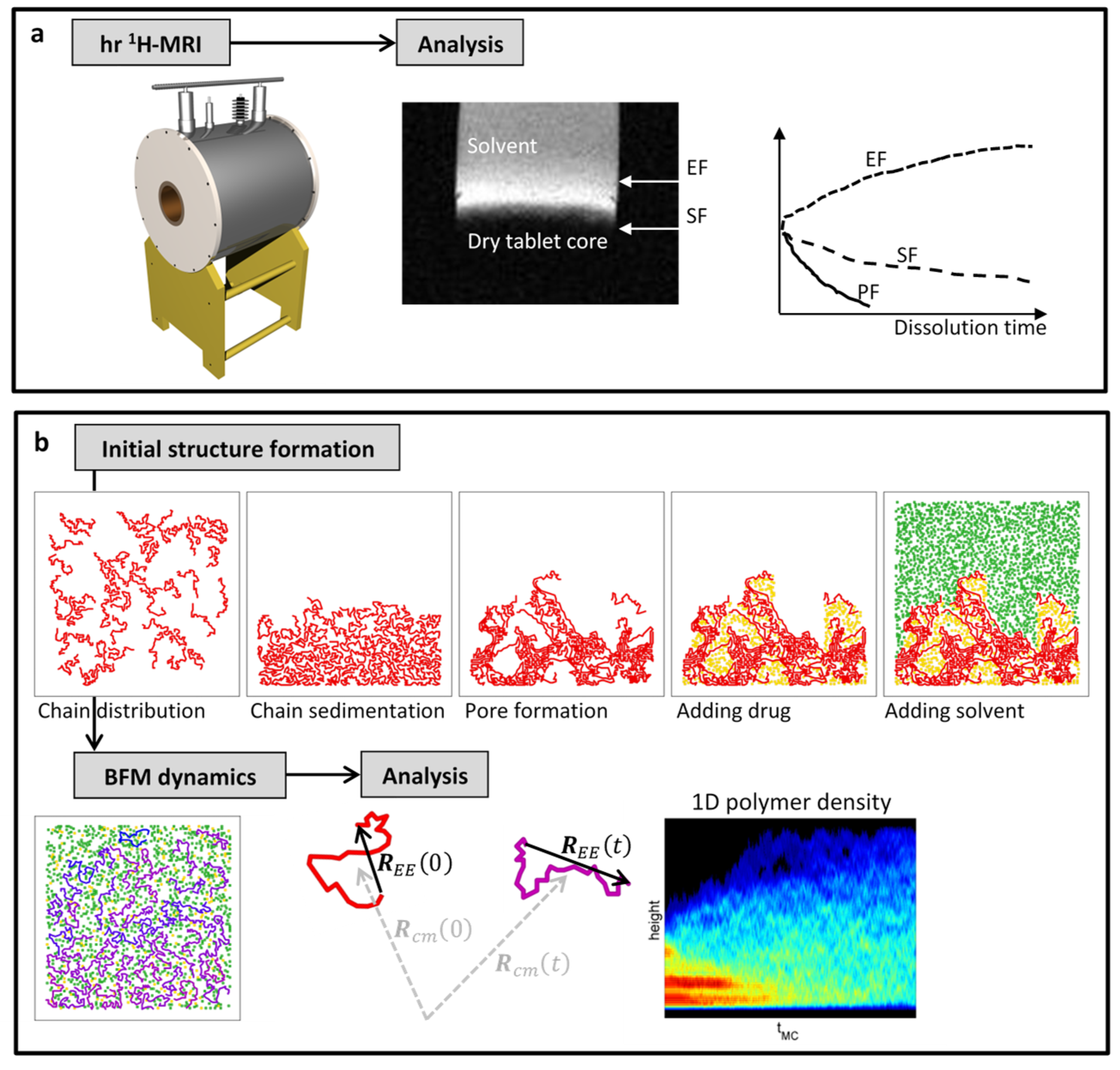
2.1. Experimental Section: Magnetic Resonance Microscopy (MRM)
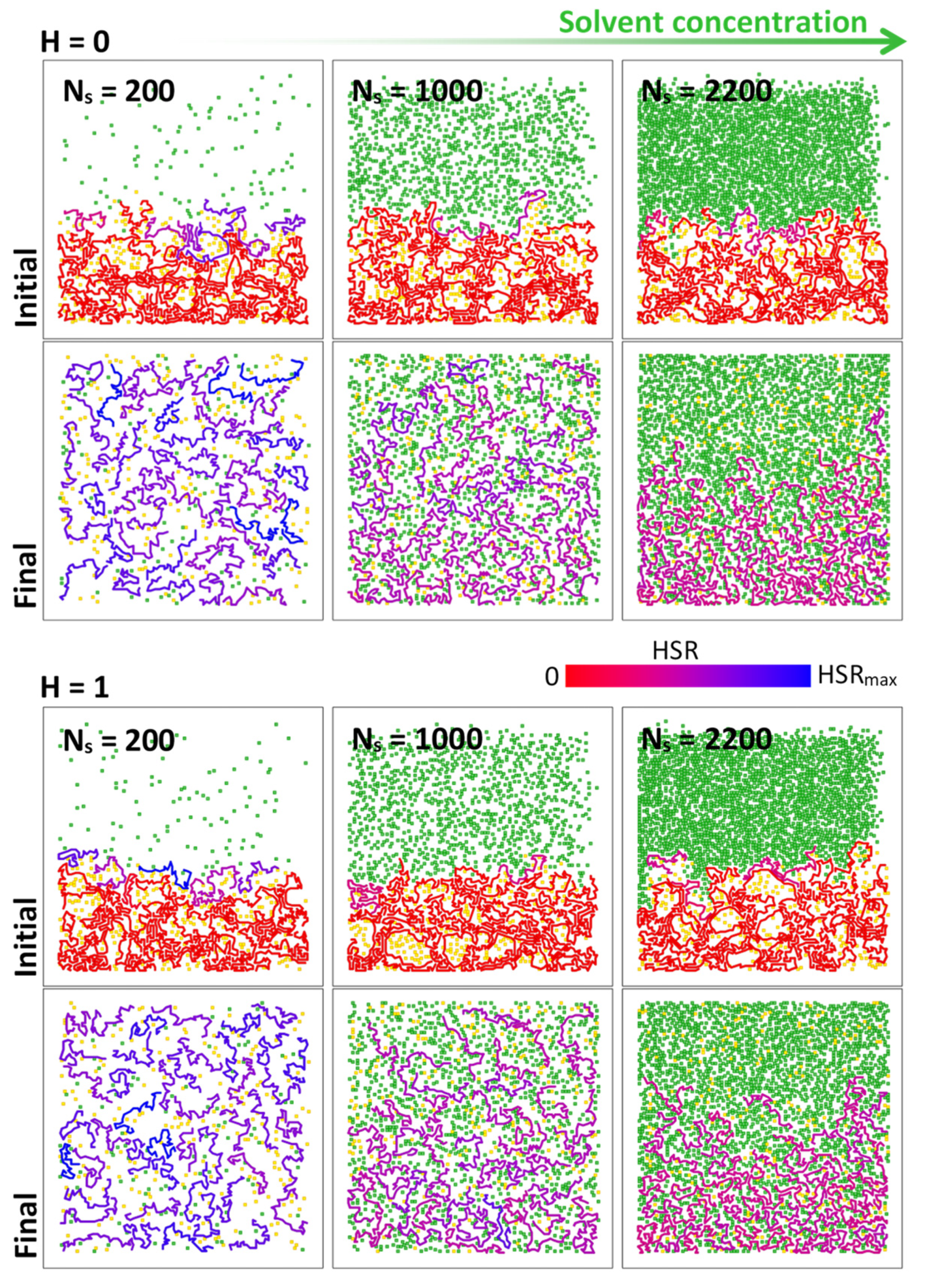
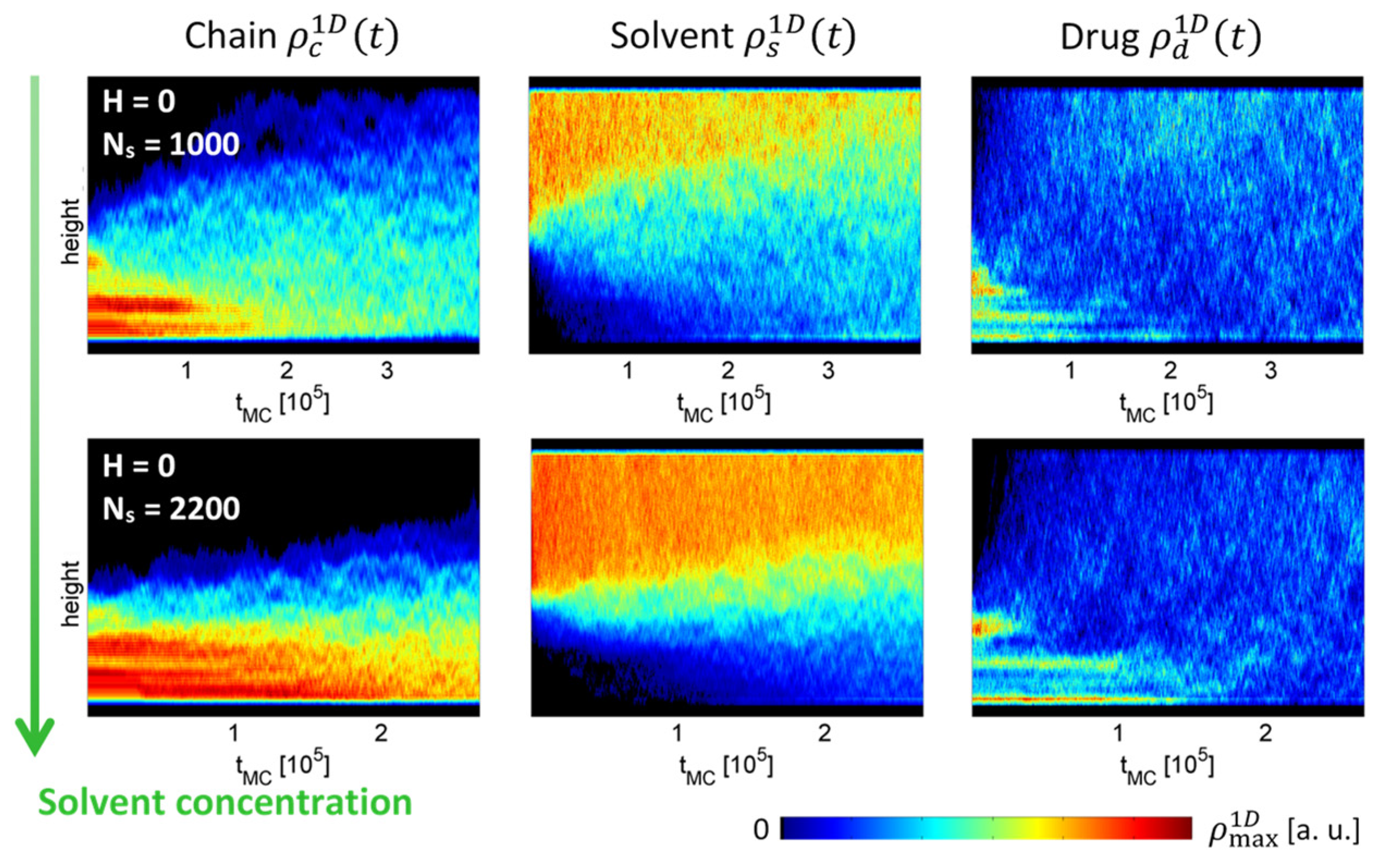
2.2. Numerical Simulations: Bond Fluctuation Model (BFM)
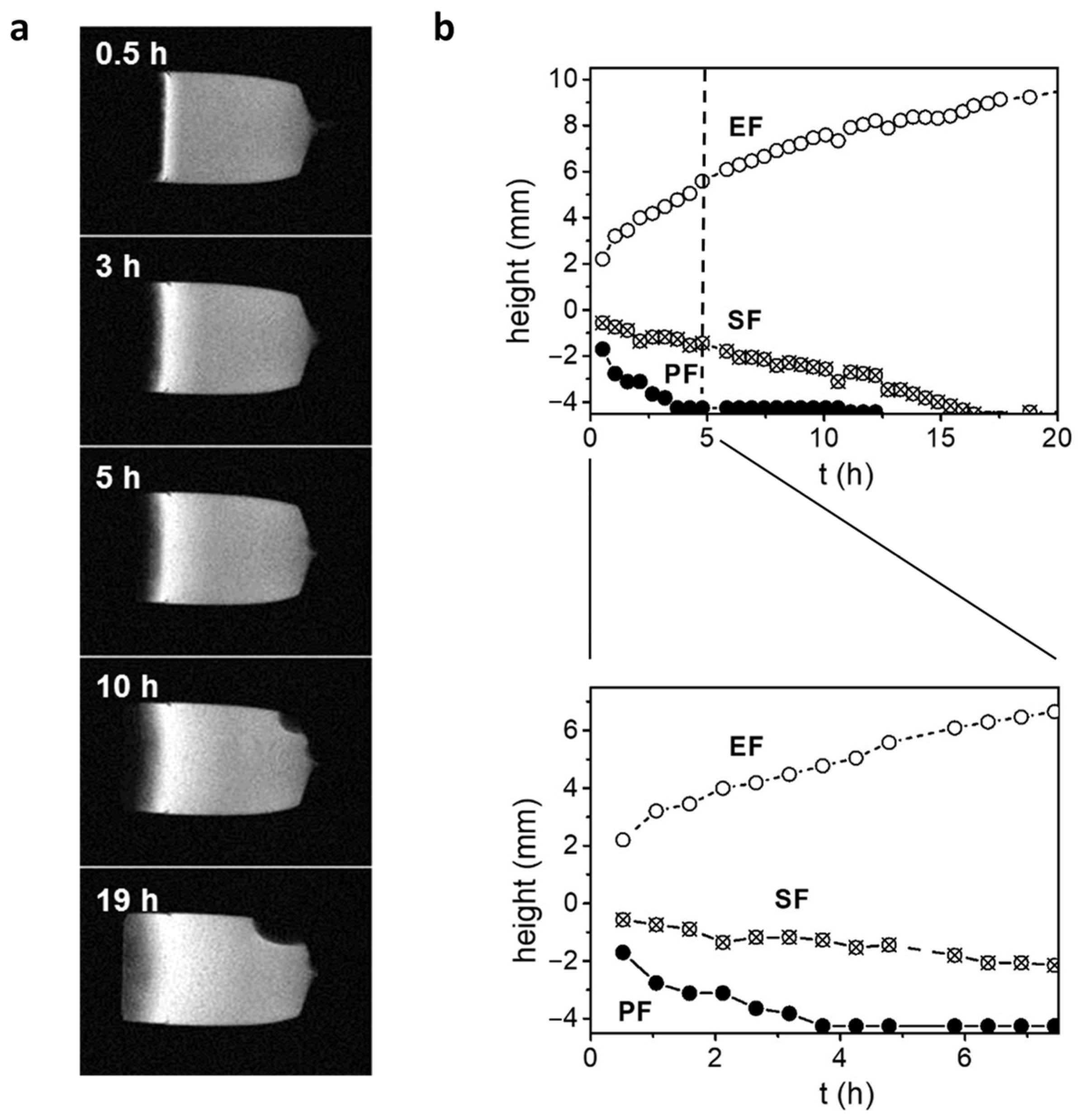
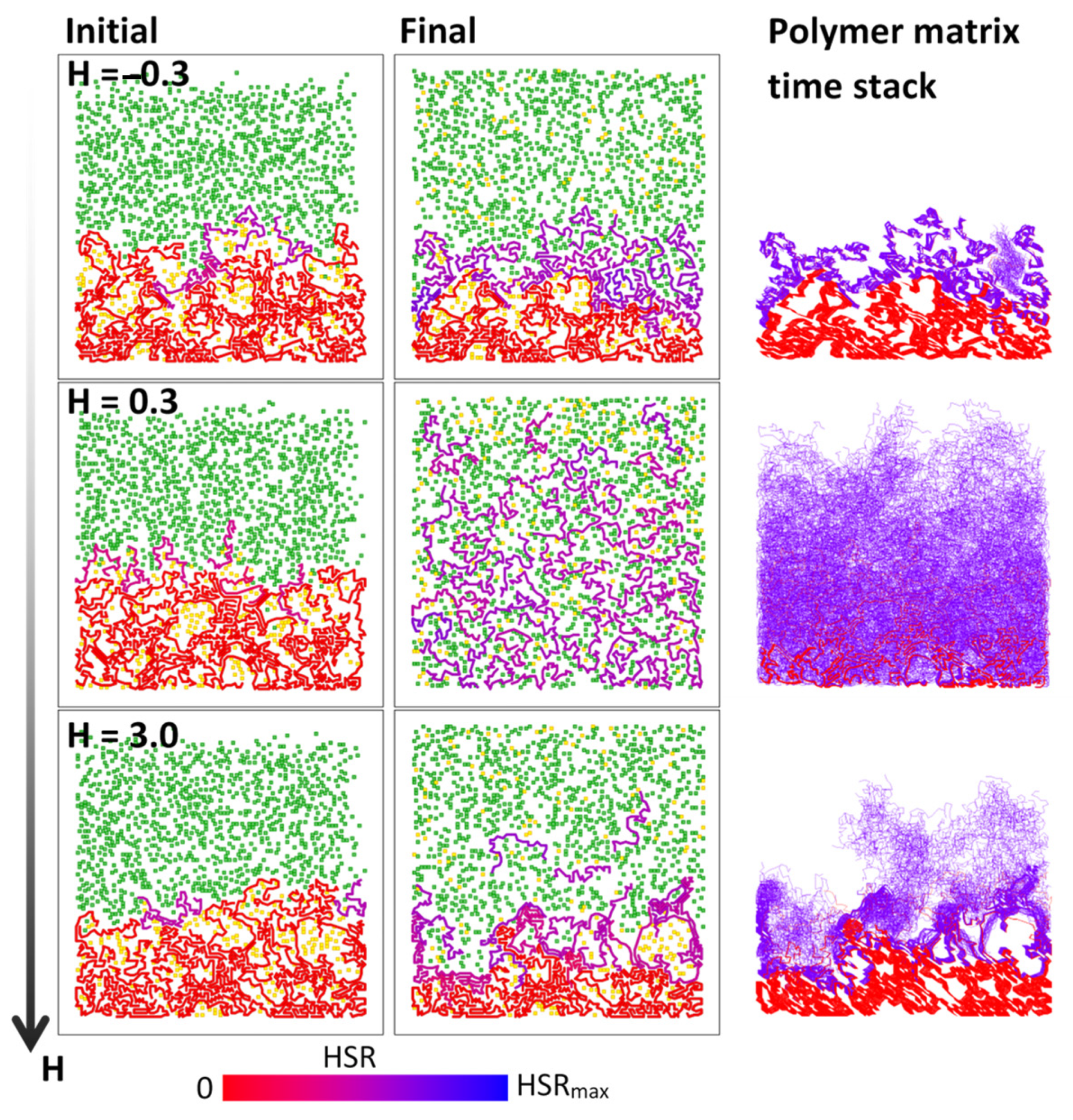
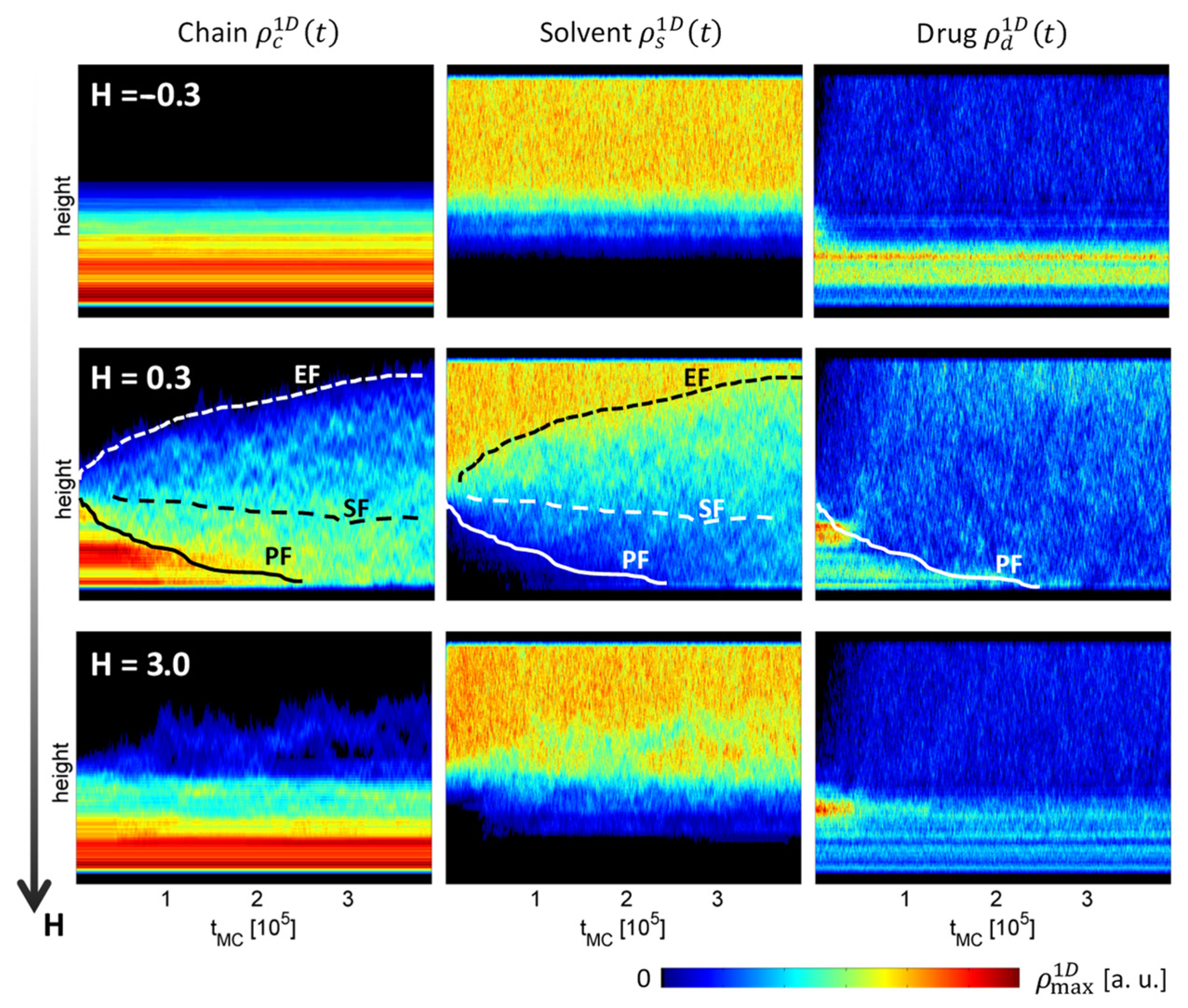
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nokhodchi, A.; Raja, S.; Patel, P.; Asare-Addo, K. The role of oral controlled release matrix tablets in drug delivery systems. BioImpacts BI 2012, 2, 175–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harland, R.S.; Gazzaniga, A.; Sangalli, M.E.; Colombo, P.; Peppas, N.A. Drug/polymer matrix swelling and dissolution. Pharm. Res. 1988, 5, 488–494. [Google Scholar] [CrossRef]
- Kumar, A.; Deepak; Sharma, S.; Srivastava, A.; Kumar, R. Synthesis of xanthan gum graft copolymer and its application for controlled release of highly water soluble Levofloxacin drug in aqueous medium. Carbohydr. Polym. 2017, 171, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Petri, D.F. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulou, E.; Kim, C.S.; Lee, S.S.; Chen, Z.; Facchetti, A.; Toney, M.F.; Loo, Y.-L. Tuning the morphology of all-polymer OPVs through altering polymer–solvent interactions. Chem. Mater. 2014, 26, 5020–5027. [Google Scholar] [CrossRef]
