Crystallization of Polylactic Acid with Organic Nucleating Agents under Quiescent Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Selection
2.2. Sample Preparation
2.2.1. Compounding
2.2.2. Quiescent Crystallization
2.2.3. Sample Etching Procedure
- In total, 5 g of sodium hydroxide (NaOH), purchased from Sigma-Aldrich (Millipore Sigma, Saint Louis, MO, USA), was dissolved in 250 mL of water to achieve a 0.5 mol/L concentration. Twenty-one clean glass bottles with lids were prepared to etch and hold the PLA samples. The liquid prepared was distributed into 21 bottles.
- Etching: the 21 PLA samples prepared with quiescent crystallization were immersed individually into the solution in glass bottles for 12 h.
- Cleaning: After etching, samples were kept in the bottles for 20 min at 25 °C in an ultrasonic bath (Branson CPX 2800H, Brookfield, CT, USA) to remove residual particles. After cleaning, the samples were removed from the etching solvent and dried with compressed air. The samples were then kept in sealed bags individually for further characterization.
2.3. Characterization Techniques
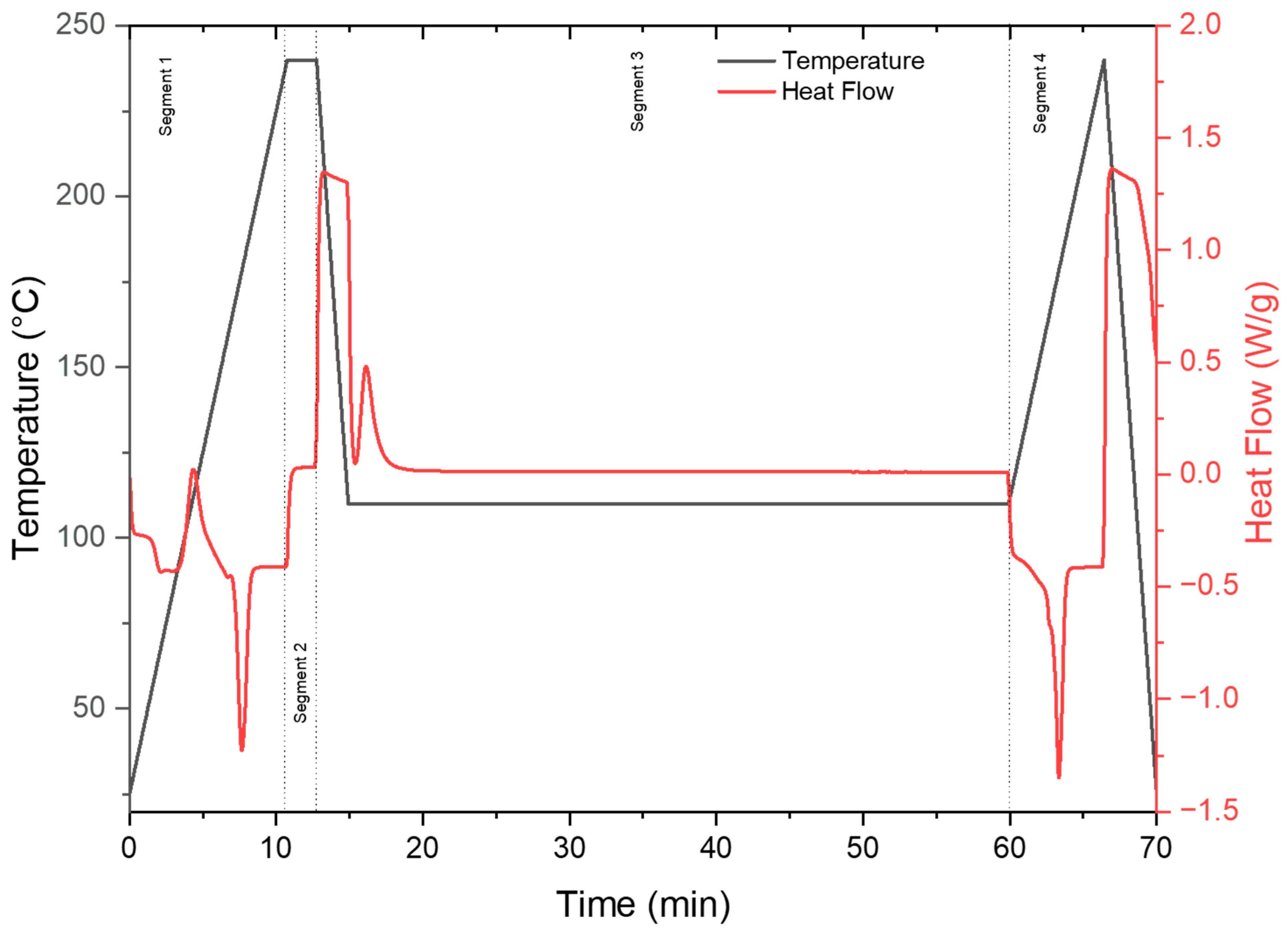
2.3.1. Isothermal Differential Scanning Calorimetry
- Segment 1: Heat the sample from 25 °C to 240 °C at 20 °C/min, followed by an isotherm at 240 °C for 3 min. This segment was intended to melt and remove all thermal history of the pellets. The 3 min isotherm ensured the complete melting of the sample.
- Segment 2: Cool the sample rapidly using the maximum cooling rate at 60 °C/min to various isothermal temperatures (80 °C, 110 °C, and 140 °C). This segment is intended to quench the PLA polymer melt to the designed isotherm temperature using the maximum cooling rate, thus minimizing the crystallization behavior during the cooling period.
- Segment 3: The abovementioned isotherm temperatures are held for 60 min. This segment was intended to capture the crystallization process of PLA even with low concentrations of additives and at low temperatures. The 60 min holding time ensures no additional crystallization at the current temperatures and concentrations.
