Comigration Behavior of Cr(VI) and Microplastics and Remediation of Microplastics-Facilitated Cr(VI) Transportation in Saturated Porous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Column and Batch Experiments
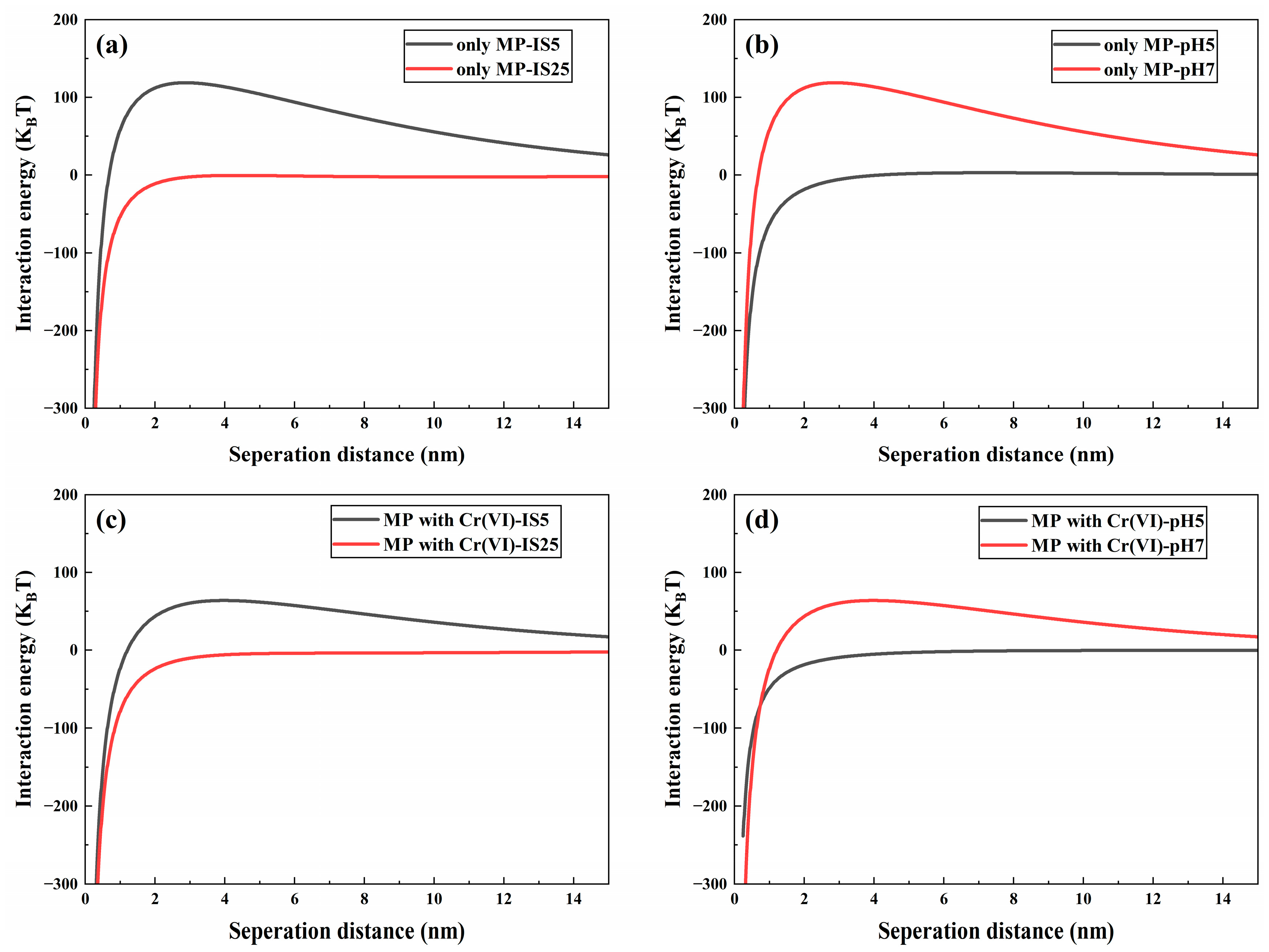
2.3. DLVO Interaction Energy Calculations
2.4. Mathematical Modeling of Cr(VI) Reactive Transport
3. Results and Discussion
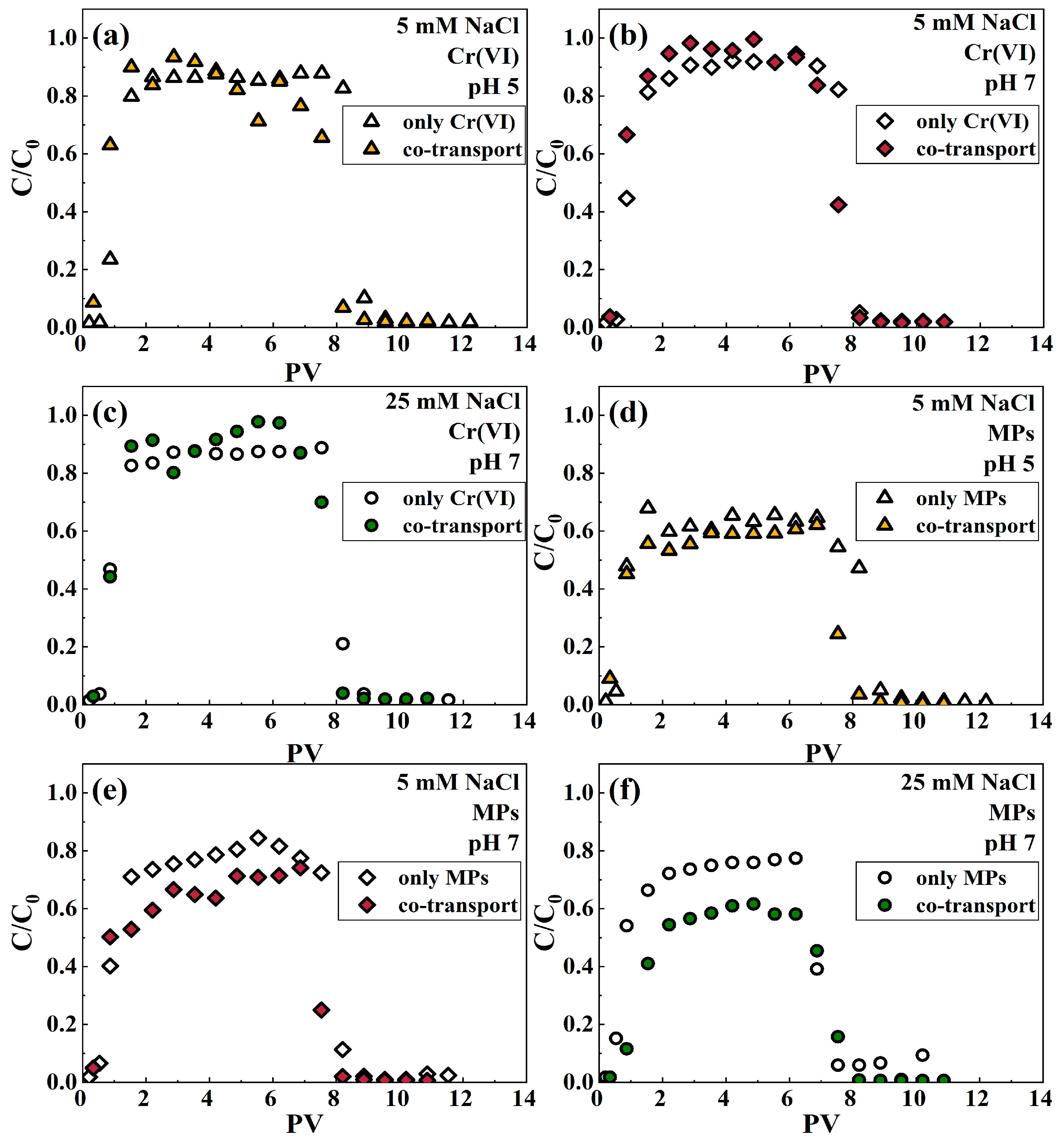
3.1. Effect of IS on the Cotransport of Cr(VI) and Microplastics
3.2. Effect of pH on the Cotransport of Cr(VI) and Microplastics
3.3. Effects of SA/NZVI-rGO Gel Beads on Cr(VI) Transport with Microplastics
