Effect of Different Mouthwashes on the Surface Microhardness and Color Stability of Dental Nanohybrid Resin Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Specimen Grouping
- Group 1: specimens immersed in artificial saliva (control).
- Group 2: specimens immersed in CHX mouthwash.
- Group 3: specimens immersed in Green Tea mouthwash.
- Group 4: specimens immersed in Colgate Optic White mouthwash.
2.3. Microhardness Test
2.4. Color Stability
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paolone, G. Direct Composites in Anteriors: A Matter of Substrate. Int. J. Esthet. Dent. 2017, 2, 2355. [Google Scholar]
- Mansurov, Z.A. Recent Achievements and Future Challenges in Nanoscience and Nanotechnology. Eurasian Chem. J. 2020, 22, 241–253. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Mousa, S.M.A.; Sherief, M.A. Effect of Incorporation of Lanthanum and Cerium-Doped Hydroxyapatite on Acrylic Bone Cement Produced from Phosphogypsum Waste. Egypt. J. Chem. 2020, 63, 1823–1832. [Google Scholar] [CrossRef]
- Hong, G.; Yang, J.; Jin, X.; Wu, T.; Dai, S.; Xie, H.; Chen, C. Mechanical Properties of Nanohybrid Resin Composites Containing Various Mass Fractions of Modified Zirconia Particles. Int. J. Nanomed. 2020, 15, 9891–9907. [Google Scholar] [CrossRef] [PubMed]
- Elkassas, D.; Arafa, A. The Innovative Applications of Therapeutic Nanostructures in Dentistry. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1543–1562. [Google Scholar] [CrossRef]
- Sonnahalli, N.; Chowdhary, R. Effect of Nanoparticles on Color Stability and Mechanical and Biological Properties of Maxillofacial Silicone Elastomer: A Systematic Review. J. Indian Prosthodont. Soc. 2020, 20, 244–254. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Saniour, S.H.; Sherief, M.A.; Zaki, D.Y. Effect of Incorporation of 20 Wt% Amorphous Nano-Hydroxyapatite Fillers in Poly Methyl Methacrylate Composite on the Compressive Strength. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 9399. [Google Scholar] [CrossRef]
- Çelik, Ç.; Arhun, N.; Yamanel, K. Clinical Evaluation of Resin-Based Composites in Posterior Restorations: A 3-Year Study. Med. Princ. Pract. 2014, 23, 453–459. [Google Scholar] [CrossRef]
- Heintze, S.D.; Loguercio, A.D.; Hanzen, T.A.; Reis, A.; Rousson, V. Clinical Efficacy of Resin-Based Direct Posterior Restorations and Glass-Ionomer Restorations—An Updated Meta-Analysis of Clinical Outcome Parameters. Dent. Mater. 2022, 38, e109–e135. [Google Scholar] [CrossRef]
- Szalewski, L.; Wójcik, D.; Sofińska-Chmiel, W.; Kuśmierz, M.; Różyło-Kalinowska, I. How the Duration and Mode of Photopolymerization Affect the Mechanical Properties of a Dental Composite Resin. Materials 2023, 16, 113. [Google Scholar] [CrossRef]
- Hamdy, T.M.; El-Korashy, S.A. Novel Bioactive Zinc Phosphate Dental Cement with Low Irritation and Enhanced Microhardness. e-J. Surf. Sci. Nanotechnol. 2018, 16, 431–435. [Google Scholar] [CrossRef]
