Evaluation of the Modification Effects of Heparin/Dalteparin on Silk Fibroin Structure and Physical Properties for Skin Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BSF Aqueous Solution
2.2. Chemical Modification of BSF with Heparin
2.3. HSF Reaction Confirmation
2.4. Secondary Structure Analysis of Film
2.5. Evaluation of Film Chemical Properties
2.6. Dynamic Viscoelasticity Evaluation
2.7. Cell Culture
2.8. Evaluation of Cell Proliferation
2.9. Statistical Analysis
3. Results
3.1. Reaction Verification
3.2. Secondary Structure Analysis
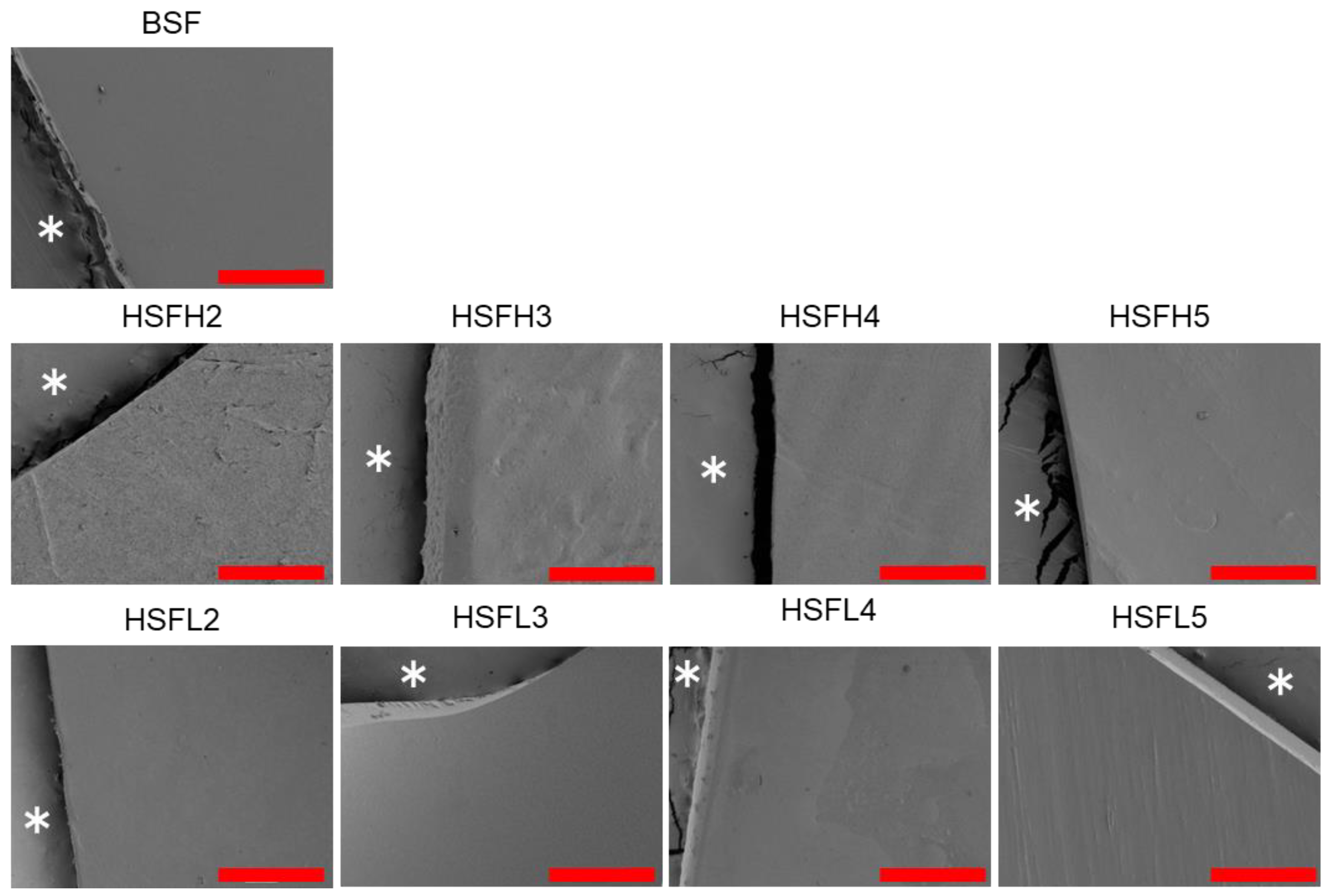
3.3. Chemical Properties of Film and Film Surface
3.4. Dynamic Viscoelasticity Evaluation
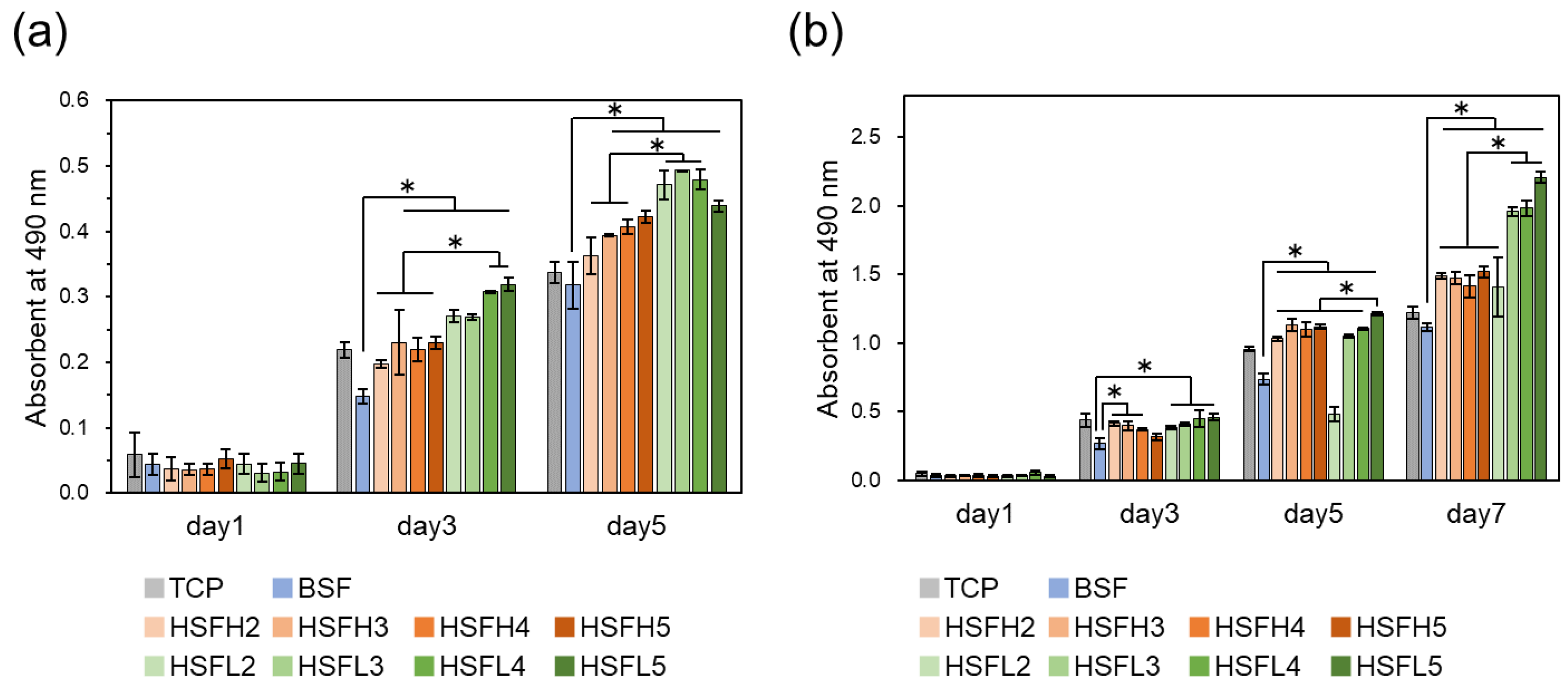
3.5. Cell Proliferation In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nosrati, H.; Heydari, M.; Khodaei, M. Cerium Oxide Nanoparticles: Synthesis Methods and Applications in Wound Healing. Mater. Today Biol. 2023, 23, 100823. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-Origin Polymers as Carriers and Scaffolds for Biomolecules and Cell Delivery in Tissue Engineering Applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- James, G.; Rheinwald, H.G. Epidermal Growth Factor and the Multiplication of Cultured Human Epidermal Keratinocytes. Nature 1977, 265, 421–424. [Google Scholar] [CrossRef]
- Idrus, R.B.H.; Rameli, M.A.B.P.; Cheong, L.K.; Xian, L.J.; Hui, C.K.; Latiff, M.B.A.; Saim, A. Bin Allogeneic Bilayered Tissue-Engineered Skin Promotes Full-Thickness Wound Healing in Ovine Model. Biomed. Res. 2014, 25, 192–198. [Google Scholar]
- Sharma, P.; Kumar, A.; Dey, A.D.; Behl, T.; Chadha, S. Stem Cells and Growth Factors-Based Delivery Approaches for Chronic Wound Repair and Regeneration: A Promise to Heal from Within. Life Sci. 2021, 268, 118932. [Google Scholar] [CrossRef]
- Hama, R.; Reinhardt, J.W.; Ulziibayar, A.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Tissue Engineering Approaches to Mimicking the Extracellular Matrix Structure for Skin Regeneration. Biomimetics 2023, 8, 130. [Google Scholar] [CrossRef]
