Electrochemical Analysis of Curcumin in Real Samples Using Intelligent Materials
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Solutions
2.2. Instrumentation
2.3. Sample Collection and Preparation
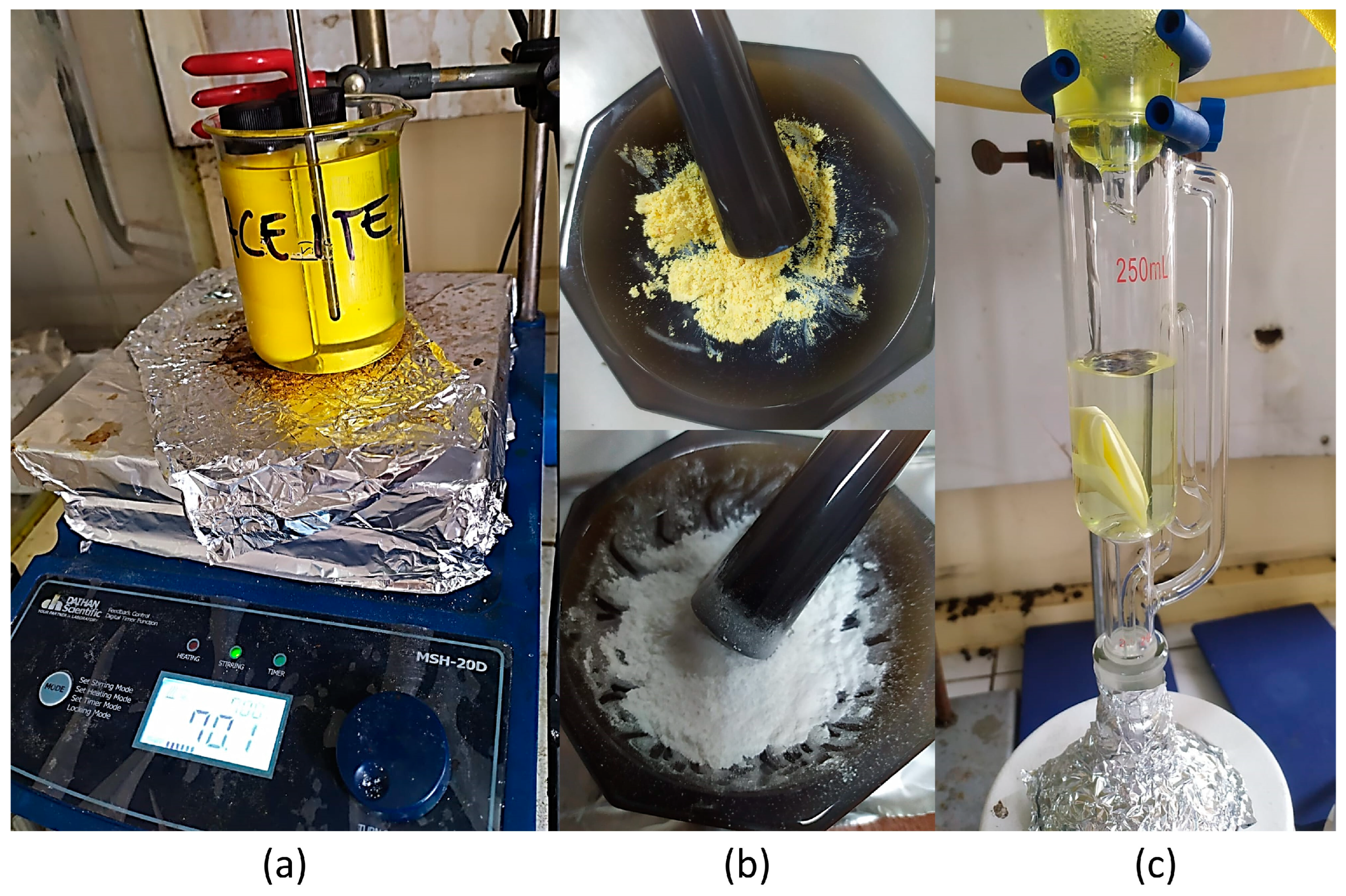
2.4. Synthesis of Molecularly Imprinted Polymer (MIP) and Molecularly Non-Imprinted Polymer (NIP) by Precipitation Method
2.5. Modification of the Glassy Carbon Electrode
2.6. Application of Modified Sensor to Real Samples
3. Results and Discussion
3.1. Textural Morphological and FTIR Characterization of the MIPs and NIPs
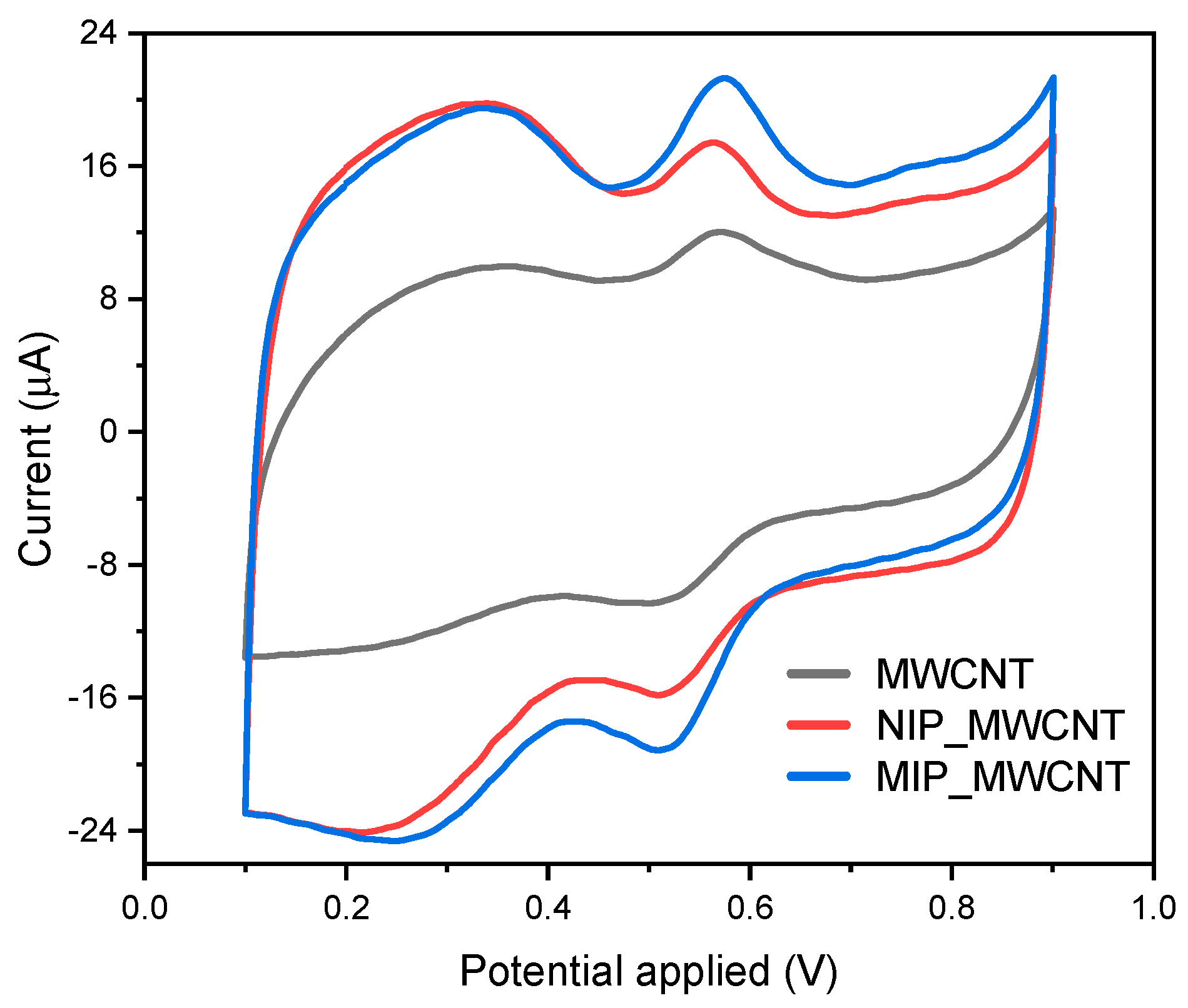
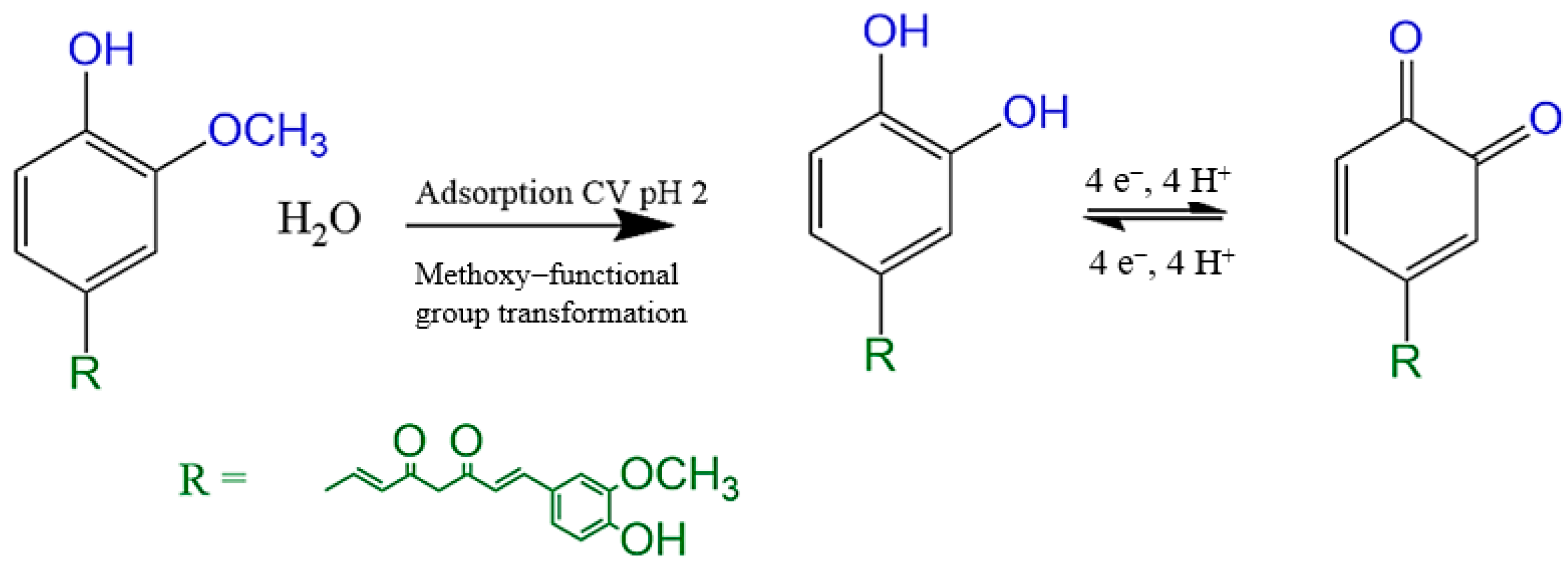
3.2. Electrochemical Behavior of Curcumin
3.3. Analysis of Selectivity
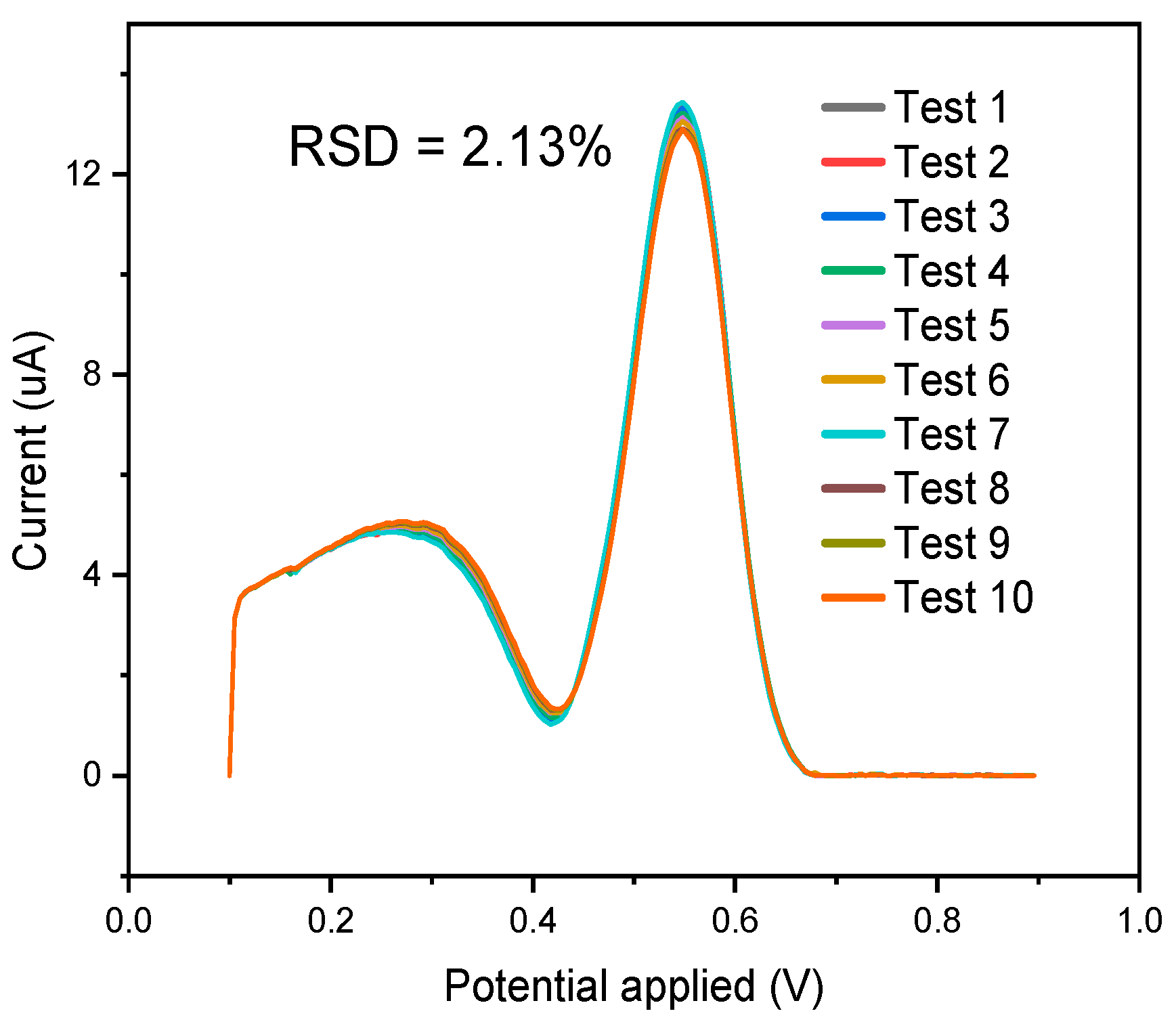
3.4. Repeatability Study
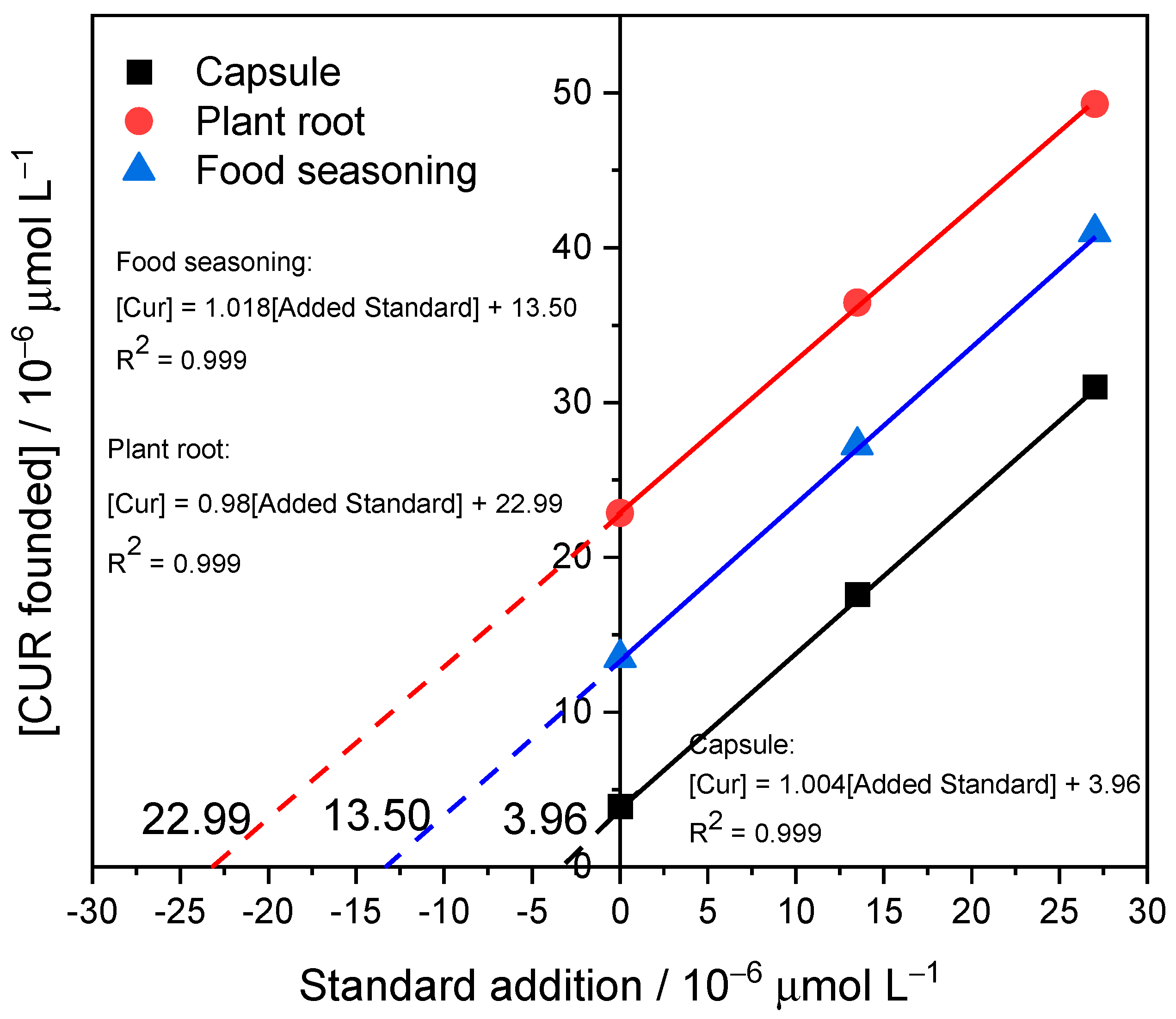
3.5. Applications to Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarado, H.-L.; Limón, D.; Calpena-Campmany, A.-C.; Mallandrich, M.; Rodríguez-Cid, L.; Aliaga-Alcalde, N.; González-Campo, A.; Pérez-García, L. Intrinsic Permeation and Anti-Inflammatory Evaluation of Curcumin, Bisdemethoxycurcumin and Bisdemethylcurcumin by a Validated HPLC-UV Method. Int. J. Mol. Sci. 2023, 24, 6640. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Salkini, A.A.; Alam, P.; Alshahrani, K.A.; Foudah, A.I.; Alqarni, M.H. A High-Performance Thin-Layer Chromatographic Method for the Simultaneous Determination of Curcumin I, Curcumin II and Curcumin III in Curcuma longa and Herbal Formulation. Separations 2022, 9, 94. [Google Scholar] [CrossRef]
