Biochar as a UV Stabilizer: Its Impact on the Photostability of Poly(butylene succinate) Biocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PBSu Biocomposites
2.3. Preparation of Films
2.4. UV Irradiation
2.5. Gel Permeation Chromatography–Size Exclusion Chromatography (GPC/SEC)
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Differential Scanning Calorimetry (DSC)
2.8. Pyrolysis–Gas Chromatography/Mass Spectrometry Analysis (Py–GC/MS)
2.9. Mechanical Properties
2.10. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Molecular Weight of PBSu and Its Biocomposites
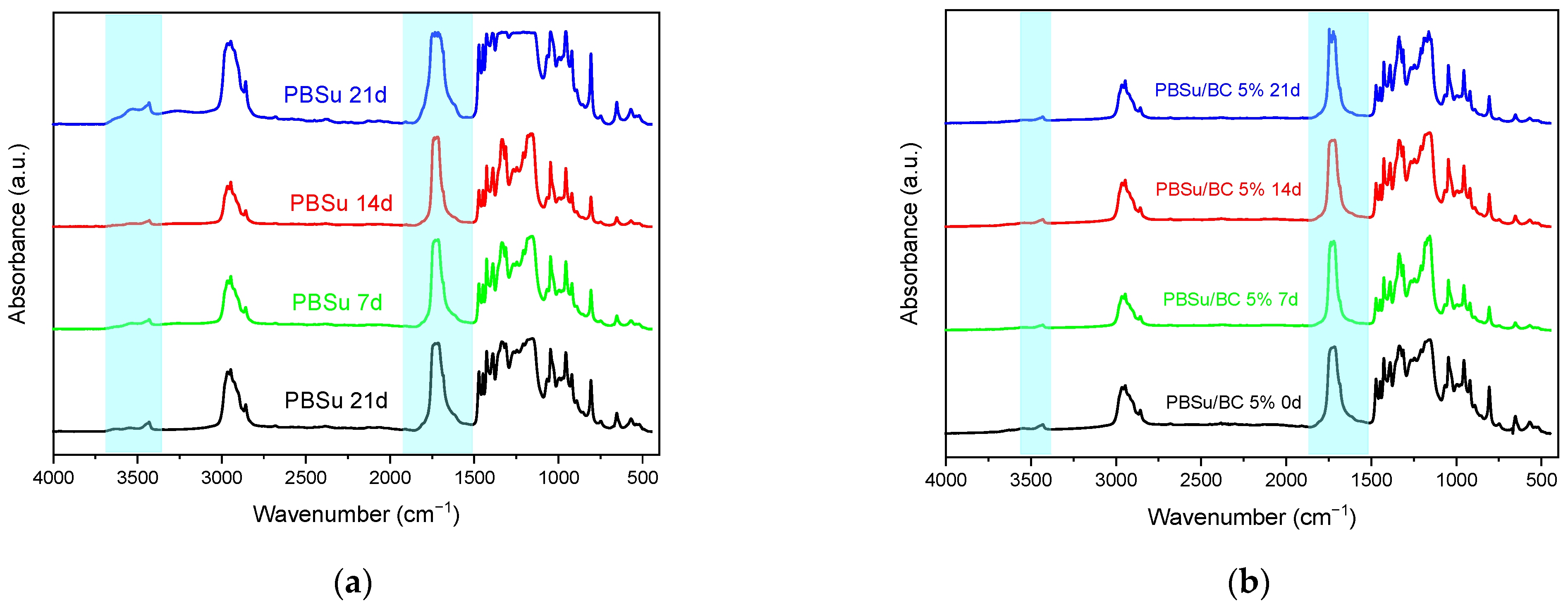
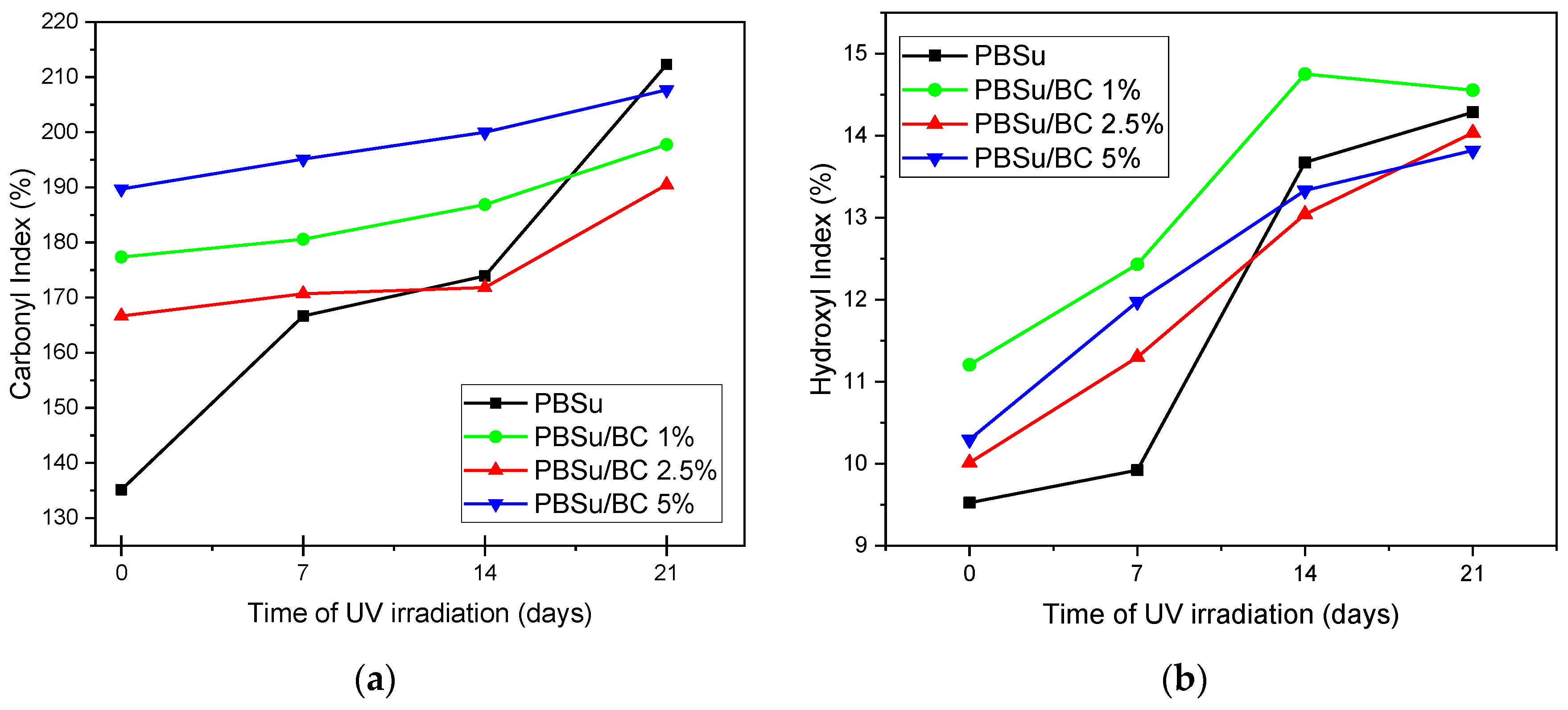
3.2. Chemical Structure Changes
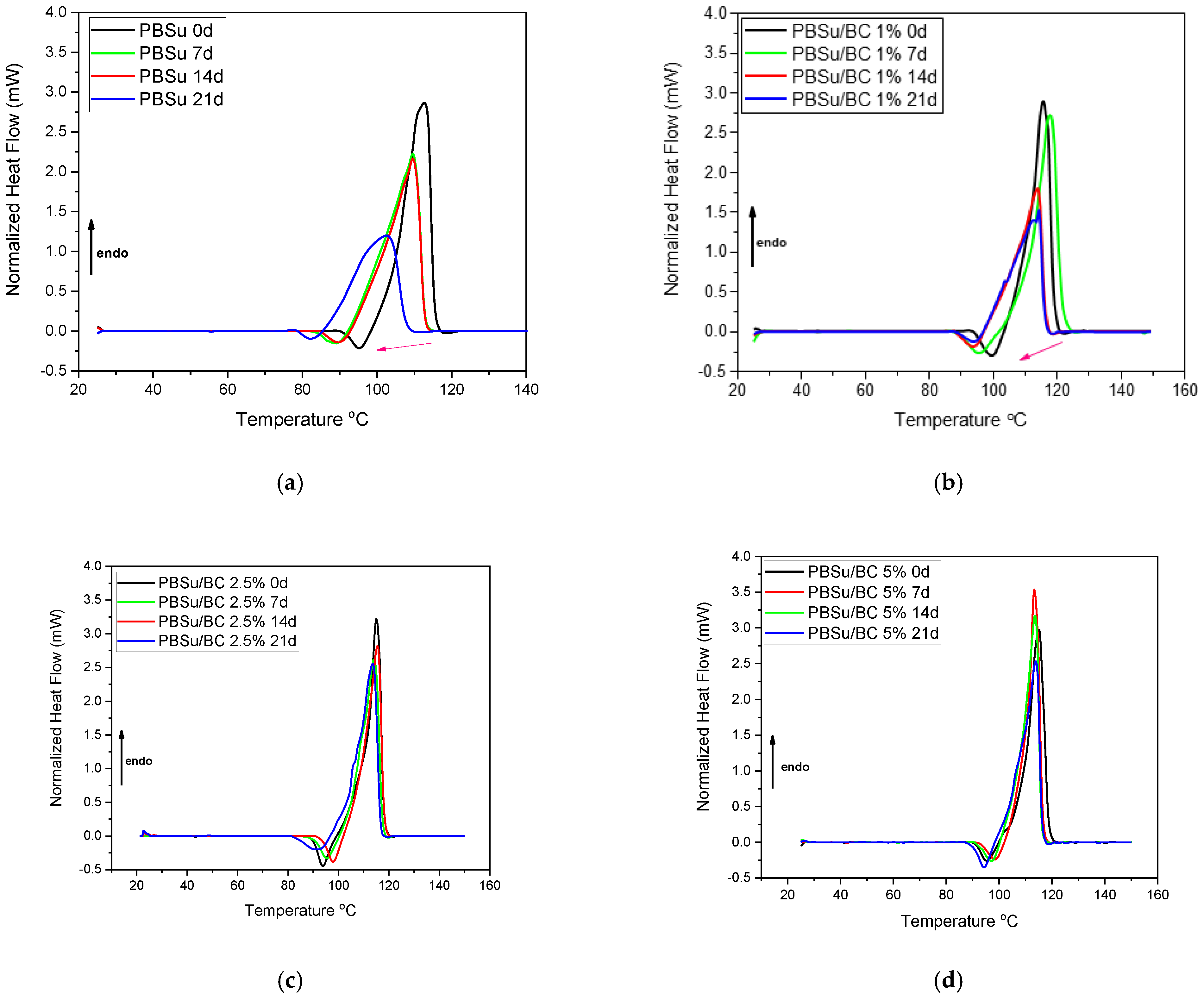
3.3. Differential Scanning Calorimetry (DSC)
3.4. Pyrolysis–Gas Chromatography/Mass Spectrometry Analysis (Py–GC/MS)
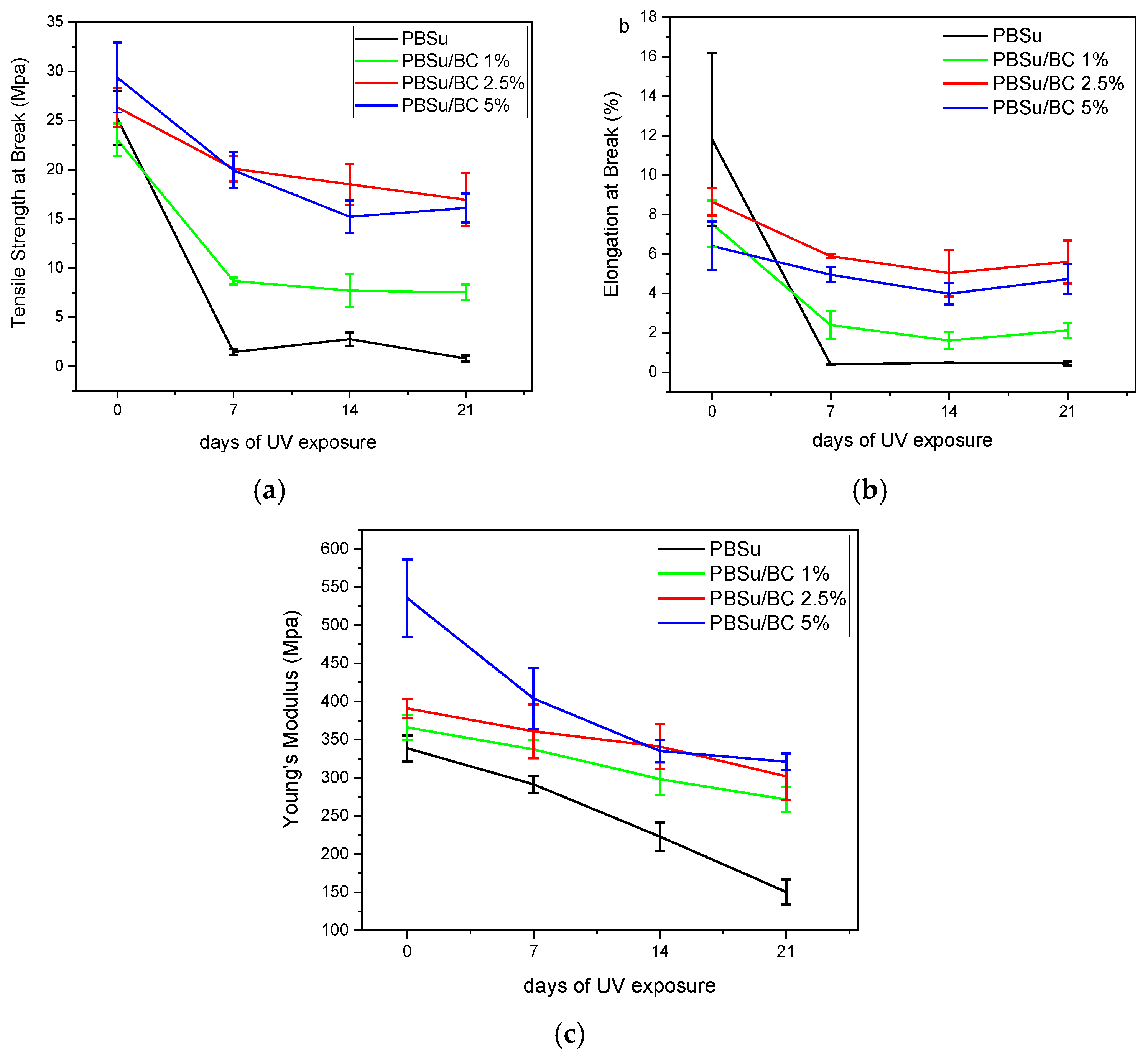
3.5. Mechanical Properties and Morphological Examination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, Y.; Zhang, B.; Zhu, Z.; Wang, G.; Mei, X.; Fang, Y.; Lu, W. Photocatalytic-Driven Self-Degradation of Polyester Microplastics Under Solar Light. J. Polym. Environ. 2023, 31, 2415–2423. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Achilias, D.S. Synthesis of Poly(Alkylene Succinate) Biodegradable Polyesters I. Mathematical Modelling of the Esterification Reaction. Polymer 2006, 47, 4851–4860. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Poly(Butylene Succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Sander, M. Biodegradation of Polymeric Mulch Films in Agricultural Soils: Concepts, Knowledge Gaps, and Future Research Directions. Environ. Sci. Technol. 2019, 53, 2304–2315. [Google Scholar] [CrossRef] [PubMed]