- Hama, R.; Aytemiz, D.; Moseti, K.O.; Kameda, T.; Nakazawa, Y. Silk Fibroin Conjugated with Heparin Promotes Epithelialization and Wound Healing. Polymers 2022, 14, 3582. [Google Scholar] [CrossRef]
- Lohmann, N.; Schirmer, L.; Atallah, P.; Wandel, E.; Ferrer, R.A.; Werner, C.; Simon, J.C.; Franz, S.; Freudenberg, U. Glycosaminoglycan-Based Hydrogels Capture Inflammatory Chemokines and Rescue Defective Wound Healing in Mice. Sci. Transl. Med. 2017, 9, eaai9044. [Google Scholar] [CrossRef]
- Ashikari-Hada, S.; Habuchi, H.; Kariya, Y.; Kimata, K. Heparin Regulates Vascular Endothelial Growth Factor165-Dependent Mitogenic Activity, Tube Formation, and Its Receptor Phosphorylation of Human Endothelial Cells: Comparison of the Effects of Heparin and Modified Heparins. J. Biol. Chem. 2005, 280, 31508–31515. [Google Scholar] [CrossRef]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Kuan, C.H.; Wu, H.C.; Tsai, J.C.; Chen, T.M.; Hsieh, D.J.; Wang, T.W. Tailored Design of Electrospun Composite Nanofibers with Staged Release of Multiple Angiogenic Growth Factors for Chronic Wound Healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Tahir, H.M.; Butt, S.A.; Ali, S.; Ahmad, A.B.; Raza, C.; Summer, M.; Hassan, A.; Nadeem, J. Silk Derived Formulations for Accelerated Wound Healing in Diabetic Mice. PeerJ 2021, 9, e10232. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mora, C.; Mrowiec, A.; García-Vizcaíno, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolás, F.J. Fibroin and Sericin from Bombyx Mori Silk Stimulate Cell Migration through Upregulation and Phosphorylation of C-Jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Fambri, L.; Migliaresi, C. Regenerated Silk Fibroin Films: Thermal and Dynamic Mechanical Analysis. Macromol. Chem. Phys. 2002, 203, 1658–1665. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Kambe, Y.; Mizoguchi, Y.; Kuwahara, K.; Nakaoki, T.; Hirano, Y.; Yamaoka, T. Beta-Sheet Content Significantly Correlates with the Biodegradation Time of Silk Fibroin Hydrogels Showing a Wide Range of Compressive Modulus. Polym. Degrad. Stab. 2020, 179, 109240. [Google Scholar] [CrossRef]
