Gold Nanoclusters Synthesized within Single-Chain Nanoparticles as Catalytic Nanoreactors in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Techniques
2.3. Procedures
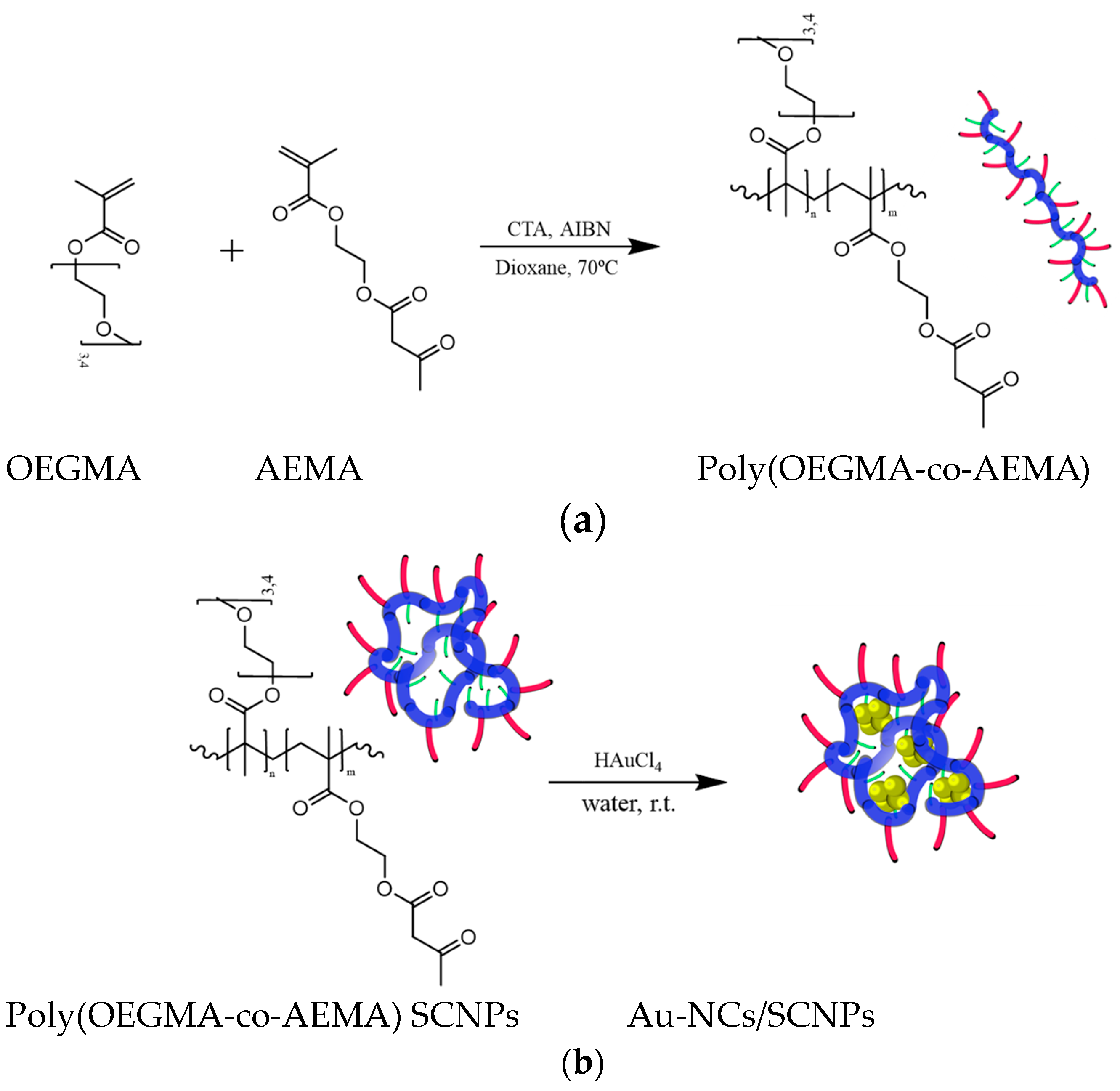
2.3.1. Procedure for the Synthesis of Poly(OEGMA-co-AEMA)
2.3.2. Procedure for the Synthesis of Gold Nanoclusters (Au-NCs) within Poly(OEGMA-co-AEMA) Single-Chain Nanoparticles (SCNPs)
2.3.3. Procedure for the Reduction of 4-Nitrophenol Catalyzed by Au-NCs/SCNPs
2.3.4. Procedure for the Reduction of Nitrobenzene Catalyzed by Au-NCs/SCNPs
2.3.5. Procedure for the Reduction of 3-(4-Nitrophenyl)-1,3-oxazolidin-2-one Catalyzed by Au-NCs/SCNPs
3. Results and Discussion
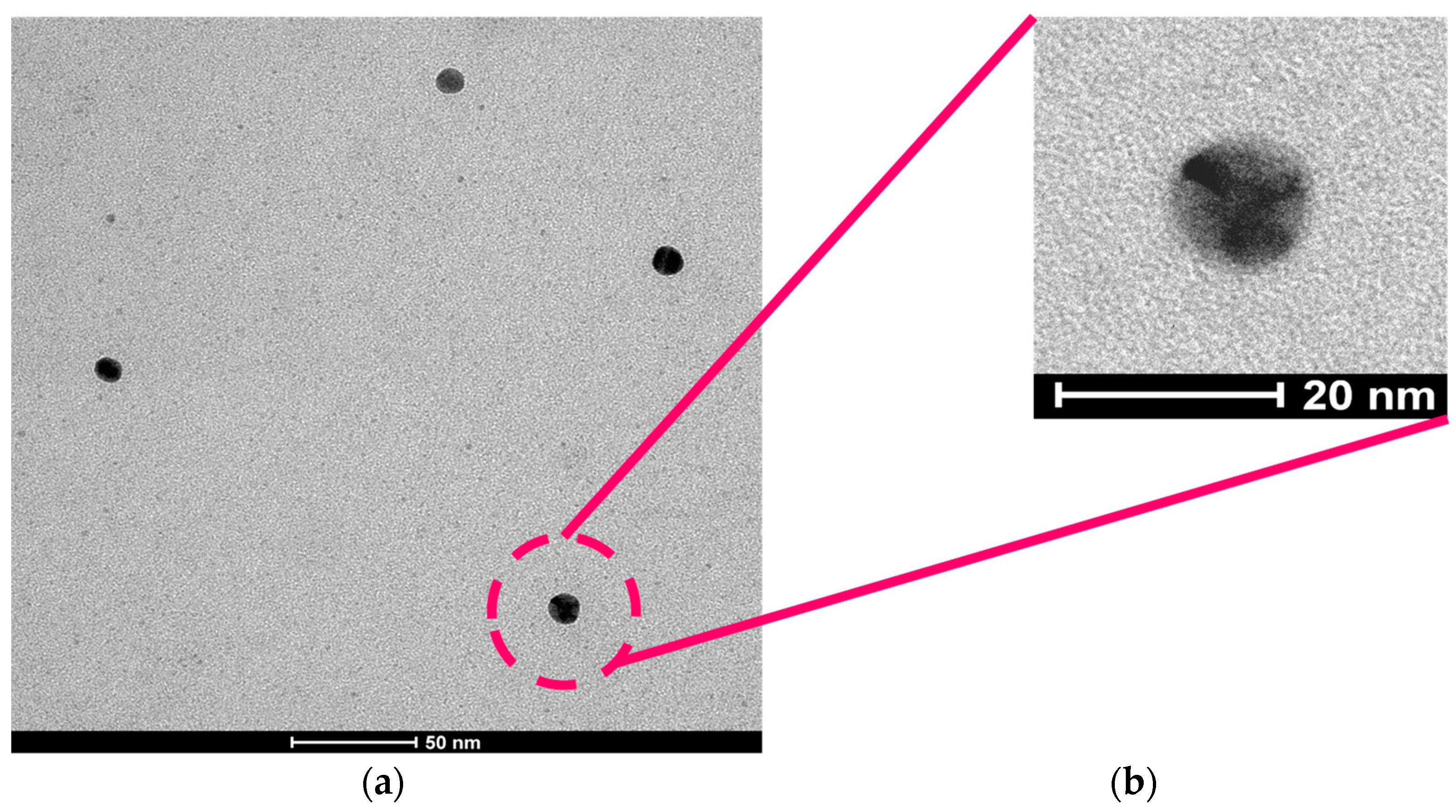
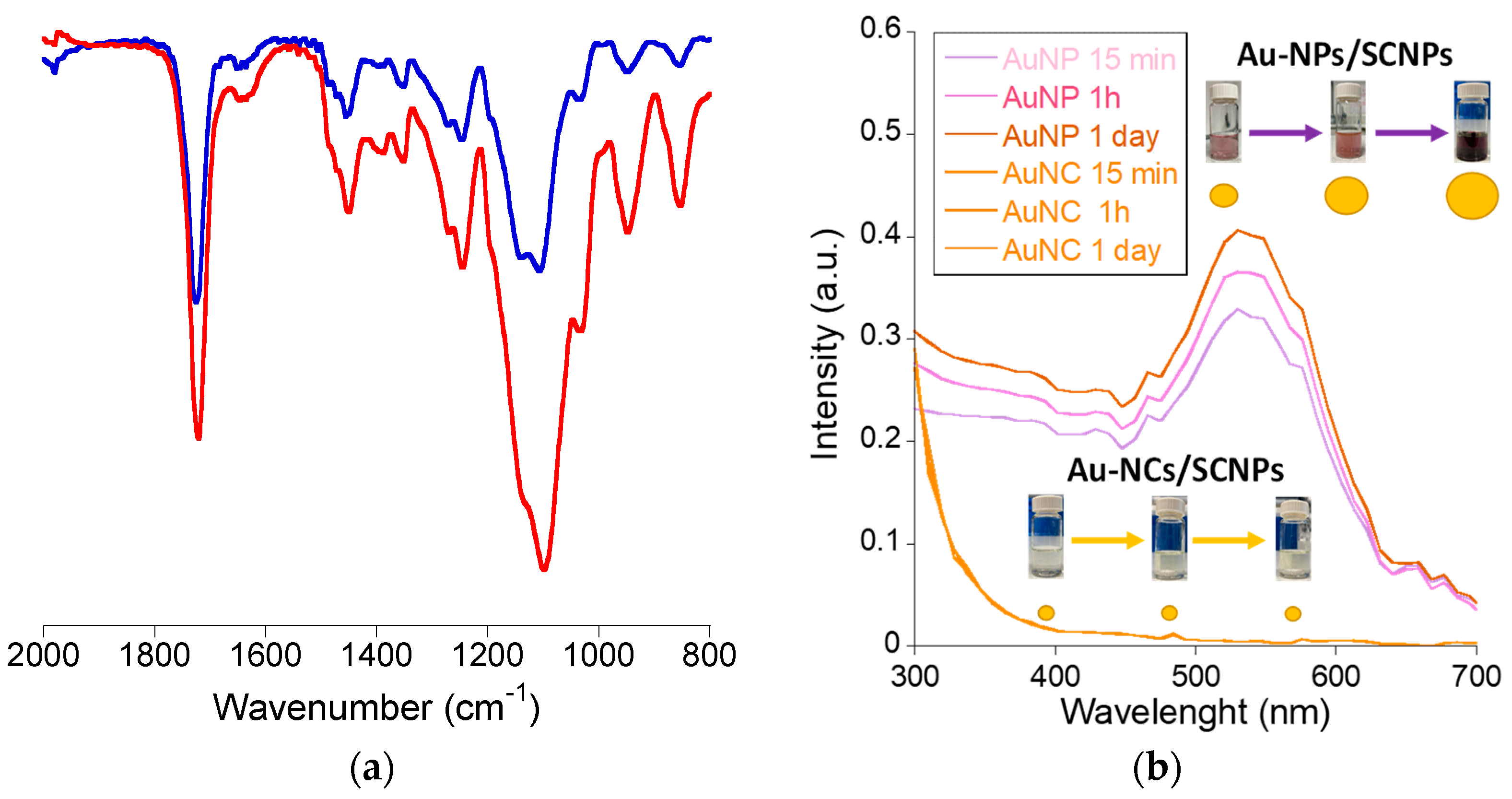
3.1. Synthesis of Gold Nanoclusters within Single-Chain Nanoparticles (Au-NCs/SCNPs)
3.2. Gold Nanoclusters within Single-Chain Nanoparticles (Au-NCs/SCNPs) as Catalytic Nanoreactors
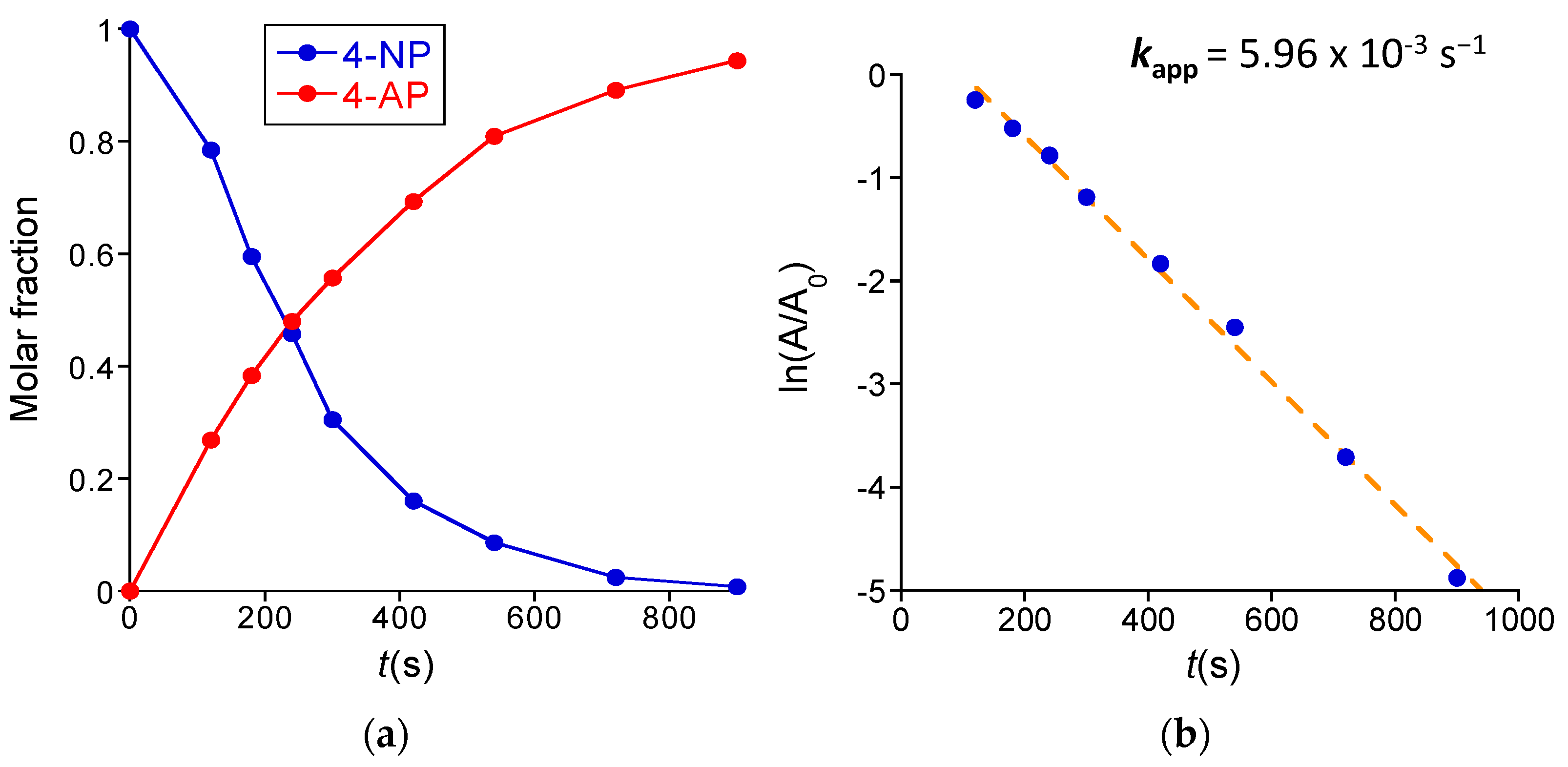
3.2.1. Reduction of 4-Nitrophenol to 4-Aminophenol Catalyzed by Au-NCs/SCNPs