- Chinelatti, M.A.; Chimello, D.T.; Ramos, R.P.; Palma-Dibb, R.G. Evaluation of the Surface Hardness of Composite Resins before and after Polishing at Different Times. J. Appl. Oral Sci. 2006, 14, 188–192. [Google Scholar] [CrossRef]
- Qaiser, S.; Devadiga, D.; Hegde, M.N. Esthetic Rehabilitation With Nanohybrid Composite: Case Report Series. J. Adv. Oral Res. 2020, 11, 251–256. [Google Scholar] [CrossRef]
- De Morais Sampaio, G.A.; Rangel Peixoto, L.; de Vasconcelos Neves, G.; do Nascimento Barbosa, D. Effect of Mouthwashes on Color Stability of Composite Resins: A Systematic Review. J. Prosthet. Dent. 2021, 126, 386–392. [Google Scholar] [CrossRef]
- Paolone, G.; Formiga, S.; De Palma, F.; Abbruzzese, L.; Chirico, L.; Scolavino, S.; Goracci, C.; Cantatore, G.; Vichi, A. Color Stability of Resin-Based Composites: Staining Procedures with Liquids—A Narrative Review. J. Esthet. Restor. Dent. 2022, 34, 865–887. [Google Scholar] [CrossRef]
- Paolone, G.; Pavan, F.; Mandurino, M.; Baldani, S.; Guglielmi, P.C.; Scotti, N.; Cantatore, G.; Vichi, A. Color Stability of Resin-Based Composites Exposed to Smoke. A Systematic Review. J. Esthet. Restor. Dent. 2023, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ardu, S.; Duc, O.; Di Bella, E.; Krejci, I.; Daher, R. Color Stability of Different Composite Resins after Polishing. Odontology 2018, 106, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Nasoohi, N.; Hadian, M.; Hoorizad, M.; Hashemi, S.; Naziri saeed, S. In-Vitro Effect of Alcohol and Non-Alcohol Mouthwash on Color Change of Two Types of Bleach Shade Composite. J. Res. Dent. Maxillofac. Sci. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Kepler, L.C.; Rodrigues, A.P.M.; Agnol, M.A.D.; Rodrigues-Junior, S.A. Effect of Whitening Mouth Rinses on the Chemical and Physical Properties of a Nanofilled Composite. Braz. J. Oral Sci. 2021, 20, 1–11. [Google Scholar] [CrossRef]
- Al-Samadani, K.H. Surface Hardness of Dental Composite Resin Restorations in Response to Preventive Agents. J. Contemp. Dent. Pract. 2016, 17, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, N.B.; Arikan, V.; Akbay Oba, A. Effect of Mouthwashes on the Discolouration of Restorative Materials Commonly Used in Paediatric Dentistry. Eur. Arch. Paediatr. Dent. 2018, 19, 147–153. [Google Scholar] [CrossRef]
- de Moraes Porto, I.C.; das Neves, L.E.; de Souza, C.K.; Parolia, A.; Barbosa dos Santos, N. A Comparative Effect of Mouthwashes with Different Alcohol Concentrations on Surface Hardness, Sorption and Solubility of Composite Resins. Oral Health Dent. Manag. 2014, 13, 502–506. [Google Scholar] [PubMed]
- Vasudevan, K.; Stahl, V. Cannabinoids Infused Mouthwash Products Are as Effective as Chlorhexidine on Inhibition of Total-Culturable Bacterial Content in Dental Plaque Samples. J. Cannabis Res. 2020, 2, 27. [Google Scholar] [CrossRef]