- Hama, R.; Ulziibayar, A.; Reinhardt, J.W.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules 2023, 13, 280. [Google Scholar] [CrossRef]
- He, S.; Shi, D.; Han, Z.; Dong, Z.; Xie, Y.; Zhang, F.; Zeng, W.; Yi, Q. Heparinized Silk Fibroin Hydrogels Loading FGF1 Promote the Wound Healing in Rats with Full-Thickness Skin Excision. Biomed. Eng. Online 2019, 18, 1–12. [Google Scholar] [CrossRef]
- Zhou, C.-Z. Fine Organization of Bombyx Mori Fibroin Heavy Chain Gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef]
- Cestari, M.; Muller, V.; Rodrigues, J.H.D.S.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Preparing Silk Fibroin Nanofibers through Electrospinning: Further Heparin Immobilization toward Hemocompatibility Improvement. Biomacromolecules 2014, 15, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Çakır, C.O.; Ozturk, M.T.; Tuzlakoglu, K. Design of Antibacterial Bilayered Silk Fibroin-Based Scaffolds for Healing of Severe Skin Damages. Mater. Technol. 2018, 33, 651–658. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, H.; Tu, C.; Xu, Z.; Ye, L.; Zhao, L.; Gu, Z.; Zhao, D.; Zhang, J.; Feng, Z. In Situ Hydrogel Dressing Loaded with Heparin and Basic Fibroblast Growth Factor for Accelerating Wound Healing in Rat. Mater. Sci. Eng. C 2020, 116, 111169. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Miyamoto, S.; Miyachi, H.; Iwaki, R.; Shoji, T.; Blum, K.; Chang, Y.C.; Kelly, J.; Reinhardt, J.W.; Nakayama, H.; et al. Improvement of a Novel Small-Diameter Tissue-Engineered Arterial Graft with Heparin Conjugation. Ann. Thorac. Surg. 2021, 111, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Ogle, M.E.; Krieger, J.R.; Tellier, L.E.; McFaline-Figueroa, J.; Temenoff, J.S.; Botchwey, E.A. Dual Affinity Heparin-Based Hydrogels Achieve Pro-Regenerative Immunomodulation and Microvascular Remodeling. ACS Biomater. Sci. Eng. 2018, 4, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K.; Nordenhem, A. Dalteparin, a Low-Molecular-Weight Heparin, Promotes Angiogenesis Mediated by Heparin-Binding VEGF-A In Vivo. Apmis 2010, 118, 949–957. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Yu, F.; Ma, L.; Pan, X.; Luo, G.; Lin, S.; Mo, X.; He, C.; Wang, H. Hyaluronic Acid/EDC/NHS-Crosslinked Green Electrospun Silk Fibroin Nanofibrous Scaffolds for Tissue Engineering. RSC Adv. 2016, 6, 99720–99728. [Google Scholar] [CrossRef]