- Segment 4: Heat the sample at 10 °C/min to 240 °C. The degree of crystallinity achieved from the isotherm was quantified from the melting peak observed during this heating segment. The degree of crystallinity (XC) was calculated using the following:
2.3.2. X-ray Diffractometry
2.3.3. Scanning Electron Microscopy
3. Results and Discussion
3.1. Thermodynamics of Crystallization
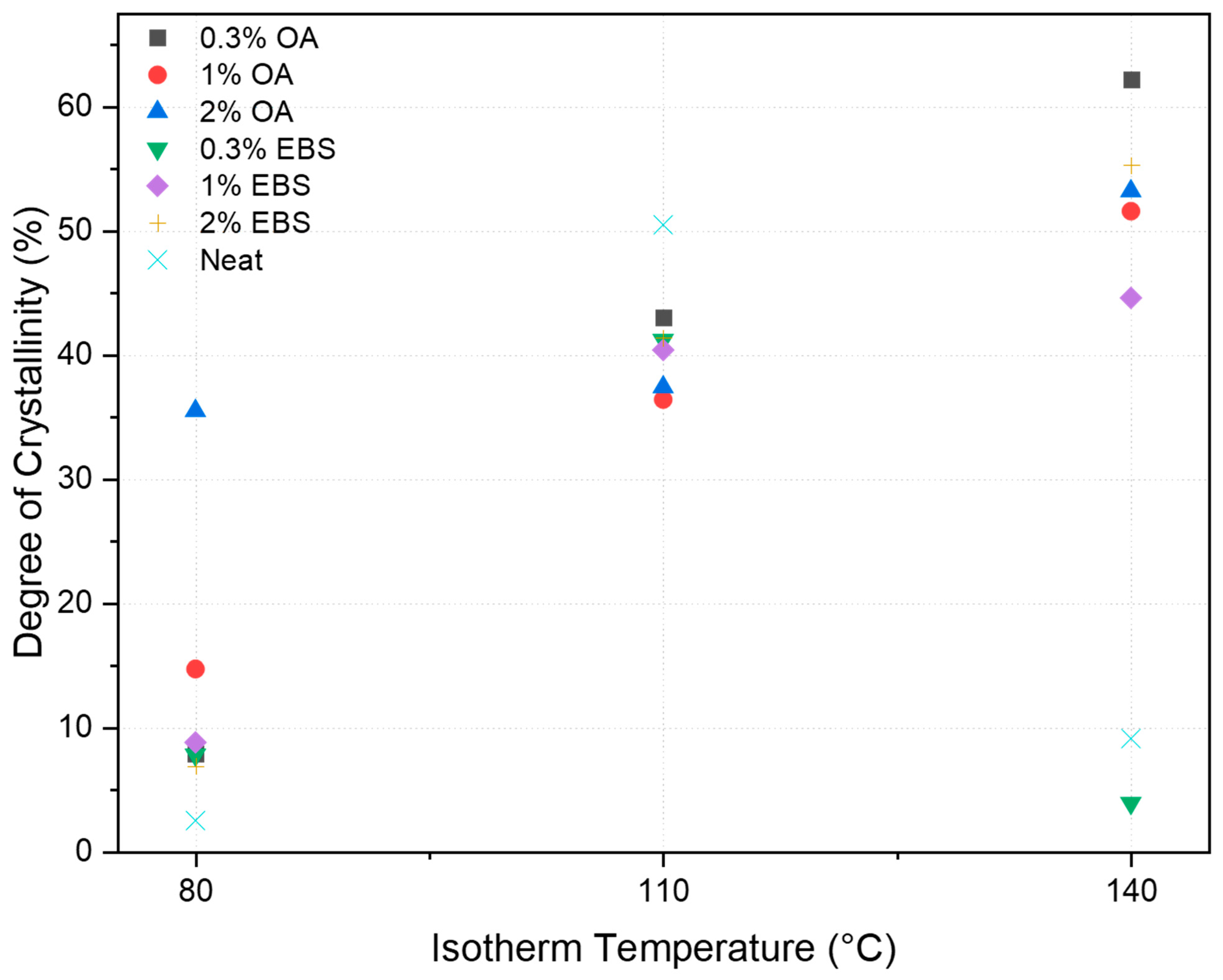
Degree of Crystallinity
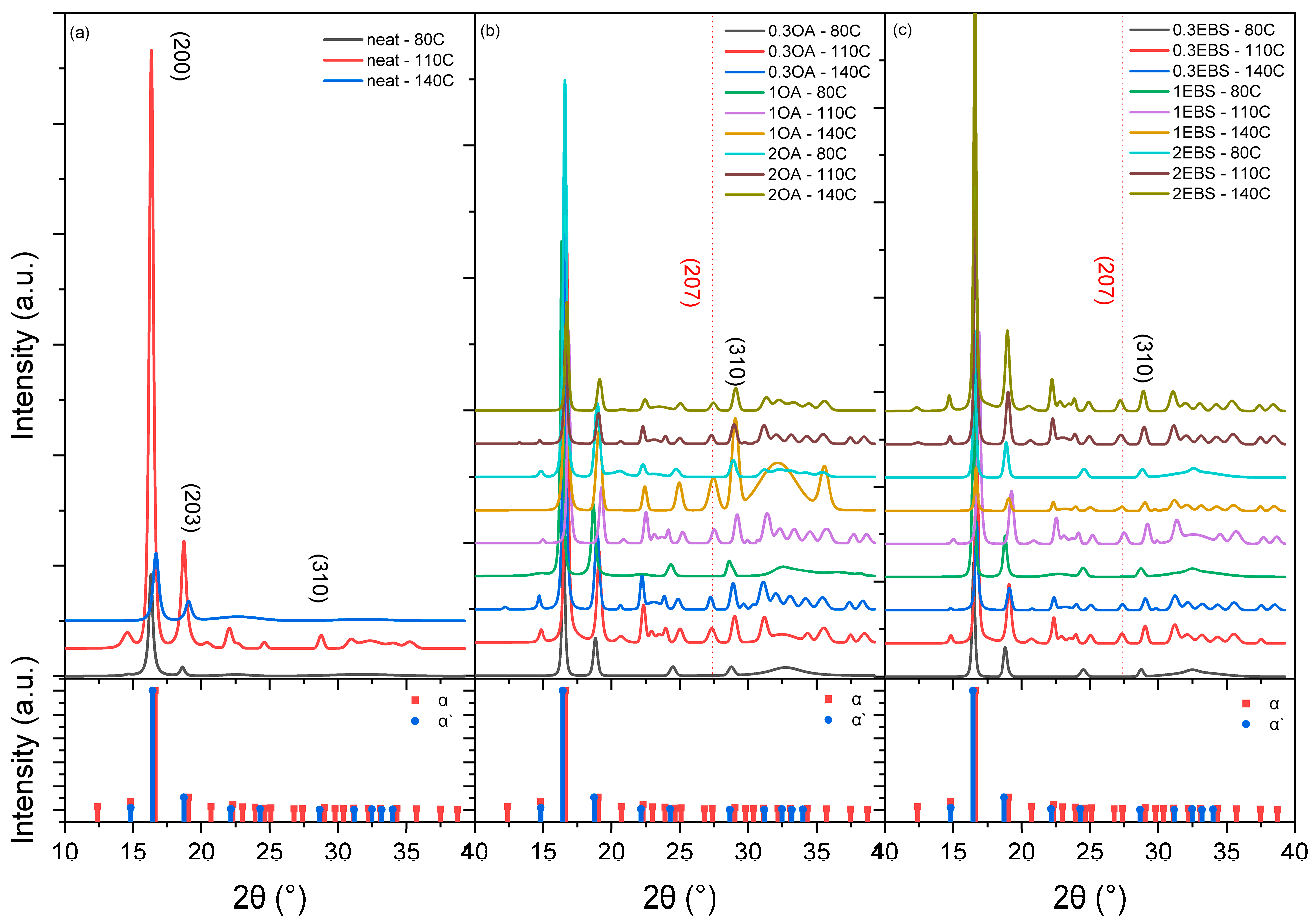
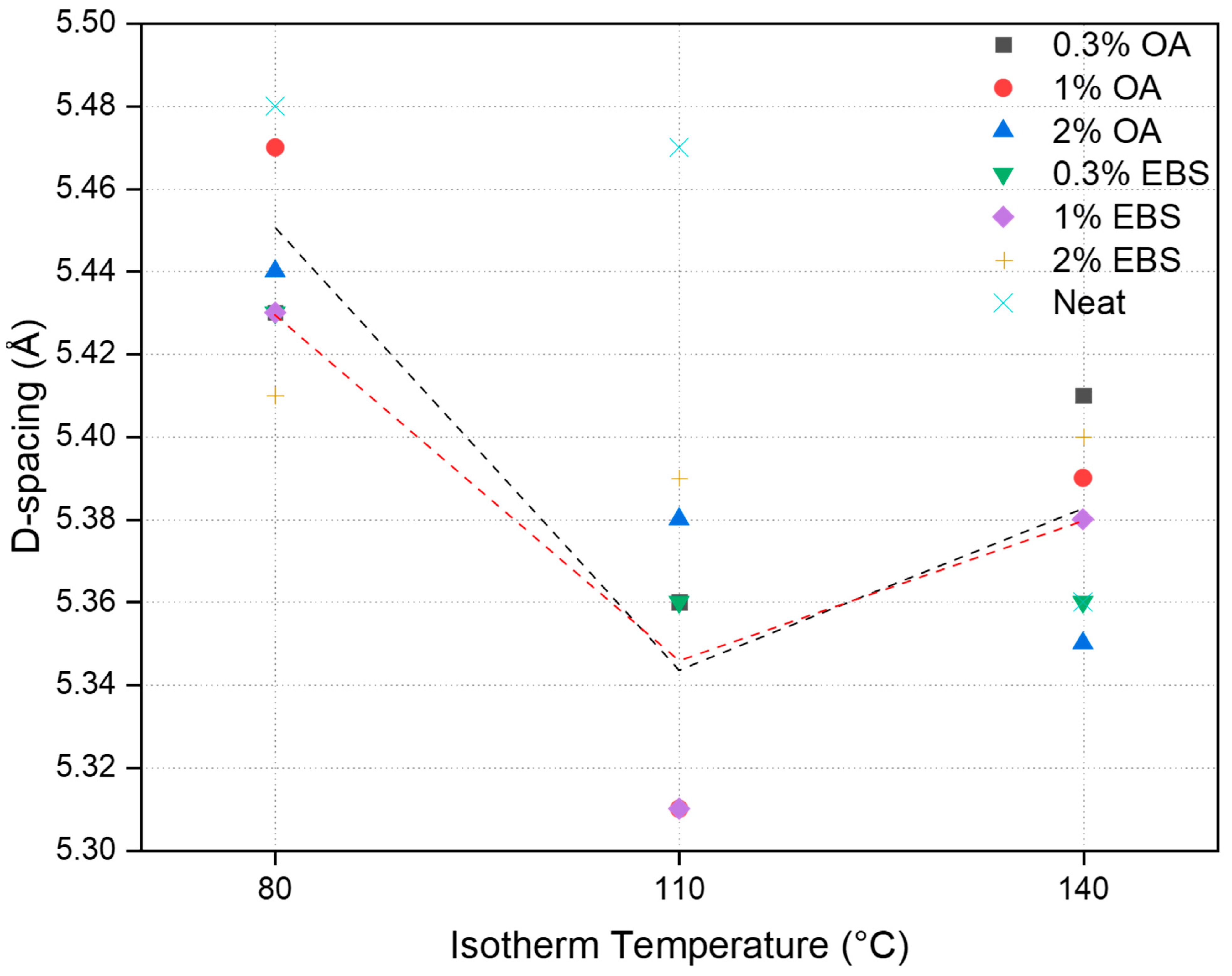
3.2. Polymorphic Analysis of Crystalline Domains
3.3. Crystallization Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.-X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2021, 7, 83–103. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, J.; Yang, X.; Ke, Y.; Ou, R.; Wang, Y.; Madbouly, S.A.; Wang, Q. From plant phenols to novel bio-based polymers. Prog. Polym. Sci. 2021, 125, 101473. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Kobayashi, J.; Asahi, T.; Ichiki, M.; Oikawa, A.; Suzuki, H.; Watanabe, T.; Fukada, E.; Shikinami, Y. Structural and optical properties of poly lactic acids. J. Appl. Phys. 1995, 77, 2957–2973. [Google Scholar] [CrossRef]
- De Santis, P.; Kovacs, A.J. Molecular conformation of poly(S-lactic acid). Biopolymers 1968, 6, 299–306. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Investigation of phase transitional behavior of poly(l-lactide)/poly(d-lactide) blend used to prepare the highly-oriented stereocomplex. Macromolecules 2007, 40, 1049–1054. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(l-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of poly(l-lactide): Molecular weight dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Brinke, G.T.; Zugenmaier, P. Crystal structure, conformation and morphology of solution-spun poly(L-lactide) fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- Puiggali, J.; Ikada, Y.; Tsuji, H.; Cartier, L.; Okihara, T.; Lotz, B. The frustrated structure of poly(l-lactide). Polymer 2000, 41, 8921–8930. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Lotz, B. Triangular Polymer Single Crystals: Stereocomplexes, Twins, and Frustrated Structures. Macromolecules 1997, 30, 6313–6322. [Google Scholar] [CrossRef]
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.-I.; Tsuji, H.; Hyon, S.-H.; Ikada, Y. Crystal structure of stereocomplex of poly(L-lactide) and poly(D-lactide). J. Macromol. Sci. Part B 1991, 30, 119–140. [Google Scholar] [CrossRef]