- Kaur, S.; Kour, K. Short Term Side Effects of 0.2% and 0.12% Chlorhexidine Mouthwash. IP Int. J. Periodontol. Implantol. 2020, 4, 138–140. [Google Scholar] [CrossRef]
- Singh, O.; Reddy, V.; Pradhan, D.; Sharma, L. Comparative Evaluation of Green Tea and Chlorhexidine Mouthwashes on Gingivitis: A Randomized Controlled Trial. J. Indian Assoc. Public Health Dent. 2019, 17, 269. [Google Scholar] [CrossRef]
- Barouti, P. Effect of Green Tea Mouthwash on Reducing Plaque and Gingivitis. J. Dent. Heal. Oral Disord. Ther. 2018, 9, 360. [Google Scholar] [CrossRef]
- Asdagh, S.; Daneshpooy, M.; Rahbar, M.; Dabaghi-Tabriz, F.; Bahramian, A.; Esmaielzadeh, M. Effect of Home Bleaching on the Color Matching of Composite Resin Restorations. Pesqui. Bras. Odontopediatria Clin. Integr. 2018, 18. [Google Scholar] [CrossRef]
- Kurtulmus-Yilmaz, S.; Cengiz, E.; Ulusoy, N.; Ozak, S.T.; Yuksel, E. The Effect of Home-Bleaching Application on the Color and Translucency of Five Resin Composites. J. Dent. 2013, 41, 7. [Google Scholar] [CrossRef]
- García-Contreras, R.; Scougall-Vilchis, R.; Acosta-Torres, L.; Arenas-Arrocena, C.; García-Garduño, R.; de la Fuente-Hernández, J. Vickers Microhardness Comparison of 4 Composite Resins with Different Types of Filler. J. Oral Res. 2015, 4, 313–320. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Abdelraouf, R.M.; Habib, N.A. Effect of Two Artificial Aging Protocols on Color and Gloss of Single-Shade versus Multi-Shade Resin Composites. BMC Oral Health 2022, 22, 321. [Google Scholar] [CrossRef]
- Comba, A.; Scotti, N.; Maravić, T.; Mazzoni, A.; Carossa, M.; Breschi, L.; Cadenaro, M. Vickers Hardness and Shrinkage Stress Evaluation of Low and High Viscosity Bulk-Fill Resin Composite. Polymers 2020, 12, 1477. [Google Scholar] [CrossRef]
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 Color-Difference Formula: Implementation Notes, Supplementary Test Data, and Mathematical Observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]
- Paravina, R.D.; Pérez, M.M.; Ghinea, R. Acceptability and Perceptibility Thresholds in Dentistry: A Comprehensive Review of Clinical and Research Applications. J. Esthet. Restor. Dent. 2019, 28, 2992. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric Materials and Films in Dentistry: An Overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef]
- Abdelraouf, R.M. Chemical Analysis and Microstructure Examination of Extended-Pour Alginate Impression versus Conventional One (Characterization of Dental Extended-Pour Alginate). Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 612–618. [Google Scholar] [CrossRef]
- Abdelraouf, R.M.; Bayoumi, R.E.; Hamdy, T.M. Effect of Powder/Water Ratio Variation on Viscosity, Tear Strength and Detail Reproduction of Dental Alginate Impression Material (In Vitro and Clinical Study). Polymers 2021, 13, 2923. [Google Scholar] [CrossRef]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- Abdelraouf, R.M.; Bayoumi, R.E.; Hamdy, T.M. Influence of Incorporating 5% Weight Titanium Oxide Nanoparticles on Flexural Strength, Micro-Hardness, Surface Roughness and Water Sorption of Dental Self-Cured Acrylic Resin. Polymers 2022, 14, 3767. [Google Scholar] [CrossRef]
