Modification and Use of Naturally Renewable Cardanol-Type Prepolymers in One-Component Silyl-Terminated Prepolymer Sealant Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Model Systems and One-Component Sealant
2.3. Testing Methods
2.3.1. Tensile Test
2.3.2. Rheology Tests
2.3.3. Aging of Materials in a Climatic Chamber
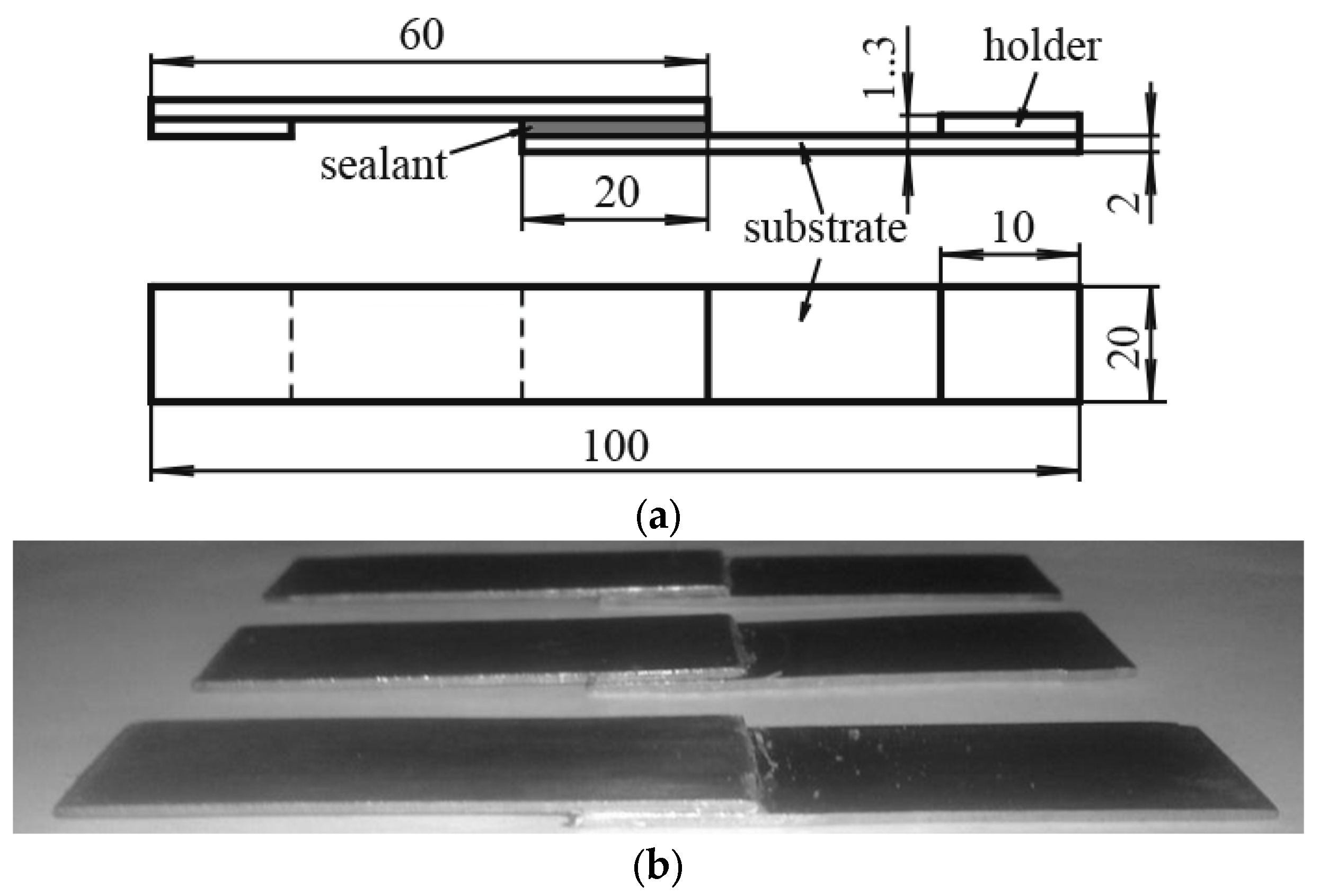