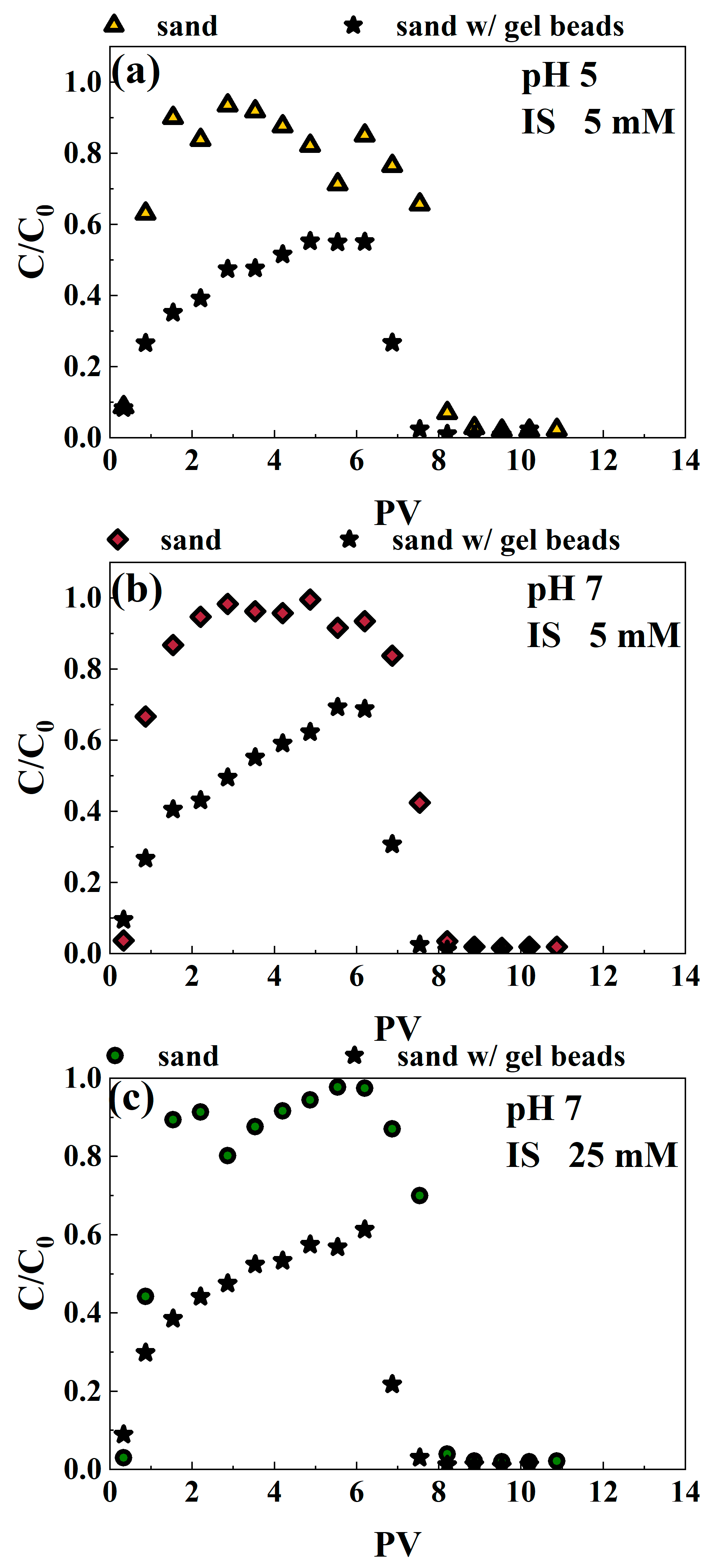
3.3.1. The Removal of Cr(VI) and MPs by Gel Beads and an Analysis of the Mechanism
3.3.2. Cr(VI) Migrates with Microplastics in the Quartz Sand Filled with Gel Beads
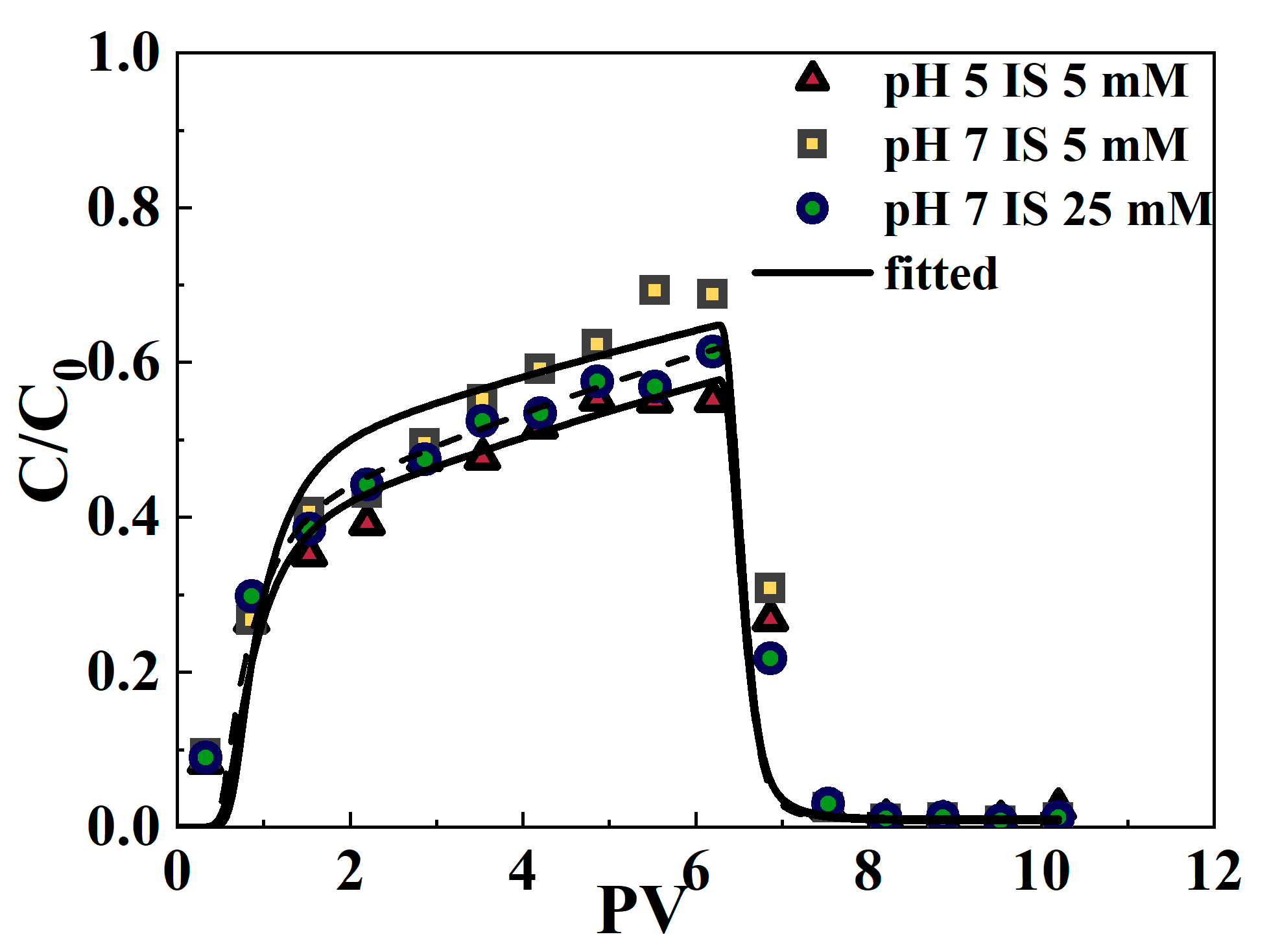
3.4. Reactive Transport Model of Cr(VI) in Filled Saturated Porous Media During Comigration with MPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, J.; Xiao, Y.; Tan, Z.; Xu, X.; Wang, Z.; Zhang, L.; Shi, Y.; Pi, K.; Qiu, G.; Yang, X. Influence of Coexisting Anions on the One-Step Electrochemical Reduction and Precipitation Removal of Cr(VI): Implications for Advanced Wastewater Treatment. J. Environ. Manag. 