3.2.2. Reduction of Nitrobenzene to Aniline Catalyzed by Au-NCs/SCNPs
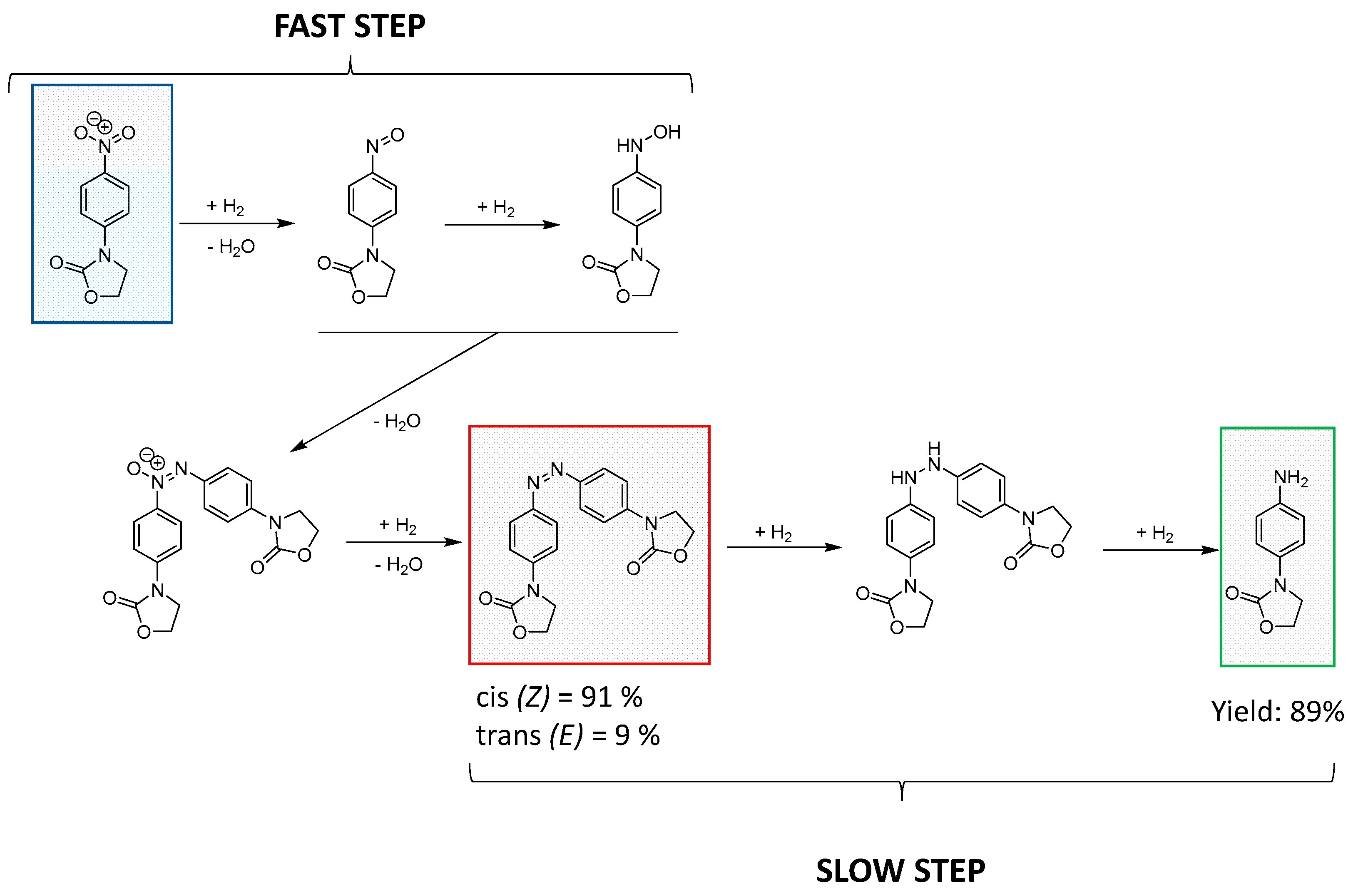
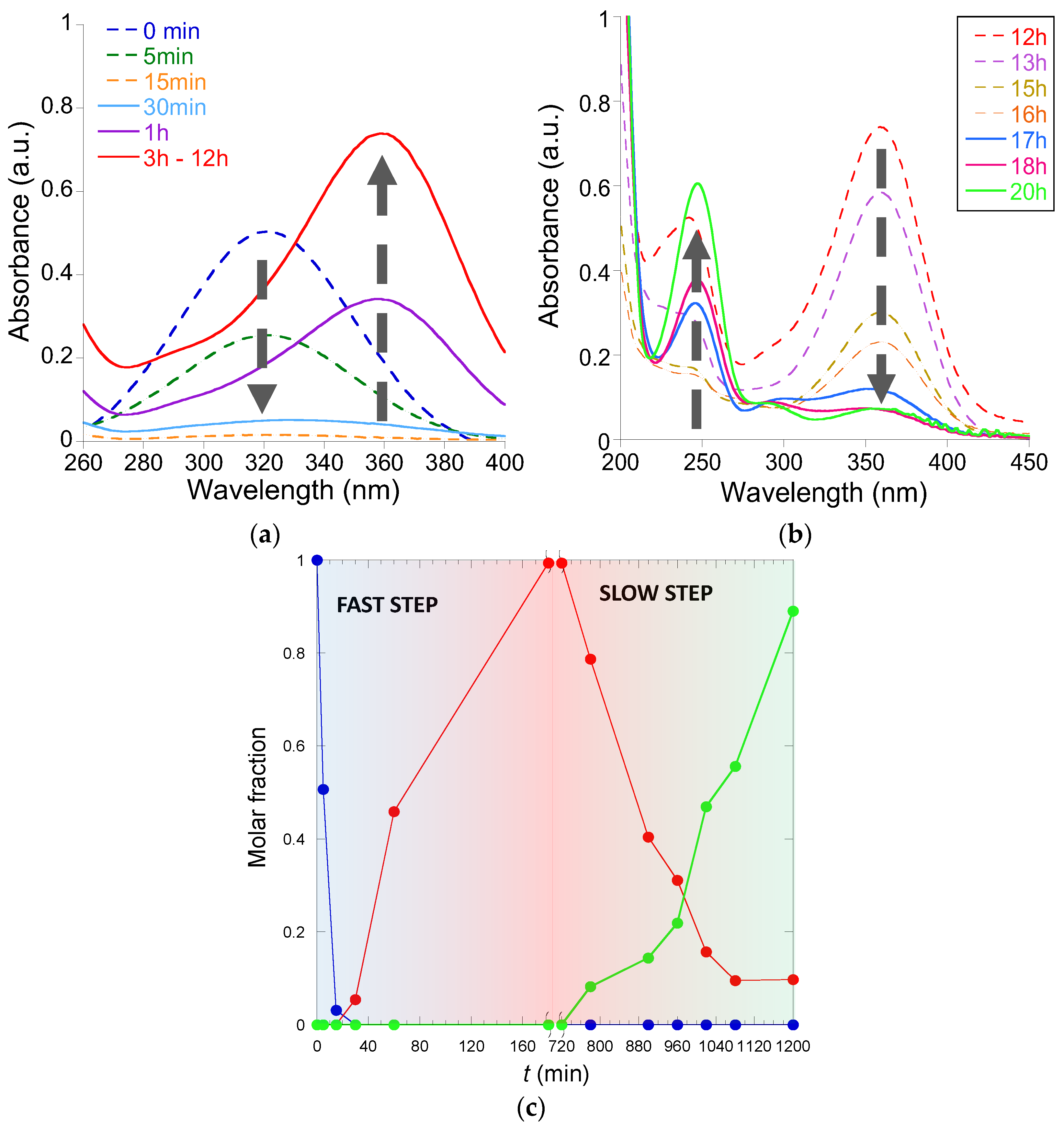
3.2.3. Reduction of 3-(4-Nitrophenyl)-1,3-oxazolidin-2-one to 3-(4-Aminophenyl)-1,3-oxazolidin-2-one Catalyzed by Au-NCs/SCNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valdez, C.E.; Smith, Q.A.; Nechay, M.R.; Alexandrova, A.N. Mysteries of metals in metalloenzymes. Acc. Chem. Res. 2014, 47, 3110–3117. [Google Scholar] [CrossRef]
- Götz, S.; Zechel, S.; Hager, M.D.; Newkome, G.R.; Schubert, U.S. Versatile Applications of Metallopolymers. Prog. Polym. Sci. 2021, 119, 101428. [Google Scholar] [CrossRef]
- Womble, C.T.; Kuepfert, M.; Cohen, A.E.; Weck, M. Multicompartment Polymeric Nanoreactors for Non-Orthogonal Cascade Catalysis. Macromol. Rapid Commun. 2019, 40, 1800580. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Sánchez, A.; Pomposo, J.A. Recent bioinspired applications of single-chain nanoparticles. Polym. Int. 2016, 65, 855–860. [Google Scholar] [CrossRef]
