Performance of Particleboard Made of Agroforestry Residues Bonded with Thermosetting Adhesive Derived from Waste Styrofoam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Waste Styrofoam-Based Adhesives
2.3. Characterization of WPS-Based Adhesives
2.4. Preparation of Particleboard
2.5. Evaluation of Particleboard Properties
2.6. Statistical Analysis
3. Results
3.1. Basic Characteristics of WPS-Based Adhesives
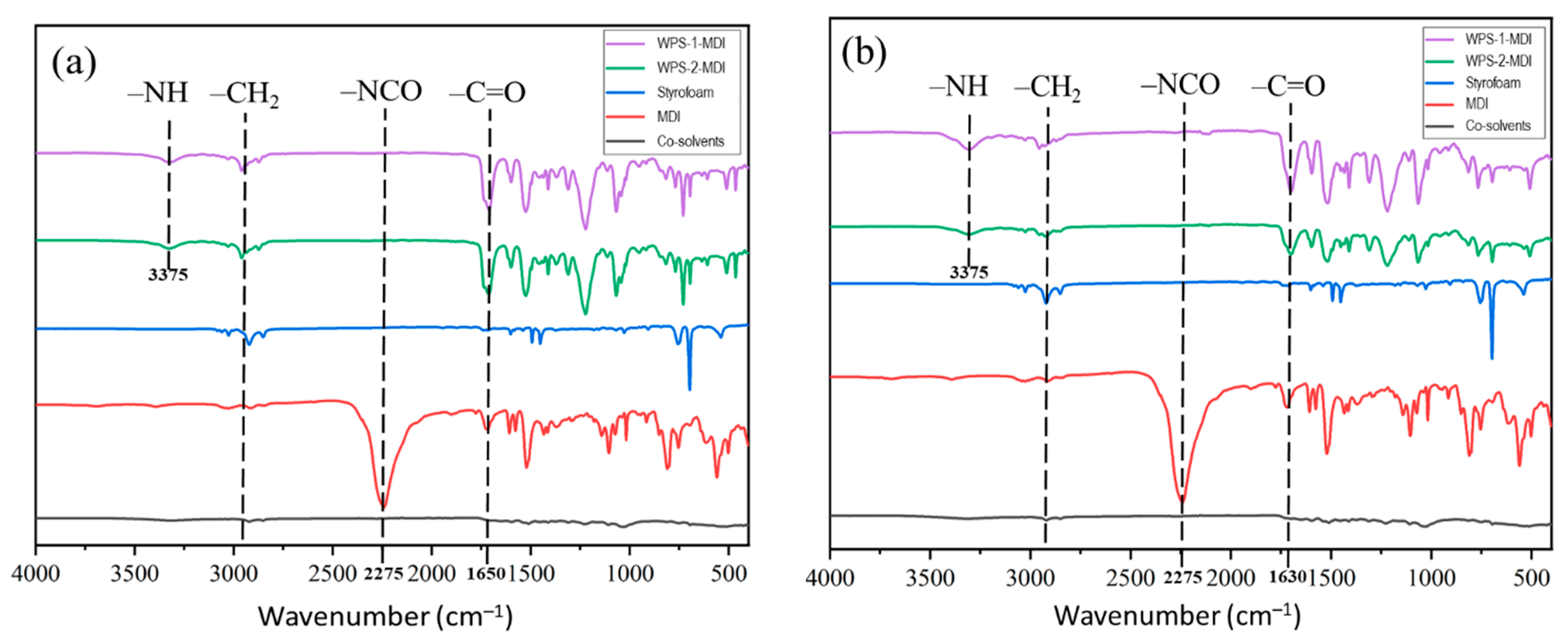
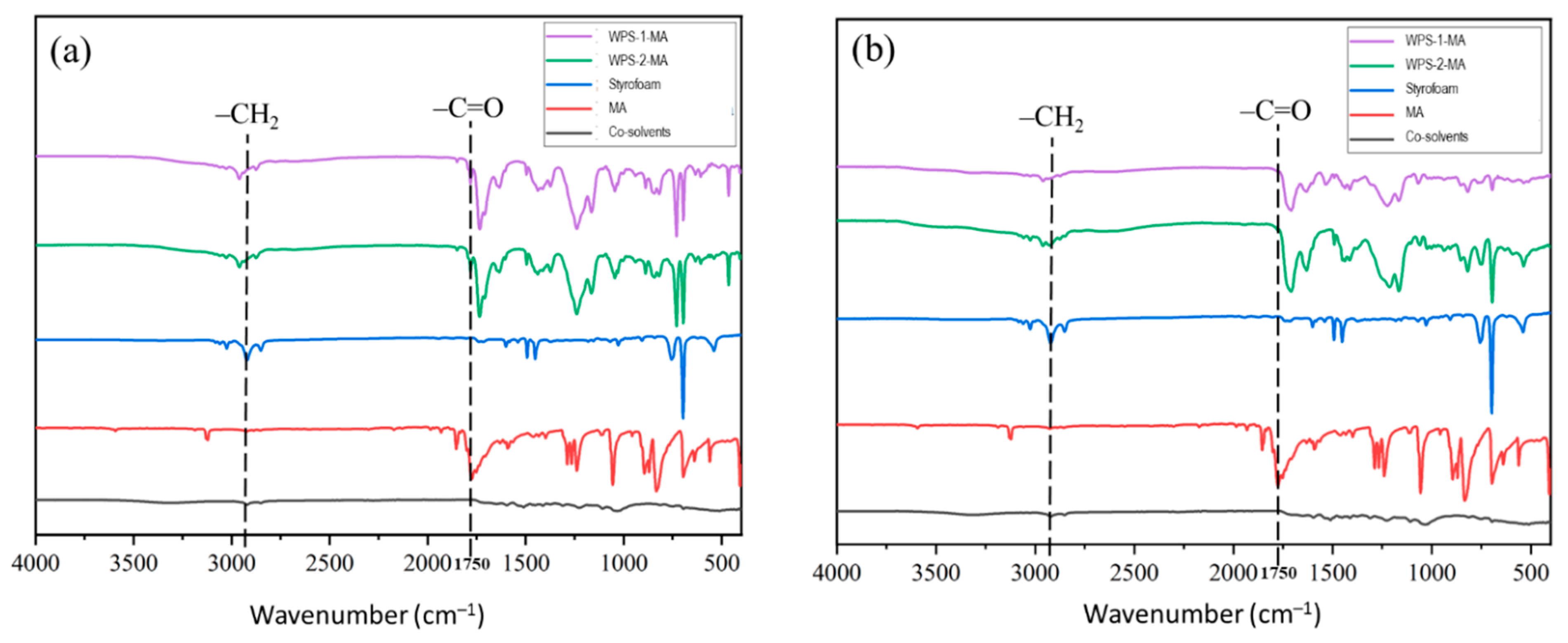
3.2. Chemical Properties of WPS-Based Adhesives
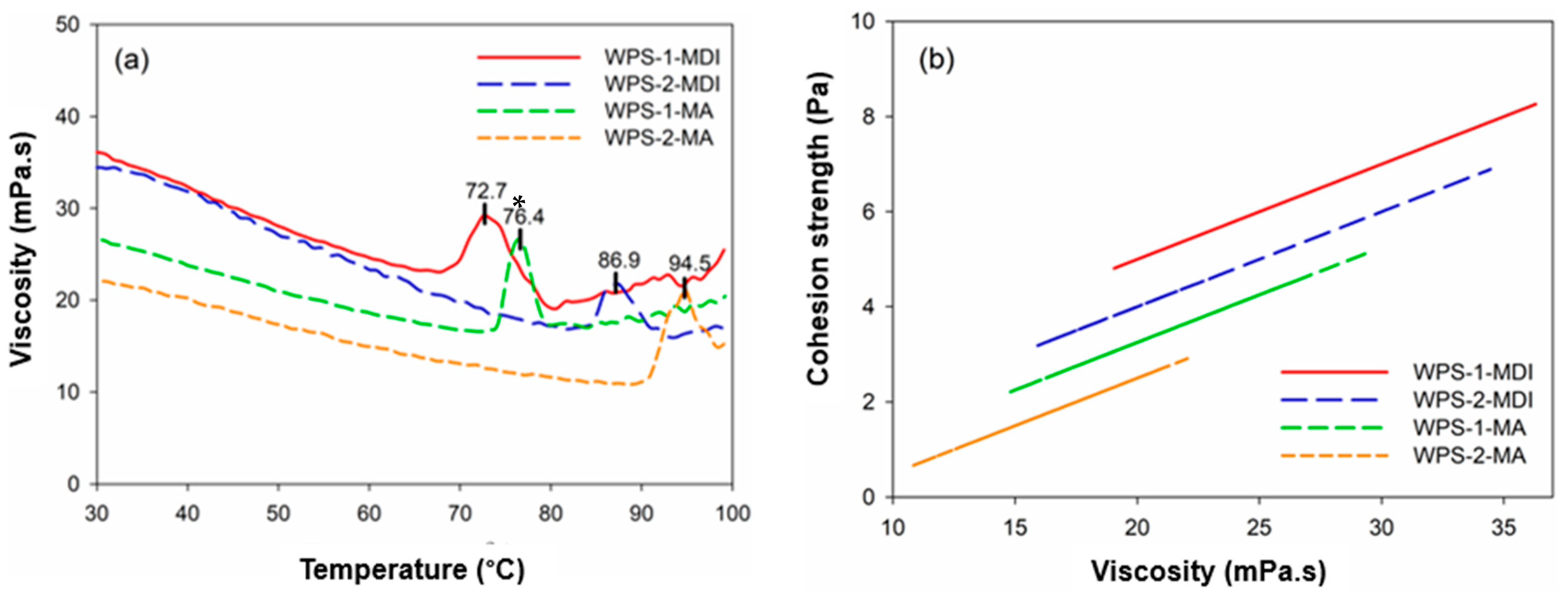
3.3. Thermo-Mechanical Properties of WPS-Based Adhesives
3.4. Morphological Characteristic of WPS-Based Adhesives
3.5. Evaluation of Particleboard Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Activation energy (Ea) | Moisture content (MC) |
| Attenuated total reflection (ATR) | Particleboard (PB) |
| Dynamic Mechanical Analysis (DMA) | Polystyrene (PS) |
| Expanded polystyrene (EPS) | Thickness swelling (TS) |
| Fourier-transform infrared (FTIR) | Urea–formaldehyde (UF) |
| Japanese Industrial Standard (JIS) | Waste polystyrene (WPS) |
| Internal bonding (IB) | Water absorption (WA) |
| Methylene diphenyl diisocyanate (MDI) | Weight by volume (w/v) |
| Maleic anhydride (MA) | Wood–Styrofoam composite (WSC) |
| Modulus of elasticity (MOE) | |
| Modulus of rupture (MOR) |
References
- Chaukura, N.; Gwenzi, W.; Bunhu, T.; Ruziwa, D.T.; Pumure, I. Potential uses and value-added products derived from waste polystyrene in developing countries: A review. Resour. Conserv. Recycl. 2016, 107, 157–165. [Google Scholar] [CrossRef]
