Compatibilization of Cellulose Nanocrystal-Reinforced Natural Rubber Nanocomposite by Modified Natural Rubber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Pre-Dispersed NR/CNC Nanocomposite
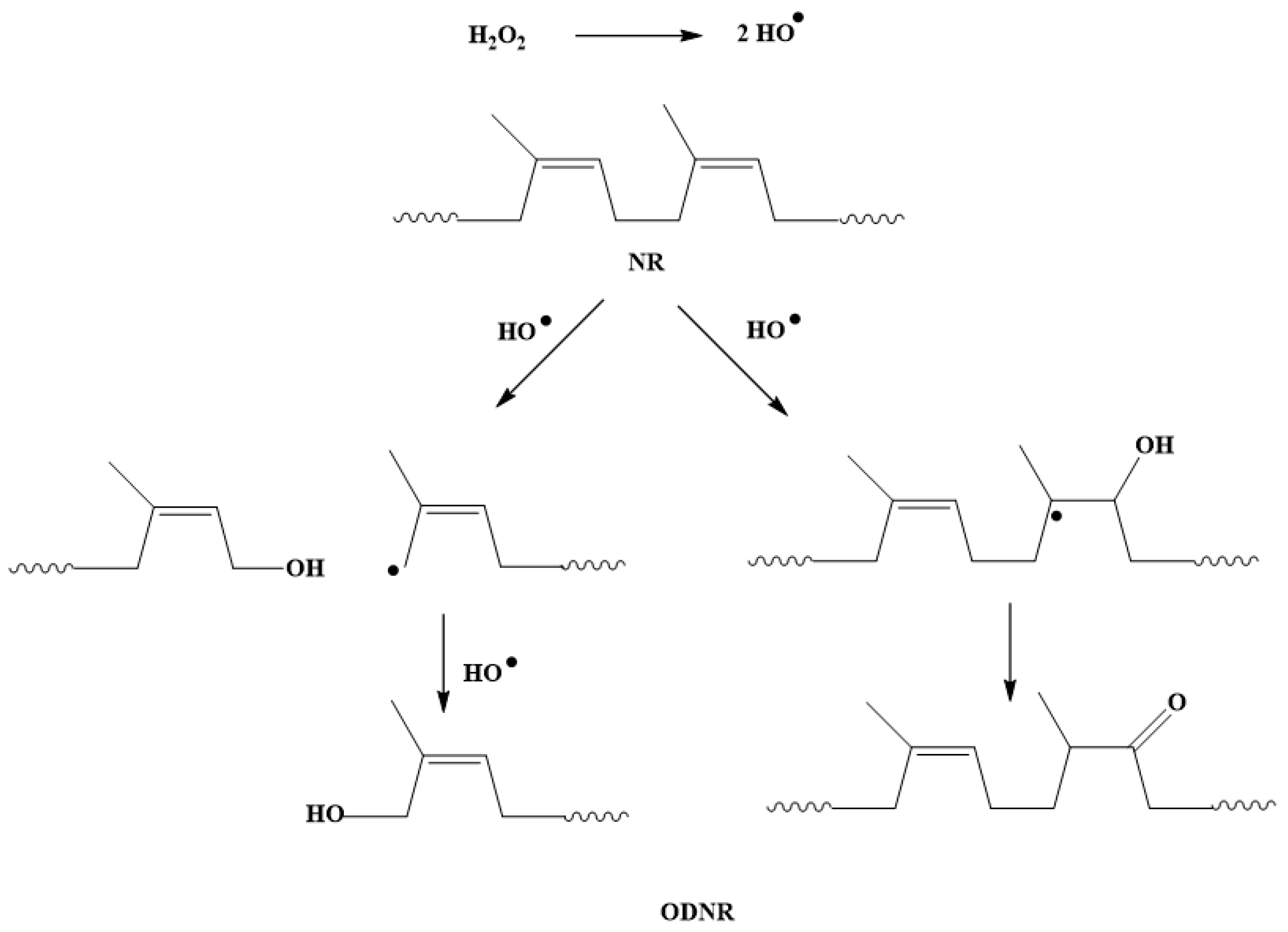
2.3. Preparation of Oxidized Degraded NR (ODNR)
2.4. Preparation of Rubber Compounding
2.5. Preparation of NR/CNC Vulcanizates
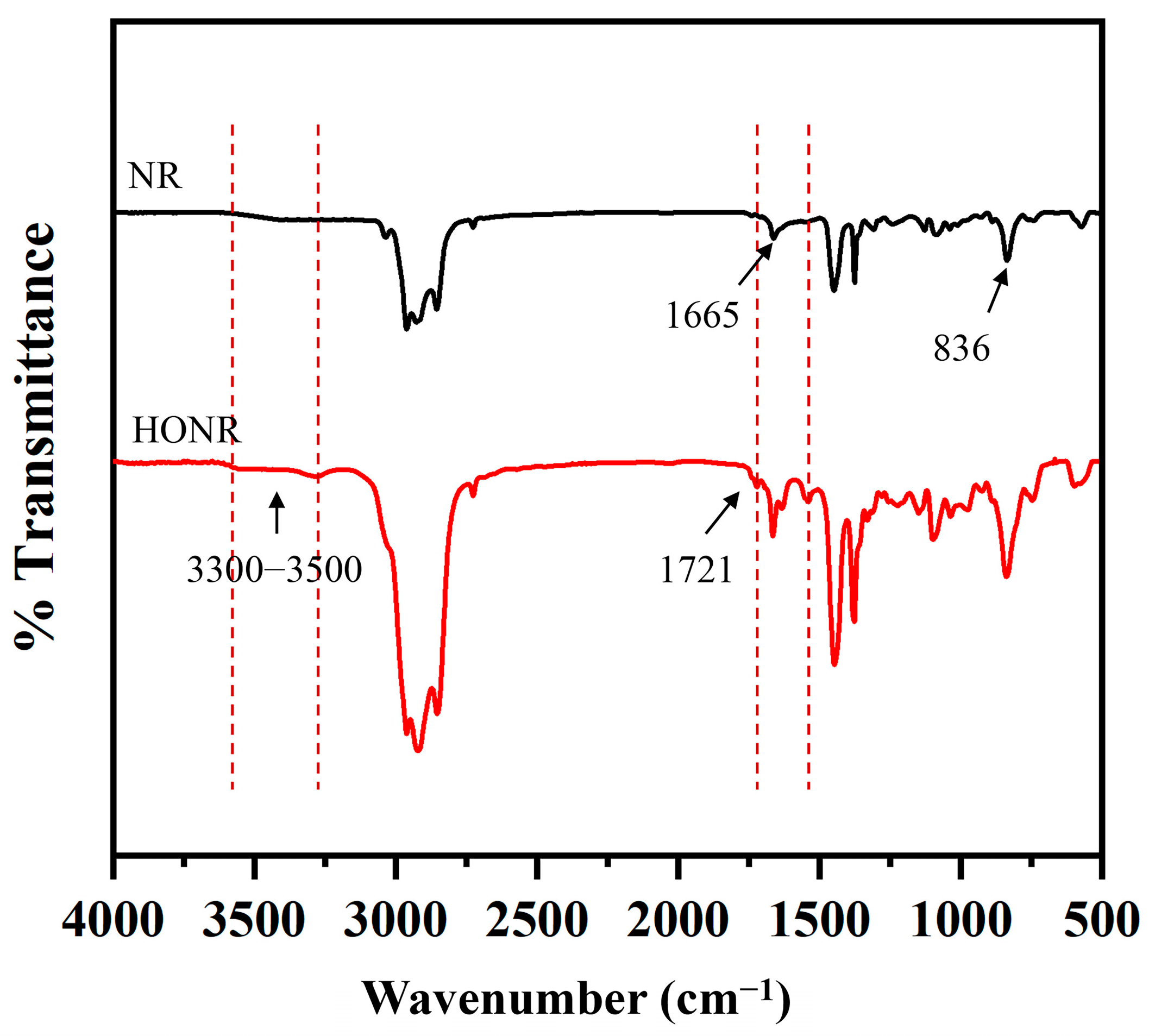
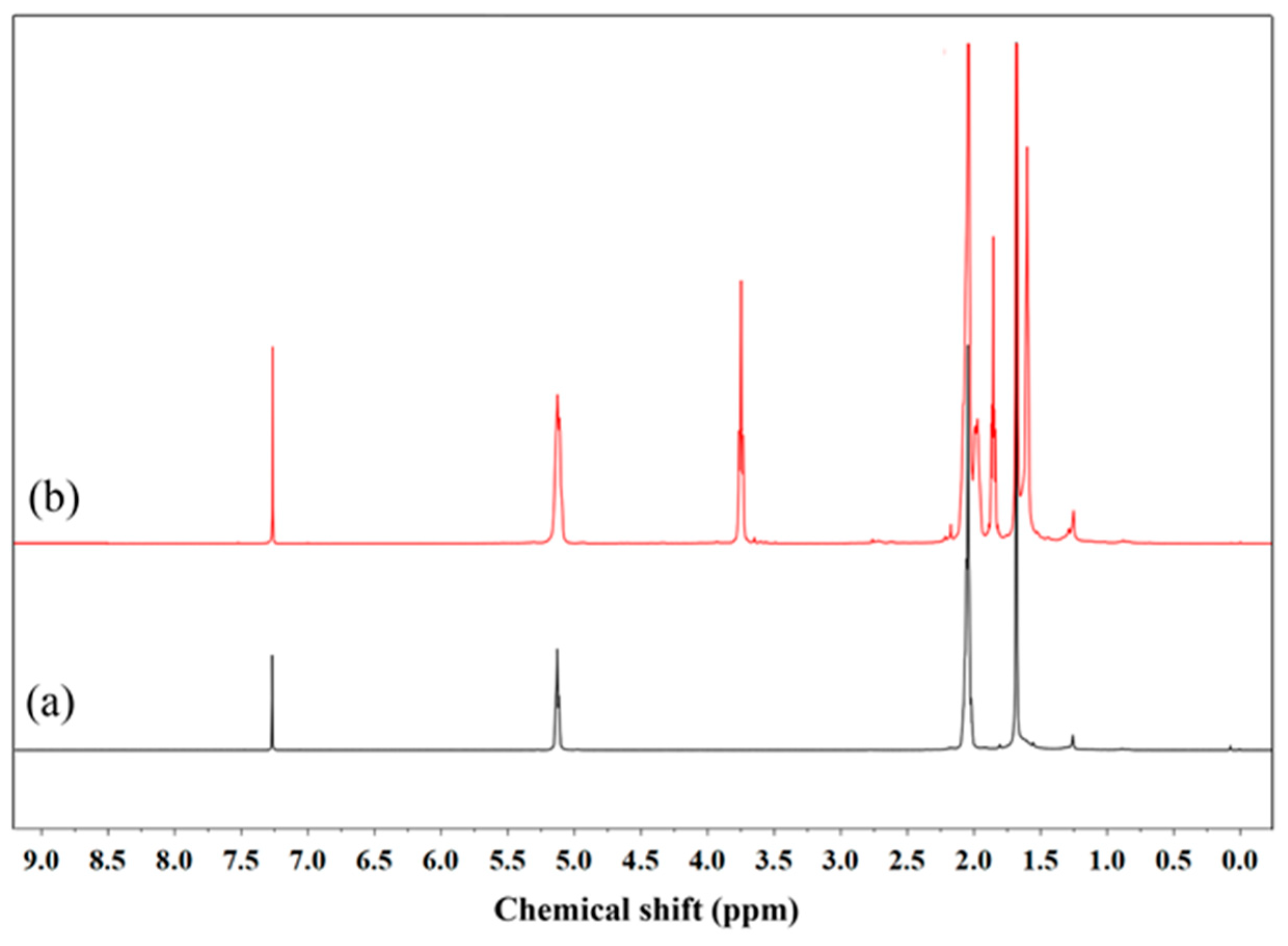
2.6. Characterization of ODNR
2.7. Characterization of Rubber Nanocomposites
3. Results and Discussion
3.1. Analysis of ODNR
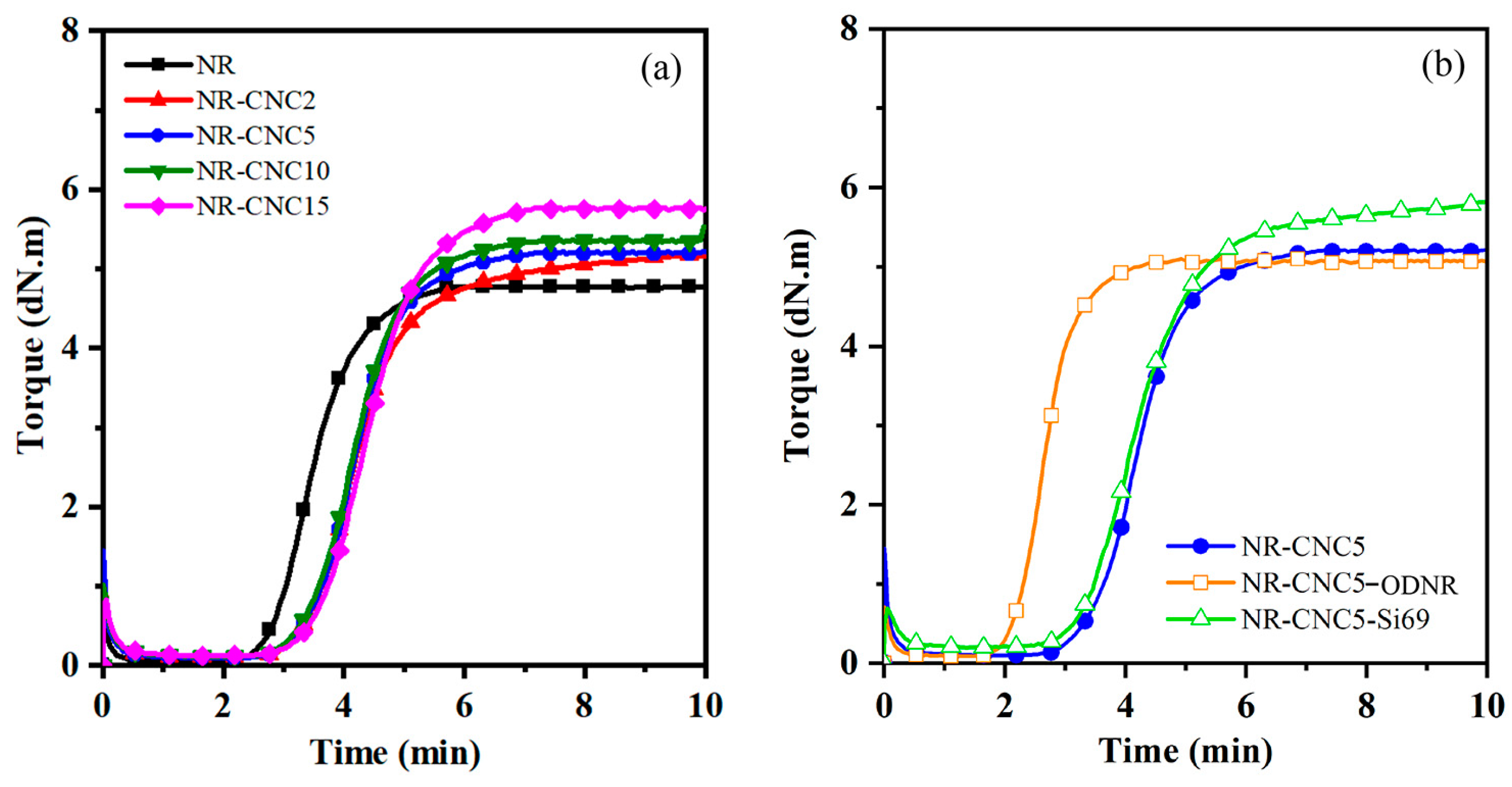
3.2. Cure Characteristics of NR/CNC Nanocomposites
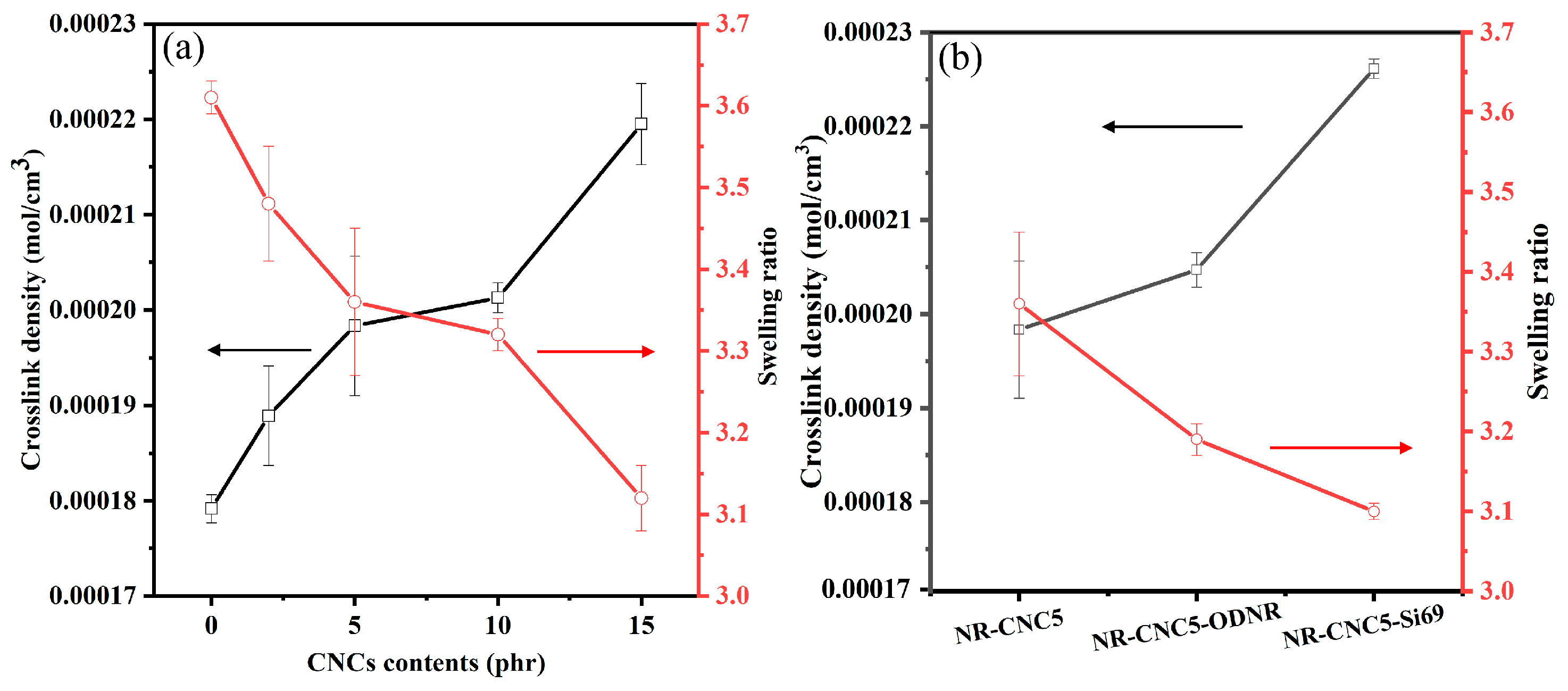
3.3. Crosslink Density of NR/CNC Nanocomposites
3.4. Mechanical Properties
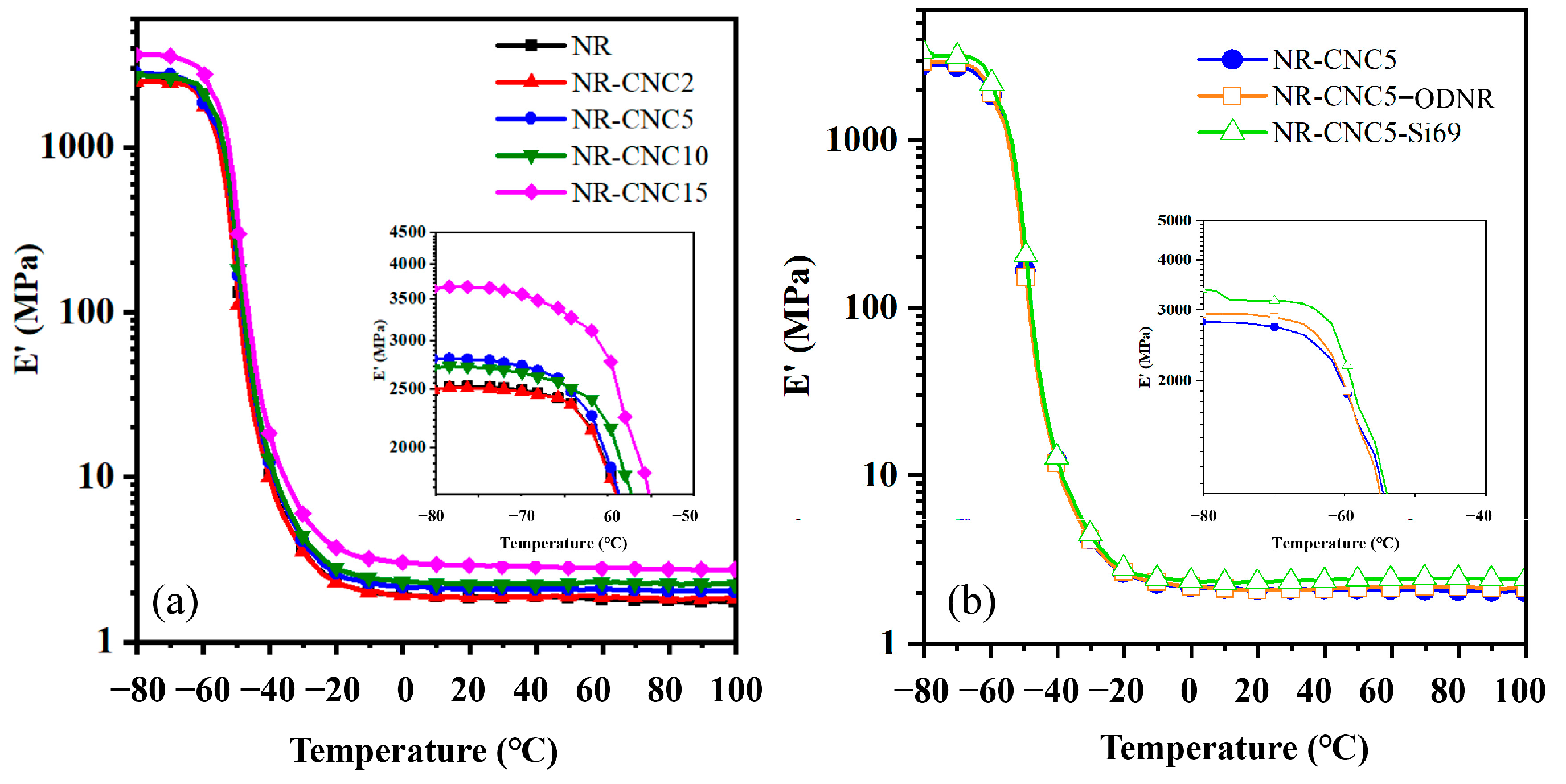
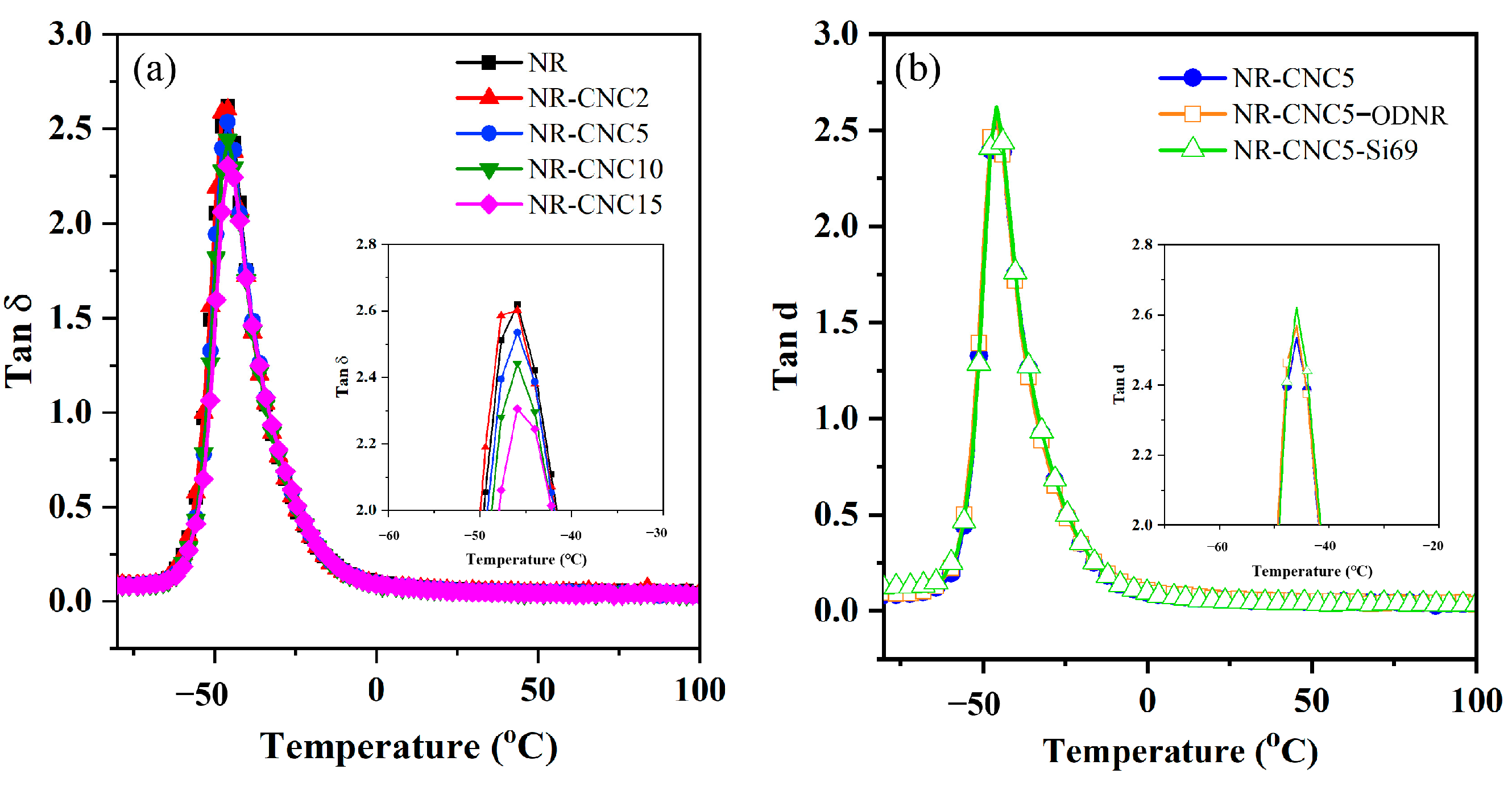
3.5. Dynamic Mechanical Properties
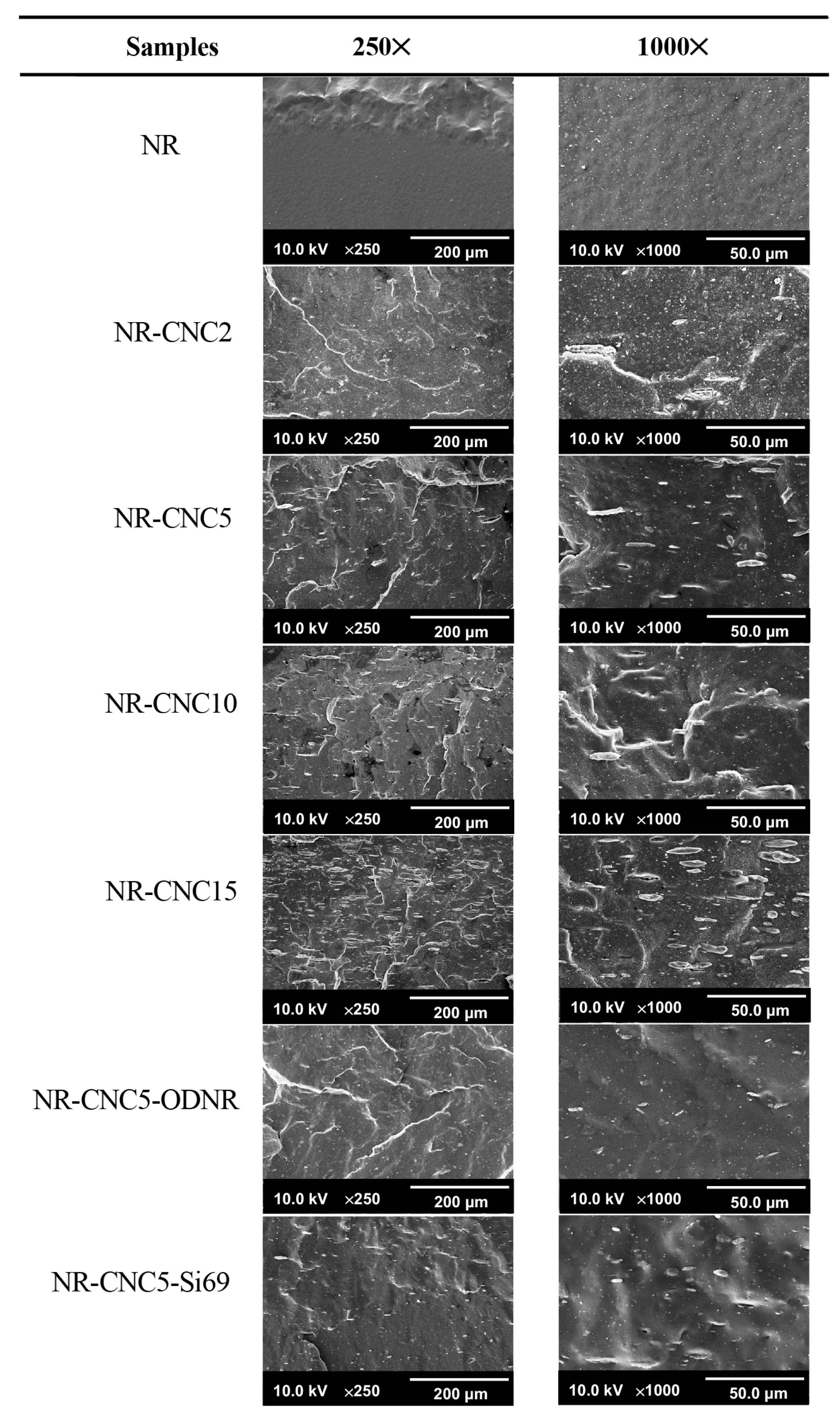
3.6. Morphology of NR/CNC Nanocomposites
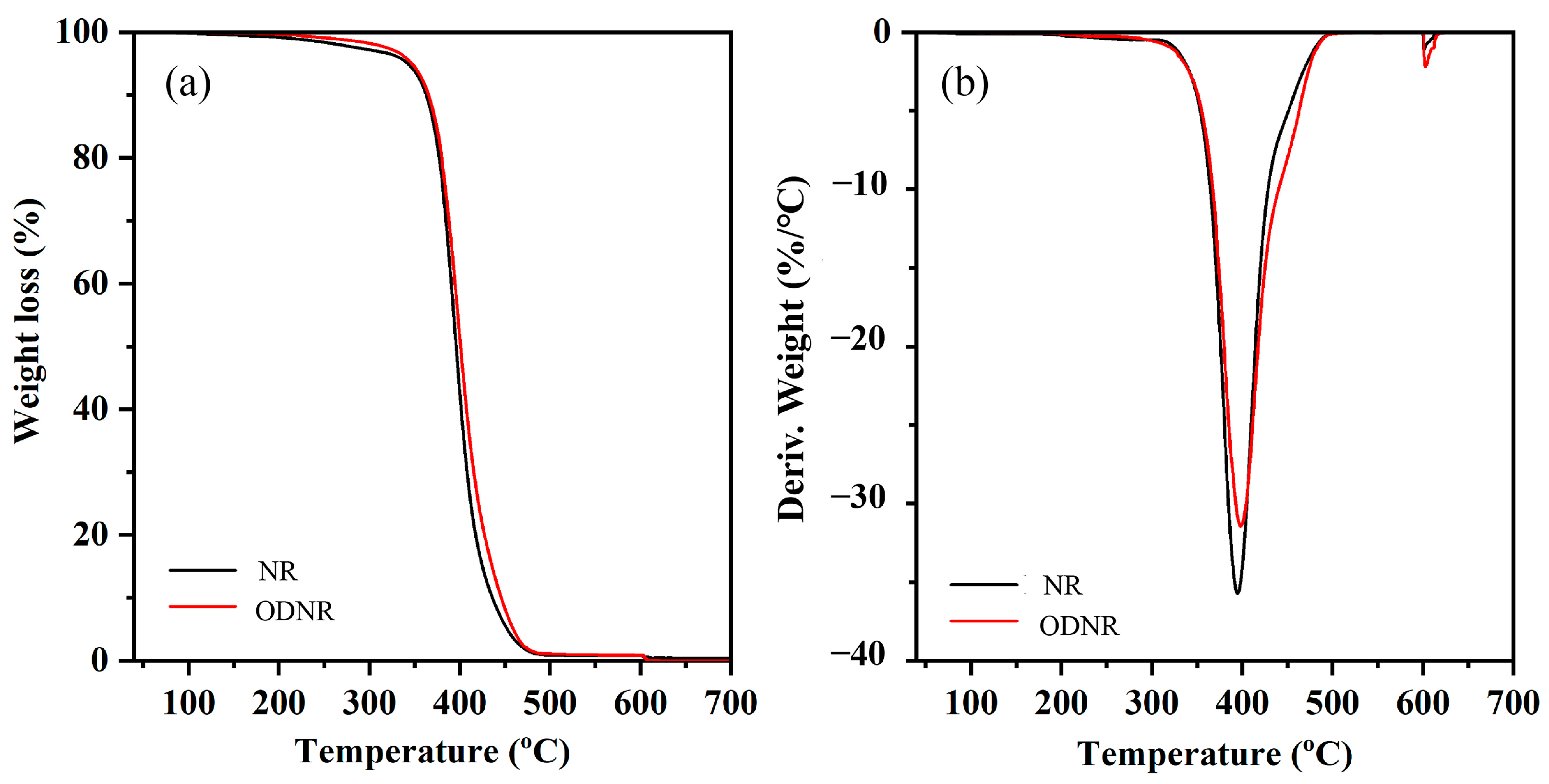
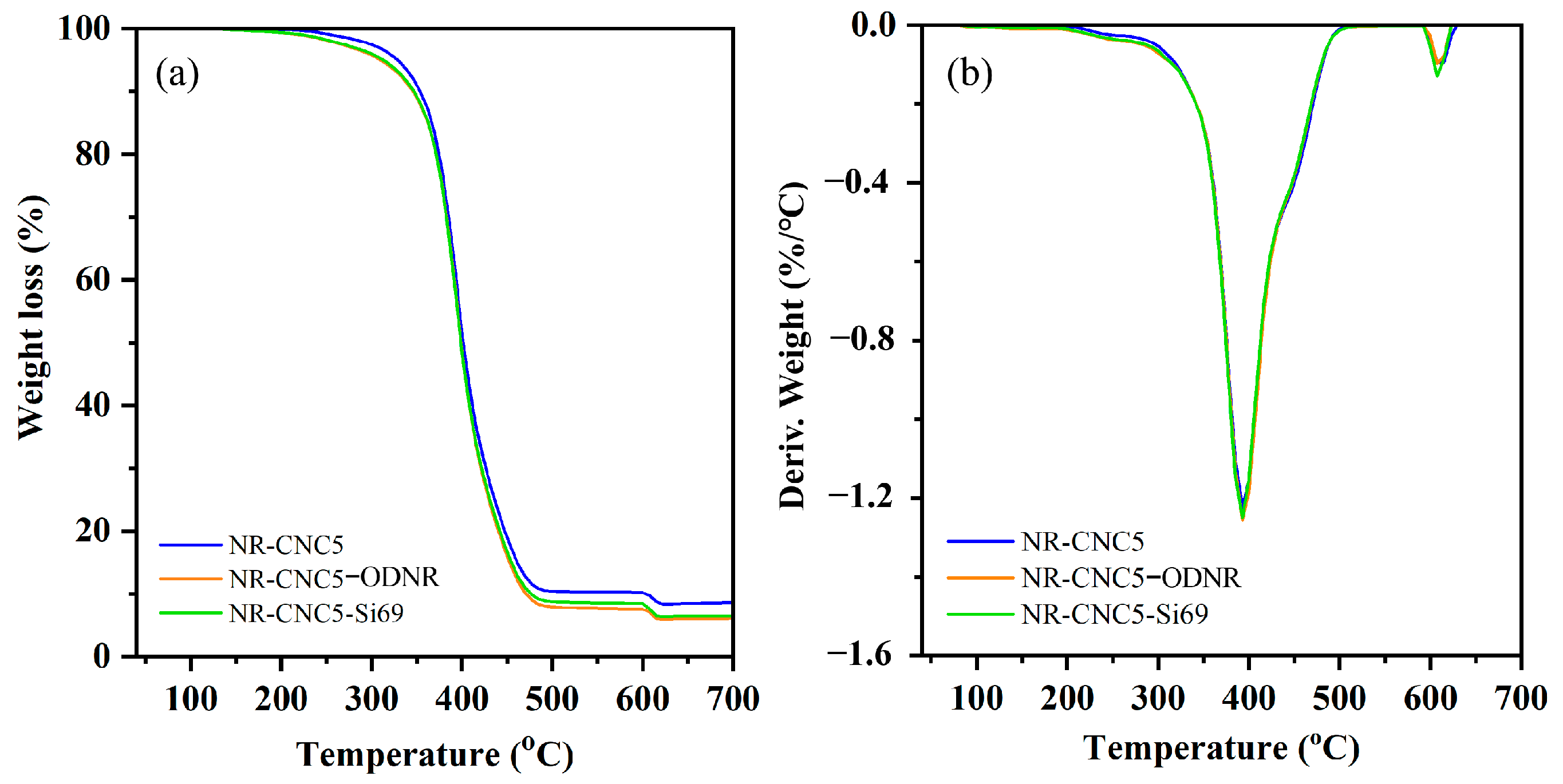
3.7. Thermal Stability of NR/CNC Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koeipudsa, N.; Chanthateyanonth, R.; Daniel, P.; Phinyocheep, P. Development of natural rubber nanocomposites reinforced with cellulose nanocrystal isolated from oil palm biomass. J. Polym. Res. 2022, 29, 403. [Google Scholar] [CrossRef]
- Lozada, E.R.; Gutiérrez, A.C.M.; Jaramillo, C.J.A.; Sánchez, J.C.; Barrera Torres, G. Vegetable cellulose fibers in natural rubber composites. Polymers 2023, 15, 2914. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2007, 15, 149–159. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Haafiz, M.K.M.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, L.; Lu, Q.; Wang, S.; Chen, X.; Huang, B. Preparation of cellulose nanocrystals and carboxylated cellulose nanocrystals from borer powder of bamboo. Cellulose 2014, 21, 1611–1618. [Google Scholar] [CrossRef]
- Syafri, E.; Kasim, A.; Abral, H.; Asben, A. Cellulose nanofibers isolation and characterization from ramie using a chemical-ultrasonic treatment. J. Nat. Fibers 2018, 16, 1145–1155. [Google Scholar] [CrossRef]
- Jantachum, P.; Phinyocheep, P. A simple method for extraction of cellulose nanocrystals from green Luffa cylindrica biomaterial and their characteristics. Polym. Int. 2023, 72, 243–251. [Google Scholar] [CrossRef]
- Ng, H.M.; Sin, L.T.; Tee, T.T.; Bee, S.-T.; Hui, D.; Low, C.Y. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Fallahi, H.; Kaynan, O.; Asadi, A. Insights into the effect of fiber–matrix interphase physiochemical- mechanical properties on delamination resistance and fracture toughness of hybrid composites. Compos. Part A Appl. Sci. Manuf. 2023, 166, 107390. [Google Scholar] [CrossRef]
- Pasquini, D.; de Morais Teixeira, E.; da Silva Curvelo, A.A.; Belgacem, M.N.; Dufresne, A. Extraction of cellulose whiskers from cassava bagasse and their applications as reinforcing agent in natural rubber. Ind. Crops Prod. 2010, 32, 486–490. [Google Scholar] [CrossRef]