- Pomposo, J.A.; Moreno, A.J.; Arbe, A.; Colmenero, J. Local Domain Size in Single-Chain Polymer Nanoparticles. ACS Omega 2018, 3, 8648–8654. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.M.; Garcia, E.S.; Chen, J.F.; Zhu, L.Y.; Alzona, A.J.; Zimmerman, S.C. Enzyme-like catalysis by single chain nanoparticles that use transition metal cofactors. Chem. Commun. 2022, 58, 985–988. [Google Scholar] [CrossRef]
- Rubio-Cervilla, J.; González, E.; Pomposo, J.A. Advances in Single-Chain Nanoparticles for Catalysis Applications. Nanomaterials 2017, 7, 341. [Google Scholar] [CrossRef]
- Rothfuss, H.; Knöfel, N.D.; Roesky, P.W.; Barner-Kowollik, C. Single-Chain Nanoparticles as Catalytic Nanoreactors. J. Am. Chem. Soc. 2018, 140, 5875–5881. [Google Scholar] [CrossRef]
- ter Huurne, G.M.; Palmans, A.R.A.; Meijer, E.W. Supramolecular Single-Chain Polymeric Nanoparticles. CCS Chem. 2019, 1, 64–82. [Google Scholar] [CrossRef]
- Verde-Sesto, E.; Arbe, A.; Moreno, A.J.; Cangialosi, D.; Alegría, A.; Colmenero, J.; Pomposo, J.A. Single-chain nanoparticles: Opportunities provided by internal and external confinement. Mater. Horiz. 2020, 7, 2292–2313. [Google Scholar] [CrossRef]