- Gotoh, Y.; Tsukada, M.; Minourat, N. Chemical Modification of Silk Fibroin with Cyanuric Chloride-Activated Poly (Ethy1ene Glycol): Analyses of Reaction Site by ’ H-NMR Spectroscopy and Conformation of the Conjugates. Bioconjugate Chem. 1993, 4, 554–559. [Google Scholar] [CrossRef]
- Dai, M.; Xu, K.; Xiao, D.; Zheng, Y.; Zheng, Q.; Shen, J.; Qian, Y.; Chen, W. In Situ Forming Hydrogel as a Tracer and Degradable Lacrimal Plug for Dry Eye Treatment. Adv. Healthc. Mater. 2022, 11, 2200678. [Google Scholar] [CrossRef]
- Aytemiz, D.; Fukuda, Y.; Higuchi, A.; Asano, A.; Nakazawa, C.T.; Kameda, T.; Yoshioka, T.; Nakazawa, Y. Compatibility Evaluation of Non-Woven Sheet Composite of Silk Fibroin and Polyurethane in the Wet State. Polymers 2018, 10, 874. [Google Scholar] [CrossRef]
- Gotoh, Y.; Niimi, S.; Hayakawa, T.; Miyashita, T. Preparation of Lactose-Silk Fibroin Conjugates and Their Application as a Scaffold for Hepatocyte Attachment. Biomaterials 2004, 25, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, Y.; Tsukada, M.; Aiba, S.; Minoura, N. Chemical Modification of Silk Fibroin with N-Acetyl-Chito-Oligosaccharides. Int. J. Biol. Macromol. 1996, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, Y.; Minoura, N.; Miyashita, T. Preparation and Characterization of Conjugates of Silk Fibroin and Chitooligosaccharides. Colloid Polym. Sci. 2002, 280, 562–568. [Google Scholar] [CrossRef]
- Ponedel’kina, I.Y.; Odinokov, V.N.; Lukina, E.S.; Tyumkina, T.V.; Khalilov, L.M.; Dzhemilev, U.M. Chemical Modification of Heparin. Russ. J. Bioorganic Chem. 2006, 32, 472–477. [Google Scholar] [CrossRef]
- Asakura, T.; Nakazawa, Y. Determination of Structures of Silk Fibroins from Silkworms and Spiders Using Solid-State NMR. Kobunshi Ronbunshu 2006, 63, 707–719. [Google Scholar] [CrossRef][Green Version]