- Francioni, M.; Kishimoto-Mo, A.W.; Tsuboi, S.; Hoshino, Y.T. Evaluation of the Mulch Films Biodegradation in Soil: A Methodological Review. Ital. J. Agron. 2022, 17, 183–194. [Google Scholar] [CrossRef]
- Mansoor, Z.; Tchuenbou-Magaia, F.; Kowalczuk, M.; Adamus, G.; Manning, G.; Parati, M.; Radecka, I.; Khan, H. Polymers Use as Mulch Films in Agriculture—A Review of History, Problems and Current Trends. Polymers 2022, 14, 5062. [Google Scholar] [CrossRef]
- Infurna, G.; Caruso, G.; Dintcheva, N.T. Sustainable Materials Containing Biochar Particles: A Review. Polymers 2023, 15, 343. [Google Scholar] [CrossRef]
- Infurna, G.; Botta, L.; Ingargiola, I.; Maniscalco, M.; Caputo, G.; Dintcheva, N.T. Biochar from Digestate Pyrolysis as a Filler for Biopolymer Blends: Effect of Blend Composition. J. Polym. Environ. 2023, 32, 1921–1936. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Iwuozor, K.O.; Emenike, E.C.; Amoloye, M.A.; Aransiola, E.S.; Motolani, F.O.; Kayode, S.H. Prospects and Problems in the Development of Biochar-Filled Plastic Composites: A Review. Funct. Compos. Struct. 2023, 5, 012002. [Google Scholar] [CrossRef]
- Hernandez-Charpak, Y.D.; Trabold, T.A.; Lewis, C.L.; Diaz, C.A. Biochar-Filled Plastics: Effect of Feedstock on Thermal and Mechanical Properties. Biomass Convers. Biorefin. 2022, 12, 4349–4360. [Google Scholar] [CrossRef]