2.3.4. Lap Shear Strength Test
2.3.5. Synthesis of a Silyl-Terminated Prepolymer Using Modified Cardanol Ultra Lite 513
Synthesis of SIL 1189 Prepolymer
Synthesis of SIL 1122 Prepolymer
3. Results
3.1. Mechanical (Tensile) Properties of SAX 520/SIL 1189 and SIL 1122 Systems
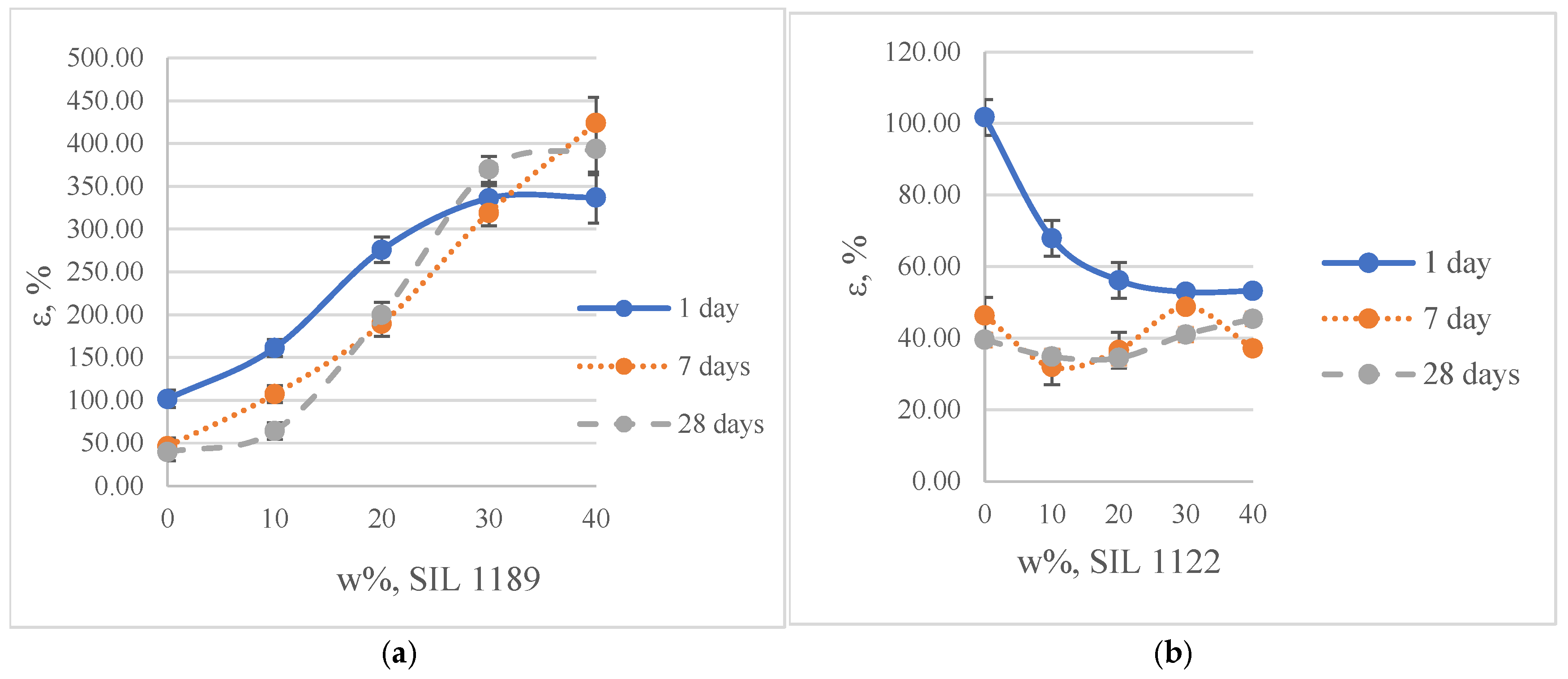
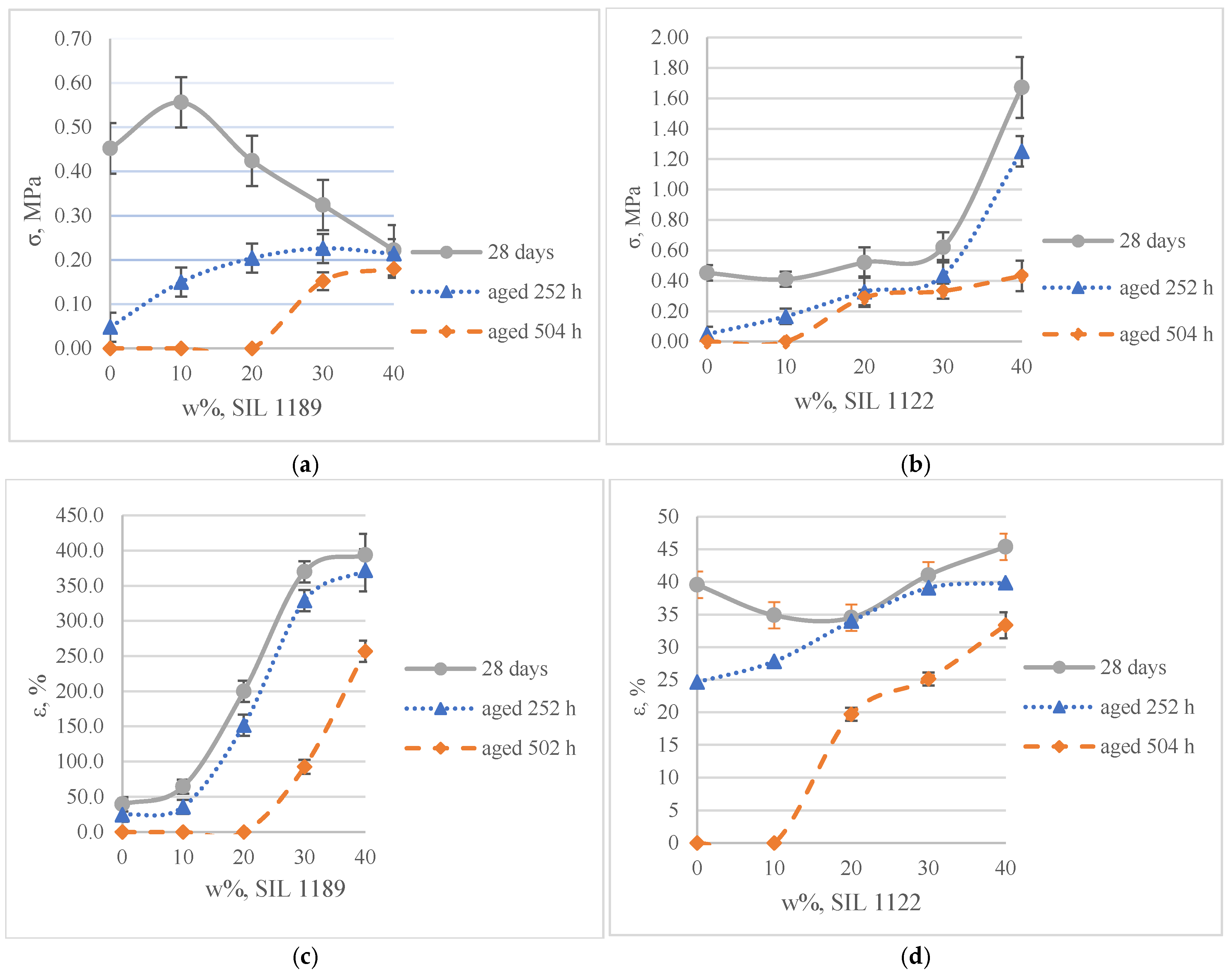