- Rios-Aguirre, S.; Gil-Garzón, M.A. Microencapsulación por secado por aspersión de compuestos bioactivos en diversas matrices: Una revisión. TecnoLógicas 2021, 24, e1836. [Google Scholar] [CrossRef]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Babu, B.V. Removal of toxic metal Cr(VI) from aqueous solutions using sawdust as adsorbent: Equilibrium, kinetics and regeneration studies. Chem. Eng. J. 2009, 150, 352–365. [Google Scholar] [CrossRef]
- Elfadil, D.; Lamaoui, A.; Della Pelle, F.; Amine, A.; Compagnone, D. Molecularly Imprinted Polymers Combined with Electrochemical Sensors for Food Contaminants Analysis. Molecules 2021, 26, 4607. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Amine, A. Recent Advances in Electrochemical Sensors Based on Molecularly Imprinted Polymers and Nanomaterials. Electroanalysis 2019, 31, 188–201. [Google Scholar] [CrossRef]
- López, R.; Khan, S.; Wong, A.; Sotomayor, M.D.P.T.; Picasso, G. Development of a New Electrochemical Sensor Based on Mag-MIP Selective Toward Amoxicillin in Different Samples. Front. Chem. 2021, 9, 615602. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A. Powder XRD Technique and its Applications in Science and Technology. J. Anal. Bioanal. Tech 2014, 5, 212. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Melamine Detection in Milk and Dairy Products: Traditional Analytical Methods and Recent Developments. Food Anal. Methods 2018, 11, 128–147. [Google Scholar] [CrossRef]