- Schleier, J.; Simons, M.; Greiff, K.; Walther, G. End-of-life treatment of EPS-based building insulation material—An estimation of future waste and review of treatment options. Resour. Conserv. Recycl. 2022, 187, 106603. [Google Scholar] [CrossRef]
- García-Barrera, L.V.; Ortega-Solís, D.L.; Soriano-Giles, G.; Lopez, N.; Romero-Romero, F.; Reinheimer, E.; Varela-Guerrero, V.; Ballesteros-Rivas, M.F. A Recycling Alternative for Expanded Polystyrene Residues Using Natural Esters. J. Polym. Environ. 2022, 30, 3832–3839. [Google Scholar] [CrossRef]
- Capricho, J.C.; Prasad, K.; Hameed, N.; Nikzad, M.; Salim, N. Upcycling Polystyrene. Polymers 2022, 14, 5010. [Google Scholar] [CrossRef] [PubMed]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent progress in ultra-low formaldehyde emitting adhesive systems and formaldehyde scavengers in wood-based panels: A review. Wood Mater. Sci. Eng. 2023, 18, 763–782. [Google Scholar] [CrossRef]
- Iswanto, A.H.; Lubis, M.A.R.; Sutiawan, J.; Al-Edrus, S.S.O.; Lee, S.H.; Antov, P.; Kristak, L.; Reh, R.; Mardawati, E.; Santoso, A.; et al. Latest Advancements in the Development of High-Performance Lignin- and Tannin-Based Non-Isocyanate Polyurethane Adhesive for Wood Composites. Polymers 2023, 15, 3864. [Google Scholar] [CrossRef]
- Dunky, M. Wood adhesives based on natural resources: A critical review: Part IV. special topics. In Progress in Adhesion and Adhesives, Volume 6; Mittal, K.L., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 761–840. ISBN 9781119846703. [Google Scholar]
- Dunky, M. Wood adhesives based on natural resources: A critical review: Part II. carbohydrate-based adhesives. In Progress in Adhesion and Adhesives; Mittal, K.L., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 337–382. ISBN 9781119846703. [Google Scholar]