- Han, L.; Pan, P.; Shan, G.; Bao, Y. Stereocomplex crystallization of high-molecular-weight poly(l-lactic acid)/poly(d-lactic acid) racemic blends promoted by a selective nucleator. Polymer 2015, 63, 144–153. [Google Scholar] [CrossRef]
- Ma, P.; Shen, T.; Xu, P.; Dong, W.; Lemstra, P.J.; Chen, M. Superior Performance of Fully Biobased Poly(lactide) via Stereocomplexation-Induced Phase Separation: Structure versus Property. ACS Sustain. Chem. Eng. 2015, 3, 1470–1478. [Google Scholar] [CrossRef]
- Zisopol, D.G.; Portoaca, A.I.; Nae, I.; Ramadan, I. A Comparative Analysis of the Mechanical Properties of Annealed PLA. Eng. Technol. Appl. Sci. Res. 2022, 12, 8978–8981. [Google Scholar] [CrossRef]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the crystallinity and mechanical properties of PLA by nucleation and annealing. Polym. Degrad. Stab. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Bhandari, S.; Lopez-Anido, R.A.; Gardner, D.J. Enhancing the interlayer tensile strength of 3D printed short carbon fiber reinforced PETG and PLA composites via annealing. Addit. Manuf. 2019, 30, 100922. [Google Scholar] [CrossRef]
- Barkhad, M.S.; Abu-Jdayil, B.; Mourad, A.H.I.; Iqbal, M.Z. Thermal Insulation and Mechanical Properties of Polylactic Acid (PLA) at Different Processing Conditions. Polymers 2020, 12, 2091. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L. The crystallization and melting processes of poly(l-lactic acid). Macromol. Symp. 2006, 234, 176–183. [Google Scholar] [CrossRef]
- Gao, P.; Kundu, A.; Alqosaibi, K.; Coulter, J. Enhanced crystallinity development of poly-lactic acid by dynamic melt manipulation. In Proceedings of the ASME 2021 International Mechanical Engineering Congress and Exposition, Online, 1–5 November 2021; pp. 1–9. [Google Scholar] [CrossRef]
- Refaa, Z.; Boutaous, M.; Xin, S.; Siginer, D.A. Thermophysical analysis and modeling of the crystallization and melting behavior of PLA with talc: Kinetics and crystalline structures. J. Therm. Anal. Calorim. 2017, 128, 687–698. [Google Scholar] [CrossRef]
- As’habi, L.; Jafari, S.H.; Khonakdar, H.A.; Häussler, L.; Wagenknecht, U.; Heinrich, G. Non-isothermal crystallization behavior of PLA/LLDPE/nanoclay hybrid: Synergistic role of LLDPE and clay. Thermochim. Acta 2013, 565, 102–113. [Google Scholar] [CrossRef]