- Neto, W.P.F.; Mariano, M.; da Silva, I.S.V.; Silverio, H.A.; Putaux, J.L.; Otaguro, H. Mechanical properties of natural rubber nanocomposites reinforced with high aspect ratio cellulose nanocrystals isolated from soy hulls. Carbohydr. Polym. 2016, 53, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Potiyaraj, P. Development of high performance microcrystalline cellulose based natural rubber composites using maleated natural rubber as compatibilizer. Cellulose 2017, 25, 1077–1087. [Google Scholar] [CrossRef]
- Somseemee, O.; Sae-Oui, P.; Siriwong, C. Reinforcement of surface-modified cellulose nanofibrils extracted from Napier grass stem in natural rubber composites. Ind. Crops Prod. 2021, 171, 113881. [Google Scholar] [CrossRef]
- Jantachum, P.; Khumpaitool, B.; Utara, S. Effect of silane coupling agent and cellulose nanocrystals loading on the properties of acrylonitrile butadiene rubber/natural rubber nanocomposites. Ind. Crops Prod. 2023, 195, 116407. [Google Scholar] [CrossRef]
- Moonart, U.; Utara, S. Effect of surface treatments and filler loading on the properties of hemp fiber/natural rubber composites. Cellulose 2019, 26, 7271–7295. [Google Scholar] [CrossRef]
- ASTM D2240-97; Standard Test Method for Rubber Property—Durometer Hardness. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2015.
- ASTM D412-98; Standard Test Methods for Vulcanized Rubber and Thermoplastic Rubbers and Thermoplastic Elastomers-Tension. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2002.
- Phinyocheep, P.; Phetphaisit, C.W.; Derouet, D.; Campistron, I.; Brosse, J.C. Chemical degradation of epoxidized natural rubber using periodic acid: Preparation of epoxidized liquid natural rubber. J. Appl. Polym. Sci. 2005, 95, 6–15. [Google Scholar] [CrossRef]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of Liquid Natural Rubber via Oxidative Degradation of Natural Rubber. Polymers 2014, 6, 2928–2941. [Google Scholar] [CrossRef]