- Mundsinger, K.; Izuagbe, A.; Tuten, B.; Roesky, P.; Barner-Kowollik, C. Single Chain Nanoparticles in Catalysis. Angew. Chem. Int. Ed. 2023, e202311734. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tremblay, L.; Lacelle, S.; Zhao, Y. Preparation of Polymer Single Chain Nanoparticles Using Intramolecular Photodimerization of Coumarin. Soft Matter 2011, 7, 2380–2386. [Google Scholar] [CrossRef]
- Fan, W.Z.; Tong, X.; Farnia, F.; Yu, B.; Zhao, Y. CO2-Responsive Polymer Single-Chain Nanoparticles and Self-Assembly for Gas-Tunable Nanoreactors. Chem. Mater. 2017, 29, 5693–5701. [Google Scholar] [CrossRef]
- Yang, Z.R.; Yang, X.; Guo, Y.H.; Kawasaki, H. A Review on Gold Nanoclusters for Cancer Phototherapy. ACS Appl. Bio Mater. 2023, 6, 4504–4517. [Google Scholar] [CrossRef]
- Mordini, D.; Mavridi-Printezi, A.; Menichetti, A.; Cantelli, A.; Li, X.K.; Montalti, M. Luminescent Gold Nanoclusters for Bioimaging: Increasing the Ligand Complexity. Nanomaterials 2023, 13, 648. [Google Scholar] [CrossRef]