- Asakura, T.; Suzuki, Y.; Nakazawa, Y.; Yazawa, K.; Holland, G.P.; Yarger, J.L. Silk Structure Studied with Nuclear Magnetic Resonance. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 69, 23–68. [Google Scholar] [CrossRef]
- Chuang, W.; Christ, M.D.; Rabenstein, D.L. Determination of the Primary Structures of Heparin- and Heparan Sulfate-Derived Oligosaccharides Using Band-Selective Experiments. Anal. Chem. 2001, 73, 2310–2316. [Google Scholar] [CrossRef]
- Asakura, T.; Okushita, K.; Williamson, M.P. Analysis of the Structure of Bombyx Mori Silk Fibroin by NMR. Macromolecules 2015, 48, 2345–2357. [Google Scholar] [CrossRef]
- Asakura, T.; Saotome, T.; Aytemiz, D.; Shimokawatoko, H.; Yagi, T.; Fukayama, T.; Ozai, Y.; Tanaka, R. Characterization of Silk Sponge in the Wet State Using 13C Solid State NMR for Development of a Porous Silk Vascular Graft with Small Diameter. RSC Adv. 2013, 4, 4427–4434. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Effect of Preadsorbed Proteins on Cell Adhesion to Polymer Surfaces. J. Colloid Interface Sci. 1993, 155, 334–339. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, K.; Kitakami, E.; Kobayashi, S.; Hoshiba, T.; Fukushima, K. Design of Biocompatible and Biodegradable Polymers Based on Intermediate Water Concept. Polym. J. 2015, 47, 114–121. [Google Scholar] [CrossRef]
- Morita, S.; Tanaka, M.; Ozaki, Y. Time-Resolved in Situ ATR-IR Observations of the Process of Sorption of Water into a Poly(2-Methoxyethyl Acrylate) Film. Langmuir 2007, 23, 3750–3761. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.N.; Tran, S.D.; Abughanam, G.; Laurenti, M.; Zuanazzi, D.; Mezour, M.A.; Xiao, Y.; Cerruti, M.; Siqueira, W.L.; Tamimi, F. Biomaterial Surface Proteomic Signature Determines Interaction with Epithelial Cells. Acta Biomater. 2017, 54, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Effect of Wettability and Surface Functional Groups on Protein Adsorption and Cell Adhesion Using Well-Defined Mixed Self-Assembled Monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Shirakata, Y.; Kimura, R.; Nanba, D.; Iwamoto, R.; Tokumaru, S.; Morimoto, C.; Yokota, K.; Nakamura, M.; Sayama, K.; Mekada, E.; et al. Heparin-Binding EGF-like Growth Factor Accelerates Keratinocyte Migration and Skin Wound Healing. J. Cell Sci. 2005, 118, 2363–2370. [Google Scholar] [CrossRef]