- Yusuf, H.; Wijiani, N.; Rahmawati, R.A.; Primaharinastiti, R.; Rijal, M.A.S.; Isadiartuti, D. Analytical method for the determination of curcumin entrapped in polymeric micellar powder using HPLC. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Anjani, Q.K.; Utomo, E.; Domínguez-Robles, J.; Detamornrat, U.; Donnelly, R.F.; Larrañeta, E. A New and Sensitive HPLC-UV Method for Rapid and Simultaneous Quantification of Curcumin and D-Panthenol: Application to In Vitro Release Studies of Wound Dressings. Molecules 2022, 27, 1759. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Ghuge, A.; Parab, M.; Al-Refaei, Y.; Khandare, A.; Dand, N.; Waghmare, N. A comparative review on High-Performance Liquid Chromatography (HPLC), Ultra Performance Liquid Chromatography (UPLC) & High-Performance Thin Layer Chromatography (HPTLC) with current updates. Curr. Issues Pharm. Med. Sci. 2022, 35, 224–228. [Google Scholar] [CrossRef]
- Karimi, M.; Mashreghi, M.; Shokooh Saremi, S.; Jaafari, M.R. Spectrofluorometric Method Development and Validation for the Determination of Curcumin in Nanoliposomes and Plasma. J. Fluoresc. 2020, 30, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Urruela, C.; Vera-López, S.; San Andrés, M.P.; Díez-Pascual, A.M. Graphene-Based Sensors for the Detection of Bioactive Compounds: A Review. Int. J. Mol. Sci. 2021, 22, 3316. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Reddy, K.R. Novel heterostructured Ru-doped TiO2/CNTs hybrids with enhanced electrochemical sensing performance for Cetirizine. Mater. Res. Express 2019, 6, 115085. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.-C.; OuYang, J.; Jin, L.; Li, H.; Zhao, C.-Y.; Zhang, L.; Wei, J.; et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, R.; Ashrafzadeh, S.; Heidari, B. pH-sensitive molecularly imprinted polymer based on graphene oxide for stimuli actuated controlled release of curcumin. J. Alloy. Compd. 2021, 857, 157603. [Google Scholar] [CrossRef]