2024, 371, 123167. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in Agricultural Soils and Crops: A Review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Vimercati, L.; Gatti, M.F.; Gagliardi, T.; Cuccaro, F.; De Maria, L.; Caputi, A.; Quarato, M.; Baldassarre, A. Environmental Exposure to Arsenic and Chromium in an Industrial Area. Environ. Sci. Pollut. Res. 2017, 24, 11528–11535. [Google Scholar] [CrossRef]
- Ambika, S.; Kumar, M.; Pisharody, L.; Malhotra, M.; Kumar, G.; Sreedharan, V.; Singh, L.; Nidheesh, P.V.; Bhatnagar, A. Modified Biochar as a Green Adsorbent for Removal of Hexavalent Chromium from Various Environmental Matrices: Mechanisms, Methods, and Prospects. Chem. Eng. J. 2022, 439, 135716. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and Toxicity of Hexavalent Chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Yang, D.; Fang, W.; Zhang, H.; Sun, H.; Gu, X.; Chen, H.; Luo, J. Effects of nZVI on the Migration and Availability of Cr(VI) in Soils under Simulated Acid Rain Leaching Conditions. J. Hazard. Mater. 2024, 476, 134985. [Google Scholar] [CrossRef]
- US EPA, O. Drinking Water. Available online: https://www.epa.gov/report-environment/drinking-water (accessed on 30 August 2024).