- Suhawati, I.; Asrul, M. Effect of reagents concentration and ratio on degradation of natural rubber latex in acidic medium. Malays. J. Anal. Sci. 2014, 18, 405–414. [Google Scholar]
- Mariano, M.; Kissi, N.E.; Dufresne, A. Cellulose nanocrystal reinforced oxidized natural rubber nanocomposites. Carbohydr. Polym. 2016, 137, 174–183. [Google Scholar] [CrossRef]
- Tomić, N.Z. Chapter 17—Thermal studies of compatibilized polymer blends. In Compatibilization of Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2020; pp. 489–510. [Google Scholar]
- Aini, N.A.M.; Othman, N.; Hussin, M.H.; Sahakaro, K.; Hayeemasae, N. Efficiency of interaction between hybrid fillers carbon black/lignin with various rubber-based compatibilizer, epoxidized natural rubber, and liquid butadiene rubber in NR/BR composites: Mechanical, flexibility and dynamical properties. Ind. Crops Prod. 2022, 185, 115167. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Silanized cereal straw as a novel, functional filler of natural rubber biocomposites. Cellulose 2019, 26, 1025–1040. [Google Scholar] [CrossRef]
- Hariwongsanupab, N.; Thanawan, S.; Amornsakchai, T.; Vallat, M.F.; Mougin, K. Improving the mechanical properties of short pineapple leaf fiber reinforced natural rubber by blending with acrylonitrile butadiene rubber. Polym. Test. 2017, 57, 94–100. [Google Scholar] [CrossRef]