- Thongchai, W.; Fukngoen, P. Synthesis of curcuminoid-imprinted polymers applied to the solid-phase extraction of curcuminoids from turmeric samples. J. Pharm. Anal. 2018, 8, 60–68. [Google Scholar] [CrossRef]
- Pesavento, M.; Merli, D.; Biesuz, R.; Alberti, G.; Marchetti, S.; Milanese, C. A MIP-based low-cost electrochemical sensor for 2-furaldehyde detection in beverages. Anal. Chim. Acta 2021, 1142, 201–210. [Google Scholar] [CrossRef]
- Khan, S.; Wong, A.; Rychlik, M.; Sotomayor, M.d.P.T. A Novel Synthesis of a Magnetic Porous Imprinted Polymer by Polyol Method Coupled with Electrochemical Biomimetic Sensor for the Detection of Folate in Food Samples. Chemosensors 2022, 10, 473. [Google Scholar] [CrossRef]
- Wulandari, M.; Nofrizal, N.; Azhar, S.; Sulaiman, S. A novel synthesis of stabilized molecularly imprinted polymer for electropolymerization to detect bismethoxycurcumin from curcuminoid. Songklanakarin J. Sci. Technol. 2022, 44, 1524–1531. [Google Scholar]
- Mohammadinejad, A.; Abouzari-Lotf, E.; Aleyaghoob, G.; Rezayi, M.; Kazemi Oskuee, R. Application of a transition metal oxide/carbon-based nanocomposite for designing a molecularly imprinted poly (l-cysteine) electrochemical sensor for curcumin. Food Chem. 2022, 386, 132845. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Giang, H.H.; Tran, T.T.N.; Van, T.-K.; Tran, T. Synthesis and Characteristics of Polymer-Mediated Curcumin Molecular Imprinting for Quantitative Determination of Curcumin in Food Samples. J. Chromatogr. A 2024, 1713, 464567. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, Z.; Tan, L.; Wang, S.; Wang, C.; Zhang, J.; Zhou, L.; Zhang, Q.; Yuan, C. Rapid measurements of curcumin from complex samples coupled with magnetic biocompatibility molecularly imprinted polymer using electrochemical detection. J. Sep. Sci. 2020, 43, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, R.; Yassari, M.; Heidari, B. Thermo-responsive molecularly imprinted polymer containing magnetic nanoparticles: Synthesis, characterization and adsorption properties for curcumin. Colloids Surf. B Biointerfaces 2018, 162, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Uzuriaga-Sánchez, R.J.; Khan, S.; Wong, A.; Picasso, G.; Pividori, M.I.; Sotomayor, M.D.P.T. Magnetically separable polymer (Mag-MIP) for selective analysis of biotin in food samples. Food Chem. 2016, 190, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.H.M.T.; Naskar, H.; Banerjee, S.; Ghatak, B.; Das, N.; Tudu, B.; Bandyopadhyay, R. Electrochemical sensor based on molecularly imprinted polymer embedded graphite electrode for detecting curcumin. Sens. Actuators A Phys. 2022, 344, 113748. [Google Scholar] [CrossRef]
- Gao, Q.; Zang, Y.; Zhang, Y.; Xie, J.; Li, J.; Gao, J.; Xue, H. Composite polymerized molecular imprinting membrane-based electrochemical sensor for sensitive determination of curcumin by using 4-pentenoyl-aminoacyl-chitosan oligosaccharide as functional monomer oligomer. J. Electroanal. Chem. 2020, 879, 114793. [Google Scholar] [CrossRef]
