The Influence of TiO2–Lignin Hybrid Fillers in Low-Density Polyethylene Composites on Photocatalytic Performance and UV-Barrier Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2–Lignin Hybrid Systems
2.3. Characteristics of TiO2–Lignin Hybrid Systems
2.4. Preparation of Polyethylene-Based Composites
2.5. Characteristics of Polyethylene-Based Composites
2.5.1. Mechanical Properties
2.5.2. Thermal Analysis
2.5.3. UV–Vis Spectrophotometry Analysis
2.5.4. Photocatalytic Properties
3. Results and Discussion
3.1. Characteristics of TiO2–Lignin Hybrid Systems and Pristine Components
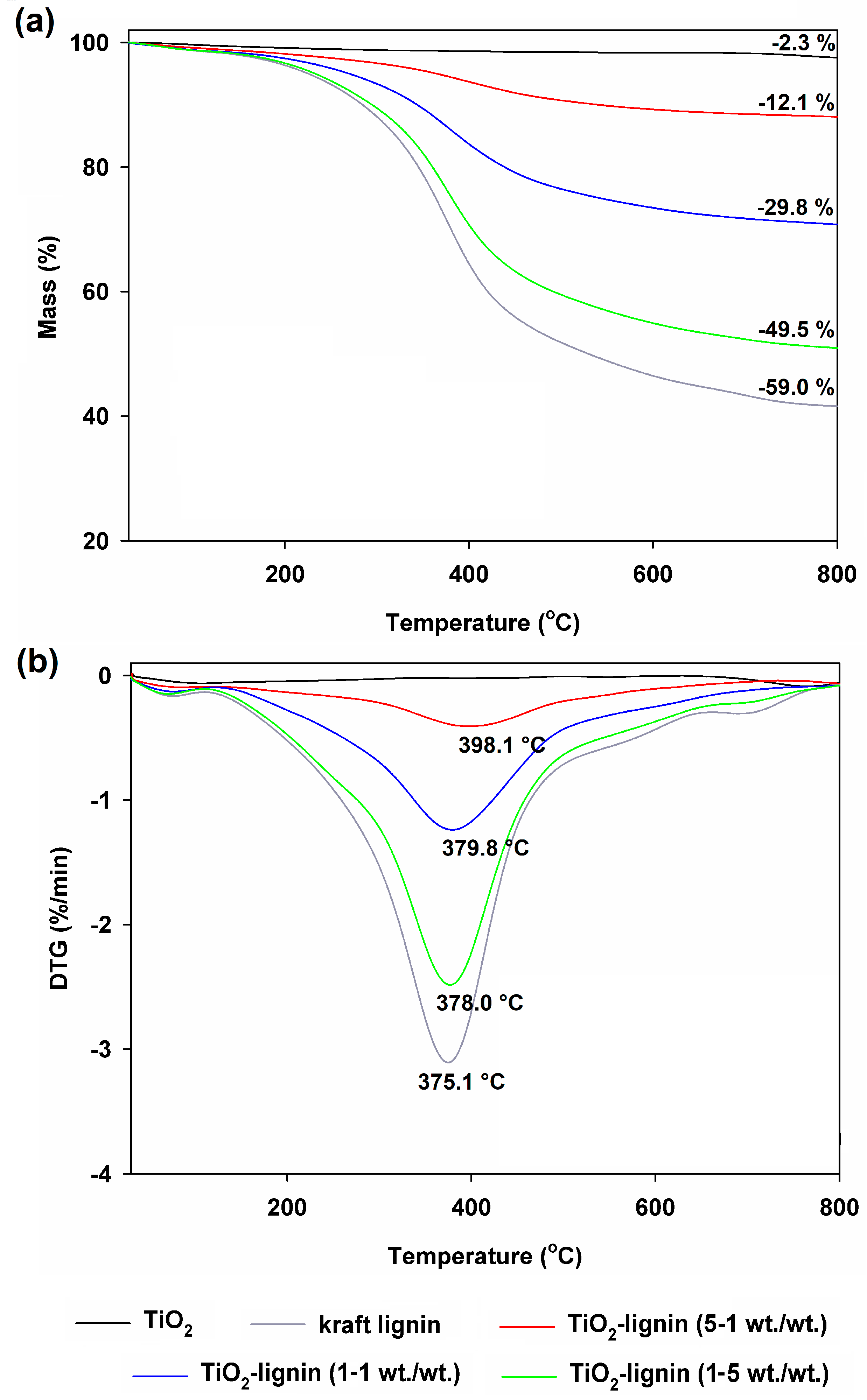
3.1.1. Thermal Stability Assessment
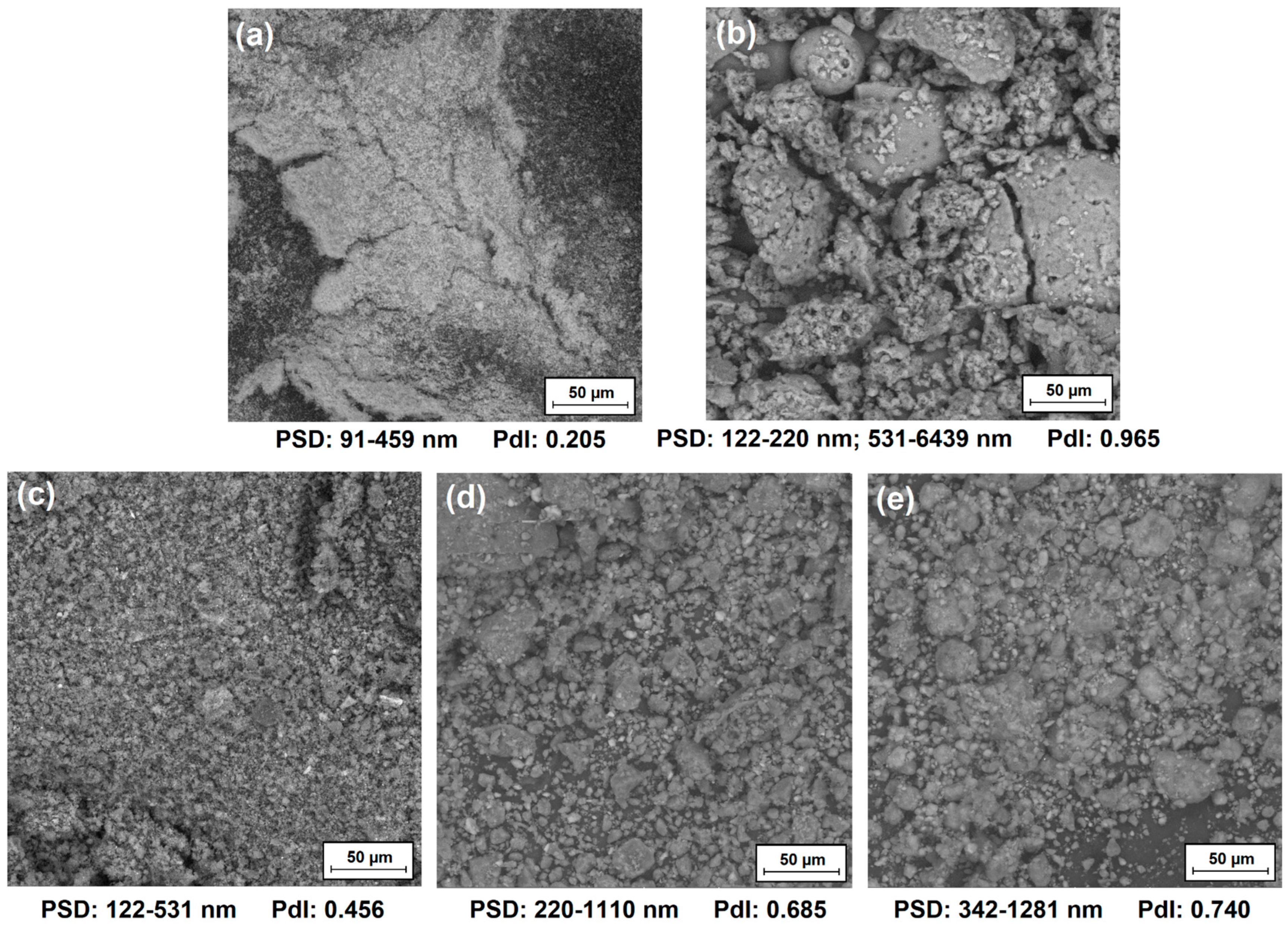
3.1.2. Characteristics of Dispersion and Morphological Properties
3.1.3. Electrokinetic Stability Assessment
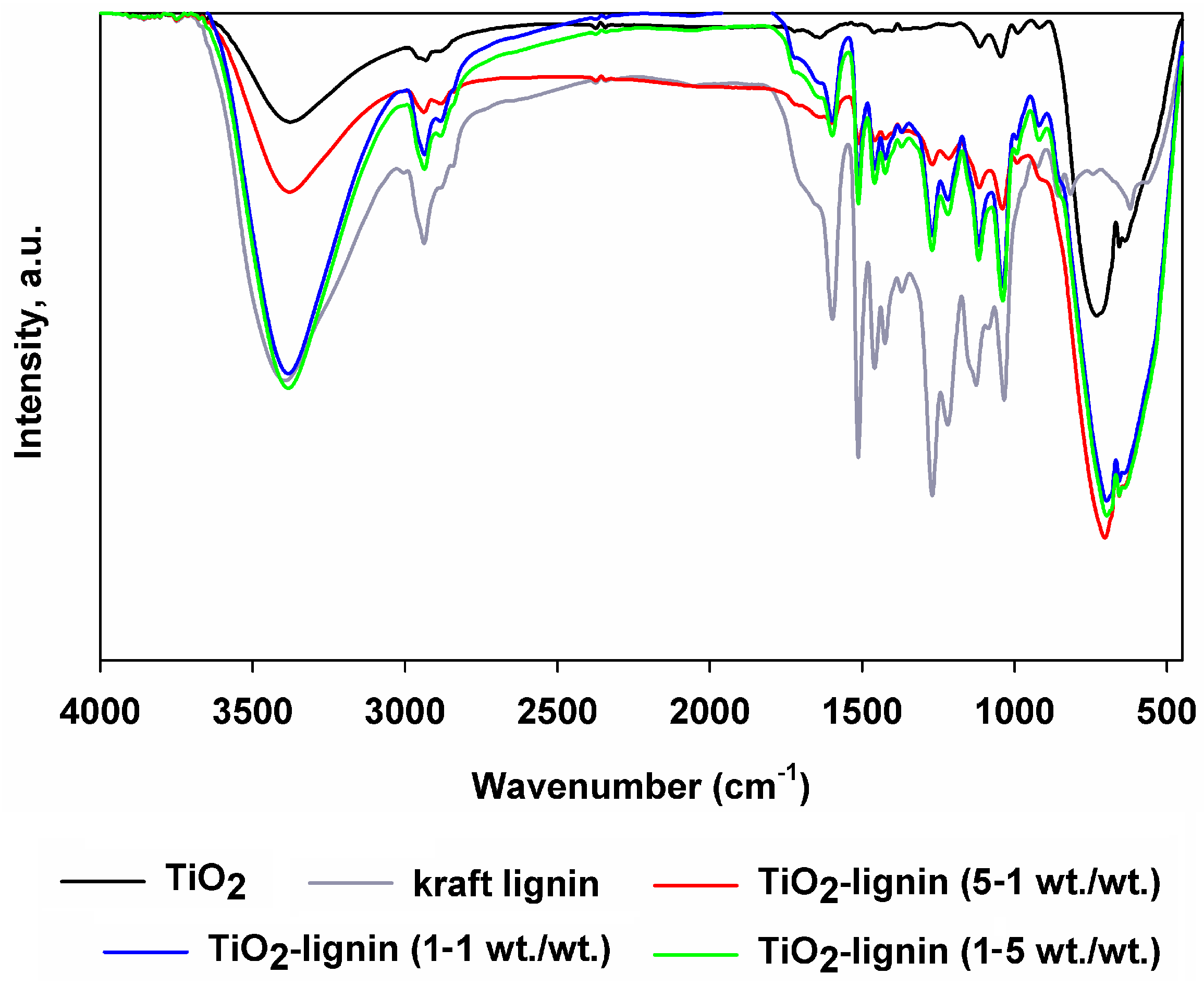
3.1.4. Fourier-Transform Infrared Spectroscopy
3.2. Characteristics of Polyethylene-Based Composites
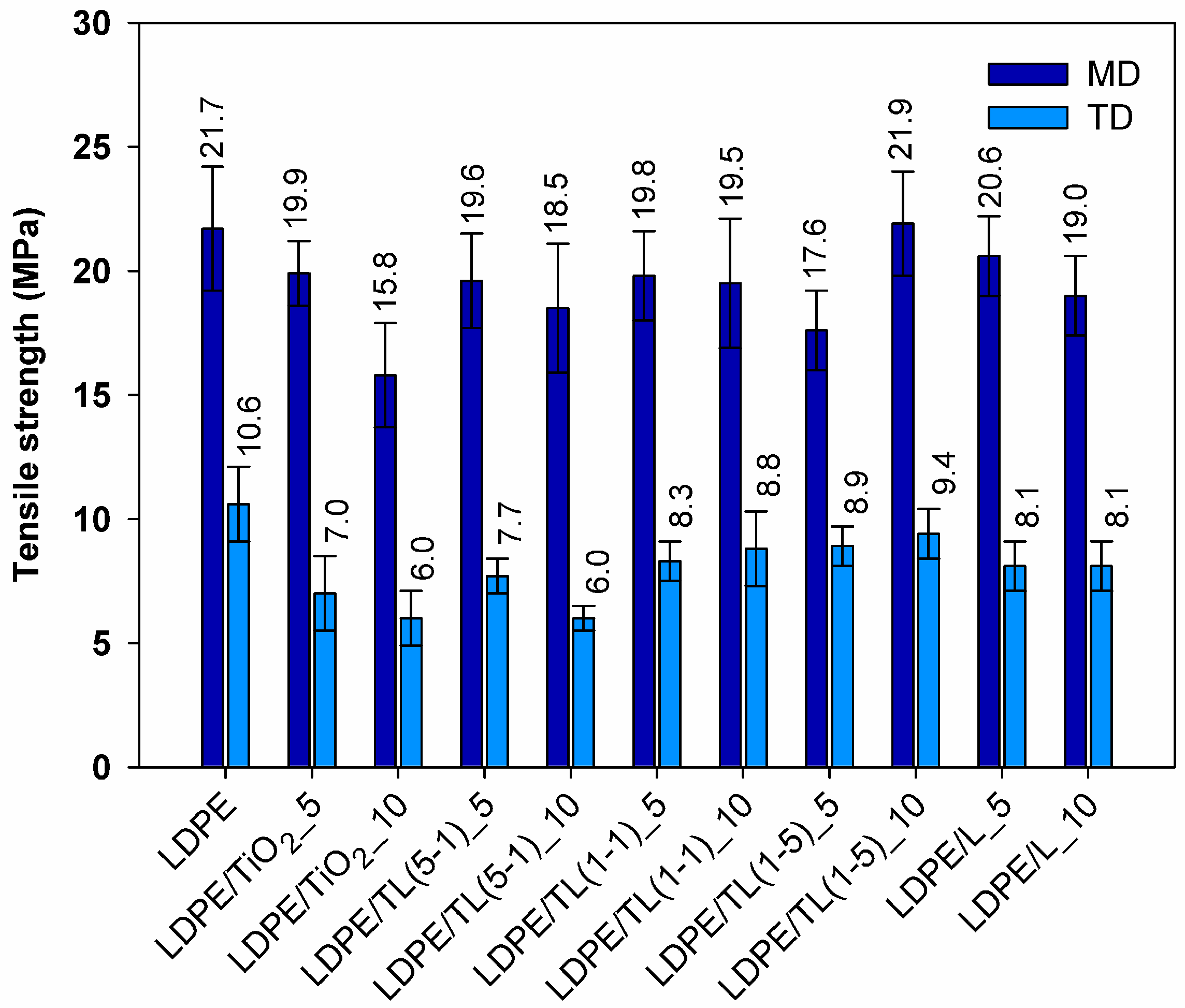
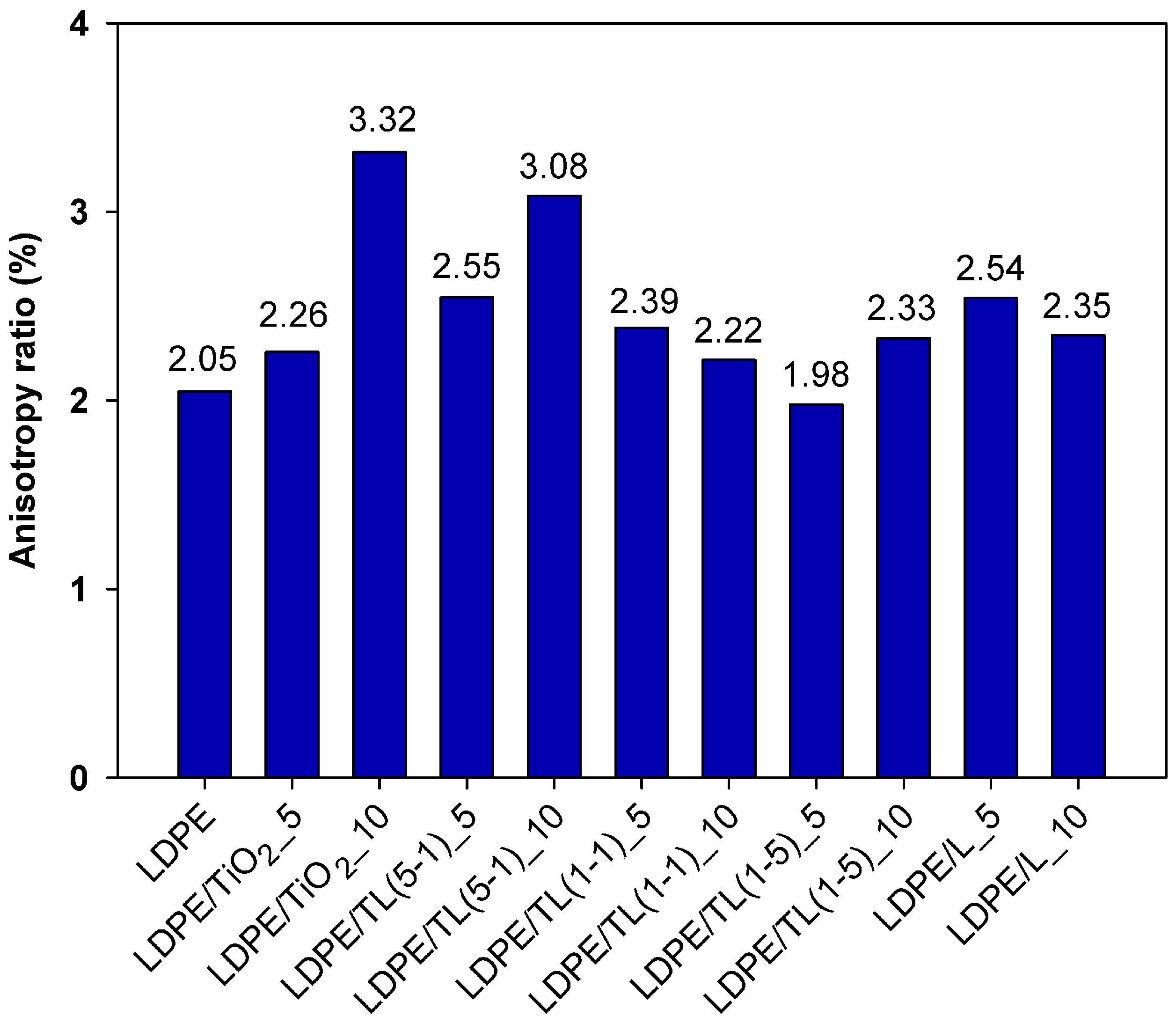
3.2.1. Mechanical Properties
3.2.2. Thermal Analysis
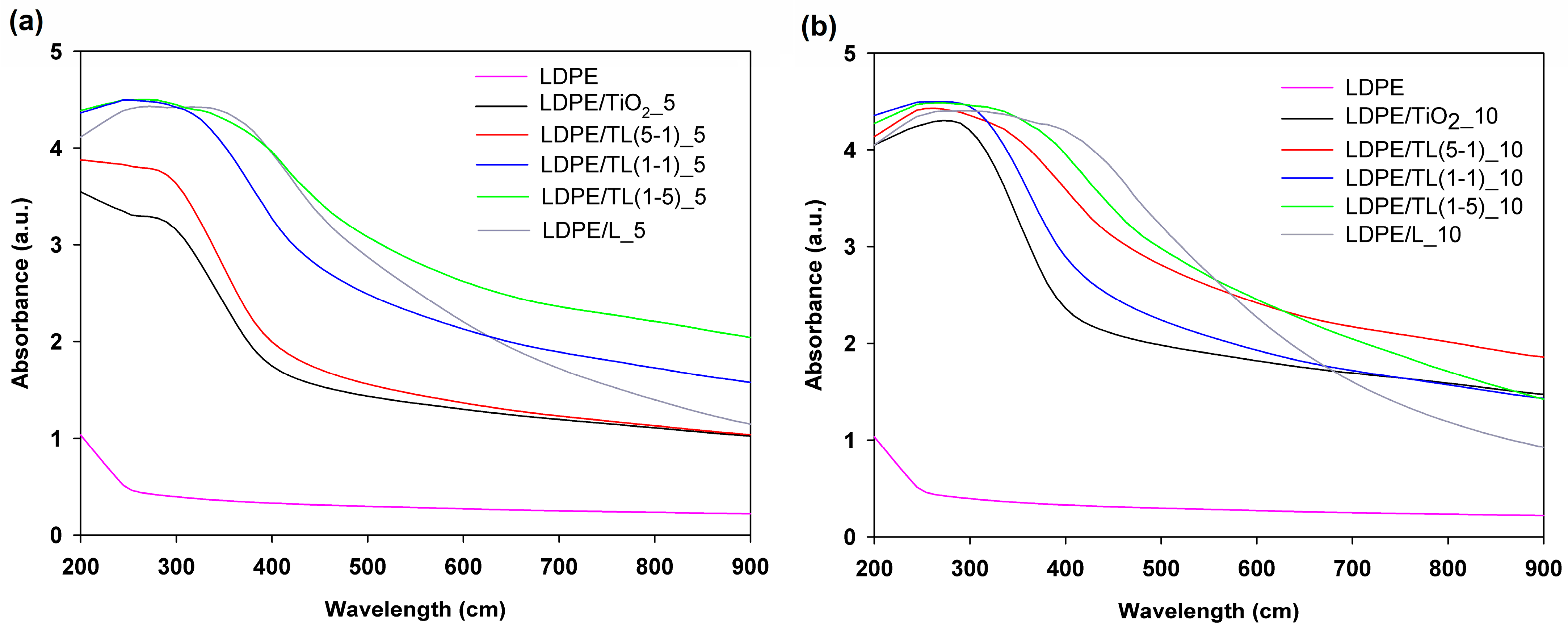
3.2.3. UV–Vis Spectrophotometry
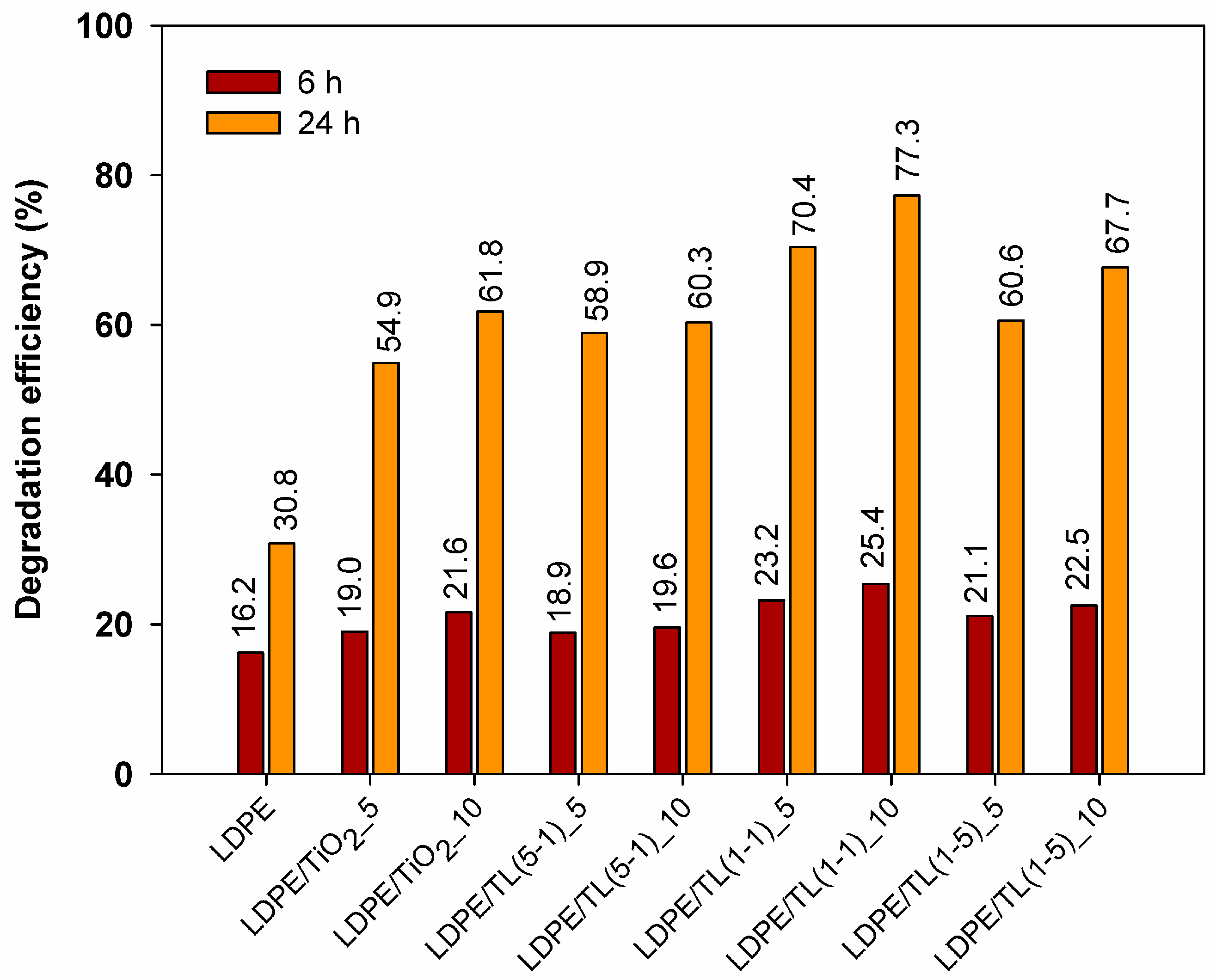