- Issam, A.M.; Poh, B.T.; Abdul Khalil, H.P.S.; Lee, W.C. Adhesion Properties of Adhesive Prepared from Waste Polystyrene. J. Polym. Environ. 2009, 17, 165–169. [Google Scholar] [CrossRef]
- Ahmetli, G.; Sen, N.; Pehlivan, E.; Durak, S. Adhesive and anticorrosive polymeric coatings obtained from modified industrial waste oligostyrenes. Prog. Org. Coat. 2006, 55, 262–267. [Google Scholar] [CrossRef]
- Demirkir, C.; Colak, S.; Aydin, I. Some technological properties of wood-styrofoam composite panels. Compos. Part B Eng. 2013, 55, 513–517. [Google Scholar] [CrossRef]
- Uttaravalli, A.N.; Dinda, S.; Gidla, B.R.; Kasturi, G.; Kasala, P.; Penta, G. Studies on development of adhesive material from post-consumer (waste) expanded polystyrene: A two-edged sword approach. Process Saf. Environ. Prot. 2021, 145, 312–320. [Google Scholar] [CrossRef]
- Wang, L.; Lagerquist, L.; Zhang, Y.; Koppolu, R.; Tirri, T.; Sulaeva, I.; von Schoultz, S.; Vähäsalo, L.; Pranovich, A.; Rosenau, T.; et al. Tailored Thermosetting Wood Adhesive Based on Well-Defined Hardwood Lignin Fractions. ACS Sustain. Chem. Eng. 2020, 8, 13517–13526. [Google Scholar] [CrossRef]
- Feghali, E.; Torr, K.M.; van de Pas, D.J.; Ortiz, P.; Vanbroekhoven, K.; Eevers, W.; Vendamme, R. Thermosetting Polymers from Lignin Model Compounds and Depolymerized Lignins. Top. Curr. Chem. 2018, 376, 32. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Park, B.-D. Enhancing the performance of low molar ratio urea–formaldehyde resin adhesives via in-situ modification with intercalated nanoclay. J. Adhes. 2021, 97, 1271–1290. [Google Scholar] [CrossRef]