- Carbinatto, F.M.; de Castro, A.D.; Evangelista, R.C.; Cury, B.S.F. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian J. Pharm. Sci. 2014, 9, 27–34. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Santi, P.; Peppas, N.A. Swellable matrices for controlled drug delivery: Gel-layer behaviour, mechanisms and optimal performance. Pharm. Sci. Technol. Today 2000, 3, 198–204. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Guo, Z.; Wu, L.; Chen, F.; de Matas, M.; Shao, Q.; Xiao, T.; York, P.; He, Y.; et al. Quantification of swelling and erosion in the controlled release of a poorly water-soluble drug using synchrotron X-ray computed microtomography. AAPS J. 2013, 15, 1025–1034. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L. Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochim. Biophys. Acta 2006, 1758, 858–867. [Google Scholar] [CrossRef]
- Wray, P.S.; Clarke, G.S.; Kazarian, S.G. Application of FTIR spectroscopic imaging to study the effects of modifying the pH microenvironment on the dissolution of ibuprofen from HPMC matrices. J. Pharm. Sci. 2011, 100, 4745–4755. [Google Scholar] [CrossRef]
- Wray, P.S.; Clarke, G.S.; Kazarian, S.G. Dissolution of tablet-in-tablet formulations studied with ATR-FTIR spectroscopic imaging. Eur. J. Pharm. Sci. 2013, 48, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Kimber, J.A.; Kazarian, S.G.; Štěpánek, F. Microstructure-based mathematical modelling and spectroscopic imaging of tablet dissolution. Comput. Chem. Eng. 2011, 35, 1328–1339. [Google Scholar] [CrossRef]