- Xu, Z.; Su, L.; Jiang, S.; Gu, W.; Peng, M.; Wang, P. Crystallization behavior and water vapor permeability of poly(lactic acid) nanocomposite under oscillatory shear. J. Appl. Polym. Sci. 2015, 132, 42321. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Crystallization behavior and morphology of polylactic acid (PLA) with aromatic sulfonate derivative. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Alzahrani, F.J.; Duhduh, A.A.; Gao, P.; Coulter, J.P. The influence of cooling rate and mold temperature on polymers crystallization kinetics in injection molding. In Proceedings of the ASME 2021 International Mechanical Engineering Congress and Exposition, Online, 1–5 November 2021; pp. 1–7. [Google Scholar] [CrossRef]
- Gao, P.; Kundu, A.; Coulter, J. Vibration-assisted injection molding: An efficient process for enhanced crystallinity development and mechanical characteristics for poly lactic acid. Int. J. Adv. Manuf. Technol. 2022, 121, 3111–3124. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Williams, J.S.; Lewis, D.N. Melt rheology of poly(lactic acid): Entanglement and chain architecture effects. J. Rheol. 1999, 43, 1141–1155. [Google Scholar] [CrossRef]
- Refaa, Z.; Boutaous, M.; Siginer, D.A. PLA crystallization kinetics and morphology development. Int. Polym. Process. 2018, 33, 336–344. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, G.-J.; Fu, Q.; Lei, J.; Jiang, W.; Hsiao, B.S.; Li, Z.-M. Formation of Shish-Kebabs in Injection-Molded Poly(l-lactic acid) by Application of an Intense Flow Field. ACS Appl. Mater. Interfaces 2012, 4, 6774–6784. [Google Scholar] [CrossRef]
- Ghosh, S.; Viana, J.; Reis, R.; Mano, J. Effect of processing conditions on morphology and mechanical properties of injection-molded poly(l-lactic acid). Polym. Eng. Sci. 2007, 47, 1141–1147. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Na, B.; Du, R.; Fu, Q.; Shen, K. Dependence of impact strength on the fracture propagation direction in dynamic packing injection molded PP/EPDM blends. Polymer 2003, 44, 4261–4271. [Google Scholar] [CrossRef]
- Volpe, V.; Foglia, F.; Pantani, R. Effect of the application of low shear rates on the crystallization kinetics of PLA. Polym. Cryst. 2020, 3, e10139. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Q.; Yang, S.; Ru, J.; Lin, J.; Xu, J.; Lei, J.; Li, Z. Flow-induced crystallization of polylactide stereocomplex under pressure. J. Appl. Polym. Sci. 2018, 135, 46378. [Google Scholar] [CrossRef]