- Gutierrez-Velasquez, E.I.; Monteiro, S.N.; Colorado, H.A. Characterization of expanded polystyrene waste as binder and coating material. Case Stud. Constr. Mater. 2022, 16, e00804. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- JIS A 5908:2003; Particleboard. Japanese Standards Association: Tokyo, Japan, 2003.
- Chanhoun, M.; Padonou, S.; Adjovi, E.C.; Olodo, E.; Doko, V. Study of the implementation of waste wood, plastics and polystyrenes for various applications in the building industry. Constr. Build. Mater. 2018, 167, 936–941. [Google Scholar] [CrossRef]
- Amin, Y.; Adji, R.P.; Lubis, M.A.R.; Nugroho, N.; Bahtiar, E.T.; Dwianto, W.; Karlinasari, L. Effect of Glue Spread on Bonding Strength, Delamination, and Wood Failure of Jabon Wood-Based Cross-Laminated Timber Using Cold-Setting Melamine-Based Adhesive. Polymers 2023, 15, 2349. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M. Innovations in Materials Manufacturing, Fabrication, and Environmental Safety; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420082166. [Google Scholar]
- Kurniawan, A.; Wirjosentono, B.; Tamrin, T.; Siregar, A.H.; Nasution, D.A. Preparation and characterisations of maleic anhydride-modified-(asphalt, natural rubber, and polystyrene) blends containing sand aggregate. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2020; Volume 2267, p. 020080. [Google Scholar]
- Tercjak, A.; Serrano, E.; Remiro, P.M.; Mondragon, I. Viscoelastic behavior of thermosetting epoxy mixtures modified with syndiotactic polystyrene during network formation. J. Appl. Polym. Sci. 2006, 100, 2348–2355. [Google Scholar] [CrossRef]
- Tribut, L.; Fenouillot, F.; Carrot, C.; Pascault, J.-P. Rheological behavior of thermoset/thermoplastic blends during isothermal curing: Experiments and modeling. Polymer 2007, 48, 6639–6647. [Google Scholar] [CrossRef]