3.2.4. Photocatalytic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vachon, J.; Assad-Alkhateb, D.; de Araujo Hsia, L.; Lora, J.H.; Baumberger, S. Effect of compatibilizers on polyethylene-eucalyptus lignin blends. J. Appl. Polym. Sci. 2023, 140, e53695. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A.; Isac-García, J.; Martín-Martínez, F.J. Lignin and Lignans as Renewable Raw Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Lai, Y.Y.; Lee, Y.M. Management strategy of plastic wastes in Taiwan. Sustain. Environ. Res. 2022, 32, 11. [Google Scholar] [CrossRef]
- Damayanti, D.; Saputri, D.R.; Marpaung, D.S.S.; Yusupandi, F.; Sanjaya, A.; Simbolon, Y.M.; Asmarani, W.; Ulfa, M.; Wu, H.S. Current prospects or plastic waste treatment. Polymers 2022, 14, 3133. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Pypeć, K.; Irska, I.; Piesowicz, E. Functional polymer hybrid nanocomposites based on polyolefins: A review. Processes 2020, 8, 1475. [Google Scholar] [CrossRef]
- Barros, C.; Miranda, S.; Castro, O.; Machado, A.V. LDPE-nanoclay films for food packaging with improved barrier properties. J. Plast. Film Sheeting 2023, 39, 304–320. [Google Scholar] [CrossRef]
- Hong, S.H.; Hwang, S.H. Construction, physical properties and foaming behavior of high-content lignin reinforced low-density polyethylene biocomposites. Polymers 2022, 14, 2688. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A. Organosolv lignin improved thermoplastic elastomeric behavior of polyethylene/polyisoprene blend. ACS Omega 2022, 7, 8483–8492. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Goliszek, M.; Podkościelna, B.; Fila, K.; Riazanova, A.V.; Aminzadeh, S.; Sevastyanova, O.; Gun’ko, V.M. Synthesis and structure characterization of polymeric nanoporous microspheres with lignin. Cellulose 2018, 25, 5843–5862. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Tekin, İ.; Hayatioğlu, N.; Ersus, S. Development of environmental friendly composite packaging films from safflower (Carthamus tinctorius L.) plant wastes. Food Biosci. 2023, 55, 102991. [Google Scholar] [CrossRef]
- Rodríguez-Fabià, S.; Zarna, C.; Chinga-Carrasco, G. A comparative study of kraft pulp fibres and the corresponding fibrillated materials as reinforcement of LDPE- and HDPE-biocomposites. Compos. Part A Appl. Sci. Manuf. 2023, 173, 107678. [Google Scholar] [CrossRef]
- Begum, T.; Follett, P.A.; Shankar, S.; Moskovchenko, L.; Salmieri, S.; Lacroix, M. Evaluation of bioactive low-density polyethylene (LDPE) nanocomposite films in combined treatment with irradiation on strawberry shelf-life extension. J. Food Sci. 2023, 88, 2141–2161. [Google Scholar] [CrossRef]