- Yazawa, K.; Ishida, K.; Masunaga, H.; Hikima, T.; Numata, K. Influence of Water Content on the β-Sheet Formation, Thermal Stability, Water Removal, and Mechanical Properties of Silk Materials. Biomacromolecules 2016, 17, 1057–1066. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Effect of Water on the Thermal Properties of Silk Fibroin. Thermochim. Acta 2007, 461, 137–144. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Dynamic Protein-Water Relationships during β-Sheet Formation. Macromolecules 2008, 41, 3939–3948. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.Y.; Park, Y.H.; Hudson, S. Structural Characteristics and Properties of the Regenerated Silk Fibroin Prepared from Formic Acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nakamura, Y.; Tamada, Y.; Kurosu, H.; Kameda, T. The Influence of Thermal Treatments on the Secondary Structure of Silk Fibroin Scaffolds and Their Interaction with Fibroblasts. PeerJ Mater. Sci. 2020, 2, e8. [Google Scholar] [CrossRef]
- Hess, S.; Van Beek, J.; Pannell, L.K. Acid Hydrolysis of Silk Fibroins and Determination of the Enrichment of Isotopically Labeled Amino Acids Using Precolumn Derivatization and High-Performance Liquid Chromatography–Electrospray Ionization–Mass Spectrometry. Anal. Biochem. 2002, 311, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Nishimura, A.; Naito, A. Stretching-Induced Conformational Transition of [3-13C]Ser- and [3-13C]Tyr-Antheraea Yamamai Silk Fibroin before Spinning Investigated with 13C Solid-State NMR Spectroscopy. Biomacromolecules 2022, 23, 5095–5105. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Ciccione, J.; Jacquet, T.; Maurel, M.; Montheil, T.; Mehdi, A.; Martinez, J.; Eymin, B.; Subra, G.; Coll, J.L. The Presence of PEG on Nanoparticles Presenting the c[RGDfK]- and/or ATWLPPR Peptides Deeply Affects the RTKs-AKT-GSK3β-ENOS Signaling Pathway and Endothelial Cells Survival. Int. J. Pharm. 