- He, Z.; Shu, T.; Su, L.; Zhang, X.J. Strategies of Luminescent Gold Nanoclusters for Chemo-/Bio-Sensing. Molecules 2019, 24, 3045. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Tian, W.J.; Liu, Y.Y. The ligand effect of atomically precise gold nanoclusters in tailoring catalytic properties. Nanoscale 2021, 13, 16847–16859. [Google Scholar] [CrossRef] [PubMed]
- Casteleiro, B.; Martinho, J.M.G.; Farinha, J.P.S. Encapsulation of gold nanoclusters: Stabilization and more. Nanoscale 2021, 13, 17199–17217. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Nasaruddin, R.R.; Chen, T.K.; Yan, N.; Xie, J.P. Roles of thiolate ligands in the synthesis, properties and catalytic application of gold nanoclusters. Coord. Chem. Rev. 2018, 368, 60–79. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Sharma, S.; Li, G. Recent progress in heterogeneous catalysis by atomically and structurally precise metal nanoclusters. Chem. Rec. 2021, 21, 879–892. [Google Scholar] [CrossRef]
- Biswas, S.; Das, S.; Negishi, Y. Advances in Cu nanocluster catalyst design: Recent progress and promising applications. Nanoscale Horiz. 2023, 8, 1509–1522. [Google Scholar] [CrossRef]