- Kulinowski, P.; Mlynarczyk, A.; Jasinski, K.; Talik, P.; Gruwel, M.L.; Tomanek, B.; Weglarz, W.P.; Dorozynski, P. Magnetic resonance microscopy for assessment of morphological changes in hydrating hydroxypropylmethylcellulose matrix tablets in situ-is it possible to detect phenomena related to drug dissolution within the hydrated matrices? Pharm. Res. 2014, 31, 2383–2392. [Google Scholar] [CrossRef]
- Mikac, U.; Sepe, A.; Kristl, J.; Baumgartner, S. A new approach combining different MRI methods to provide detailed view on swelling dynamics of xanthan tablets influencing drug release at different pH and ionic strength. J. Control. Release 2010, 145, 247–256. [Google Scholar] [CrossRef]
- Nott, K.P. Magnetic resonance imaging of tablet dissolution. Eur. J. Pharm. Biopharm. 2010, 74, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Kulinowski, P.; Hudy, W.; Mendyk, A.; Juszczyk, E.; Węglarz, W.P.; Jachowicz, R.; Dorożyński, P. The relationship between the evolution of an internal structure and drug dissolution from controlled-release matrix tablets. AAPS PharmSciTech 2016, 17, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Göpferich, A. Polymer bulk erosion. Macromolecules 1997, 30, 2598–2604. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Li, Q. Mathematical modeling of degradation for bulk-erosive polymers: Applications in tissue engineering scaffolds and drug delivery systems. Acta Biomater. 2011, 7, 1140–1149. [Google Scholar] [CrossRef]