- Meynie, L.; Fenouillot, F.; Pascault, J.-P. Influence of the gel on the morphology of a thermoset polymerized into a thermoplastic matrix, under shear. Polymer 2004, 45, 5101–5109. [Google Scholar] [CrossRef]
- Kim, M.; Park, B.D. Effects of synthesis method, melamine content and GPC parameter on the molecular weight of melamine-urea-formaldehyde resins. J. Korean Wood Sci. Technol. 2021, 49, 1–13. [Google Scholar] [CrossRef]
- Park, S.; Jeong, B.; Park, B.-D. A Comparison of Adhesion Behavior of Urea-Formaldehyde Resins with Melamine-Urea-Formaldehyde Resins in Bonding Wood. Forests 2021, 12, 1037. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, J.; Kang, T.J.; Choi, H.J.; Kim, J. In Situ Compatibilizer Reinforced Interface between an Amorphous Polymer (Polystyrene) and a Semicrystalline Polymer (Polyamide Nylon 6). Macromolecules 2007, 40, 5953–5958. [Google Scholar] [CrossRef]
- Ferrándiz-Mas, V. Design of bespoke lightweight cement mortars containing waste expanded polystyrene by experimental statistical methods. Mater. Des. 2016, 89, 901–912. [Google Scholar] [CrossRef]
- Beran, R.; Zárybnická, L.; Machová, D.; Večeřa, M.; Kalenda, P. Wood adhesives from waste-free recycling depolymerisation of flexible polyurethane foams. J. Clean. Prod. 2021, 305, 127142. [Google Scholar] [CrossRef]
- Hwan Oh, K.; Seo, Y.; Man Hong, S. Interfacial Adhesion between an Amorphous Polymer (Polystyrene) and a Semi-crystalline Polymer (Polyamide-nylon 6). In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2011; Volume 1353, pp. 756–760. [Google Scholar]
- Dillard, D.A.; Yamaguchi, T. Special Tests. In Handbook of Adhesion Technology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1–2, pp. 593–612. ISBN 9783319554112. [Google Scholar]