- Espinoza-Torres, S.; López, R.; Sotomayor, M.D.P.T.; Tuesta, J.C.; Picasso, G.; Khan, S. Synthesis, Characterization, and Evaluation of a Novel Molecularly Imprinted Polymer (MIP) for Selective Quantification of Curcumin in Real Food Sample by UV-Vis Spectrophotometry. Polymers 2023, 15, 3332. [Google Scholar] [CrossRef]
- Zheng, L.; Song, J. Curcumin multi-wall carbon nanotubes modified glassy carbon electrode and its electrocatalytic activity towards oxidation of hydrazine. Sens. Actuators B Chem. 2009, 135, 650–655. [Google Scholar] [CrossRef]
- Dinesh, B.; Shalini Devi, K.S.; Kumar, A.S. Curcumin-quinone immobilised carbon black modified electrode prepared by in-situ electrochemical oxidation of curcumin-phytonutrient for mediated oxidation and flow injection analysis of sulfide. J. Electroanal. Chem. 2017, 804, 116–127. [Google Scholar] [CrossRef]
- Jara-Cornejo, E.; Khan, S.; Vega-Chacón, J.; Wong, A.; da Silva Neres, L.C.; Picasso, G.; Sotomayor, M.D.P.T. Biomimetic Material for Quantification of Methotrexate Using Sensor Based on Molecularly Imprinted Polypyrrole Film and MWCNT/GCE. Biomimetics 2023, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhai, H.Y.; Pan, Y.F.; Li, K. A simple and sensitive sensor based on a molecularly imprinted polymer-modified carbon paste electrode for the determination of curcumin in foods. RSC Adv. 2017, 7, 22913–22918. [Google Scholar] [CrossRef]
- Kar, S.; Naskar, H.; Tudu, B.; Bandyopadhyay, R. Application of a Polytrimethoxysilane Based Molecularly Imprinted Polymer (MIP) Electrode towards Discrimination of Different Types of Turmeric Powde. Carbon Sci. Technol. 2018, 10, 8–16. [Google Scholar]