- Infurna, G.; Botta, L.; Maniscalco, M.; Morici, E.; Caputo, G.; Marullo, S.; D’Anna, F.; Dintcheva, N.T. Biochar Particles Obtained from Agricultural Carob Waste as a Suitable Filler for Sustainable Biocomposite Formulations. Polymers 2022, 14, 3075. [Google Scholar] [CrossRef]
- Kane, S.; Ryan, C. Biochar from Food Waste as a Sustainable Replacement for Carbon Black in Upcycled or Compostable Composites. Compos. Part C Open Access 2022, 8, 100274. [Google Scholar] [CrossRef]
- Mandal, S.; Pu, S.; Adhikari, S.; Ma, H.; Kim, D.H.; Bai, Y.; Hou, D. Progress and Future Prospects in Biochar Composites: Application and Reflection in the Soil Environment. Crit. Rev. Environ. Sci. Technol. 2021, 51, 219–271. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Tengku Yasim-Anuar, T.A.; Yee-Foong, L.N.; Lawal, A.A.; Ahmad Farid, M.A.; Mohd Yusuf, M.Z.; Hassan, M.A.; Ariffin, H. Emerging Application of Biochar as a Renewable and Superior Filler in Polymer Composites. RSC Adv. 2022, 12, 13938–13949. [Google Scholar] [CrossRef] [PubMed]
- Das, O.; Sarmah, A.K.; Bhattacharyya, D. Biocomposites from Waste Derived Biochars: Mechanical, Thermal, Chemical, and Morphological Properties. Waste Manag. 2016, 49, 560–570. [Google Scholar] [CrossRef]
- Vidal, J.L.; Yavitt, B.M.; Wheeler, M.D.; Kolwich, J.L.; Donovan, L.N.; Sit, C.S.; Hatzikiriakos, S.G.; Jalsa, N.K.; MacQuarrie, S.L.; Kerton, F.M. Biochar as a Sustainable and Renewable Additive for the Production of Poly(ε-Caprolactone) Composites. Sustain. Chem. Pharm. 2022, 25, 100586. [Google Scholar] [CrossRef]