3.1.1. Mechanical (Tensile) and Aging Properties of SAX 520/SIL 1189 and SIL 1122 Model Systems
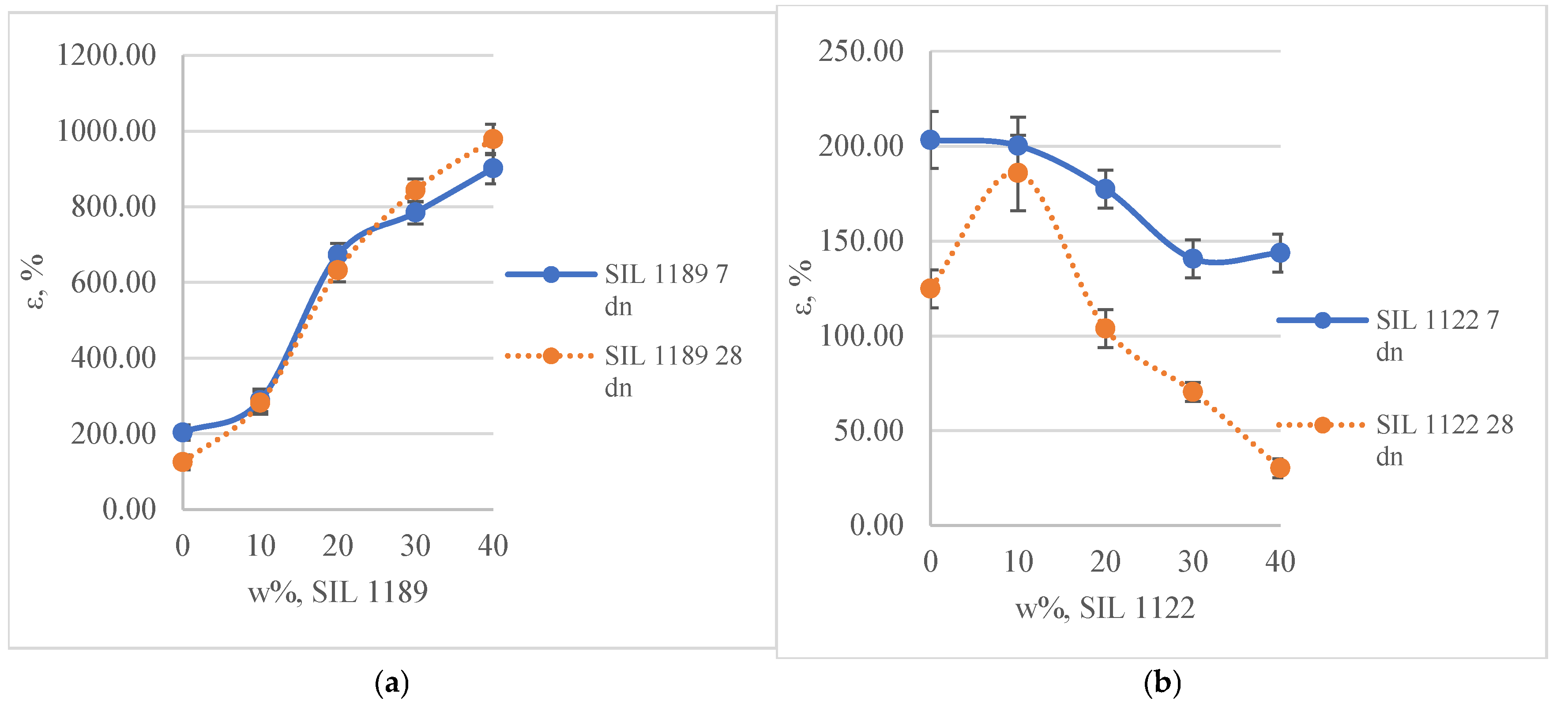
3.1.2. Tensile, Rheological and Adhesive Properties of Full Systems
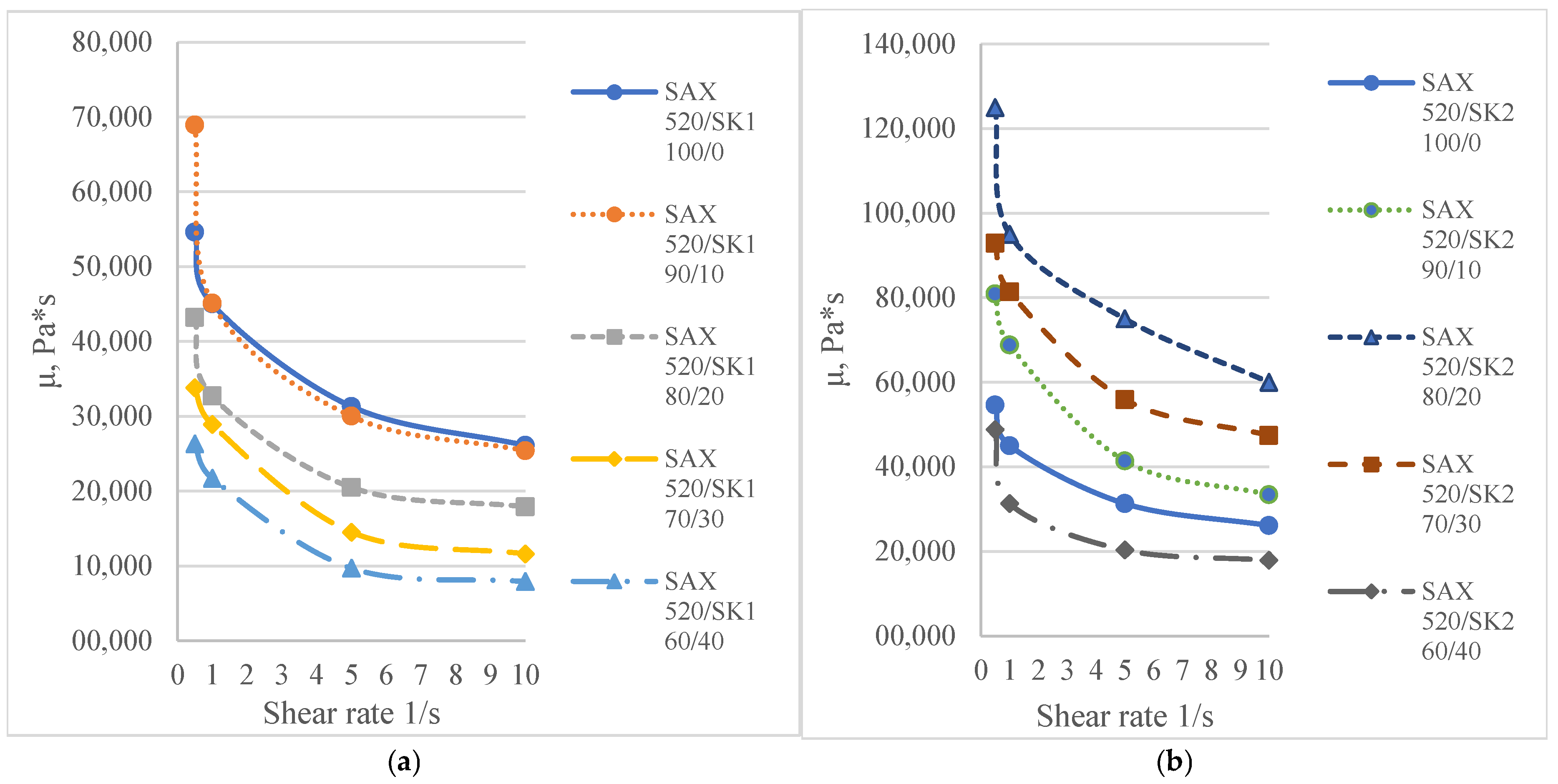
3.1.3. Full-System Rheological Properties
3.1.4. Lap Shear Strength of Adhesive Joint of SAX 520/SIL 1189 and SIL 1122 Full Systems
4. Conclusions
- (1)
- The test results for the model systems showed that by modifying the Ultra Lite 513 molecule with the silane Dynasilan 1189, it is possible to make the polymer structure more elastic, but by adding Dynasylan 1122, it is possible to increase the tensile strength of the material. It is possible to increase the elasticity of the material by 894% by adding 40% of SIL 1189 to the commercial prepolymer SAX 520 after 28 days at 23 °C and 50% RH, but even at this concentration the material becomes too soft and sticky. By adding SIL 1122 to the material, it is possible to increase the tensile strength of the material by 171% by replacing 40% of the SAX 520 prepolymer after 28 days at 23 °C and 50% RH.