- Kolstad, J.J. Crystallization kinetics of poly(L-lactide-co-meso-lactide). J. Appl. Polym. Sci. 1996, 62, 1079–1091. [Google Scholar] [CrossRef]
- Shieh, Y.; Twu, Y.; Su, C.; Lin, R.; Liu, G. Crystallization kinetics study of poly(L-lactic acid)/carbon nanotubes nanocomposites. J. Polym. Sci. Part B 2010, 48, 983–989. [Google Scholar] [CrossRef]
- Tang, H.; Chen, J.-B.; Wang, Y.; Xu, J.-Z.; Hsiao, B.S.; Zhong, G.-J.; Li, Z.-M. Shear flow and carbon nanotubes synergistically induced nonisothermal crystallization of poly(lactic acid) and its application in injection molding. Biomacromolecules 2012, 13, 3858–3867. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Chen, T.; Yang, C.-L.; Li, Z.-M.; Mao, Y.-M.; Zeng, B.-Q.; Hsiao, B.S. Isothermal crystallization of poly(l-lactide) induced by graphene nanosheets and carbon nanotubes: A comparative study. Macromolecules 2010, 43, 5000–5008. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, Z. Effect of Orotic Acid on the Crystallization Kinetics and Morphology of Biodegradable Poly(L-lactide) as an Efficient Nucleating Agent. Ind. Eng. Chem. Res. 2011, 50, 12299–12303. [Google Scholar] [CrossRef]
- Gao, P.; Masato, D.; Kundu, A.; Coulter, J.P. An Investigation on the Efficacy of Orotic Acid as a Bio-Nucleating Agent for Poly-Lactic Acid under Quiescent Condition and Injection Molding. Micromachines 2022, 13, 2186. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Hodgson, P.D.; Wen, C. Study on the Role of Stearic Acid and Ethylene-bis-stearamide on the Mechanical Alloying of a Biomedical Titanium Based Alloy. Met. Mater. Trans. A 2010, 41, 1409–1420. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, H.; Xiong, Z.; Xiong, Y.; Xu, W. Flowability and Mechanical and Thermal Properties of Nylon 6/Ethylene bis-Stearamide/Carboxylic Silica Composites. J. Macromol. Sci. Part B 2011, 50, 2255–2270. [Google Scholar] [CrossRef]
- ASTM D792-20; Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM D1238-10; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D5225-22; Standard Test Method for Measuring Solution Viscosity of Polymers with a Differential Viscometer. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D3418-21; Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2021.