- Wang, X.; Li, L.; Yan, X.; Meng, X.; Chen, Y. Processes of Chromium (VI) Migration and Transformation in Chromate Production Site: A Case Study from the Middle of China. Chemosphere 2020, 257, 127282. [Google Scholar] [CrossRef]
- Huang, D.; Khan, N.A.; Wang, G.; Carroll, K.C.; Brusseau, M.L. The Co-Transport of PFAS and Cr(VI) in Porous Media. Chemosphere 2022, 286, 131834. [Google Scholar] [CrossRef]
- Walker, T.R. Calling for a Decision to Launch Negotiations on a New Global Agreement on Plastic Pollution at UNEA5.2. Mar. Pollut. Bull. 2022, 176, 113447. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Pico, Y. Nano- and Microplastic Analysis: Focus on Their Occurrence in Freshwater Ecosystems and Remediation Technologies. Trends Anal. Chem. 2019, 17. [Google Scholar] [CrossRef]
- Cole, M. Microplastics as Contaminants in the Marine Environment: A Review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating Microplastic Trophic Transfer in Marine Top Predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef]
- Okeke, E.S.; Okoye, C.O.; Atakpa, E.O.; Ita, R.E.; Nyaruaba, R.; Mgbechidinma, C.L.; Akan, O.D. Microplastics in Agroecosystems-Impacts on Ecosystem Functions and Food Chain. Resour. Conserv. Recycl. 2022, 177, 105961. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and Micro- Plastics in Soil-Plant System: Effects of Plastic Mulch Film Residues on Wheat (Triticum Aestivum) Growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Lahiva, E. Earthworms Ingest Microplastic Fibres and Nanoplastics with Effects on Egestion Rate and Long-Term Retention. Sci. Total Environ. 2022, 807, 151022. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and Quantification of Macro- and Microplastics on an Agricultural Farmland. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Guo, J.-J. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Ren, Z.; Gui, X.; Wei, Y.; Chen, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Chemical and Photo-Initiated Aging Enhances Transport Risk of Microplastics in Saturated Soils: Key Factors, Mechanisms, and Modeling. Water Res. 2021, 202, 117407. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic Pollution of Lakeshore Sediments from Remote Lakes in Tibet Plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, J.; Mishra, P.P. Microplastics in Polar Regions: An Early Warning to the World’s Pristine Ecosystem. Sci. Total Environ. 2021, 784, 147149. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Yi, K.; He, L.; Han, P.; Tong, M. Transport and Deposition of Microplastic Particles in Saturated Porous Media: Co-Effects of Clay Particles and Natural Organic Matter. Environ. Pollut. 2021, 287, 117585. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Wang, X.; Cai, L.; Tong, M.; Kim, H. Transport and Retention Behaviors of Titanium Dioxide Nanoparticles in Iron Oxide-Coated Quartz Sand: Effects of pH, Ionic Strength, and Humic Acid. Colloids Surf. Physicochem. Eng. Asp. 2014, 454, 119–127. [Google Scholar] [CrossRef]
- Lee, S.; Ko, I.-W.; Yoon, I.-H.; Kim, D.-W.; Kim, K.-W. Colloid Mobilization and Heavy Metal Transport in the Sampling of Soil Solution from Duckum Soil in South Korea. Environ. Geochem. Health 2019, 41, 469–480. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, Y.; Wang, Y.; Song, Z.; Wu, Y.; Ma, W.; Hou, Y.; Zhang, W.; Yang, Y. Influence of Soil Colloids on Ni Adsorption and Transport in the Saturated Porous Media: Effects of pH, Ionic Strength, and Humic Acid. Appl. Sci. 2022, 12, 6591. [Google Scholar] [CrossRef]
- Li, M.; Wu, D.; Wu, D.; Guo, H.; Han, S. Influence of Polyethylene-Microplastic on Environmental Behaviors of Metals in Soil. Environ. Sci. Pollut. Res. 2021, 28, 28329–28336. [Google Scholar] [CrossRef]
- Abduro Ogo, H.; Tang, N.; Li, X.; Gao, X.; Xing, W. Combined Toxicity of Microplastic and Lead on Submerged Macrophytes. Chemosphere 2022, 295, 133956. [Google Scholar] [CrossRef]