- Veytskin, Y.; Bobko, C.; Castorena, C.; Kim, Y.R. Nanoindentation investigation of asphalt binder and mastic cohesion. Constr. Build. Mater. 2015, 100, 163–171. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, M.; Lin, L. Investigation of bulk and in situ mechanical properties of coupling agents treated wood plastic composites. Polym. Test. 2017, 58, 292–299. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M.; Sultan, M.T.H.; Alothman, O.Y.; Abdullah, L.C. Thermomechanical and dynamic mechanical properties of bamboo/woven kenaf mat reinforced epoxy hybrid composites. Compos. Part B Eng. 2019, 163, 165–174. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.-D. Determination of Crystallinity of Thermosetting Urea-Formaldehyde Resins Using Deconvolution Method. Macromol. Res. 2020, 28, 615–624. [Google Scholar] [CrossRef]
- Kyriakou-Tziamtzi, C.; Vlachopoulos, A.; Zamboulis, A.; Bikiaris, D.N.; Achilias, D.S.; Chrissafis, K. Kinetic evaluation of the crosslinking of a low-temperature cured biobased epoxy-diamine structure. Prog. Org. Coat. 2023, 174, 107285. [Google Scholar] [CrossRef]
- Xian-qing, X.; Ying-ying, Y.; Yi-ting, N.; Liang-ting, Z. Development of a cornstarch adhesive for laminated veneer lumber bonding for use in engineered wood flooring. Int. J. Adhes. Adhes. 2020, 98, 102534. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.D. Direct measurement of surface adhesion between thin films of nanocellulose and urea–formaldehyde resin adhesives. Cellulose 2021, 28, 8459–8481. [Google Scholar] [CrossRef]