- Matus, M.F.; Häkkinen, H. Understanding ligand-protected noble metal nanoclusters at work. Nature Rev. Mater. 2023, 8, 372–389. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, H.C.; Gan, Y.H.; Wu, B.D.; Chen, Z.H.; Wei, S.S.; Zhang, G.Y.; Zhang, S.J.; Pan, B.C.; Chen, C.C. An all-in-one approach for synthesis and functionalization of nano colloidal gold with acetylacetone. Nanotechnology 2022, 33, 075605. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Arbe, A.; Kohlbrecher, J.; Colmenero, J.; Pomposo, J.A. Efficient Synthesis of Single-Chain Globules Mimicking the Morphology and Polymerase Activity of Metalloenzymes. Macromol. Rapid Commun. 2015, 36, 1592–1597. [Google Scholar] [CrossRef]
- Robles-Hernández, B.; González, E.; Pomposo, J.A.; Colmenero, J.; Alegría, A. Water dynamics and self-assembly of single-chain nanoparticles in concentrated solutions. Soft Matter 2020, 16, 9738–9745. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic Analysis of the Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- Yamamoto, H.; Yano, H.; Kouchi, H.; Obora, Y.; Arakawa, R.; Kawasaki, H. N,N-Dimethylformamide-stabilized gold nanoclusters as a catalyst for the reduction of 4-nitrophenol. Nanoscale 2012, 4, 4148–4154. [Google Scholar] [CrossRef] [PubMed]
- Vetráková, L.; Ladányi, V.; Al Anshori, J.; Dvorák, P.; Wirz, J.; Heger, D. The absorption spectrum of cis-azobenzene. Photochem. Photobiol. Sci. 2017, 16, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Noschese, A.; Buonerba, A.; Canton, P.; Milione, S.; Capacchione, C.; Grassi, A. Highly efficient and selective reduction of nitroarenes into anilines catalyzed by gold nanoparticles incarcerated in a nanoporous polymer matrix: Role of the polymeric support and insight into the reaction mechanism. J. Catal. 2016, 340, 30–40. [Google Scholar] [CrossRef]




| Material Type | Reaction Time, t | DLS Hydrodynamic Diameter, Dh (nm) 2 |
|---|---|---|
| Poly(OEGMA-co-AEMA) 1 | 0 min. | 11.0 |
| Au-NCs/SCNPs | 1 min. | 11.0 |
| Au-NCs/SCNPs | 15 min. | 10.7 |
| Au-NCs/SCNPs | 1 h | 11.3 |