- Grassi, M.; Grassi, G. Mathematical modelling and controlled drug delivery: Matrix systems. Curr. Drug Deliv. 2005, 2, 97–116. [Google Scholar] [CrossRef]
- Lao, L.L.; Peppas, N.A.; Boey, F.Y.; Venkatraman, S.S. Modeling of drug release from bulk-degrading polymers. Int. J. Pharm. 2011, 418, 28–41. [Google Scholar] [CrossRef] [PubMed]
- von Burkersroda, F.; Schedl, L.; Gopferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef]
- Ariane, M.; Allouche, M.H.; Bussone, M.; Giacosa, F.; Bernard, F.; Barigou, M.; Alexiadis, A. Discrete multi-physics: A mesh-free model of blood flow in flexible biological valve including solid aggregate formation. PLoS ONE 2017, 12, e0174795. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Y.; Hentschke, R. Computer simulation study on the swelling of a model polymer network by a chainlike solvent. Phys. Rev. E 2002, 65, 041807. [Google Scholar] [CrossRef]
- Frenning, G.; Brohede, U.; Stromme, M. Finite element analysis of the release of slowly dissolving drugs from cylindrical matrix systems. J. Control. Release 2005, 107, 320–329. [Google Scholar] [CrossRef]
- Carmesin, I.; Kremer, K. The bond fluctuation method: A new effective algorithm for the dynamics of polymers in all spatial dimensions. Macromolecules 1988, 21, 2819–2823. [Google Scholar] [CrossRef]