- Tanpichai, S.; Boonmahitthisud, A.; Soykeabkaew, N.; Ongthip, L. Review of the recent developments in all-cellulose nanocomposites: Properties and applications. Carbohydr. Polym. 2022, 286, 119192. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Rydzkowski, T.; Thakur, V.K.; Borysiak, S. Enzymatic engineering of nanometric cellulose for sustainable polypropylene nanocomposites. Ind. Crops Prod. 2021, 161, 113188. [Google Scholar] [CrossRef]
- Venkatarajan, S.; Athijayamani, A. An overview on natural cellulose fiber reinforced polymer composites. Mater. Today Proc. 2021, 37, 3620–3624. [Google Scholar] [CrossRef]
- Scholten, P.B.V.; Özen, M.B.; Söyler, Z.; Thomassin, J.-M.; Wilhelm, M.; Detrembleur, C.; Meier, M.A.R. Rheological and mechanical properties of cellulose/LDPE composites using sustainable and fully renewable compatibilisers. J. Appl. Polym. Sci. 2020, 137, 48744. [Google Scholar] [CrossRef]
- Suzuki, H.; Kubo, Y.; Sekiguchi, Y.; Kobayashi, M.; Kumada, A.; Sato, M. Direct measurement of charge trap depth in polymer nanocomposites. J. Phys. D Appl. Phys. 2023, 56, 325301. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abd El-Aziz, M.E.; Morsi, S.M.M. Development and evaluation of antimicrobial LDPE/TiO2 nanocomposites for food packaging applications. Polym. Bull. 2023, 80, 5417–5431. [Google Scholar] [CrossRef]