- Hidayat, W.; Aprilliana, N.; Asmara, S.; Bakri, S.; Hidayati, S.; Banuwa, I.S.; Lubis, M.A.R.; Iswanto, A.H. Performance of eco-friendly particleboard from agro-industrial residues bonded with formaldehyde-free natural rubber latex adhesive for interior applications. Polym. Compos. 2022, 43, 2222–2233. [Google Scholar] [CrossRef]
- Hong, M.K.; Lubis, M.A.R.; Park, B.D. Effect of panel density and resin content on properties of medium density fiberboard. J. Korean Wood Sci. Technol. 2017, 45, 444–455. [Google Scholar] [CrossRef]
- Xiong, X.; Niu, Y.; Yuan, Y.; Zhang, L. Study on dimensional stability of veneer rice straw particleboard. Coatings 2020, 10, 558. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y. Novel lignocellulosic hybrid particleboard composites made from rice straws and coir fibers. Mater. Des. 2014, 55, 19–26. [Google Scholar] [CrossRef]

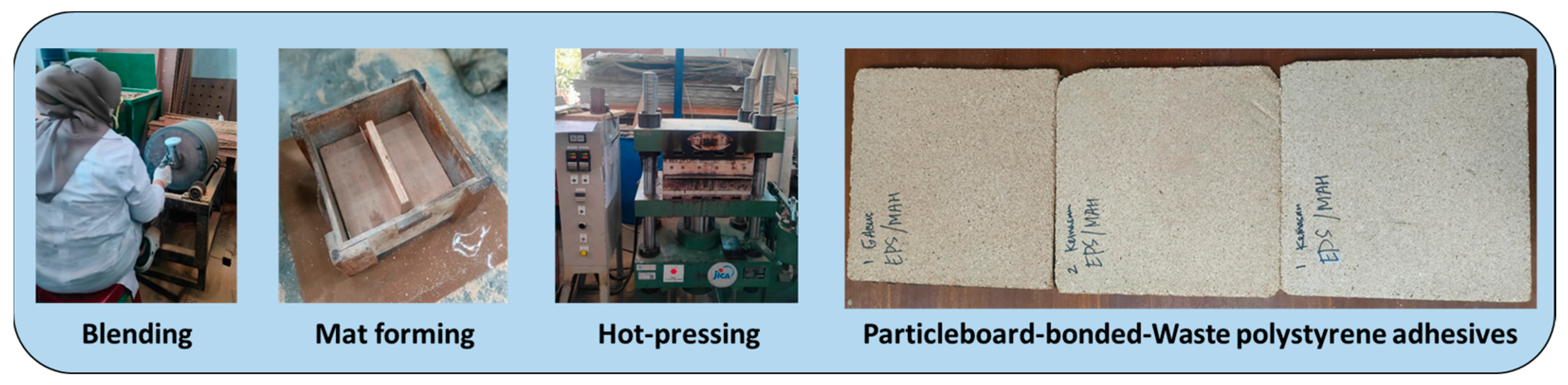


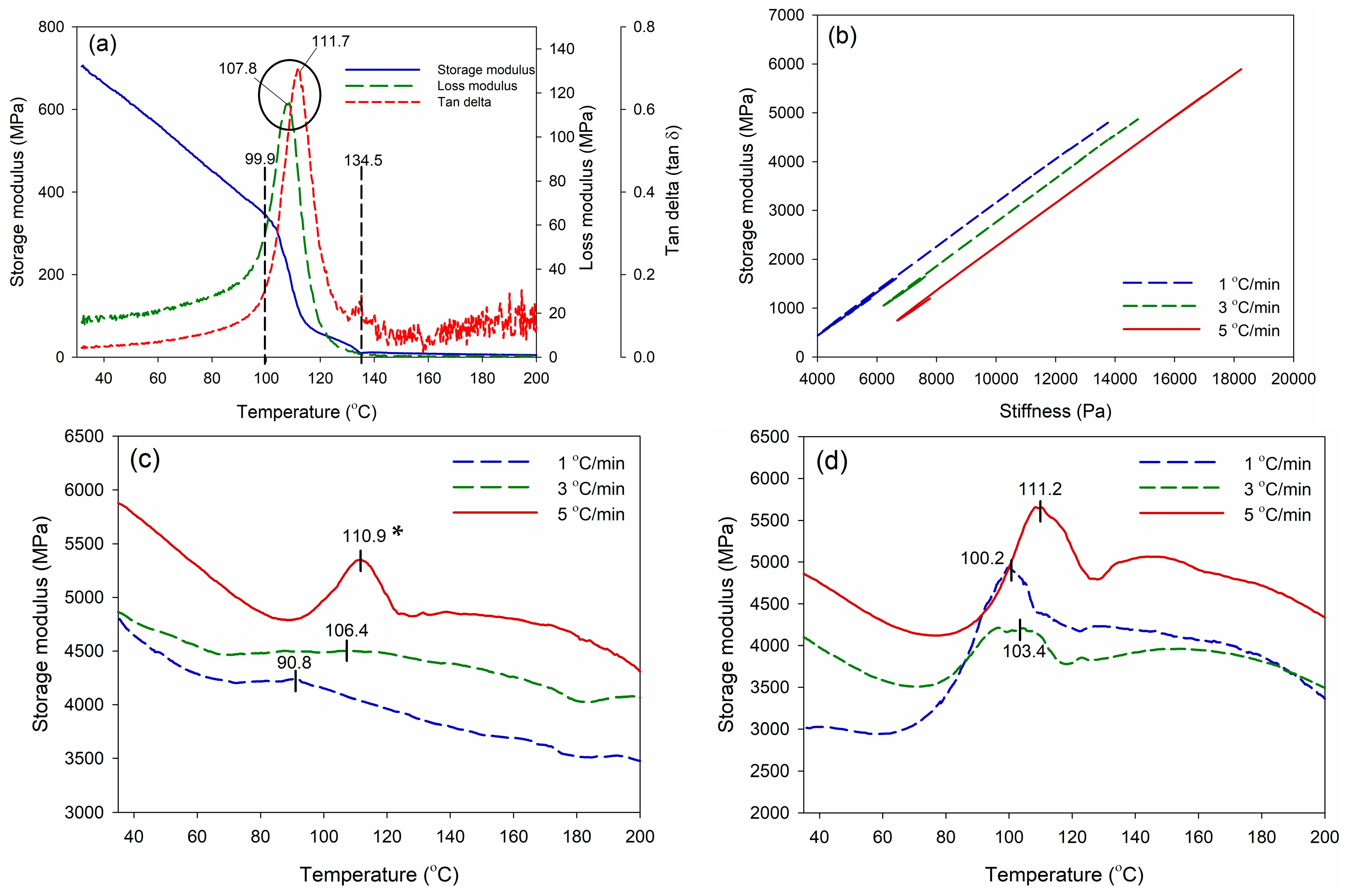


| Adhesive Type | Solids Content (%) | Average Viscosity at 25 °C (mPa·s) | Gel Time at 100 °C (min) | pH |
|---|---|---|---|---|
| UF resins | 58.4 ± 1.7 | 252.4 ± 5.1 | 3.5 ± 0.5 | 8.2 ± 0.3 |
| WPS-1-MDI | 25.9 ± 1.1 | 65.2 ± 2.3 | 6.4 ± 0.3 | 6.8 ± 0.2 |
| WPS-2-MDI | 26.7 ± 1.2 | 68.6 ± 3.8 | 8.6 ± 0.5 | 6.7 ± 0.2 |
| WPS-1-MA | 12.4 ± 1.2 | 24.8 ± 0.6 | 10.2 ± 0.4 | 2.6 ± 0.1 |
| WPS-2-MA | 13.6 ± 1.5 | 26.7 ± 0.4 | 13.5 ± 0.7 | 2.5 ± 0.1 |
| Type of Adhesive | Heating Rates (°C/min) | Tp (°C) | Ea (kJ/mole) | R2 |
|---|---|---|---|---|
| WPS-MDI | 1 | 90.8 | 83.4 | 0.9860 |
| 3 | 106.4 | |||
| 5 | 110.9 | |||
| WPS-MA | 1 | 100.2 | 150.8 | 0.8075 |
| 3 | 103.4 | |||
| 5 | 111.2 |
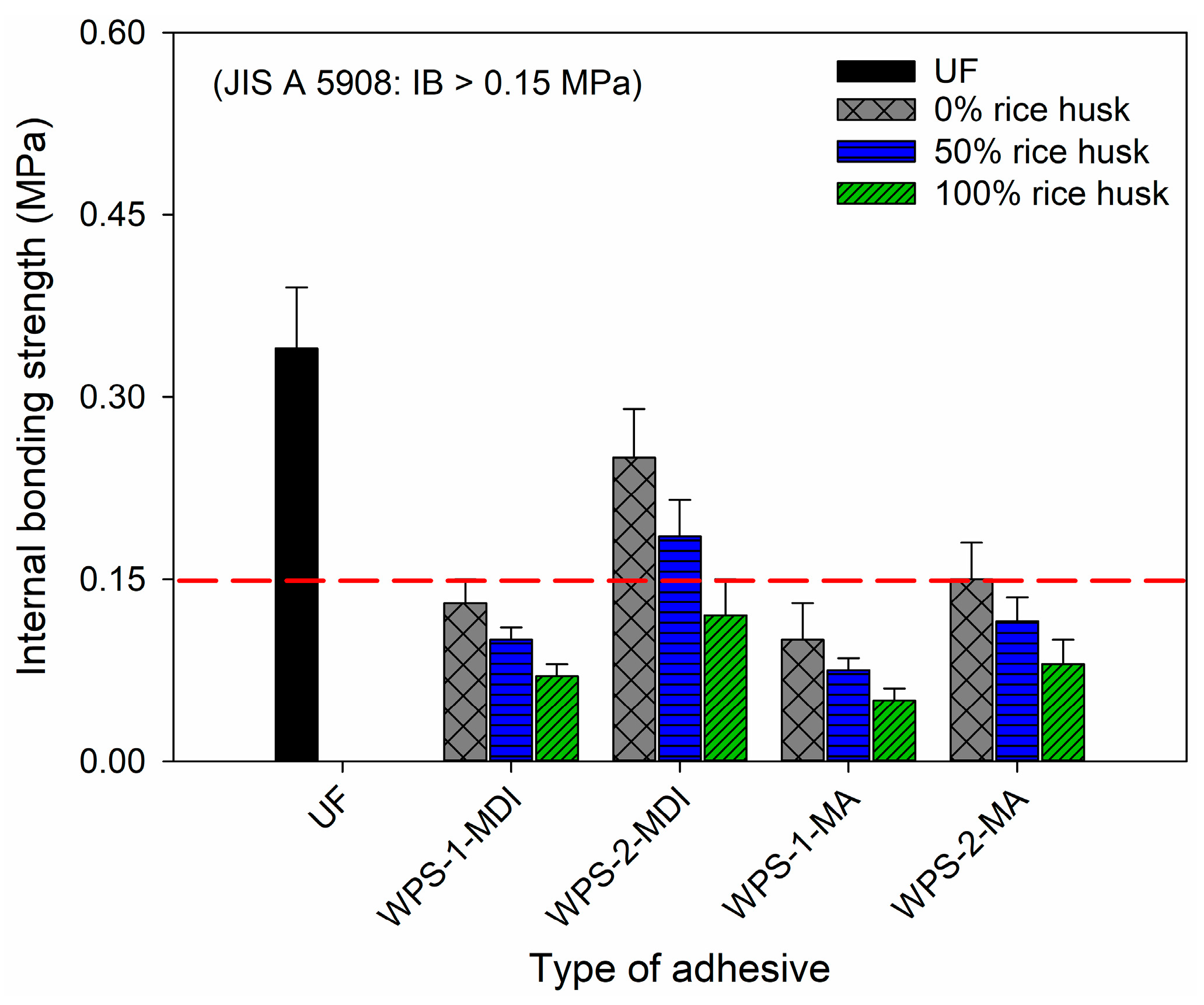
| Adhesive Type | Particleboard Properties | |||
|---|---|---|---|---|
| Density (g/cm3) | Moisture Content (%) | Thickness Swelling (%) | Water Absorption (%) | |