- (2)
- Both modified prepolymers (SIL 1189 and SIL 1122) increased the climatic resistance of the material, and increasing their concentration improves the climatic resistance. As the concentration of the modified prepolymers increased, the deviation of the mechanical properties from the initial tensile strength decreased, indicating that the modified prepolymers protect the overall model system.
- (3)
- In the developed full adhesive system, the tensile strength properties increased both for systems without and with the prepolymers SIL 1189 and 1122. The compositions with the prepolymer SIL 1189 reached their maximum tensile strength at a 30% polymer concentration (2.92 MPa); at this concentration, they also had high deformation (843%). When the SIL 1122 prepolymer was added to the composition, the increase in adhesive tensile strength was higher (3.23 MPa at SIL 1122 40%) compared to SIL 1189, but at higher concentrations, a decrease in deformation (30.2% at SIL 1122 40%) was also observed, indicating that the prepolymer acted as a cross-linker of the system.
- (4)
- The lap shear strength of the adhesive joint of the prepolymers based on SIL 1189 and SIL 1122 in the commercially available SAX 520 material was determined. It was established that the highest values of this parameter (1.565 and 1.375 MPa) are for the SAX 520/SIL 1189 system with 10% prepolymer (1) and the SAX 520/SIL 1122 system with 20%. It was established that the strongest adhesive joints are mainly destroyed cohesively due to their high level of interaction with metal substrates.
- (5)
- The synthesized prepolymers SIL 1189 and SIL 1122 have approximately 100 times lower viscosity compared to the commercially available SAX 520, which means that when integrating the synthesized prepolymers into the system, the viscosity of the adhesives decreases, which potentially allows increasing the content of fillers in the material, thus potentially reducing the cost of the adhesive.
- (6)
- The developed compositions are competitive with commercially available adhesives and also significantly improve the mechanical, rheological and climatic properties of the typical commercially available prepolymer SAX 520.
- (7)
- Synthesized prepolymers of the cardanol type are more expensive than commercial products, but their effect, even at low prepolymer concentrations (20–40% of the prepolymer composition), on the material’s mechanical properties and climatic resistance is very significant. The effect of the synthesized cardanol-based prepolymers makes them a different class of material, with a higher price than typical sealants and adhesives used in construction. This type of material could potentially be used as a structural adhesive or in the automotive industry. In these industries, the material added value is higher; therefore, despite the price increase caused by the synthesized cardanol prepolymer, the profit obtained will be higher.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Heale, F.L.; Page, K.; Wixey, J.; Taylor, P. Inexpensive and non-toxic water repellent coatings comprising SiO2 nanoparticles and long chain fatty acids. RSC Adv. 2018, 8, 27064–27072. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Rua, R.; Silvestre, A.J.D.; Gandini, A. Polymers and copolymers from fatty acid-based monomers. Ind. Crop. Prod. 2010, 32, 97–104. [Google Scholar] [CrossRef]
- Gusain, R.; Khan, A.; Khatri, O.P. Fatty acid-derived ionic liquids as renewable lubricant additives: Effect of chain length and unsaturation. J. Mol. Liq. 2020, 301, 112322. [Google Scholar] [CrossRef]
- Hamnas, A.; Unnikrishnan, G. Bio-lubricants from vegetable oils: Characterization, modifications, applications and challenges—Review. Renew. Sustain. Energy Rev. 2023, 182, 113413. [Google Scholar] [CrossRef]
- Briou, B.; Jego, L.; Miguel, T.D.D.; Duguet, N.; Caillol, S. Eco-friendly synthesis of cardanol-based AB monomer for formaldehyde-free phenolic thermosets. RSC Sustain. 2023, 1, 994–1005. [Google Scholar] [CrossRef]
- Mora, A.S.; Tayouo, R.; Boutevin, B.; David, G.; Caillol, S. Synthesis of biobased reactive hydroxyl amines by amination reaction of cardanol-based epoxy monomers. Eur. Polym. J. 2019, 118, 429–436. [Google Scholar] [CrossRef]
- Kalita, D.J.; Tarnavchyk, I.; Kalita, H.; Chisholm, B.J.; Webster, D.C. Bio-based coating resins derived from cardanol using carbocationic polymerization and their evaluation as one-component alkyd-type coatings. Prog. Org. Coat. 2023, 174, 107252. [Google Scholar] [CrossRef]
- Jia, P.; Song, F.; Li, Q.; Xia, H. Recent Development of Cardanol Based Polymer Materials-A Review. J. Renew. Mater. 2019, 7, 601–619. [Google Scholar] [CrossRef]
- Available online: https://www.cardolite.com (accessed on 20 September 2024).