2019, 568, 118507. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Agrawal, G.; Thakur, S.; Thakur, V.K.; Tanaka, M.; Mishra, Y.K.; Christie, G.; Mostafavi, E.; Boukherroub, R.; et al. Polysaccharides, Proteins, and Synthetic Polymers Based Multimodal Hydrogels for Various Biomedical Applications: A Review. Int. J. Biol. Macromol. 2023, 247, 125606. [Google Scholar] [CrossRef]
- Tanaka, M.; Kobayashi, S.; Murakami, D.; Aratsu, F.; Kashiwazaki, A.; Hoshiba, T.; Fukushima, K. Design of Polymeric Biomaterials: The “Intermediate Water Concept”. Bull. Chem. Soc. Jpn. 2019, 92, 2043–2057. [Google Scholar] [CrossRef]
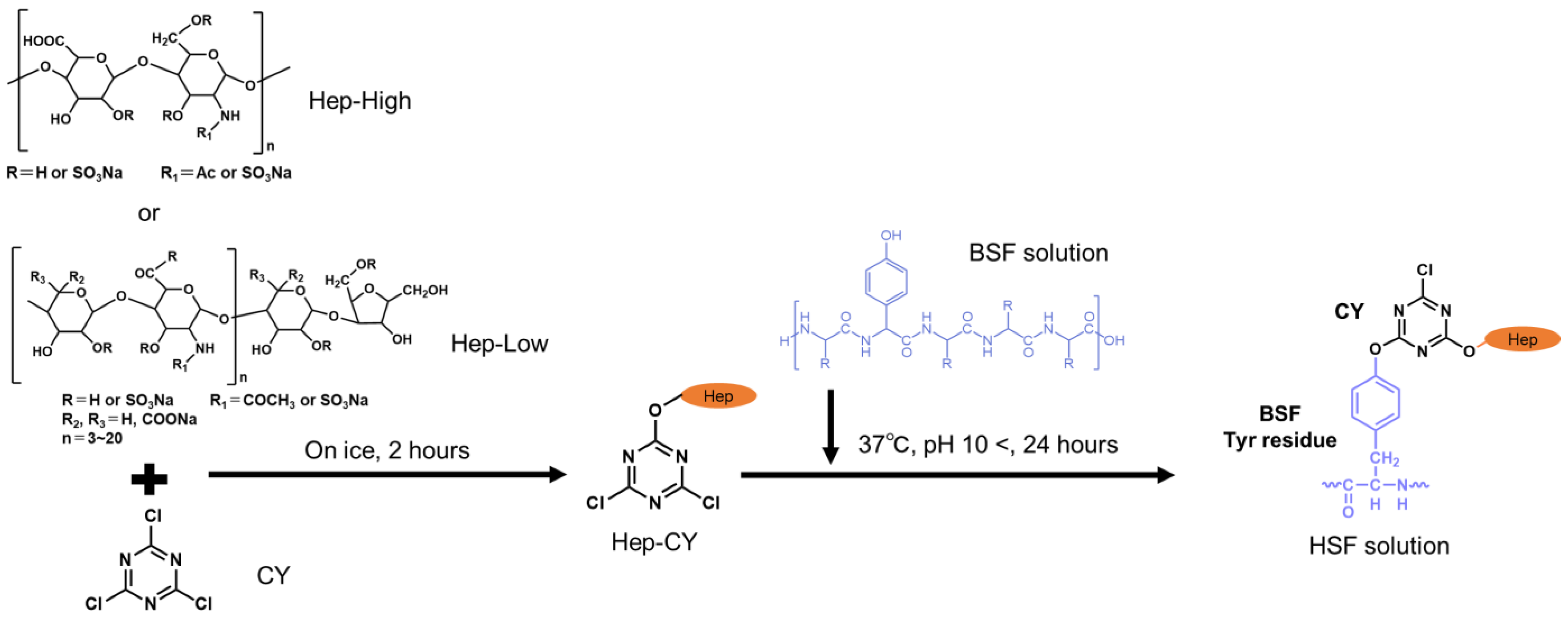





| Target Sites in BSF (mol) | Cyanuric Chloride (mol) | Heparin Sodium (mol) | |
|---|---|---|---|
| HSFH2, HSFL2 | 20 | 20 | 2 |
| HSFH3, HSFL3 | 20 | 20 | 3 |
| HSFH4, HSFL4 | 20 | 20 | 4 |
| HSFH5, HSFL5 | 20 | 20 | 5 |
| HSFH (mol%) | HSFL (mol%) | |
|---|---|---|
| HSF2 | 7.03 | 8.19 |
| HSF3 | 7.16 | 8.35 |
| HSF4 | 8.65 | 10.07 |
| HSF5 | 10.10 | 11.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hama, R.; Nakazawa, Y. Evaluation of the Modification Effects of Heparin/Dalteparin on Silk Fibroin Structure and Physical Properties for Skin Wound Healing. Polymers 2024, 16, 321. https://doi.org/10.3390/polym16030321
Hama R, Nakazawa Y. Evaluation of the Modification Effects of Heparin/Dalteparin on Silk Fibroin Structure and Physical Properties for Skin Wound Healing. Polymers. 2024; 16(3):321. https://doi.org/10.3390/polym16030321
Chicago/Turabian StyleHama, Rikako, and Yasumoto Nakazawa. 2024. "Evaluation of the Modification Effects of Heparin/Dalteparin on Silk Fibroin Structure and Physical Properties for Skin Wound Healing" Polymers 16, no. 3: 321. https://doi.org/10.3390/polym16030321
APA StyleHama, R., & Nakazawa, Y. (2024). Evaluation of the Modification Effects of Heparin/Dalteparin on Silk Fibroin Structure and Physical Properties for Skin Wound Healing. Polymers, 16(3), 321. https://doi.org/10.3390/polym16030321