- David, I.G.; Iorgulescu, E.E.; Popa, D.E.; Buleandra, M.; Cheregi, M.C.; Noor, H. Curcumin Electrochemistry—Antioxidant Activity Assessment, Voltammetric Behavior and Quantitative Determination, Applications as Electrode Modifier. Antioxidants 2023, 12, 1908. [Google Scholar] [CrossRef]





| Amounts | Concentration (mM) | Molar Ratio | ||
|---|---|---|---|---|
| Curcumin | 0.1 mmol | 2.5 | %T | |
| Acrylamide | 0.4 mmol | 10.0 | (Acrylamide (g) + EGDMA(g))/V(mL) | 2.55 |
| EGDMA | 5.0 mmol | 125.0 | %C | |
| ABCVA | 100 mg | - | EGDMA/(Acrylamide + EGDMA) | 0.926 |
| Acetonitrile | 40 mL | - | Template:(Acrylamide + EGDMA) | 1:54 |
| Polymer | BET Surface Area (m2 g−1) | Micropore Area (m2 g−1) | Mesopore Area (m2 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| MIP | 48.9 | 8.1 | 40.8 | 40.1 |
| NIP | 10.9 | 1.5 | 9.4 | 43.0 |
| Sample | Added Concentration (µmol L−1) | HPLC Method | Electrochemical Method | % Recovery of Proposed Method * | %Error ** |
|---|---|---|---|---|---|
| Sample Concentration | Sample Concentration | ||||
| (µmol L−1) | (µmol L−1) | ||||
| Capsule | 0 | 3.81 | 3.92 | 103.98 | 3.53 |
| 13.5 | 17.4 | 17.6 | |||
| 27 | 30.89 | 31.03 | |||
| Plant root | 0 | 22.92 | 22.87 | 100.33 | −2.18 |
| 13.5 | 37.09 | 36.46 | |||
| 27 | 47.73 | 49.29 | |||
| Food seasoning | 0 | 13.67 | 13.51 | 98.78 | −1.41 |
| 13.5 | 27.32 | 27.24 | |||
| 27 | 40.81 | 41.01 |
| Material | Analyte/ Real Sample | Method | LOD/ %Recovery | Ref. |
|---|---|---|---|---|
| MIP-CPE Sensor | Curcumin/ Curcuma powder and cookies | CV | 10.1 nmol L−1 90.77~105.7% | [34] |
| PAA-MIP/ G Sensor | Curcumin/ Turmeric powder and capsules | DPV | 0.04 µmol L−1 >99.00% | [28] |