- Yantaboot, K.; Amornsakchai, T. Effect of mastication time on the low strain properties of short pineapple leaf fiber reinforced natural rubber composites. Polym. Test. 2017, 57, 31–37. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B. The effects of aspect ratio of cellulose nanocrystals on the properties of all CNC films: Tunicate and wood CNCs. Carbohydr. Polym. 2023, 5, 100311. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Teramoto, Y. Recent advances in nanocellulose composites with polymers: A guide for choosing partners and how to incorporate them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef]
- Kazemi, H.; Mighri, F.; Park, K.W.; Frikha, S.; Rodrigue, D. Natural rubber biocomposites reinforced with cellulose nanocrystals/lignin hybrid fillers. Polym. Compos. 2022, 43, 5442–5453. [Google Scholar] [CrossRef]
- Hosseinmardi, A.; Amiralian, N.; Martin, D.J.; Annamalai, P.K. Achieving ultra-tear resistant high-performance natural rubber nanocomposite via bio-inspired lignocellulosic compatibilization. Ind. Crops Prod. 2024, 207, 117729. [Google Scholar] [CrossRef]
- Srisuwan, L.; Jarukumjorn, K.; Suppakarn, N. Effect of silane treatment methods on physical properties of rice husk flour/natural rubber composites. Adv. Mater. Sci. Eng. 2018, 2018, 4583974. [Google Scholar] [CrossRef]
- Maslowski, M.; Miedzianowska, J.; Strzelec, K. Natural Rubber Composites Filled with Crop Residues as an Alternative to Vulcanizates with Common Fillers. Polymers 2019, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Lopattananon, N.; Panawarangkul, K.; Sahakaro, K.; Ellis, B. Performance of pineapple leaf fiber–natural rubber composites: The effect of fiber surface treatments. J. Appl. Polym. Sci. 2006, 102, 1974–1984. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Sainumsai, W.; Toki, S.; Amnuaypornsri, S.; Nimpaiboon, A.; Sakdapipanich, J.; Rong, L. Dependence of the Onset of Strain-Induced Crystallization of Natural Rubber and Its Synthetic Analogue on Crosslink and Entanglement by Using Synchrotron X-ray. Rubber Chem. Technol. 2017, 90, 728–742. [Google Scholar] [CrossRef]
- Cao, X.; Xu, C.; Wang, Y.; Liu, Y.; Liu, Y.; Chen, Y. New nanocomposite materials reinforced with cellulose nanocrystals in nitrile rubber. Polym. Test. 2013, 32, 819–826. [Google Scholar] [CrossRef]
- Wongsorat, W.; Suppakarn, N.; Jarukumjorn, K. Effects of compatibilizer type and fiber loading on mechanical properties and cure characteristics of sisal fiber/natural rubber composites. J. Compos. Mater. 2013, 48, 2401–2411. [Google Scholar] [CrossRef]
- Intapun, J.; Rungruang, T.; Suchat, S.; Cherdchim, B.; Hiziroglu, S. The characteristics of natural rubber composites with Klason lignin as a green reinforcing filler: Thermal stability, mechanical and dynamical properties. Polymers 2021, 13, 1109. [Google Scholar] [CrossRef]
- Bendahou, A.; Kaddami, H.; Dufresne, A. Investigation on the effect of cellulosic nanoparticles’ morphology on the properties of natural rubber-based nanocomposites. Eur. Polym. J. 2010, 46, 609–620. [Google Scholar] [CrossRef]
- Bokobza, L. Elastomer nanocomposites: Effect of filler-matrix and filler-filler interactions. Polymers 2023, 15, 2900. [Google Scholar] [CrossRef]
- Dayo, A.Q.; Gao, B.C.; Wang, J.; Liu, W.B.; Derradji, M.; Shah, A.H.; Babar, A.A. Natural hemp fiber reinforced polybenzoxazine composites: Curing behavior, mechanical and thermal properties. Compos. Sci. Technol. 2017, 144, 114–124. [Google Scholar] [CrossRef]