- Guo, A.; Pan, C.; Su, X.; Zhou, X.; Bao, Y. Combined Effects of Oxytetracycline and Microplastic on Wheat Seedling Growth and Associated Rhizosphere Bacterial Communities and Soil Metabolite Profiles. Environ. Pollut. 2022, 302, 119046. [Google Scholar] [CrossRef]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics Influence the Adsorption and Desorption Characteristics of Cd in an Agricultural Soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef]
- Yao, J.; Wang, H.; Ma, C.; Cao, Y.; Chen, W.; Gu, L.; He, Q.; Liu, C.; Xiong, J.; Ma, J.; et al. Cotransport of Thallium(I) with Polystyrene Plastic Particles in Water-Saturated Porous Media. J. Hazard. Mater. 2022, 422, 126910. [Google Scholar] [CrossRef]
- Dehmani, Y.; Ba Mohammed, B.; Oukhrib, R.; Dehbi, A.; Lamhasni, T.; Brahmi, Y.; El-Kordy, A.; Franco, D.S.P.; Georgin, J.; Lima, E.C.; et al. Adsorption of Various Inorganic and Organic Pollutants by Natural and Synthetic Zeolites: A Critical Review. Arab. J. Chem. 2024, 17, 105474. [Google Scholar] [CrossRef]
- Islam, M.M.; Mohana, A.A.; Rahman, M.A.; Rahman, M.; Naidu, R.; Rahman, M.M. A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution. Toxics 2023, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, H.; Bian, K.; Wang, H.; Wang, C. A Critical Review of Control and Removal Strategies for Microplastics from Aquatic Environments. J. Environ. Chem. Eng. 2021, 9, 105463. [Google Scholar] [CrossRef]
- Lodge, J.P. Critical Reviews in Environmental Science and Technology. Atmos. Environ. 1994, 28, 753–754. [Google Scholar] [CrossRef]
- Azzam, A.M.; El-Wakeel, S.T.; Mostafa, B.B.; El-Shahat, M.F. Removal of Pb, Cd, Cu and Ni from Aqueous Solution Using Nano Scale Zero Valent Iron Particles. J. Environ. Chem. Eng. 2016, 4, 2196–2206. [Google Scholar] [CrossRef]
- Dongsheng, Z.; Wenqiang, G.; Guozhang, C.; Shuai, L.; Weizhou, J.; Youzhi, L. Removal of Heavy Metal Lead(II) Using Nanoscale Zero-Valent Iron with Different Preservation Methods. Adv. Powder Technol. 2019, 30, 581–589. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Liang, F.; Zhang, W. Heavy Metal Removal Using Nanoscale Zero-Valent Iron (nZVI): Theory and Application. J. Hazard. Mater. 2017, 322, 163–171. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on Nano Zerovalent Iron (nZVI): From Synthesis to Environmental Applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Yuan, F.; Yue, L.; Zhao, H.; Wu, H. Study on the Adsorption of Polystyrene Microplastics by Three-Dimensional Reduced Graphene Oxide. Water Sci. Technol. 2020, 81, 2163–2175. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Y.; Liu, J.; Wang, Y.; Cui, Q.; Song, P.; Zhang, X.; Zhang, C. Hierarchically Structured Hydrogel Actuator for Microplastic Pollutant Detection and Removal. Chem. Mater. 2022, 34, 5165–5175. [Google Scholar] [CrossRef]
- Peng, G. Engineering 3D Graphene-like Carbon-Assembled Layered Double Oxide for Efficient Microplastic Removal in a Wide pH Range. J. Hazard. Mater. 2022, 433, 128672. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Z.; Zheng, H.; Chen, L.; Li, F. Biodegradable and Re-Usable Sponge Materials Made from Chitin for Efficient Removal of Microplastics. J. Hazard. Mater. 2021, 420, 126599. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Safari, E.; Baghdadi, M.; Janmohammadi, M. Enhanced Adsorption of Heavy Metals in Groundwater Using Sand Columns Enriched with Graphene Oxide: Lab-Scale Experiments and Process Modeling. J. Water Process Eng. 2021, 40, 101961. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An Overview of Preparation and Applications of Stabilized Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Calderon, B.; Fullana, A. Heavy Metal Release Due to Aging Effect during Zero Valent Iron Nanoparticles Remediation. Water Res. 2015, 83, 1–9. [Google Scholar] [CrossRef]
- Jabeen, H.; Kemp, K.C.; Chandra, V. Synthesis of Nano Zerovalent Iron Nanoparticles – Graphene Composite for the Treatment of Lead Contaminated Water. J. Environ. Manag. 2013, 130, 429–435. [Google Scholar] [CrossRef]