- Hamdy, T.M. Polymers and Ceramics Biomaterials in Orthopedics and Dentistry: A Review Article. Egypt. J. Chem. 2018, 61, 723–730. [Google Scholar] [CrossRef]
- Abdelraouf, R.M.; Mohammed, M.; Abdelgawad, F. Evaluation of Shear-Bond-Strength of Dental Self-Adhering Flowable Resin-Composite versus Total-Etch One to Enamel and Dentin Surfaces: An in-Vitro Study. Open Access Maced. J. Med. Sci. 2019, 7, 2162–2166. [Google Scholar] [CrossRef]
- Tamer, M. Hamdy Interfacial Microscopic Examination and Chemical Analysis of Resin-Dentin Interface of Self-Adhering Flowable Resin Composite. F1000Research 2017, 8, 214. [Google Scholar] [CrossRef]
- Hamdy, T.M. Polymerization Shrinkage in Contemporary Resin-Based Dental Composites: A Review Article. Egypt. J. Chem. 2021, 64, 3087–3092. [Google Scholar] [CrossRef]
- James, V.; Balagopal, S.I.V.; Varghese, S.C.J.; Veign, G.; Valogol, M. The evaluation of the effect of various mouth rinse on the discoloration of different composite resins. J. Biomed. Pharm. Res. 2019, 8, 648. [Google Scholar] [CrossRef]
- Bozoğullari, H.N.; Çitir Yücel, H. Effect of Mouthwashes on Color Stability of Artificial Gingival Materials Used in Implant Retained Hybrid Prostheses. Selcuk Dent. J. 2021, 3, 2982. [Google Scholar] [CrossRef]
- Lopes, R.G.; Oliveira-Reis, B.; Maluly-Proni, A.T.; Silva, M.H.T.; Briso, A.L.F.; dos Santos, P.H. Influence of Green Tea Extract in the Color of Composite Resin Restorations. J. Mech. Behav. Biomed. Mater. 2019, 100, 408. [Google Scholar] [CrossRef] [PubMed]
- Al-Samadani, K.H. The Effect of Preventive Agents (Mouthwashes/Gels) on the Color Stability of Dental Resin-Based Composite Materials. Dent. J. 2017, 5, 18. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary PH: A Diagnostic Biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.T.; Sedarousy, M.; Hiltz, G.S. The PH of Tooth-Whitening Products. J. Can. Dent. Assoc. 2000, 66, 421–426. [Google Scholar]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.Y. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef]
- Mohammadi, Z. Chlorhexidine Gluconate, Its Properties and Applications in Endodontics. Iran. Endod. J. 2008, 2, 113–125. [Google Scholar]
- Abdelnabi, A.; Hamza, N.K.; El-Borady, O.M.; Hamdy, T.M. Effect of Different Formulations and Application Methods of Coral Calcium on Its Remineralization Ability on Carious Enamel. Open Access Maced. J. Med. Sci. 2020, 8, 94–99. [Google Scholar] [CrossRef]
- Meurman, J.H.; ten Cate, J.M. Pathogenesis and Modifying Factors of Dental Erosion. Eur. J. Oral Sci. 1996, 104, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Poppolo Deus, F.; Ouanounou, A. Mouthwashes and Their Use in Dentistry: A Review. Oral Health 2021, 1, 22–34. [Google Scholar]