- Haris, N.I.N.; Hassan, M.Z.; Ilyas, R.; Suhot, M.A.; Sapuan, S.; Dolah, R.; Mohammad, R.; Asyraf, M.R.M. Dynamic mechanical properties of natural fiber reinforced hybrid polymer composites: A review. J. Mater. Res. Technol. 2022, 19, 167–182. [Google Scholar] [CrossRef]
- Low, D.Y.S.; Supramaniam, J.; Soottitantawat, A.; Charinpanitkul, T.; Tanthapanichakoon, W.; Tan, K.W.; Tang, S.Y. Recent Developments in Nanocellulose-Reinforced Rubber Matrix Composites: A Review. Polymers 2021, 13, 550. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gardner, D.J.; Han, Y.; Kiziltas, A.; Cai, Z.; Tshabalala, M.A. Influence of drying method on the material properties of nanocellulose I: Thermostability and crystallinity. Cellulose 2013, 20, 2379–2392. [Google Scholar] [CrossRef]
- Alias, N.F.; Ismail, H.; Ishak, K.M.K. Poly(lactic acid)/natural rubber/kenaf biocomposites production using poly(methyl methacrylate) and epoxidized natural rubber as co-compatibilizers. Iran. Polym. J. 2021, 30, 737–749. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Reid, M.S.; Bras, J.; Heux, L.; Godoy-Vargas, J.; Panga, M.K.R. Insight into thermal stability of cellulose nanocrystals from new hydrolysis methods with acid blends. Cellulose 2018, 26, 507–528. [Google Scholar] [CrossRef]


| Ingredients | Amount (phr) | ||||||
|---|---|---|---|---|---|---|---|
| NR | NR-CNC2 | NR-CNC5 | NR-CNC10 | NR-CNC15 | NR-CNC5-ODNR | NR-CNC5-Si69 | |
| NR | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| CNCs | 0 | 2 | 5 | 10 | 15 | 5 | 5 |
| ODNR | - | - | - | - | - | 5 | - |
| Si69 | - | - | - | - | - | - | 0.25 |