- Mikešová, J.; Hašek, J.; Tishchenko, G.; Morganti, P. Rheological study of chitosan acetate solutions containing chitin nanofibrils. Carbohydr. Polym. 2014, 112, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lai, Z.; Jin, X.; Sun, T.; Liu, H.; Qi, H. Multifunctional Liquid-Free Ionic Conductive Elastomer Fabricated by Liquid Metal Induced Polymerization. Adv. Funct. Mater. 2021, 31, 2101957. [Google Scholar] [CrossRef]
- Al Hosni, A.S.; Pittman, J.K.; Robson, G.D. Microbial degradation of four biodegradable polymers in soil and compost demonstrating polycaprolactone as an ideal compostable plastic. Waste Manag. 2019, 97, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic Degradation Efficiency of Postconsumer Polyethylene Terephthalate Packaging Determined by Their Polymer Microstructure. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.M.; Veeraraghavan, V.G.; Rubin, H.; Winchell, P.G. The approximation of symmetric X-ray peaks by Pearson type VII distributions. J. Appl. Crystallogr. 1977, 10, 66–68. [Google Scholar] [CrossRef]
- Prevey, P.S. The Use of Person VII Distribution Functions in X-Ray Diffraction Residual Stress Measurement. Adv. X-ray Anal. 1985, 29, 103–111. [Google Scholar] [CrossRef]
- Zhuo, R.; Zhang, Y.; Li, G.; Shao, C.; Wang, Y.; Liu, C.; Li, Q.; Cao, W.; Shen, C. Structural evolution of poly(lactic acid) upon uniaxial stretching investigated by in situ infrared spectroscopy. Vib. Spectrosc. 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Salač, J.; Šerá, J.; Jurča, M.; Verney, V.; Marek, A.A.; Koutný, M. Photodegradation and biodegradation of poly(lactic) acid containing orotic acid as a nucleation agent. Materials 2019, 12, 481. [Google Scholar] [CrossRef]
- Wen, H.; Wang, Y.; Wang, D.; de Claville Christiansen, J.; Yu, D.; Jiang, S.; Chen, C. Evaluation of Relationship Between Crystallization Structure and Thermal-Mechanical Performance of PLA with MCC Addition. ChemistrySelect 2019, 4, 10174–10180. [Google Scholar] [CrossRef]
- Cunha, B.L.C.; Bahú, J.O.; Xavier, L.F.; Crivellin, S.; de Souza, S.D.A.; Lodi, L.; Jardini, A.L.; Filho, R.M.; Schiavon, M.I.R.B.; Concha, V.O.C.; et al. Lactide: Production Routes, Properties, and Applications. Bioengineering 2022, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, A.; Nowacka, M. Supramolecular Interactions in Hybrid Polylactide Blends—The Structures, Mechanisms and Properties. Molecules 2020, 25, 3351. [Google Scholar] [CrossRef] [PubMed]

| Properties | Ingeo 2500HP | ASTM Standard |
|---|---|---|
| Specific gravity | 1.24 | D792 [44] |
| MFR, g/10 min (210 °C, 2.16 kg) | 8 | D1238 [45] |