- Landau, D.P.; Binder, K. A Guide to Monte Carlo Simulations in Statistical Physics; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005; p. 449. [Google Scholar]
- Sommer, J.-U.; Werner, M.; Baulin, V.A. Critical adsorption controls translocation of polymer chains through lipid bilayers and permeation of solvent. Europhys. Lett. 2012, 98, 18003. [Google Scholar] [CrossRef][Green Version]
- Rabbel, H.; Werner, M.; Sommer, J.-U. Interactions of amphiphilic triblock copolymers with lipid membranes: Modes of interaction and effect on permeability examined by generic Monte Carlo simulations. Macromolecules 2015, 48, 4724–4732. [Google Scholar] [CrossRef]
- Li, H.; Qian, C.-J.; Wang, C.; Luo, M.-B. Critical adsorption of a flexible polymer confined between two parallel interacting surfaces. Phys. Rev. E 2013, 87, 012602. [Google Scholar] [CrossRef]
- Wengenmayr, M.; Dockhorn, R.; Sommer, J.-U. Multicore unimolecular structure formation in single dendritic–linear copolymers under selective solvent conditions. Macromolecules 2016, 49, 9215–9227. [Google Scholar] [CrossRef]
- Bajd, F.; Sersa, I. A Bond-Fluctuation Model of Translational Dynamics of Chain-like Particles through Mucosal Scaffolds. Biophys. J. 2018, 114, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.A. Simulating the co-encapsulation of drugs in a “smart” core-shell-shell polymer nanoparticle. Eur. Phys. J. E 2014, 37, 14. [Google Scholar] [CrossRef] [PubMed]
