Disposable Polyaniline/m-Phenylenediamine-Based Electrochemical Lactate Biosensor for Early Sepsis Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Lactate Biosensor Fabrication
2.3. Electrochemical Measurement
2.4. Optimization Studies
2.5. Selectivity, Reproducibility, Long-Term and Storage Stability
2.6. Real Sample Analysis
3. Results and Discussion
3.1. Characterization of the Lactate Biosensor
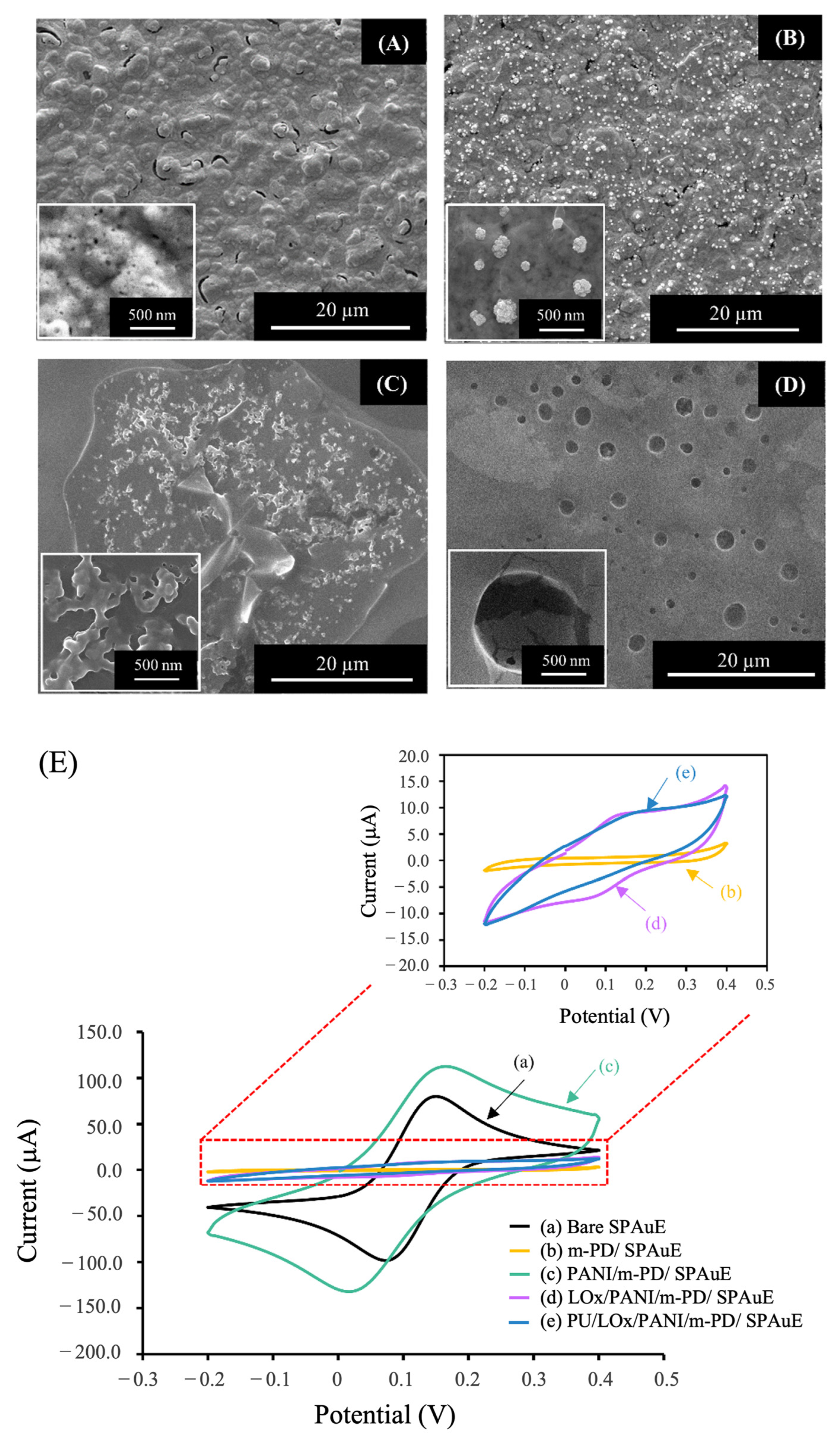
3.1.1. Surface Morphology
3.1.2. Electrochemical Characterization
3.2. Optimization Studies
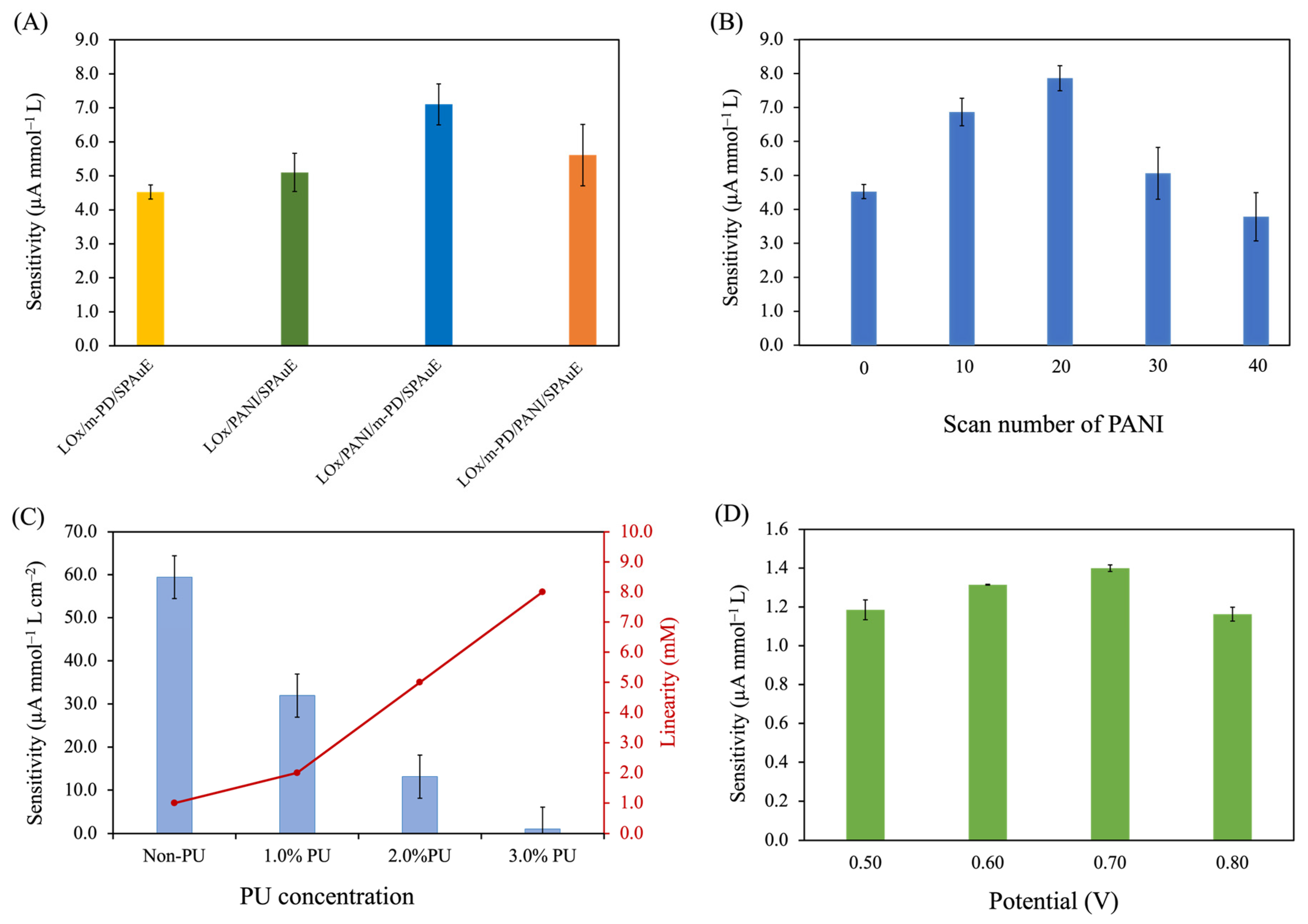
3.2.1. Effect of Electrode Modification Strategies
3.2.2. Effect of the PANI Layer
3.2.3. Effect of PU Concentrations
3.2.4. Effect of Operational Potential for Lactate Determination
3.3. Analytical Performances of the Optimized PU/LOx/PANI/m-PD/SPAuE
3.3.1. Linearity and Detection Limit
3.3.2. Selectivity
3.3.3. Reproducibility and Long-Term and Storage Stability
3.3.4. Comparison with Other Sensors
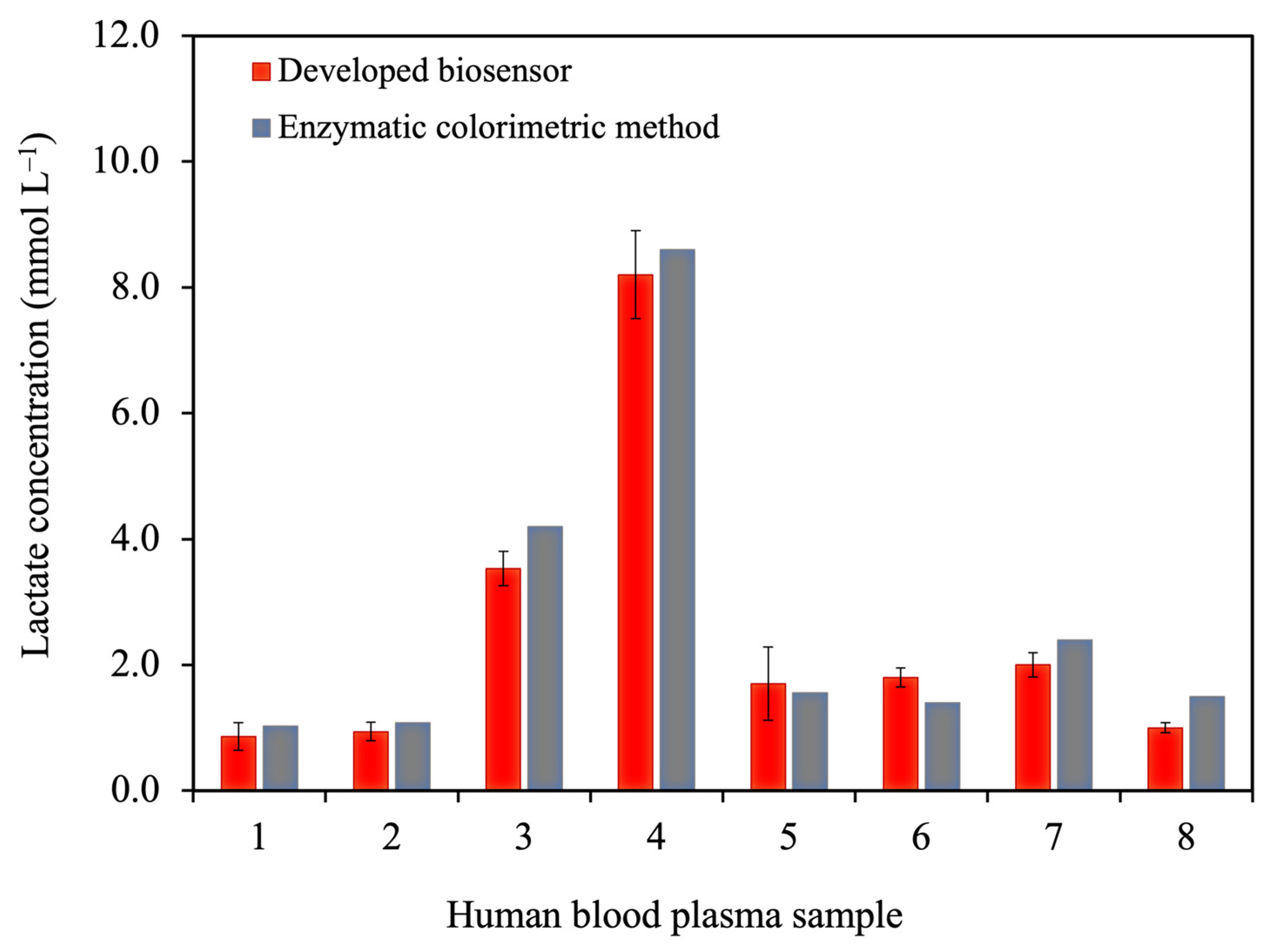
3.3.5. Analysis of Human Blood Plasma Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keep, J.W.; Messmer, A.S.; Sladden, R.; Burrell, N.; Pinate, R.; Tunnicliff, M.; Glucksman, E. National Early Warning Score at Emergency Department Triage May Allow Earlier Identi Fi Cation of Patients with Severe Sepsis and Septic Shock: A Retrospective Observational Study. Emerg. Med. J. 2016, 33, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Rakpraisuthepsiri, N.; Surabenjawong, U.; Limsuwat, C.; Lertvipapath, P. Efficacy of a Modified Sepsis System on the Mortality Rate of Septic Shock Patients in the Emergency Department of Siriraj Hospital. J. Health Sci. Med. Res. 2022, 40, 543–550. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 Update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Crapnell, R.D.; Tridente, A.; Banks, C.E.; Dempsey-Hibbert, N.C. Evaluating the Possibility of Translating Technological Advances in Non-Invasive Continuous Lactate Monitoring into Critical Care. Sensors 2021, 21, 879. [Google Scholar] [CrossRef] [PubMed]