- Bendaoued, A.; Messaoud, M.; Harzallah, O.; Bistac, S.; Salhi, R. Nano-TiO2 effect on thermal, rheological and structural properties of thermoplastic polypropylene nanocomposites. J. Mater. Res. Technol. 2022, 17, 2313–2325. [Google Scholar] [CrossRef]
- Sahu, M.; Satapathy, A. Processing and characterization of TiO2 filled polymer composites. Mater. Today Proc. 2021, 44, 4945–4951. [Google Scholar] [CrossRef]
- Bula, K.; Klapiszewski, Ł.; Piasecki, A.; Jesionowski, T. The role of inorganic-organic bio-fillers containing kraft lignin in improvement in functional properties of polyethylene. Materials 2021, 14, 2114. [Google Scholar] [CrossRef] [PubMed]
- Bula, K.; Jędrzejczak, P.; Ajnbacher, D.; Collins, M.N.; Klapiszewski, Ł. Design and characterization of functional TiO2-lignin fillers used in rotational molded polyethylene containers. Int. J. Biol. Macromol. 2023, 246, 125626. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Malik, P.; Jain, P. Biopolymer reinforced nanocomposites: A comprehensive review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Kavuncuoglu, H.; Yalcin, H.; Dogan, M. Development of (TiO2-ZnO)/LDPE based active nanocomposite films and detection of migration to minced beef during storage using response surface methodology. Food Chem. 2023, 402, 134278. [Google Scholar] [CrossRef] [PubMed]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of organic-inorganic hybrid