- Papadopoulou, K.; Klonos, P.A.; Kyritsis, A.; Mašek, O.; Wurzer, C.; Tsachouridis, K.; Anastasiou, A.D.; Bikiaris, D.N. Synthesis and Study of Fully Biodegradable Composites Based on Poly(Butylene Succinate) and Biochar. Polymers 2023, 15, 1049. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, K.; Tarani, E.; Chrissafis, K.; Mašek, O.; Bikiaris, D.N. Non-Isothermal Crystallization Kinetics of PBSu/Biochar Fitting Methods. Polymers 2023, 15, 1603. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, K.; Tarani, E.; Ainali, N.M.; Chrissafis, K.; Wurzer, C.; Mašek, O.; Bikiaris, D.N. The Effect of Biochar Addition on Thermal Stability and Decomposition Mechanism of Poly(Butylene Succinate) Bionanocomposites. Molecules 2023, 28, 5330. [Google Scholar] [CrossRef]
- Ho, M.P.; Lau, K.T.; Wang, H.; Hui, D. Improvement on the Properties of Polylactic Acid (PLA) Using Bamboo Charcoal Particles. Compos. Part B Eng. 2015, 81, 14–25. [Google Scholar] [CrossRef]
- Karimi, S.; Helal, E.; Gutierrez, G.; Moghimian, N.; Madinehei, M.; David, E.; Samara, M.; Demarquette, N. A Review on Graphene’s Light Stabilizing Effects for Reduced Photodegradation of Polymers. Crystals 2021, 11, 3. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sorrentino, A. Photo-Oxidative Stabilization of Carbon Nanotubes on Polylactic Acid. Polym. Degrad. Stab. 2013, 98, 963–971. [Google Scholar] [CrossRef]
- Botta, L.; Teresi, R.; Titone, V.; Salvaggio, G.; La Mantia, F.P.; Lopresti, F. Use of Biochar as Filler for Biocomposite Blown Films: Structure-Processing-Properties Relationships. Polymers 2021, 13, 3953. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sotoudehniakarani, F.; Yu, Z.; Morrell, J.J.; Cappellazzi, J.; McDonald, A.G. Evaluation of Corrugated Cardboard Biochar as Reinforcing Fiber on Properties, Biodegradability and Weatherability of Wood-Plastic Composites. Polym. Degrad. Stab. 2019, 168, 108955. [Google Scholar] [CrossRef]