- Panja, D.; Barkema, G.T. Simulations of two-dimensional unbiased polymer translocation using the bond fluctuation model. J. Chem. Phys. 2010, 132, 014902. [Google Scholar] [CrossRef] [PubMed]
- Hastings, W.K. Monte Carlo sampling methods using Markov chains and their applications. Biometrika 1970, 57, 97–109. [Google Scholar] [CrossRef]
- Liao, Q.; Carrillo, J.-M.Y.; Dobrynin, A.V.; Rubinstein, M. Rouse dynamics of polyelectrolyte solutions: molecular dynamics study. Macromolecules 2007, 40, 7671–7679. [Google Scholar] [CrossRef]
- Rissanou, A.N.; Harmandaris, V. Dynamics of various polymer–graphene interfacial systems through atomistic molecular dynamics simulations. Soft Matter 2014, 10, 2876–2888. [Google Scholar] [CrossRef]
- Skačej, G.; Zannoni, C. Molecular simulations shed light on supersoft elasticity in polydomain liquid crystal elastomers. Macromolecules 2014, 47, 8824–8832. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Modeling of drug release from swellable polymers. Eur. J. Pharm. Biopharm. 2000, 49, 47–58. [Google Scholar] [CrossRef]
- Everaers, R.; Sukumaran, S.K.; Grest, G.S.; Svaneborg, C.; Sivasubramanian, A.; Kremer, K. Rheology and microscopic topology of entangled polymeric liquids. Science 2004, 303, 823–826. [Google Scholar] [CrossRef]