materials: Prehistory, art, science, and advanced applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Zielińska, D.; Siwińska-Ciesielczyk, K.; Bula, K.; Jesionowski, T.; Borysiak, S. TiO2/nanocellulose hybrids as functional additives for advanced polypropylene nanocomposites. Ind. Crops Prod. 2022, 176, 114314. [Google Scholar] [CrossRef]
- Bula, K.; Klapiszewski, Ł.; Jesionowski, T. Effect of processing conditions and functional silica/lignin content on the properties of bio-based composite thin sheet film. Polym. Test. 2019, 77, 105911. [Google Scholar] [CrossRef]
- Bula, K.; Kubicki, G.; Jesionowski, T.; Klapiszewski, Ł. MgO-Lignin Dual Phase Filler as an Effective Modifier of Polyethylene Film Properties. Materials 2020, 13, 809. [Google Scholar] [CrossRef]
- Bula, K.; Kubicki, G.; Kubiak, A.; Jesionowski, T.; Klapiszewski, Ł. Influence of MgO-Lignin Dual Component Additives on Selected Properties of Low Density Polyethylene. Polymers 2020, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Bula, K.; Klapiszewski, Ł.; Jesionowski, T. A novel functional silica/lignin hybrid material as a potential bio-based polypropylene filler. Polym. Compos. 2016, 36, 913–922. [Google Scholar] [CrossRef]
- PN-EN ISO 527-3:2019-01; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. ISO: Geneva, Switzerland, 2019.