- Pontefract, H.; Hughes, J.; Kemp, K.; Yates, R.; Newcombe, R.G.; Addy, M. The Erosive Effects of Some Mouthrinses on Enamel: A Study in Situ. J. Clin. Periodontol. 2001, 28, 319–324. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–Remineralization Dynamics in Teeth and Bone. Int. J. Nanomedicine 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Donovan, T.; Nguyen-Ngoc, C.; Abd Alraheam, I.; Irusa, K. Contemporary Diagnosis and Management of Dental Erosion. J. Esthet. Restor. Dent. 2021, 33, 78–87. [Google Scholar] [CrossRef]
- Ramírez-Vargas, G.G.; Mendoza, J.E.M.Y.; Aliaga-Mariñas, A.S.; Ladera-Castañeda, M.I.; Cervantes-Ganoza, L.A.; Cayo-Rojas, C.F. Effect of Polishing on the Surface Microhardness of Nanohybrid Composite Resins Subjected to 35% Hydrogen Peroxide: An in Vitro Study. J. Int. Soc. Prev. Community Dent. 2021, 11, 216–221. [Google Scholar] [CrossRef]
- Münchow, E.A.; Ferreira, A.C.A.; Machado, R.M.M.; Ramos, T.S.; Rodrigues-Junior, S.A.; Zanchi, C.H. Effect of Acidic Solutions on the Surface Degradat Ion of a Micro-Hybrid Composite Resin. Braz. Dent. J. 2014, 25, 321–326. [Google Scholar] [CrossRef]
- Szalewski, L.; Wójcik, D.; Bogucki, M.; Szkutnik, J.; Różyło-Kalinowska, I. The Influence of Popular Beverages on Mechanical Properties of Composite Resins. Materials 2021, 14, 3097. [Google Scholar] [CrossRef]
- Rahman, K.N.; Damiyanti, M.; Irawan, B.; Noerdin, A. Effect of Green Tea (Camellia Sinensis) Solution on Color Change of Silorane- and Methacrylate-Based Composite Resins. J. Phys. Conf. Ser. 2018, 1, 1073. [Google Scholar] [CrossRef]
- Kusmita, L.; Puspitaningrum, I.; Limantara, L. Identification, Isolation and Antioxidant Activity of Pheophytin from Green Tea (Camellia Sinensis (L.) Kuntze). Procedia Chem. 2015, 14, 232–238. [Google Scholar] [CrossRef]
- Baig, A.R.; Shori, D.D.; Shenoi, P.R.; Ali, S.N.; Shetti, S.; Godhane, A. Mouthrinses Affect Color Stability of Composite. J. Conserv. Dent. 2016, 19, 355–359. [Google Scholar] [CrossRef]
- Bagis, B.; Baltacioglu, E.; Özcan, M.; Ustaomer, S. Evaluation of Chlorhexidine Gluconate Mouthrinse-Induced Staining Using a Digital Colorimeter: An In Vivo Study; Quintessence Int.: New Malden, UK, 2011; Volume 42. [Google Scholar]
- Guerra, E.; Gosetti, F.; Marengo, E.; Llompart, M.; Garcia-Jares, C. Study of Photostability of Three Synthetic Dyes Commonly Used in Mouthwashes. Microchem. J. 2019, 146, 776–781. [Google Scholar] [CrossRef]
- Torres, C.R.G.; Crastechini, E.; Feitosa, F.A.; Pucci, C.R.; Borges, A.B. Influence of PH on the Effectiveness of Hydrogen Peroxide Whitening. Oper. Dent. 2014, 39, E261–E268. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, J.Y.T.; Sato, F.; Bianchi, G.; Baesso, M.L.; Santana, R.G.; Pascotto, R.C. Color Stability over Time of Three Resin-Based Restorative Materials Stored Dry and in Artificial Saliva. J. Esthet. Restor. Dent. 2014, 26, 279–287. [Google Scholar] [CrossRef] [PubMed]