- Jing, Q.; You, W.; Qiao, S.; Ma, Y.; Ren, Z. Comprehensive Understanding of Adsorption and Reduction on 2,4-DCP and Cr(VI) Removal Process by NZVI-rGO: Performance and Mechanism. J. Water Process Eng. 2023, 51, 103413. [Google Scholar] [CrossRef]
- Bezbaruah, A.N.; Krajangpan, S.; Chisholm, B.J.; Khan, E.; Elorza Bermudez, J.J. Entrapment of Iron Nanoparticles in Calcium Alginate Beads for Groundwater Remediation Applications. J. Hazard. Mater. 2009, 166, 1339–1343. [Google Scholar] [CrossRef]
- Kuang, Y.; Du, J.; Zhou, R.; Chen, Z.; Megharaj, M.; Naidu, R. Calcium Alginate Encapsulated Ni/Fe Nanoparticles Beads for Simultaneous Removal of Cu (II) and Monochlorobenzene. J. Colloid Interface Sci. 2015, 447, 85–91. [Google Scholar] [CrossRef]
- Kamata, H.; Akagi, Y.; Kayasuga-Kariya, Y.; Chung, U.; Sakai, T. “Nonswellable” Hydrogel Without Mechanical Hysteresis. Science 2014, 343, 873–875. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F.; Chen, H.; Zheng, J.; Ma, J.; Chen, J. Alginate/Graphene Double-Network Nanocomposite Hydrogel Beads with Low-Swelling, Enhanced Mechanical Properties, and Enhanced Adsorption Capacity. J. Mater. Chem. A 2016, 4, 10885–10892. [Google Scholar] [CrossRef]
- Modrogan, C.; Pandele, A.M.; Bobirică, C.; Dobrotǎ, D.; Dăncilă, A.M.; Gârleanu, G.; Orbuleţ, O.D.; Borda, C.; Gârleanu, D.; Orbeci, C. Synthesis, Characterization and Sorption Capacity Examination for a Novel Hydrogel Composite Based on Gellan Gum and Graphene Oxide (GG/GO). Polymers 2020, 12, 1182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qi, Y.; Wang, Y.; Xue, Y.; Xu, P.; Li, Z.; Li, Q. Morphology and Thermal Properties of Calcium Alginate/Reduced Graphene Oxide Composites. Polymers 2018, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Ma, Y.; He, J.; Ren, Z. Highly Stable, Mechanically Enhanced, and Easy-to-Collect Sodium Alginate/NZVI-rGO Gel Beads for Efficient Removal of Cr(VI). Polymers 2023, 15, 3764. [Google Scholar] [CrossRef] [PubMed]
- van Oss, C.J. Acid—Base Interfacial Interactions in Aqueous Media. Colloids Surf. Physicochem. Eng. Asp. 1993, 78, 1–49. [Google Scholar] [CrossRef]
- Montalvo, D.; Vanderschueren, R.; Fritzsche, A.; Meckenstock, R.U.; Smolders, E. Efficient Removal of Arsenate from Oxic Contaminated Water by Colloidal Humic Acid-Coated Goethite: Batch and Column Experiments. J. Clean. Prod. 2018, 189, 510–518. [Google Scholar] [CrossRef]
- Jiang, S.; Pang, L.; Buchan, G.D.; Šimůnek, J.; Noonan, M.J.; Close, M.E. Modeling Water Flow and Bacterial Transport in Undisturbed Lysimeters under Irrigations of Dairy Shed Effluent and Water Using HYDRUS-1D. Water Res. 2010, 44, 1050–1061. [Google Scholar] [CrossRef]
- Šimůnek, J.; van Genuchten, M.T. Modeling Nonequilibrium Flow and Transport Processes Using HYDRUS. Vadose Zone J. 2008, 7, 782–797. [Google Scholar] [CrossRef]
- Gu, X.; Mo, H.; Wang, L.; Zhang, L.; Ding, Z. Co-Transport of Cr(VI) and Bentonite Colloid in Saturated Porous Media. Bull. Environ. Contam. Toxicol. 2022, 110, 30. [Google Scholar] [CrossRef]
- Tong, M.; He, L.; Rong, H.; Li, M.; Kim, H. Transport Behaviors of Plastic Particles in Saturated Quartz Sand without and with Biochar/Fe3O4-Biochar Amendment. Water Res. 2020, 169, 115284. [Google Scholar] [CrossRef]
- Ghiasi, B.; Niksokhan, M.H.; Mahdavi Mazdeh, A. Co-Transport of Chromium(VI) and Bentonite Colloidal Particles in Water-Saturated Porous Media: Effect of Colloid Concentration, Sand Gradation, and Flow Velocity. J. Contam. Hydrol. 2020, 234, 103682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, T.; Wu, Y. Influence of Mineral Colloids and Humic Substances on Uranium(VI) Transport in Water-Saturated Geologic Porous Media. J. Contam. Hydrol. 2014, 170, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, Y.; Luo, H.; Yang, J. Characteristic of Adsorption, Desorption, and Co-Transport of Vanadium on Humic Acid Colloid. Ecotoxicol. Environ. Saf. 2020, 190, 110087. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Su, F.; Wang, Y.; Peng, L.; Liu, D. Adsorption Behaviour of Microplastics on the Heavy Metal Cr(VI) before and after Ageing. Chemosphere 2022, 302, 134865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tong, J.; Hu, B.X.; Wei, W. Adsorption and Desorption for Dynamics Transport of Hexavalent Chromium (Cr(VI)) in Soil Column. Environ. Sci. Pollut. Res. 2018, 25, 459–468. [Google Scholar] [CrossRef]