- Dugar, S.; Choudhary, C.; Duggal, A. Sepsis and Septic Shock: Guideline-Based Management. Crit. Care Nephrol. Third Ed. 2019, 87, 500–504.e1. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.M.; Lee, J.; Lee, Y.-S.; Lee, J.H.; Lim, K.S.; Huh, J.W.; Hong, S.-B.; Lim, C.-M.; Koh, Y.; Kim, W.Y. Lactate Level Versus Lactate Clearance for Predicting Mortality in Patients With Septic Shock Defined by Sepsis-3. Crit. Care Med. 2018, 46, e489–e495. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Narwal, V.; Batra, B. Biosensors and Bioelectronics Determination of Lactic Acid with Special Emphasis on Biosensing Methods: A Review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef]
- Rassaei, L.; Olthuis, W.; Tsujimura, S.; Ernst, J.R.; Sudhölter, A.; van den, B. Lactate Biosensors: Current Status and Outlook. Anal. Bioanal. Chem. 2013, 406, 123–137. [Google Scholar] [CrossRef]
- Samaraweera, S.A.; Gibbons, B.; Gour, A.; Sedgwick, P. Arterial versus Venous Lactate: A Measure of Sepsis in Children. Eur. J. Pediatr. 2017, 176, 1055–1060. [Google Scholar] [CrossRef]
- Kiatamornrak, P.; Boobphahom, S.; Lertussavavivat, T.; Rattanawaleedirojn, P.; Chailapakul, O.; Rodthongkum, N.; Srisawat, N. A Portable Blood Lactate Sensor with a Non-Immobilized Enzyme for Early Sepsis Diagnosis. Analyst 2022, 147, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors Based on Electrochemical Lactate Detection: A Comprehensive Review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Bradley, Z.; Bhalla, N. Point-of-Care Diagnostics for Sepsis Using Clinical Biomarkers and Microfluidic Technology. Biosens. Bioelectron. 2023, 227, 115181. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Zhu, M.; Kuang, Z.; Li, X.; Xu, F.; Miao, S.; Zhang, Z.; Lou, X.; Li, H.; et al. Electrochemical Biosensors for Whole Blood Analysis: Recent Progress, Challenges, and Future Perspectives. Chem. Rev. 2023, 123, 7953–8039. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Topolnikova, Y.V.; Soldatkin, O.O. Advances in the Biosensors for Lactate and Pyruvate Detection for Medical Applications: A Review. TrAC Trends Anal. Chem. 2019, 110, 160–172. [Google Scholar] [CrossRef]