| Mouthwashes | Manufacturer | Composition |
|---|---|---|
| Artificial Saliva | Sigma, Sigma-Aldrich CO., Missouri City, TX, USA | Potassium chloride, sodium bicarbonate, sodium phosphate, potassium thiocyanate, and lactic acid |
| Oradex antibacterial mouthwash | Cavico Sdn Bhd., Selangor, Malaysia | Chlorhexidine gluconate (0.12%) |
| LISTERINE® Green Tea | Johnson and Johnson S.p.A, Rome, Italy | Aqua, Propylene Glycol, Sorbitol, poloxamer 407, Sodium Lauryl Sulfate, Sodium Saccharin, Aroma, Eucalyptol, Benzoid Acid, Sodium Benzoate, Methyl Salicylate, thymol, Sodium Fluoride, Menthol, Sucralose, Camellia Sinensis Leaf Extracts, Caffeine, CI 47005, CI 42053, contains sodium fluoride (220 ppm F) |
| Colgate Optic White, Whitening Mouthwash | Colgate-Palmolive Co., New York, NY, USA | Water, Glycerin, Propylene Glycol, Sorbitol, Hydrogen Peroxide, Polysorbate 20, Sodium Acrylates/Methacryloylethyl Phosphate Copolymer, Phosphoric Acid, Citric Acid, Flavor, PVM/MA Copolymer, Sodium Saccharin |
| Artificial Saliva | Chlorhexidine Mouthwash | Green Tea Mouthwash | Bleaching Mouthwash | p Value | |
|---|---|---|---|---|---|
| pH | 7 a ± 0.04 | 3.9 c ± 0.1 | 4.2 b ± 0.07 | 7.1 a ± 0.1 | p ≤ 0.006 * |
| Artificial Saliva | Chlorhexidine Mouthwash | Green Tea Mouthwash | Bleaching Mouthwash | p Value | |
|---|---|---|---|---|---|
| Microhardness of Resin Composite | 78.5 a ± 1.4 | 78.4 a ± 0.7 | 73.5 a ± 2.1 | 67.4 b ± 2.8 | p = 0.002 |
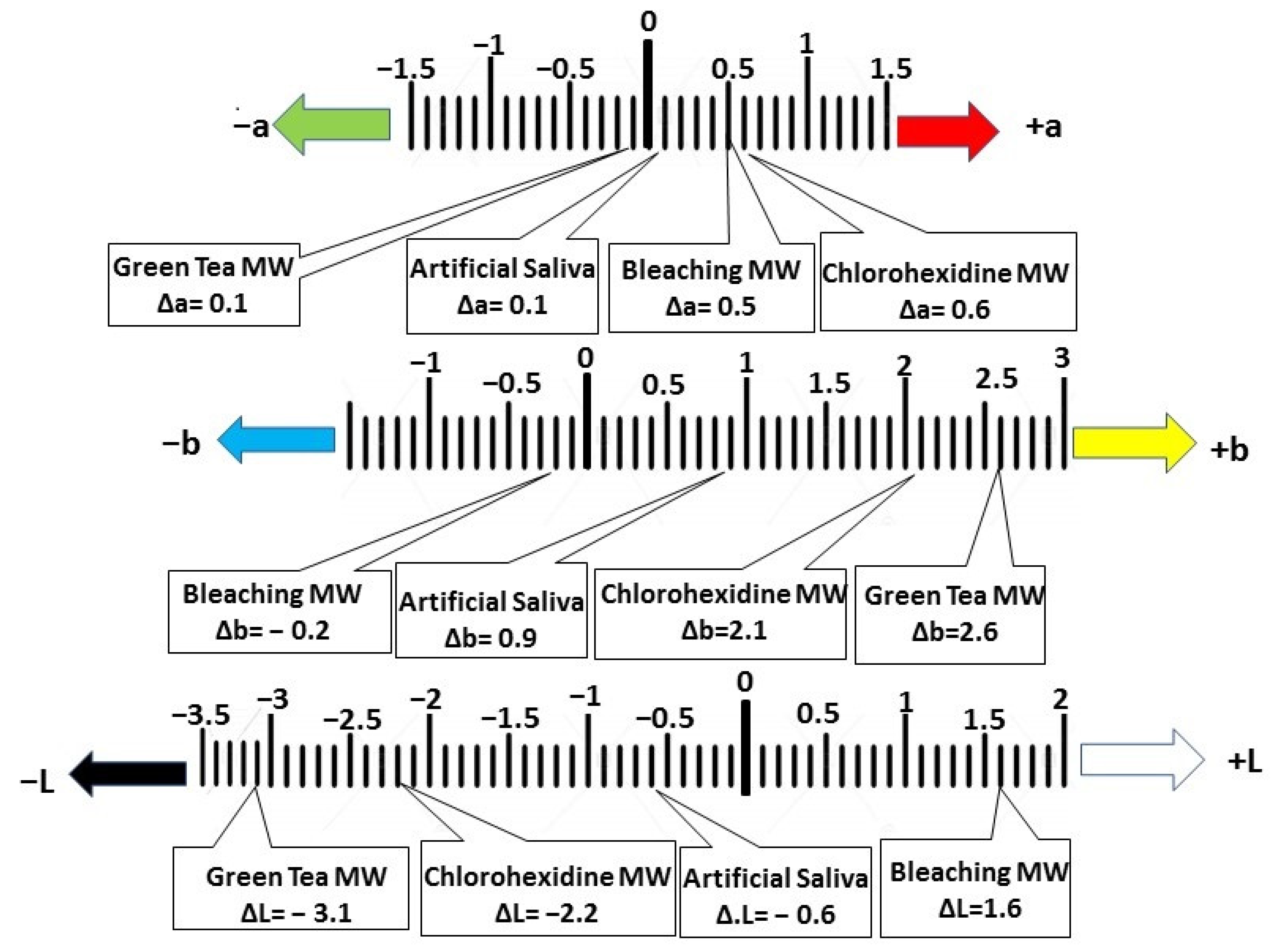
| Color Change (ΔE00) of Resin Composite | 0.9 a ± 0.04 | 2.6 c ± 0.08 | 3.2 d ± 0.1 | 1.6 b ± 0.07 | p = 0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdy, T.M.; Abdelnabi, A.; Othman, M.S.; Bayoumi, R.E.; Abdelraouf, R.M. Effect of Different Mouthwashes on the Surface Microhardness and Color Stability of Dental Nanohybrid Resin Composite. Polymers 2023, 15, 815. https://doi.org/10.3390/polym15040815
Hamdy TM, Abdelnabi A, Othman MS, Bayoumi RE, Abdelraouf RM. Effect of Different Mouthwashes on the Surface Microhardness and Color Stability of Dental Nanohybrid Resin Composite. Polymers. 2023; 15(4):815. https://doi.org/10.3390/polym15040815
Chicago/Turabian StyleHamdy, Tamer M., Ali Abdelnabi, Maha S. Othman, Rania E. Bayoumi, and Rasha M. Abdelraouf. 2023. "Effect of Different Mouthwashes on the Surface Microhardness and Color Stability of Dental Nanohybrid Resin Composite" Polymers 15, no. 4: 815. https://doi.org/10.3390/polym15040815
APA StyleHamdy, T. M., Abdelnabi, A., Othman, M. S., Bayoumi, R. E., & Abdelraouf, R. M. (2023). Effect of Different Mouthwashes on the Surface Microhardness and Color Stability of Dental Nanohybrid Resin Composite. Polymers, 15(4), 815. https://doi.org/10.3390/polym15040815