- PN-EN ISO 11358-1:2022-09; Plastics—Thermogravimetry (TG) of Polymers—Part 1: General Principles. ISO: Geneva, Switzerland, 2022.
- Rajput, R.B.; Mane, R.S.; Jamble, S.N.; Jha, N.; Kale, R.B. N-doped carbon/TiO2 composites with enhanced photocatalytic performance for the removal of organic pollutants. J. Phys. Chem. Solids 2024, 184, 111677. [Google Scholar] [CrossRef]
- Eldoma, M.A.; Alaswad, S.O.; Mahmoud, M.A.; Qudsieh, I.Y.; Hassan, M.; Bakarther, O.Y.; Elawadi, G.A.; Abouatiaa, A.F.F.; Alomar, M.S.; Elhassan, M.S.; et al. Enhancing photocatalytic performance of Co-TiO2 and Mo-TiO2-based catalysts through defect engineering and doping: A study on the degradation of organic pollutants under UV light. J. Photochem. Photobiol. 2024, 446, 115164. [Google Scholar] [CrossRef]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Gupta, P. Catalytic valorization of kraft lignin into feedstock chemicals with methyltrioxorhenium (MTO) catalyst in microbial electrochemical cell. Int. J. Biol. Macromol. 2024, 254, 127631. [Google Scholar] [CrossRef]
- Zeng, Q.; Du, Z.; Luo, L. Selective preparation of monomers from hydrotreatment of lignin using isopropanol over Ru-Pd/HZSM-5 catalysts. Biomass Convers. Biorefinery 2023, 13, 10701–10710. [Google Scholar] [CrossRef]
- Blindheim, F.H.; Ruwoldt, J. The Effect of Sample Preparation Techniques on Lignin Fourier Transform Infrared Spectroscopy. Polymers 2023, 15, 2901. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Chancelier, L.; Diallo, A.O.; Santini, C.C.; Marlair, G.; Gutel, T.; Mailley, S.; Len, C. Targeting adequate thermal stability and fire safety in selecting ionic liquid-based electrolytes for energy storage. Phys. Chem. Chem. Phys. 2014, 16, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Svorcik, V.S.; Kolarova, K.; Slepicka, P.; Mackova, A.; Novotna, M.; Hnatowicz, V. Modification of surface properties of high and low density polyethylene by Ar plasma discharge. Polym. Degrad. Stab. 2006, 91, 1219–1225. [Google Scholar] [CrossRef]
- Toh, K.; Nakano, S.; Yokoyama, H.; Ebe, K.; Gotoh, K.; Noda, H. Anti-deterioration effect of lignin as an ultraviolet absorbent in polypropylene and polyethylene. Polym. J. 2005, 37, 633–635. [Google Scholar] [CrossRef][Green Version]
- Qian, Y.; Qiu, X.; Zhu, S. Sunscreen performance of lignin from different technical resources and their general synergistic effect with synthetic sunscreens. ACS Sustain. Chem. Eng. 2016, 4, 4029–4035. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation and characterization of agar/lignin/silver nanoparticles composite films with ultraviolet light barrier and antibacterial properties. Food Hydrocoll. 2017, 71, 76–84. [Google Scholar] [CrossRef]
- Han, R.; Coey, J.D.; O’Rourke, C.; Bamford, C.G.G.; Mills, A. Flexible, disposable photocatalytic plastic films for the destruction of viruses. J. Photochem. Photobiol. 2022, 235, 112551. [Google Scholar] [CrossRef]
- Osorio-Vargas, P.; Pais-Ospina, D.; Marin-Silva, D.A.; Pinotti, A.; Damonte, L.; Cánneva, A.; Danadelli, J.A.; da Costa, L.P.; Pizzio, L.R.; Torres, C.C.; et al. TiO2 nanorods doped with g-C3N4-polyethylene composite coating for self-cleaning applications. Mater. Chem. Phys. 2022, 288, 126356. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; Pais-Ospina, D.; Marín-Silva, D.A.; Pinotti, A.; Damonte, L.; Pizzio, L.R.; Osorio-Vargas, P.; Rengifo-Herrera, J.A. Facile photocatalytic immobilization strategy for P-25 TiO2 nanoparticles on low density polyethylene films and their UV-A photo-induced super hydrophilicity and photocatalytic activity. Catal. Today 2021, 372, 11–19. [Google Scholar] [CrossRef]