| Relative viscosity (in 1.0 g/dL chloroform, 30 °C) | 4.0 | D5225 [46] |
| Highest crystallization melting point *, °C | 160–180 | D3418 [47] |
| Batch Name | Nucleating Agent | Concentration (wt.%) |
|---|---|---|
| Neat PLA | N/A | N/A |
| PLA-0.3OA | Orotic acid | 0.3 |
| PLA-1OA | Orotic acid | 1 |
| PLA-2OA | Orotic acid | 2 |
| PLA-0.3EBS | EBS | 0.3 |
| PLA-1EBS | EBS | 1 |
| PLA-2EBS | EBS | 2 |
| PLA Batch | Degree of Crystallinity (%) | ||
|---|---|---|---|
| Isotherm Temperature | |||
| 80 °C | 110 °C | 140 °C | |
| Neat PLA | 2.5 | 50.5 | 9.1 |
| PLA-0.3OA | 7.9 | 43.0 | 62.2 |
| PLA-1OA | 14.7 | 36.4 | 51.6 |
| PLA-2OA | 35.5 | 37.4 | 53.2 |
| PLA-0.3EBS | 7.8 | 41.2 | 3.9 |
| PLA-1EBS | 8.8 | 40.4 | 44.6 |
| PLA-2EBS | 6.9 | 41.4 | 55.3 |
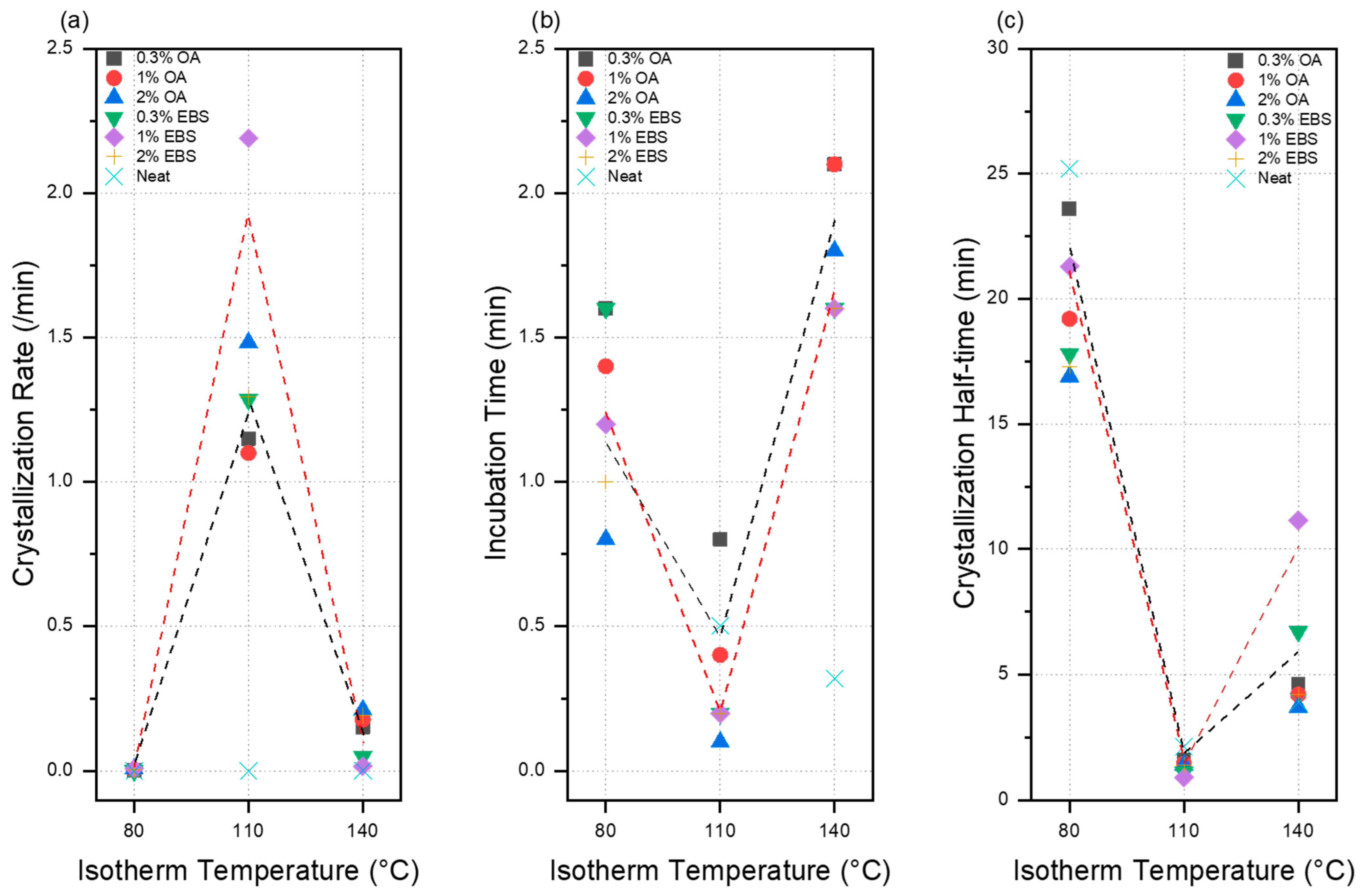
| Blends | Isotherm Temperature (°C) | Crystallization Rate (/min−n) | Avrami Index | Incubation Time (min) | t1/2 (min) |
|---|---|---|---|---|---|
| Neat PLA | 80 | 0.0001 ± 2.92 × 10−6 | 1.0 ± 1.2 × 10−4 | 5.86 ± 2.2 × 10−4 | 34.2 |
| Neat PLA | 110 | 0.0012 ± 4.13 × 10−4 | 3.3 ± 0.02 | 0.5 ± 0.02 | 2.14 |
| Neat PLA | 140 | 0.002 ± 1.63 × 10−4 | 1.2 ± 0.002 | 0.32 ± 0.07 | 3.9 |
| PLA-0.3OA | 80 | 3.6 × 10−4 ± 3.7 × 10−6 | 2.0 ± 0.03 | 1.6 ± 0.2 | 23.6 |
| PLA-1OA | 80 | 0.0029 ± 3.4 × 10−5 | 1.9 ± 0.004 | 1.4 ± 0.03 | 19.2 |
| PLA-2OA | 80 | 0.00814 ± 6.5 × 10−5 | 1.7 ± 0.003 | 0.8 ± 0.01 | 16.9 |
| PLA-0.3OA | 110 | 1.149 ± 0.015 | 1.7 ± 0.02 | 0.8 ± 0.008 | 1.61 |
| PLA-1OA | 110 | 1.099 ± 0.029 | 1.8 ± 0.05 | 0.4 ± 0.01 | 1.45 |
| PLA-2OA | 110 | 1.482 ± 0.026 | 2.2 ± 0.04 | 0.1 ± 0.01 | 1.4 |
| PLA-0.3OA | 140 | 0.152 ± 0.006 | 2.2 ± 0.03 | 2.1 ± 0.02 | 4.6 |
| PLA-1OA | 140 | 0.179 ± 0.007 | 2.0 ± 0.03 | 2.1 ± 0.02 | 4.2 |
| PLA-2OA | 140 | 0.211 ± 0.017 | 2.3 ± 0.06 | 1.8 ± 0.04 | 3.7 |
| PLA-0.3EBS | 80 | 8.7 × 10−4 ± 1.1 × 10−5 | 2.7 ± 0.3 | 1.6 ± 0.2 | 17.8 |
| PLA-1EBS | 80 | 0.0084 ± 7.7 × 10−4 | 1.7 ± 0.01 | 1.2 ± 0.1 | 21.3 |
| PLA-2EBS | 80 | 0.0047 ± 0.0024 | 2.0 ± 0.1 | 1.0 ± 0.1 | 17.3 |
| PLA-0.3EBS | 110 | 1.285 ± 0.018 | 2.4 ± 0.02 | 0.2 ± 0.01 | 1.1 |
| PLA-1EBS | 110 | 2.190 ± 0.059 | 2.1 ± 0.1 | 0.2 ± 0.02 | 0.9 |
| PLA-2EBS | 110 | 1.294 ± 0.129 | 2.4 ± 0.1 | 0.2 ± 0.05 | 1.4 |
| PLA-0.3EBS | 140 | 0.050 ± 3.45 × 10−4 | 2.1 ± 0.2 | 1.6 ± 0.02 | 6.7 |
| PLA-1EBS | 140 | 0.017 ± 0.004 | 1.9 ± 0.02 | 1.6 ± 0.02 | 11.15 |
| PLA-2EBS | 140 | 0.193 ± 0.001 | 1.8 ± 0.01 | 1.6 ± 0.01 | 4.2 |
| Sample | Isotherm Temperature (°C) | 2θ (°) | D-Spacing (Å) |
|---|---|---|---|
| Neat PLA | 80 | 16.31 | 5.48 |
| Neat PLA | 110 | 16.34 | 5.47 |
| Neat PLA | 140 | 16.67 | 5.36 |
| PLA-0.3OA | 80 | 16.49 | 5.43 |
| PLA-0.3OA | 110 | 16.70 | 5.36 |