- Lappala, A.; Terentjev, E.M. “Raindrop” Coalescence of Polymer Chains during Coil−Globule Transition. Macromolecules 2013, 46, 1239–1247. [Google Scholar] [CrossRef]
- Pawlik, G.; Mitus, A.C. Complex Monte Carlo Light-Driven Dynamics of Monomers in Functionalized Bond Fluctuation Model Polymer Chains. Materials 2023, 16, 4373. [Google Scholar] [CrossRef]
- Kipcak, A.S.; Ismail, O.; Doymaz, I.; Piskin, S. Modeling and Investigation of the Swelling Kinetics of Acrylamide-Sodium Acrylate Hydrogel. J. Chem. 2014, 2014, 281063. [Google Scholar] [CrossRef]
- Mencer, H.J.; Gomzi, Z. Swelling Kinetics of Polymer-Solvent Systems. Eur. Polym. J. 1994, 30, 33–36. [Google Scholar] [CrossRef]
- Mezhuev, Y.O.; Varankin, A.V.; Luss, A.L.; Dyatlov, V.A.; Tsatsakis, A.M.; Shtilman, M.I.; Korshak, Y.V. Immobilization of dopamine on the copolymer of N-vinyl-2-pyrrolidone and allyl glycidyl ether and synthesis of new hydrogels. Polym. Int. 2020, 69, 1275–1282. [Google Scholar] [CrossRef]
- Finch, C.A. Chemistry and Technology of Water-Soluble Polymers; Springer: New York, NY, USA, 1983. [Google Scholar]
- Desai, D.; Wong, B.; Huang, Y.; Tang, D.; Hemenway, J.; Paruchuri, S.; Guo, H.; Hsieh, D.; Timmins, P. Influence of dissolution media pH and USP1 basket speed on erosion and disintegration characteristics of immediate release metformin hydrochloride tablets. Pharm. Dev. Technol. 2015, 20, 540–545. [Google Scholar] [CrossRef]