- Mašek, O.; Buss, W.; Sohi, S. Standard Biochar Materials. Environ. Sci. Technol. 2018, 52, 9543–9544. [Google Scholar] [CrossRef]
- Mašek, O.; Buss, W.; Roy-Poirier, A.; Lowe, W.; Peters, C.; Brownsort, P.; Mignard, D.; Pritchard, C.; Sohi, S. Consistency of Biochar Properties over Time and Production Scales: A Characterisation of Standard Materials. J. Anal. Appl. Pyrolysis 2018, 132, 200–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.; Wu, S.; Qi, Z.; Xu, J.; Guo, B. Synthesis, Physical Properties and Photodegradation of Functional Poly(Butylene Succinate) Covalently Linking UV Stabilizing Moieties in Molecular Chains. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 524, 160–168. [Google Scholar] [CrossRef]
- Phua, Y.J.; Lau, N.S.; Sudesh, K.; Chow, W.S.; Mohd Ishak, Z.A. Biodegradability Studies of Poly(Butylene Succinate)/Organo-Montmorillonite Nanocomposites under Controlled Compost Soil Conditions: Effects of Clay Loading and Compatibiliser. Polym. Degrad. Stab. 2012, 97, 1345–1354. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Artificial Ageing, Chemical Resistance, and Biodegradation of Biocomposites from Poly(Butylene Succinate) and Wheat Bran. Materials 2021, 14, 7580. [Google Scholar] [CrossRef]