- Caillol, S. The future of cardanol as small giant for biobased aromatic polymers and additives. Eur. Polym. J. 2023, 193, 112096. [Google Scholar] [CrossRef]
- Mubofu, E.B.; Mgaya, J. Chemical Valorization of Cashew Nut Shell Waste. Top. Curr. Chem. 2018, 376, 8. [Google Scholar] [CrossRef] [PubMed]
- Silyl Modified Polymer Adhesive and Sealant Systems. Available online: https://www.adhesivesandcoatings.com/adhesives/about-adhesives/modified-silane-adhesives-and-sealants (accessed on 24 September 2024).
- Gadhave, R.V.; Gadhave, C.R.; Dhawale, P.V. Silane terminated prepolymers: An alternative to silicones and polyurethanes. Open J. Polym. Chem. 2021, 11, 31–54. [Google Scholar] [CrossRef]
- Pape, P.G. 29—Adhesion Promoters: Silane Coupling Agents. In Applied Plastics Engineering Handbook; Elsevier, William Andrew: Amsterdam, The Netherlands, 2011; pp. 503–517. [Google Scholar]
- Petrunin, M.A.; Maksaeva, L.B.; Gladkikh, N.A.; Yurasova, T.A.; Kotenev, V.A.; Tsivadze, A.Y. Adsorption of Organosilanes on the Surface of Inorganic Materials: 2. Adsorption on the Surface of Metals. Prot. Met. Phys. Chem. Surf. 2022, 58, 217–243. [Google Scholar] [CrossRef]
- Rusanova, S.N.; Sof’ina, S.Y.; Temnikova, N.E.; Gerasimov, V.K.; Chalykh, A.E.; Starostina, I.A.; Mezhevich, Z.V.; Stoyanov, O.V. The Effect of the Phase Structure of Silane-Modified Ethylene Copolymers on Their Surface Energy and Adhesion Properties. Polym. Sci. Ser. D 2020, 13, 258–264. [Google Scholar] [CrossRef]
- Prosvirnina, A.P.; Bugrov, A.N.; Dobrodumov, A.V.; Vlasova, E.N.; Fedotova, V.S.; Nikolaeva, A.L.; Vorobiov, V.K.; Sokolova, M.P.; Smirnov, M.A. Bacterial cellulose nanofibers modification with 3-(trimethoxysilyl)propyl methacrylate as a crosslinking and reinforcing agent for 3D printable UV-curable inks. J. Mater. Sci. 2022, 57, 20543–20557. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Strzelec, K. Improving performance of natural rubber composites by the application of functional biofiller: Horsetail modified with silane coupling agents. Cellulose 2023, 30, 10175–10198. [Google Scholar] [CrossRef]
- Berzins, R.; Merijs-Meri, R.; Zicans, J. Comparison of Two-Component Silyl-Terminated Polyether/Epoxy Resin Model and Complete Systems and Evaluation of Their Mechanical, Rheological and Adhesive Properties. Polymers 2022, 14, 2421. [Google Scholar] [CrossRef] [PubMed]





| Raw Material Class | Trade Name | Raw Material Structure Formula |
|---|---|---|
| Prepolymer | SAX 520 (Kaneka, Westerlo-Oevel, Belgium) |  |
| Prepolymer | Ultra Lite 513 (Cardolite, Gent, Belgium) |  |
| Silane | Dynasylan 1189 (Evonik, Marl, Germany) |  |
| Silane | Dynasylan 1122 (Evonik, Marl, Germany) |  |
| Drying agent | Dynasylan VTMO (Evonik, Marl, Germany) | 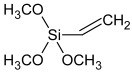 |