- Kilic, N.M.; Singh, S.; Keles, G.; Cinti, S.; Kurbanoglu, S.; Odaci, D. Novel Approaches to Enzyme-Based Electrochemical Nanobiosensors. Biosensors 2023, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Soldatkina, O.V.; Kucherenko, I.S.; Pyeshkova, V.M.; Alekseev, S.A.; Soldatkin, O.O.; Dzyadevych, S.V. Improvement of Amperometric Transducer Selectivity Using Nanosized Phenylenediamine Films. Nanoscale Res. Lett. 2017, 12, 594. [Google Scholar] [CrossRef]
- Ming, T.; Lan, T.; Yu, M.; Wang, H.; Deng, J.; Kong, D.; Yang, S.; Shen, Z. Platinum Black/Gold Nanoparticles/Polyaniline Modified Electrochemical Microneedle Sensors for Continuous In Vivo Monitoring of PH Value. Polymers 2023, 15, 2796. [Google Scholar] [CrossRef]
- Banjar, M.F.; Joynal Abedin, F.N.; Fizal, A.N.S.; Muhamad Sarih, N.; Hossain, M.S.; Osman, H.; Khalil, N.A.; Ahmad Yahaya, A.N.; Zulkifli, M. Synthesis and Characterization of a Novel Nanosized Polyaniline. Polymers 2023, 15, 4565. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, C.; Ji, Z.; Ma, W.; Wang, H. A Solid Ionic Lactate Biosensor Using Doped Graphene-like Membrane of Au-EVIMC-Titania Nanotubes-Polyaniline. Biosens. Bioelectron. 2018, 118, 97–101. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Vahabi, H.; Saeb, M.R.; Mozafari, M. Application of Polyaniline and Its Derivatives; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128179154. [Google Scholar]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Evaluation of Polyaniline and Polypyrrole Nanocomposites Based on Glucose Oxidase and Gold Nanostructures. Polymers 2020, 12, 3026. [Google Scholar] [CrossRef]
- Neampet, S.; Ruecha, N.; Qin, J.; Wonsawat, W.; Chailapakul, O.; Rodthongkum, N. A Nanocomposite Prepared from Platinum Particles, Polyaniline and a Ti3C2 MXene for Amperometric Sensing of Hydrogen Peroxide and Lactate. Microchim. Acta 2019, 186, 752. [Google Scholar] [CrossRef] [PubMed]