- Mikac, U.; Sepe, A.; Baumgartner, S.; Kristl, J. The Influence of High Drug Loading in Xanthan Tablets and Media with Different Physiological pH and Ionic Strength on Swelling and Release. Mol. Pharm. 2016, 13, 1147–1157. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajd, F.; Mikac, U.; Mohorič, A.; Serša, I. The Effect of Polymer–Solvent Interaction on the Swelling of Polymer Matrix Tablets: A Magnetic Resonance Microscopy Study Complemented by Bond Fluctuation Model Simulations. Polymers 2024, 16, 601. https://doi.org/10.3390/polym16050601
Bajd F, Mikac U, Mohorič A, Serša I. The Effect of Polymer–Solvent Interaction on the Swelling of Polymer Matrix Tablets: A Magnetic Resonance Microscopy Study Complemented by Bond Fluctuation Model Simulations. Polymers. 2024; 16(5):601. https://doi.org/10.3390/polym16050601
Chicago/Turabian StyleBajd, Franci, Urša Mikac, Aleš Mohorič, and Igor Serša. 2024. "The Effect of Polymer–Solvent Interaction on the Swelling of Polymer Matrix Tablets: A Magnetic Resonance Microscopy Study Complemented by Bond Fluctuation Model Simulations" Polymers 16, no. 5: 601. https://doi.org/10.3390/polym16050601
APA StyleBajd, F., Mikac, U., Mohorič, A., & Serša, I. (2024). The Effect of Polymer–Solvent Interaction on the Swelling of Polymer Matrix Tablets: A Magnetic Resonance Microscopy Study Complemented by Bond Fluctuation Model Simulations. Polymers, 16(5), 601. https://doi.org/10.3390/polym16050601