| PLA-0.3OA | 140 | 16.55 | 5.41 |
| PLA-1OA | 80 | 16.34 | 5.47 |
| PLA-1OA | 110 | 16.85 | 5.31 |
| PLA-1OA | 140 | 16.61 | 5.39 |
| PLA-2OA | 80 | 16.45 | 5.44 |
| PLA-2OA | 110 | 16.63 | 5.38 |
| PLA-2OA | 140 | 16.72 | 5.35 |
| PLA-0.3EBS | 80 | 16.47 | 5.43 |
| PLA-0.3EBS | 110 | 16.69 | 5.36 |
| PLA-0.3EBS | 140 | 16.70 | 5.36 |
| PLA-1EBS | 80 | 16.46 | 5.43 |
| PLA-1EBS | 110 | 16.86 | 5.31 |
| PLA-1EBS | 140 | 16.64 | 5.38 |
| PLA-2EBS | 80 | 16.55 | 5.41 |
| PLA-2EBS | 110 | 16.61 | 5.39 |
| PLA-2EBS | 140 | 16.57 | 5.40 |
| OA | 0.3% | 1% | 2% |
| 80 °C | 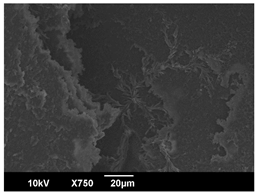 | 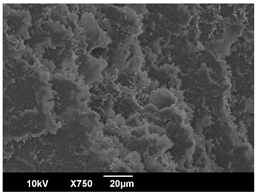 | 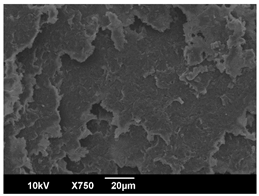 |
| 110 °C |  | 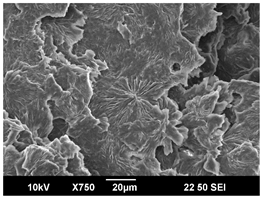 | 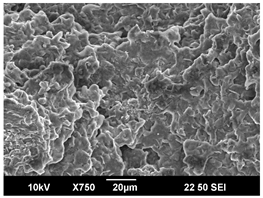 |
| 140 °C | 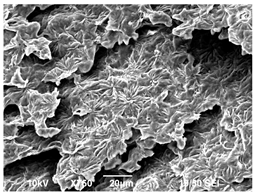 |  |  |
| EBS | 0.3% | 1% | 2% |
| 80 °C | 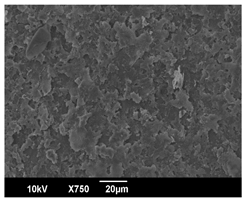 | 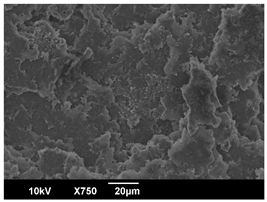 | 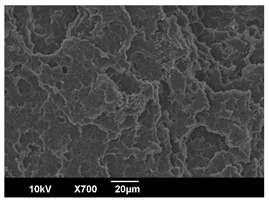 |
| 110 °C |  | 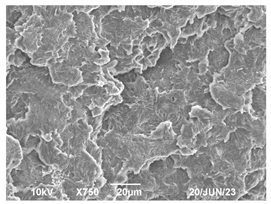 | 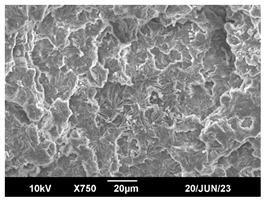 |
| 140 °C | 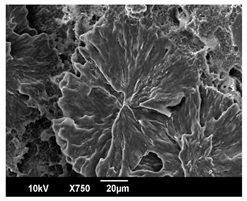 | 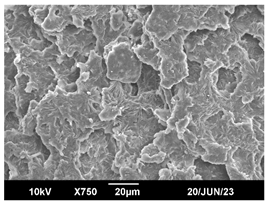 | 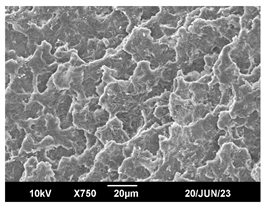 |
| Neat PLA, 80 °C | Neat PLA, 110 °C | Neat PLA, 140 °C |
 |  | 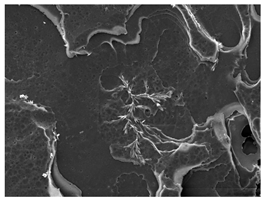 |
| Isotherm Temperature | |||
|---|---|---|---|
| 80 °C | 110 °C | 140 °C | |
| PLA-0.3OA | 7.8 ± 1.0 μm | 22.6 ± 5.2 μm | 11.7 ± 1.4 μm |
| PLA-1OA | 9.2 ± 0.6 μm | 15.6 ± 2.4 μm | 7.8 ± 0.7 μm |
| PLA-2OA | 8.8 ± 1.0 μm | 10.8 ± 2.0 μm | 6.5 ± 1.0 μm |
| PLA-0.3EBS | 7.2 ± 2.4 μm | 8.0 ± 2.0 μm | 40.0 ± 5.2 μm |
| PLA-1EBS | 9.3 ± 0.5 μm | 9.0 ± 1.5 μm | 11.5 ± 1.2 μm |
| PLA-2EBS | 10.0 ± 1.5 μm | 9.0 ± 1.2 μm | 8.0 ± 0.5 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Alanazi, S.; Masato, D. Crystallization of Polylactic Acid with Organic Nucleating Agents under Quiescent Conditions. Polymers 2024, 16, 320. https://doi.org/10.3390/polym16030320
Gao P, Alanazi S, Masato D. Crystallization of Polylactic Acid with Organic Nucleating Agents under Quiescent Conditions. Polymers. 2024; 16(3):320. https://doi.org/10.3390/polym16030320
Chicago/Turabian StyleGao, Peng, Saeed Alanazi, and Davide Masato. 2024. "Crystallization of Polylactic Acid with Organic Nucleating Agents under Quiescent Conditions" Polymers 16, no. 3: 320. https://doi.org/10.3390/polym16030320
APA StyleGao, P., Alanazi, S., & Masato, D. (2024). Crystallization of Polylactic Acid with Organic Nucleating Agents under Quiescent Conditions. Polymers, 16(3), 320. https://doi.org/10.3390/polym16030320