| Au-NCs/SCNPs | 1 day | 11.2 |
| Au-NCs/SCNPs | 1 week | 11.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinacho-Olaciregui, J.; Verde-Sesto, E.; Taton, D.; Pomposo, J.A. Gold Nanoclusters Synthesized within Single-Chain Nanoparticles as Catalytic Nanoreactors in Water. Polymers 2024, 16, 378. https://doi.org/10.3390/polym16030378
Pinacho-Olaciregui J, Verde-Sesto E, Taton D, Pomposo JA. Gold Nanoclusters Synthesized within Single-Chain Nanoparticles as Catalytic Nanoreactors in Water. Polymers. 2024; 16(3):378. https://doi.org/10.3390/polym16030378
Chicago/Turabian StylePinacho-Olaciregui, Jokin, Ester Verde-Sesto, Daniel Taton, and José A. Pomposo. 2024. "Gold Nanoclusters Synthesized within Single-Chain Nanoparticles as Catalytic Nanoreactors in Water" Polymers 16, no. 3: 378. https://doi.org/10.3390/polym16030378
APA StylePinacho-Olaciregui, J., Verde-Sesto, E., Taton, D., & Pomposo, J. A. (2024). Gold Nanoclusters Synthesized within Single-Chain Nanoparticles as Catalytic Nanoreactors in Water. Polymers, 16(3), 378. https://doi.org/10.3390/polym16030378