- Fujimoto, E.; Fujimaki, T. Effects of Pendant Methyl Groups and Lengths of Methylene Segments in Main-Chains on Photodegradation of Aliphatic Polyesters. Polym. J. 1999, 31, 645–650. [Google Scholar] [CrossRef]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the Carbonyl Index of Polyethylene and Polypropylene Using Specified Area under Band Methodology with ATR-FTIR Spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Carroccio, S.; Rizzarelli, P.; Puglisi, C.; Montaudo, G. MALDI Investigation of Photooxidation in Aliphatic Polyesters: Poly(Butylene Succinate). Macromolecules 2004, 37, 6576–6586. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Koumentakou, I.; Lykidou, S.; Nikolaidis, N. Innovative Skin Product O/W Emulsions Containing Lignin, Multiwall Carbon Nanotubes and Graphene Oxide Nanoadditives with Enhanced Sun Protection Factor and UV Stability Properties. Appl. Nano 2022, 3, 1–15. [Google Scholar] [CrossRef]
- Gorrasi, G.; Milone, C.; Piperopoulos, E.; Lanza, M.; Sorrentino, A. Hybrid Clay Mineral-Carbon Nanotube-PLA Nanocomposite Films. Preparation and Photodegradation Effect on Their Mechanical, Thermal and Electrical Properties. Appl. Clay Sci. 2013, 71, 49–54. [Google Scholar] [CrossRef]
- Llana-Ruíz-Cabello, M.; Pichardo, S.; Jiménez-Morillo, N.T.; Abad, P.; Guillamón, E.; González-Vila, F.J.; Cameán, A.M.; González-Pérez, J.A. Characterisation of a Bio-Based Packaging Containing a Natural Additive from Allium Spp. Using Analytical Pyrolysis and Carbon Stable Isotopes. J. Anal. Appl. Pyrolysis 2016, 120, 334–340. [Google Scholar] [CrossRef]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging Effects on Low- and High-Density Polyethylene, Polypropylene and Polystyrene under UV Irradiation: An Insight into Decomposition Mechanism by Py-GC/MS for Microplastic Analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- De