- Yuqing, M.; Jianrong, C.; Xiaohua, W. Using Electropolymerized Non-Conducting Polymers to Develop Enzyme Amperometric Biosensors. Trends Biotechnol. 2004, 22, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.A.; De Vries, M.G.; Cremers, T.I.F.H.; Westerink, B.H.C. The Role of Surface Availability in Membrane-Induced Selectivity for Amperometric Enzyme-Based Biosensors. Sens. Actuators B Chem. 2016, 223, 679–688. [Google Scholar] [CrossRef]
- Phonklam, K.; Thongkhao, P.; Phairatana, T. The Stability of Gold Nanoparticles-Prussian Blue Based Sensors for Biosensor Applications in Clinical Diagnosis. J. Health Sci. Med. Res. 2022, 20, 859. [Google Scholar] [CrossRef]
- Huang, L.; Jia, Z.; Liu, H.; Pi, X.; Zhou, J. Design of a Sandwich Hierarchically Porous Membrane with Oxygen Supplement Function for Implantable Glucose Sensor. Appl. Sci. 2020, 10, 2848. [Google Scholar] [CrossRef]
- Sarika, C.; Rekha, K.; Narasimha Murthy, B. Studies on Enhancing Operational Stability of a Reusable Laccase-Based Biosensor Probe for Detection of Ortho-Substituted Phenolic Derivatives. 3 Biotech 2015, 5, 911–924. [Google Scholar] [CrossRef]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical Sensors and Biosensors Based on the Use of Polyaniline and Its Nanocomposites: A Review on Recent Advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef]
- Rattu, G.; Khansili, N.; Maurya, V.K.; Krishna, P.M. Lactate Detection Sensors for Food, Clinical and Biological Applications: A Review. Environ. Chem. Lett. 2020, 19, 1135–1152. [Google Scholar] [CrossRef]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in Quality in the Analytical Laboratory. II. Analytical Method Validation and Quality Assurance. TrAC—Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- AOAC. Appendix F: Guidelines for Standard Method Performance Requirements. In AOAC Official Methods of Analysis; AOAC Off. Methods Anal: Rockville, MD, USA, 2016; pp. 1–17. [Google Scholar]
- Thvenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification (Technical Report). Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Wang, N.; Burugapalli, K.; Song, W.; Halls, J.; Moussy, F.; Ray, A.; Zheng, Y. Electrospun Fibro-Porous Polyurethane Coatings for Implantable Glucose Biosensors. Biomaterials 2013, 34, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Schott, H. Swelling Kinetics of Polymers. J. Macromol. Sci. Part B 1992, 31, 1–9. [Google Scholar] [CrossRef]
- Fang, L.; Liang, B.; Yang, G.; Hu, Y.; Zhu, Q.; Ye, X. Biosensors and Bioelectronics A Needle-Type Glucose Biosensor Based on PANI Nano Fi Bers and PU/E-PU Membrane for Long-Term Invasive Continuous Monitoring. Biosens. Bioelectron. 2017, 97, 196–202. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Kim, K.-H. Functionalization and Customization of Polyurethanes for Biosensing Applications: A State-of-the-Art Review. TrAC Trends Anal. Chem. 2020, 126, 115881. [Google Scholar] [CrossRef]
- Boobphahom, S.; Rattanawaleedirojn, P.; Boonyongmaneerat, Y.; Rengpipat, S.; Chailapakul, O.; Rodthongkum, N. TiO2 Sol/Graphene Modified 3D Porous Ni Foam: A Novel Platform for Enzymatic Electrochemical Biosensor. J. Electroanal. Chem. 2019, 833, 133–142. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Soldatkin, O.O.; Topolnikova, Y.V.; Dzyadevych, S.V.; Soldatkin, A.P. Novel Multiplexed Biosensor System for the Determination of Lactate and Pyruvate in Blood Serum. Electroanalysis 2019, 31, 1608–1614. [Google Scholar] [CrossRef]
- Dagar, K.; Pundir, C.S. An Improved Amperometric L-Lactate Biosensor Based on Covalent Immobilization of Microbial Lactate Oxidase onto Carboxylated Multiwalled Carbon Nanotubes/Copper Nanoparticles/Polyaniline Modified Pencil Graphite Electrode. Enzym. Microb. Technol. 2017, 96, 177–186. [Google Scholar] [CrossRef]
- Cunha-Silva, H.; Arcos-Martinez, M.J. Dual Range Lactate Oxidase-Based Screen Printed Amperometric Biosensor for Analysis of Lactate in Diversified Samples. Talanta 2018, 188, 779–787. [Google Scholar] [CrossRef]