- Chotpantarat, S.; Kiatvarangkul, N. Facilitated Transport of Cadmium with Montmorillonite KSF Colloids under Different pH Conditions in Water-Saturated Sand Columns: Experiment and Transport Modeling. Water Res. 2018, 146, 216–231. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, C.; Yang, X.; Liu, L.; Wang, X.; Yin, W.; Li, Y.C.; Wang, S.; Fu, W. Preparation of Highly-Conductive Pyrogenic Carbon-Supported Zero-Valent Iron for Enhanced Cr(VI) Reduction. J. Hazard. Mater. 2020, 396, 122712. [Google Scholar] [CrossRef]
- Li, H.; Ren, Z.; Huang, D.; Jing, Q.; Tang, H. Removal of Hexavalent Chromium in Aqueous Solution by Cellulose Filter Paper Loaded with Nano-Zero-Valent Iron: Performance Investigation and Numerical Modeling. Int. J. Environ. Res. Public Health 2023, 20, 1867. [Google Scholar] [CrossRef]
- Cai, L.; He, L.; Peng, S.; Li, M.; Tong, M. Influence of titanium dioxide nanoparticles on the transport and deposition of microplastics in quartz sand. Environ. Pollut. 2019, 253, 351–357. [Google Scholar] [CrossRef]
- Bergendahl, J.; Grasso, D. Prediction of colloid detachment in a model porous media: Hydrodynamics. Chem. Eng. Sci. 2000, 55, 1523–1532. [Google Scholar] [CrossRef]
- van Oss, C.J. Hydrophobicity of biosurfaces—Origin, quantitative determination and interaction energies. Colloids Surf. B Biointerfaces Hydrophobicity 1995, 5, 91–110. [Google Scholar] [CrossRef]

| Medium | Condition | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| KL (L/mg) | b (mg/g) | R2 | KF (L/g) | n | R2 | ||
| Quartz Sand | pH 5 IS5 | 0.0023 | 0.3976 | 0.9406 | 0.0008 | 0.9844 | 0.9028 |
| pH 7 IS5 | 0.0025 | 0.3779 | 0.9425 | 0.0009 | 1.0148 | 0.8974 | |
| pH 7 IS25 | 0.0026 | 0.3223 | 0.9610 | 0.0007 | 0.9819 | 0.8810 | |
| SA/NZVI-rGO gel beads | pH 5 IS5 | 0.1774 | 12.1309 | 0.9909 | 2.6411 | 2.2743 | 0.9683 |
| pH 7 IS5 | 0.1983 | 12.5629 | 0.9895 | 2.8576 | 2.2608 | 0.9944 | |
| pH 7 IS25 | 0.2434 | 11.7162 | 0.9603 | 2.9654 | 2.3725 | 0.9667 | |
| Condition | Medium | f | a (min−1) | RMSE | R2 |
|---|---|---|---|---|---|
| pH 5 IS5 | Quartz Sand | 0.0230 × 10−1 | 0.4283 × 10−2 | 0.0707 | 0.9425 |
| SA/NZVI-rGO gel beads | 0.5750 × 10−8 | 0.1194 × 10−5 | |||
| pH 7 IS5 | Quartz Sand | 0.2973 × 10−1 | 0.4125 × 10−2 | 0.0749 | 0.9303 |
| SA/NZVI-rGO gel beads | 0.1788 × 10−4 | 0.1591 × 10−3 | |||
| pH 7 IS25 | Quartz Sand | 0.2173 × 10−1 | 0.5358 × 10−2 | 0.0554 | 0.9671 |
| SA/NZVI-rGO gel beads | 0.9645 × 10−4 | 0.1046 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Ma, Y.; Jing, Q.; Ren, Z. Comigration Behavior of Cr(VI) and Microplastics and Remediation of Microplastics-Facilitated Cr(VI) Transportation in Saturated Porous Media. Polymers 2024, 16, 3271. https://doi.org/10.3390/polym16233271
Yang Z, Ma Y, Jing Q, Ren Z. Comigration Behavior of Cr(VI) and Microplastics and Remediation of Microplastics-Facilitated Cr(VI) Transportation in Saturated Porous Media. Polymers. 2024; 16(23):3271. https://doi.org/10.3390/polym16233271
Chicago/Turabian StyleYang, Zijiang, Yuheng Ma, Qi Jing, and Zhongyu Ren. 2024. "Comigration Behavior of Cr(VI) and Microplastics and Remediation of Microplastics-Facilitated Cr(VI) Transportation in Saturated Porous Media" Polymers 16, no. 23: 3271. https://doi.org/10.3390/polym16233271
APA StyleYang, Z., Ma, Y., Jing, Q., & Ren, Z. (2024). Comigration Behavior of Cr(VI) and Microplastics and Remediation of Microplastics-Facilitated Cr(VI) Transportation in Saturated Porous Media. Polymers, 16(23), 3271. https://doi.org/10.3390/polym16233271