| Adhesion promoter | Dynasylan Glymo (Evonik, Marl, Germany) |  |
| Filler | Omycarb 1TVA (Omya, Oftringen, Switzerland) | Coated natural calcium carbonate |
| Filler | Hakuenka CCR-S10 (Omya, Oftringen, Switzerland) | Coated precipitated calcium carbonate |
| Catalyst | Tibcat 318 (TIB chemicals, Mannheim, Germany) |  |
| Raw Materials | Mass, g | ||||
|---|---|---|---|---|---|
| SAX 520 | 90 | 81 | 72 | 63 | 54 |
| Synthesized SIL prepolymer (SIL 1189 or 1122) | 0 | 9 | 18 | 27 | 36 |
| Hakuenka CCR-S10 | 10 | 10 | 10 | 10 | 10 |
| Tibcat 318 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Raw Materials | Mass, g | |||
|---|---|---|---|---|
| SAX 520 | 36 | 32 | 28 | 24 |
| SIL 1189/1122 | 4 | 8 | 12 | 16 |
| Dynasylan VTMO | 0.8 | 0.8 | 0.8 | 0.8 |
| Dynasylan Glymo | 0.2 | 0.2 | 0.2 | 0.2 |
| Omycarb 1TVA | 10 | 10 | 10 | 10 |
| Hakuenka CCR-S10 | 43.6 | 43.6 | 43.6 | 43.6 |
| Tibcat 318 | 0.4 | 0.4 | 0.4 | 0.4 |
| SK1 (SIL 1189) | SK2 (SIL 1122) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 100_0 | 90_10 | 80_20 | 70_30 | 60_40 | 90_10 | 80_20 | 70_30 | 60_40 | |
| Shear rate (1/s) | μ, Pa*s | ||||||||
| 0.5 | 54,600 | 68,900 | 43,200 | 33,800 | 26,300 | 80,900 | 12,5000 | 92,900 | 48,500 |
| 1 | 45,300 | 45,100 | 32,700 | 28,900 | 21,700 | 68,800 | 95,000 | 81,400 | 31,300 |
| 5 | 31,300 | 30,000 | 20,500 | 14,500 | 9700 | 41,500 | 75,000 | 55,900 | 20,400 |
| 10 | 26,100 | 25,400 | 17,900 | 11,600 | 7900 | 33,100 | 59,100 | 47,800 | 17,900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berzins, R.; Meri, R.M.; Zicans, J.; Abele, A.; Sytar, V.; Kabat, O.; Klymenko, A.; Mitina, N. Modification and Use of Naturally Renewable Cardanol-Type Prepolymers in One-Component Silyl-Terminated Prepolymer Sealant Systems. Polymers 2024, 16, 3511. https://doi.org/10.3390/polym16243511
Berzins R, Meri RM, Zicans J, Abele A, Sytar V, Kabat O, Klymenko A, Mitina N. Modification and Use of Naturally Renewable Cardanol-Type Prepolymers in One-Component Silyl-Terminated Prepolymer Sealant Systems. Polymers. 2024; 16(24):3511. https://doi.org/10.3390/polym16243511
Chicago/Turabian StyleBerzins, Ritvars, Remo Merijs Meri, Janis Zicans, Agnese Abele, Volodymyr Sytar, Oleh Kabat, Anton Klymenko, and Nataliia Mitina. 2024. "Modification and Use of Naturally Renewable Cardanol-Type Prepolymers in One-Component Silyl-Terminated Prepolymer Sealant Systems" Polymers 16, no. 24: 3511. https://doi.org/10.3390/polym16243511
APA StyleBerzins, R., Meri, R. M., Zicans, J., Abele, A., Sytar, V., Kabat, O., Klymenko, A., & Mitina, N. (2024). Modification and Use of Naturally Renewable Cardanol-Type Prepolymers in One-Component Silyl-Terminated Prepolymer Sealant Systems. Polymers, 16(24), 3511. https://doi.org/10.3390/polym16243511