| UF | 0.81 ± 0.02 | 5.23 ± 0.74 | 30.5 ± 0.51 | 65.7 ± 0.61 |
| WPS-1-MDI | 0.75 ± 0.02 | 5.32 ± 0.46 | 42.5 ± 0.55 | 73.2 ± 0.60 |
| WPS-2-MDI | 0.78 ± 0.02 | 5.36 ± 0.53 | 45.1 ± 0.45 | 77.5 ± 0.65 |
| WPS-1-MA | 0.70 ± 0.01 | 5.52 ± 0.56 | 51.5 ± 0.60 | 76.4 ± 0.66 |
| WPS-2-MA | 0.73 ± 0.01 | 5.58 ± 0.43 | 53.7 ± 0.75 | 79.3 ± 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karliati, T.; Lubis, M.A.R.; Dungani, R.; Maulani, R.R.; Hadiyane, A.; Rumidatul, A.; Antov, P.; Savov, V.; Lee, S.H. Performance of Particleboard Made of Agroforestry Residues Bonded with Thermosetting Adhesive Derived from Waste Styrofoam. Polymers 2024, 16, 543. https://doi.org/10.3390/polym16040543
Karliati T, Lubis MAR, Dungani R, Maulani RR, Hadiyane A, Rumidatul A, Antov P, Savov V, Lee SH. Performance of Particleboard Made of Agroforestry Residues Bonded with Thermosetting Adhesive Derived from Waste Styrofoam. Polymers. 2024; 16(4):543. https://doi.org/10.3390/polym16040543
Chicago/Turabian StyleKarliati, Tati, Muhammad Adly Rahandi Lubis, Rudi Dungani, Rijanti Rahaju Maulani, Anne Hadiyane, Alfi Rumidatul, Petar Antov, Viktor Savov, and Seng Hua Lee. 2024. "Performance of Particleboard Made of Agroforestry Residues Bonded with Thermosetting Adhesive Derived from Waste Styrofoam" Polymers 16, no. 4: 543. https://doi.org/10.3390/polym16040543
APA StyleKarliati, T., Lubis, M. A. R., Dungani, R., Maulani, R. R., Hadiyane, A., Rumidatul, A., Antov, P., Savov, V., & Lee, S. H. (2024). Performance of Particleboard Made of Agroforestry Residues Bonded with Thermosetting Adhesive Derived from Waste Styrofoam. Polymers, 16(4), 543. https://doi.org/10.3390/polym16040543