Falco, F.; Nacci, T.; Durndell, L.; Thompson, R.C.; Degano, I.; Modugno, F. A Thermoanalytical Insight into the Composition of Biodegradable Polymers and Commercial Products by EGA-MS and Py-GC-MS. J. Anal. Appl. Pyrolysis 2023, 171, 105937. [Google Scholar] [CrossRef]
- Bikiaris, R.D.; Ainali, N.M.; Christodoulou, E.; Nikolaidis, N.; Lambropoulou, D.A.; Papageorgiou, G.Z. Thermal Stability and Decomposition Mechanism of Poly(Alkylene Succinate)S. Macromol 2022, 2, 58–77. [Google Scholar] [CrossRef]
- Chrysafi, I.; Ainali, N.M.; Xanthopoulou, E.; Zamboulis, A.; Bikiaris, D.N. Thermal Degradation Mechanism and Decomposition Kinetic Studies of Poly(Ethylene Succinate)/Hemp Fiber Composites. J. Compos. Sci. 2023, 7, 216. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Chrissafis, K.; Paraskevopoulos, K.M.; Triantafyllidis, K.S.; Antonakou, E.V. Investigation of Thermal Degradation Mechanism of an Aliphatic Polyester Using Pyrolysis-Gas Chromatography-Mass Spectrometry and a Kinetic Study of the Effect of the Amount of Polymerisation Catalyst. Polym. Degrad. Stab. 2007, 92, 525–536. [Google Scholar] [CrossRef]
- Wattanawong, N.; Aht-Ong, D. Antibacterial Activity, Thermal Behavior, Mechanical Properties and Biodegradability of Silver Zeolite/Poly(Butylene Succinate) Composite Films. Polym. Degrad. Stab. 2021, 183, 109459. [Google Scholar] [CrossRef]
- Bartoli, M.; Arrigo, R.; Malucelli, G.; Tagliaferro, A.; Duraccio, D. Recent Advances in Biochar Polymer Composites. Polymers 2022, 14, 2506. [Google Scholar] [CrossRef]