- Bravo, I.; Gutiérrez-Sánchez, C.; García-Mendiola, T.; Revenga-Parra, M.; Pariente, F.; Lorenzo, E. Enhanced Performance of Reagent-Less Carbon Nanodots Based Enzyme Electrochemical Biosensors. Sensors 2019, 19, 5576. [Google Scholar] [CrossRef] [PubMed]



| Electrode Materials | Linear Range (mmol L−1) | Detection Limit (µmol L−1) | Sensitivity (µA·mmol−1 L cm−2) | Stability (Days) | Samples | Ref. |
|---|---|---|---|---|---|---|
| LOx/MWCNTs/CuNPs/PANI/PEG | 0.0010–2.5 | 0.25 | NR | 140 | Blood plasma | [41] |
| LOx-Cu-MOF/CS/Pt/SPCE | 0.00075–1.0 4.0–50.0 | 0.75 | 116.26 1.64 | 50 | Sweat, saliva, wine | [42] |
| LOx/TiO2 sol gel-Gr/Ni foam | 0.050–10.0 | 19 | NR | 8 | Commercial rabbit serum | [39] |
| LOx-PVA-SbQ/m-PD/Pt disk | 0.005–1.0 | 5 | 81.6 | 14 | Human blood serum | [40] |
| LOx/Pt/PANI/MXene/SPCE | 0.005–5.0 | 5 | 0.62 | 30 | Milk samples | [24] |
| LOx-CNDs/SPAuE | 0.003–0.50 | 0.9 | 39.52 | NR | Human blood serum | [43] |
| PU/LOx/PANI/m-PD/SPAuE | 0.20–5.0 | 7.9 | 12.17 | >70 | Human blood plasma | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongkhao, P.; Numnuam, A.; Khongkow, P.; Sangkhathat, S.; Phairatana, T. Disposable Polyaniline/m-Phenylenediamine-Based Electrochemical Lactate Biosensor for Early Sepsis Diagnosis. Polymers 2024, 16, 473. https://doi.org/10.3390/polym16040473
Thongkhao P, Numnuam A, Khongkow P, Sangkhathat S, Phairatana T. Disposable Polyaniline/m-Phenylenediamine-Based Electrochemical Lactate Biosensor for Early Sepsis Diagnosis. Polymers. 2024; 16(4):473. https://doi.org/10.3390/polym16040473
Chicago/Turabian StyleThongkhao, Piromya, Apon Numnuam, Pasarat Khongkow, Surasak Sangkhathat, and Tonghathai Phairatana. 2024. "Disposable Polyaniline/m-Phenylenediamine-Based Electrochemical Lactate Biosensor for Early Sepsis Diagnosis" Polymers 16, no. 4: 473. https://doi.org/10.3390/polym16040473
APA StyleThongkhao, P., Numnuam, A., Khongkow, P., Sangkhathat, S., & Phairatana, T. (2024). Disposable Polyaniline/m-Phenylenediamine-Based Electrochemical Lactate Biosensor for Early Sepsis Diagnosis. Polymers, 16(4), 473. https://doi.org/10.3390/polym16040473