| Properties | NR | NR-CNC2 | NR-CNC5 | NR-CNC10 | NR-CNC15 | NR-CNC5-ODNR | NR-CNC5-Si69 |
|---|---|---|---|---|---|---|---|
| MH (dNm) | 4.51 | 5.10 | 5.35 | 5.46 | 6.99 | 5.71 | 6.08 |
| ML (dNm) | 0.06 | 0.10 | 0.10 | 0.12 | 0.13 | 0.09 | 0.19 |
| MH-ML (dNm) | 4.45 | 5.00 | 5.25 | 5.34 | 6.86 | 5.62 | 5.89 |
| ts2 (min) | 2.80 | 3.16 | 3.25 | 3.32 | 3.62 | 2.05 | 3.36 |
| tc90 (min) | 3.87 | 5.37 | 5.42 | 5.51 | 5.56 | 3.24 | 5.44 |
| CRI (min−1) | 93.45 | 45.24 | 46.08 | 45.66 | 51.54 | 84.03 | 48.08 |
| Samples | T5% (°C) | Tmax (°C) |
|---|---|---|
| NR | 195 | 392 |
| NR-CNC2 | 203 | 393 |
| NR-CNC5 | 202 | 393 |
| NR-CNC10 | 199 | 390 |
| NR-CNC15 | 199 | 390 |
| NR-CNC5-ODNR | 205 | 393 |
| NR-CNC5-Si69 | 207 | 393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantachum, P.; Phinyocheep, P. Compatibilization of Cellulose Nanocrystal-Reinforced Natural Rubber Nanocomposite by Modified Natural Rubber. Polymers 2024, 16, 363. https://doi.org/10.3390/polym16030363
Jantachum P, Phinyocheep P. Compatibilization of Cellulose Nanocrystal-Reinforced Natural Rubber Nanocomposite by Modified Natural Rubber. Polymers. 2024; 16(3):363. https://doi.org/10.3390/polym16030363
Chicago/Turabian StyleJantachum, Punyarat, and Pranee Phinyocheep. 2024. "Compatibilization of Cellulose Nanocrystal-Reinforced Natural Rubber Nanocomposite by Modified Natural Rubber" Polymers 16, no. 3: 363. https://doi.org/10.3390/polym16030363
APA StyleJantachum, P., & Phinyocheep, P. (2024). Compatibilization of Cellulose Nanocrystal-Reinforced Natural Rubber Nanocomposite by Modified Natural Rubber. Polymers, 16(3), 363. https://doi.org/10.3390/polym16030363