| Sample | Weight Percentage | Filler Used | ||
|---|---|---|---|---|
| LDPE | Filler | PE-g-MAH | ||
| LDPE | 98 | 0 | 2 | - |
| LDPE/TiO2_5 | 93 | 5 | T (TiO2) | |
| LDPE/TiO2_10 | 88 | 10 | ||
| LDPE/TL(5-1)_5 | 93 | 5 | TL(5-1) | |
| LDPE/TL(5-1)_10 | 88 | 10 | ||
| LDPE/TL(1-1)_5 | 93 | 5 | TL(1-1) | |
| LDPE/TL(1-1)_10 | 88 | 10 | ||
| LDPE/TL(1-5)_5 | 93 | 5 | TL(1-5) | |
| LDPE/TL(1-5)_10 | 88 | 10 | ||
| LDPE/L_5 | 93 | 5 | L (lignin) | |
| LDPE/L_10 | 88 | 10 | ||
| Sample | pH | ||||
|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | |
| Zeta Potential (mV) | |||||
| TiO2 | 25.2 | −8.9 | −18.4 | −28.5 | −30.1 |
| kraft lignin | −6.5 | −20.1 | −23.3 | −26.1 | −30.8 |
| TiO2-lignin (5-1 wt./wt.) | 18.3 | −12.3 | −26.1 | −30.5 | −36.6 |
| TiO2-lignin (1-1 wt./wt.) | −1.3 | −28.5 | −32.3 | −36.2 | −37.1 |
| TiO2-lignin (1-5 wt./wt.) | −10.3 | −30.1 | −36.1 | −38.2 | −40.8 |
| Sample | Tonset | Tmax | Td5 | Td30 | Ts |
|---|---|---|---|---|---|
| °C | |||||
| LDPE | 466.9 | 476.2 | 429.8 | 466.0 | 221.2 |
| LDPE/TiO2_5 | 460.3 | 477.2 | 443.0 | 472.9 | 225.9 |
| LDPE/TiO2_10 | 469.8 | 479.3 | 450.2 | 474.7 | 227.8 |
| LDPE/TL(5-1)_5 | 461.3 | 476.9 | 441.7 | 472.2 | 225.4 |
| LDPETL(5-1)_10 | 471.6 | 482.8 | 440.6 | 471.7 | 225.0 |
| LDPE/TL(1-1)_5 | 470.4 | 478.1 | 439.5 | 470.2 | 224.4 |
| LDPE/TL(1-1)_10 | 459.2 | 483.1 | 437.0 | 469.5 | 223.7 |
| LDPE/TL(1-5)_5 | 475.4 | 484.4 | 437.0 | 469.5 | 223.7 |
| LDPE/TL(1-5)_10 | 466.0 | 481.5 | 433.1 | 470.2 | 223.1 |
| LDPE/L_5 | 459.9 | 486.1 | 431.0 | 469.2 | 222.4 |
| LDPE/L_10 | 467.1 | 485.1 | 423.0 | 468.9 | 220.7 |
| Sample | k1 (1/min) | R2 |
|---|---|---|
| LDPE | 2.69 × 10−4 | 0.962 |
| LDPE/TiO2_5 | 5.55 × 10−4 | 0.999 |
| LDPE/TiO2_10 | 6.69 × 10−4 | 0.999 |
| LDPE/TL(5-1)_5 | 6.15 × 10−4 | 0.999 |
| LDPE/TL(5-1)_10 | 6.39 × 10−4 | 0.999 |
| LDPE/TL(1-1)_5 | 8.39 × 10−4 | 0.999 |
| LDPE/TL(1-1)_10 | 1.02 × 10−3 | 0.997 |
| LDPE/TL(1-5)_5 | 6.47 × 10−4 | 0.999 |
| LDPE/TL(1-5)_10 | 7.80 × 10−4 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejczak, P.; Cegłowski, M.; Bula, K.; Klapiszewski, Ł. The Influence of TiO2–Lignin Hybrid Fillers in Low-Density Polyethylene Composites on Photocatalytic Performance and UV-Barrier Properties. Polymers 2024, 16, 474. https://doi.org/10.3390/polym16040474
Jędrzejczak P, Cegłowski M, Bula K, Klapiszewski Ł. The Influence of TiO2–Lignin Hybrid Fillers in Low-Density Polyethylene Composites on Photocatalytic Performance and UV-Barrier Properties. Polymers. 2024; 16(4):474. https://doi.org/10.3390/polym16040474
Chicago/Turabian StyleJędrzejczak, Patryk, Michał Cegłowski, Karol Bula, and Łukasz Klapiszewski. 2024. "The Influence of TiO2–Lignin Hybrid Fillers in Low-Density Polyethylene Composites on Photocatalytic Performance and UV-Barrier Properties" Polymers 16, no. 4: 474. https://doi.org/10.3390/polym16040474
APA StyleJędrzejczak, P., Cegłowski, M., Bula, K., & Klapiszewski, Ł. (2024). The Influence of TiO2–Lignin Hybrid Fillers in Low-Density Polyethylene Composites on Photocatalytic Performance and UV-Barrier Properties. Polymers, 16(4), 474. https://doi.org/10.3390/polym16040474