| GCE/CuCo2O4/N-CNTs/P-GO/MIP (L-Cys) | Curcumin/ Serum | DPV | 30 nmol L−1 80.00–110.87% | [23] |
| MIP-CPE Sensor | Curcumin/ Turmeric powder | CV | 0.02 µmol L−1 1–100 µmol L−1 | [35] |
| CUR-MIP/GCE | Curcumin/ Turmeric extract | DPV | 5.0 nmol L−1 0.1–2.0 mmol L−1 | [36] |
| MIP/MWCNT/GCE Sensor | Curcumin/ Capsule, plant root, and food seasoning | CV/DPV | 0.136 µmol L−1 87.5–102.6% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jara-Cornejo, E.; Peña-Bedón, E.; Torres Moya, M.; Espinoza-Torres, S.; Sotomayor, M.D.P.T.; Picasso, G.; Tuesta, J.C.; López, R.; Khan, S. Electrochemical Analysis of Curcumin in Real Samples Using Intelligent Materials. Polymers 2024, 16, 366. https://doi.org/10.3390/polym16030366
Jara-Cornejo E, Peña-Bedón E, Torres Moya M, Espinoza-Torres S, Sotomayor MDPT, Picasso G, Tuesta JC, López R, Khan S. Electrochemical Analysis of Curcumin in Real Samples Using Intelligent Materials. Polymers. 2024; 16(3):366. https://doi.org/10.3390/polym16030366
Chicago/Turabian StyleJara-Cornejo, Eduardo, Erick Peña-Bedón, Mahely Torres Moya, Sergio Espinoza-Torres, Maria D. P. T. Sotomayor, Gino Picasso, Juan C. Tuesta, Rosario López, and Sabir Khan. 2024. "Electrochemical Analysis of Curcumin in Real Samples Using Intelligent Materials" Polymers 16, no. 3: 366. https://doi.org/10.3390/polym16030366
APA StyleJara-Cornejo, E., Peña-Bedón, E., Torres Moya, M., Espinoza-Torres, S., Sotomayor, M. D. P. T., Picasso, G., Tuesta, J. C., López, R., & Khan, S. (2024). Electrochemical Analysis of Curcumin in Real Samples Using Intelligent Materials. Polymers, 16(3), 366. https://doi.org/10.3390/polym16030366