- Fazal, T.; Razzaq, A.; Javed, F.; Hafeez, A.; Rashid, N.; Amjad, U.S.; Ur Rehman, M.S.; Faisal, A.; Rehman, F. Integrating Adsorption and Photocatalysis: A Cost Effective Strategy for Textile Wastewater Treatment Using Hybrid Biochar-TiO2 Composite. J. Hazard. Mater. 2020, 390, 121623. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Wang, Y.; Dionysiou, D.D.; Zhou, D. Photogeneration of Reactive Oxygen Species from Biochar Suspension for Diethyl Phthalate Degradation. Appl. Catal. B Environ. 2017, 214, 34–45. [Google Scholar] [CrossRef]
- Gonçalves, M.G.; da Silva Veiga, P.A.; Fornari, M.R.; Peralta-Zamora, P.; Mangrich, A.S.; Silvestri, S. Relationship of the Physicochemical Properties of Novel ZnO/Biochar Composites to Their Efficiencies in the Degradation of Sulfamethoxazole and Methyl Orange. Sci. Total Environ. 2020, 748, 141381. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Recent Progress in Biochar-Supported Photocatalysts: Synthesis, Role of Biochar, and Applications. RSC Adv. 2018, 8, 14237–14248. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Rao, F.; He, L.; Yang, S.; Bao, Y.; Huang, C.; Bao, M.; Chen, Y. Evaluation of Biochar Properties Exposing to Solar Radiation: A Promotion on Surface Activities. Chem. Eng. J. 2020, 384, 123353. [Google Scholar] [CrossRef]
- Liu, W.; Ren, X. Study on the Application of the Narrow Distribution and Controlled Molecular Weight Hindered Amine Lighter Stabilizers in Poly(Butylene Succinate). J. Macromol. Sci. Part A Pure Appl. Chem. 2019, 56, 535–543. [Google Scholar] [CrossRef]
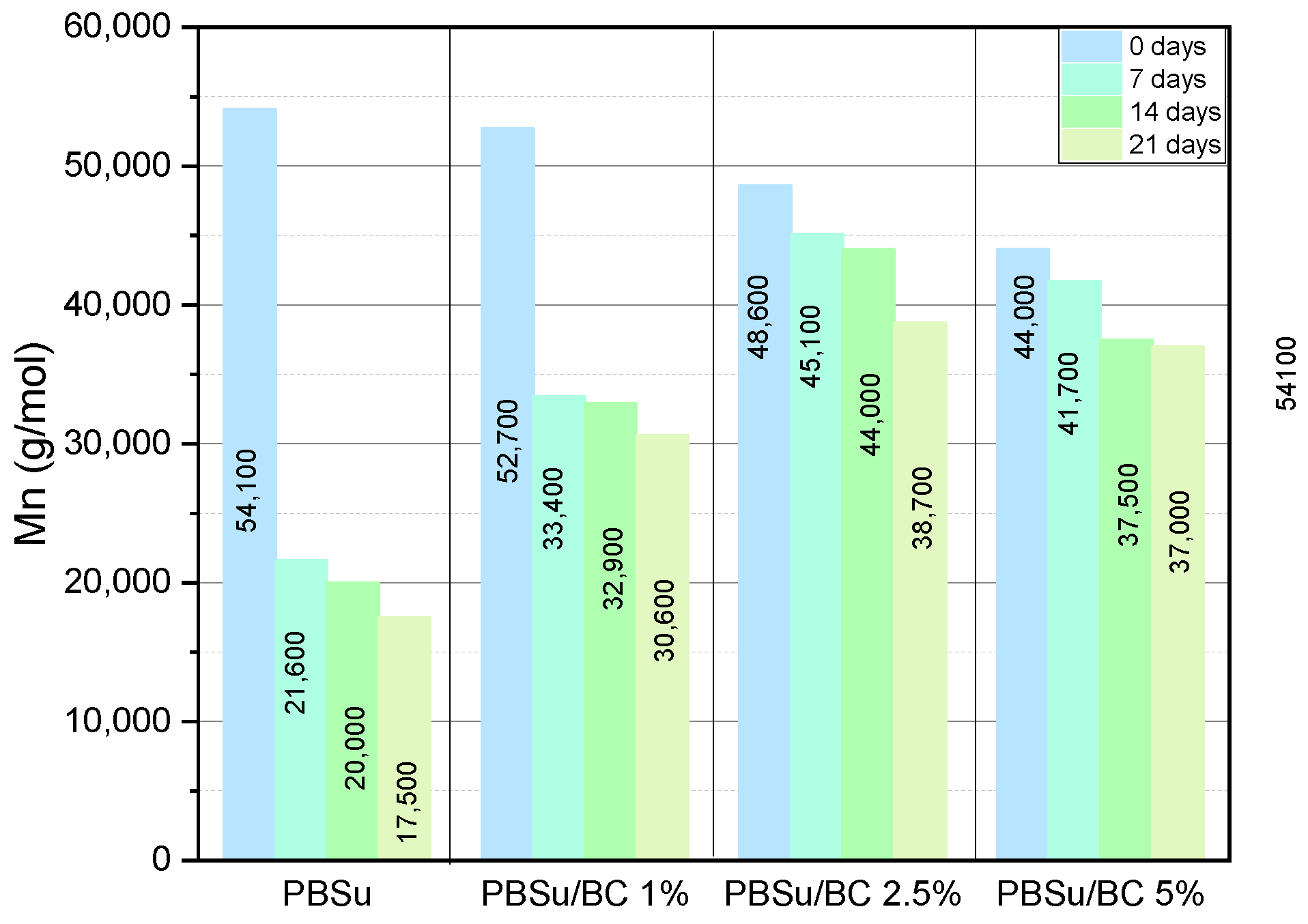
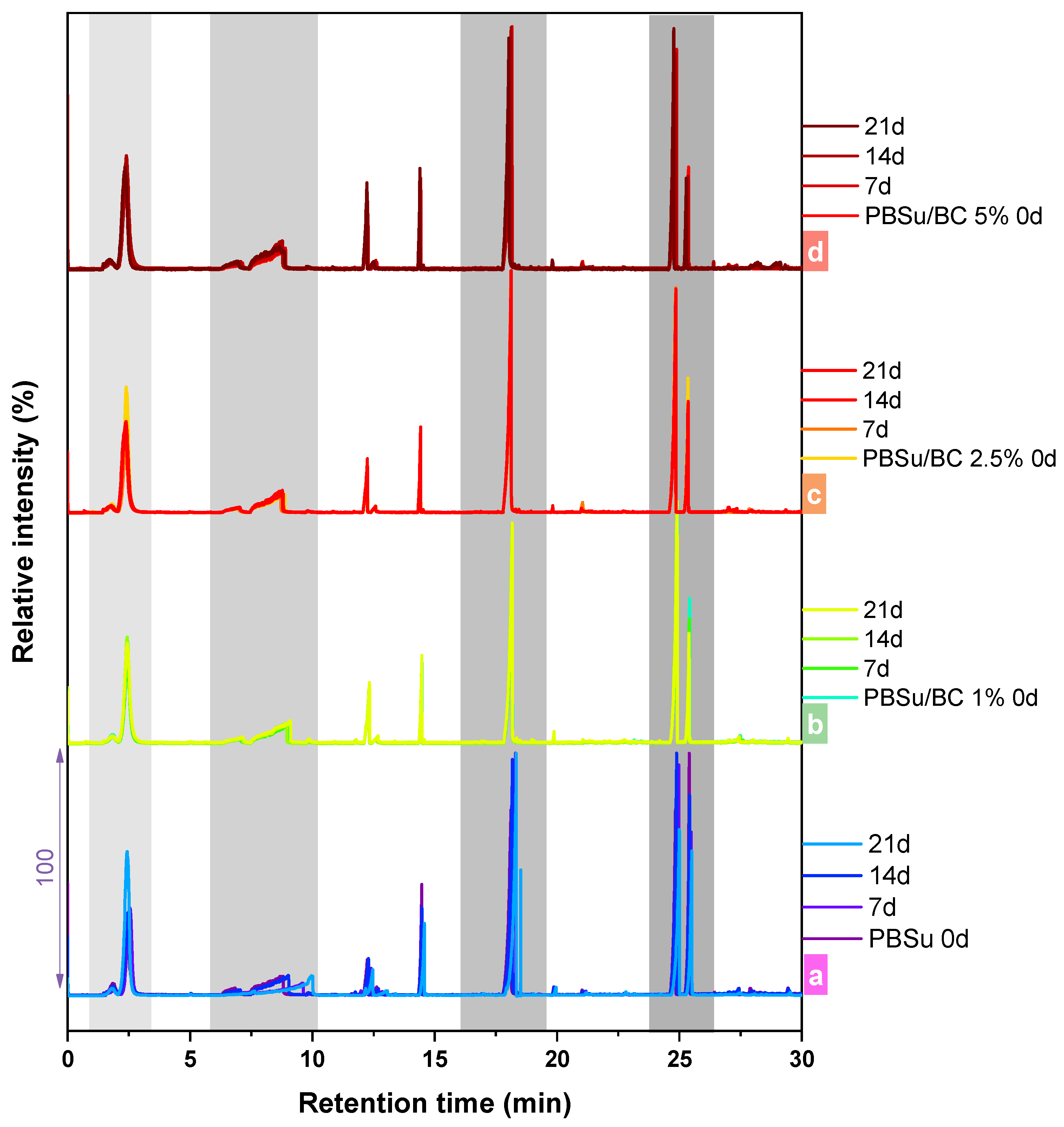
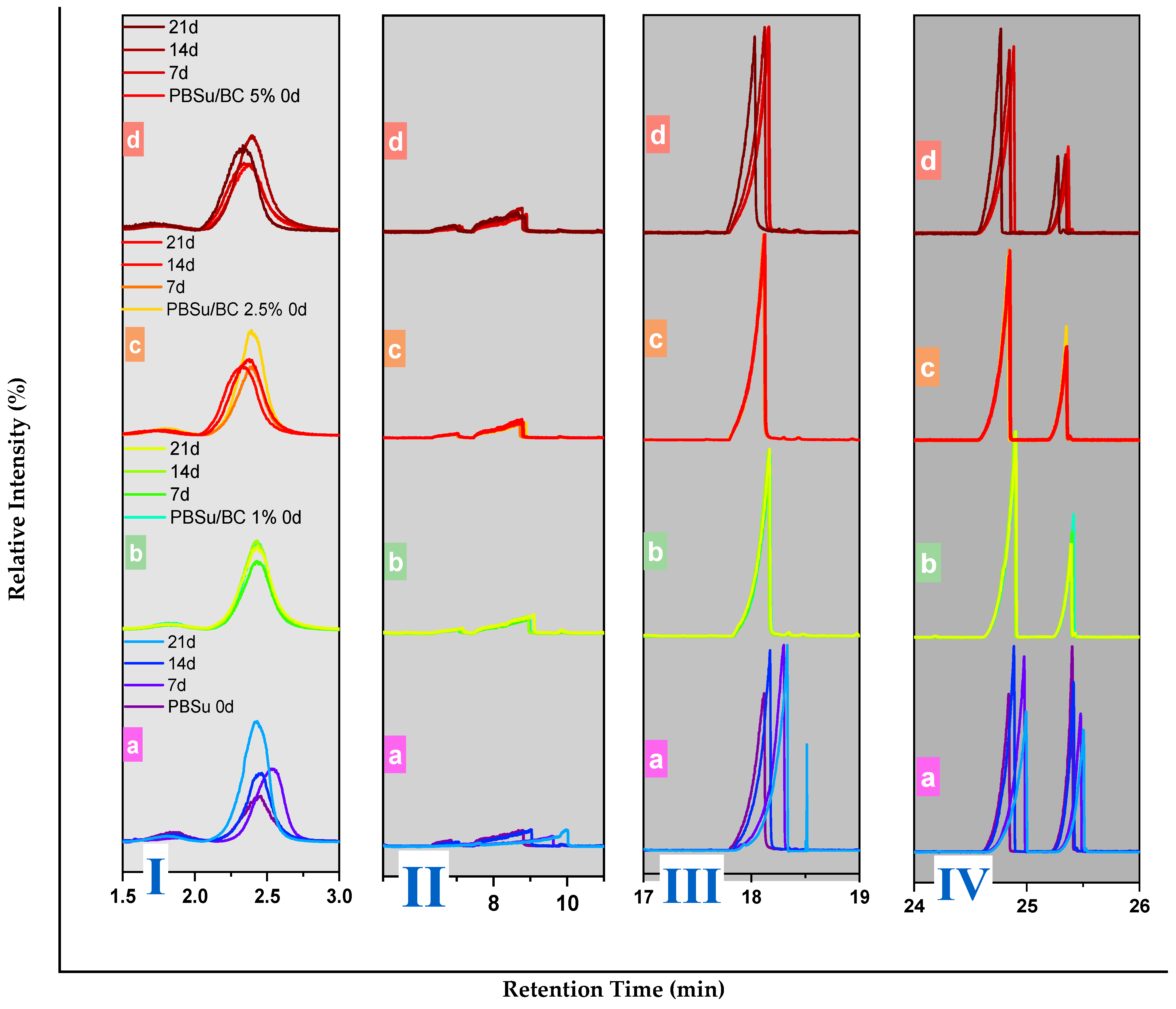
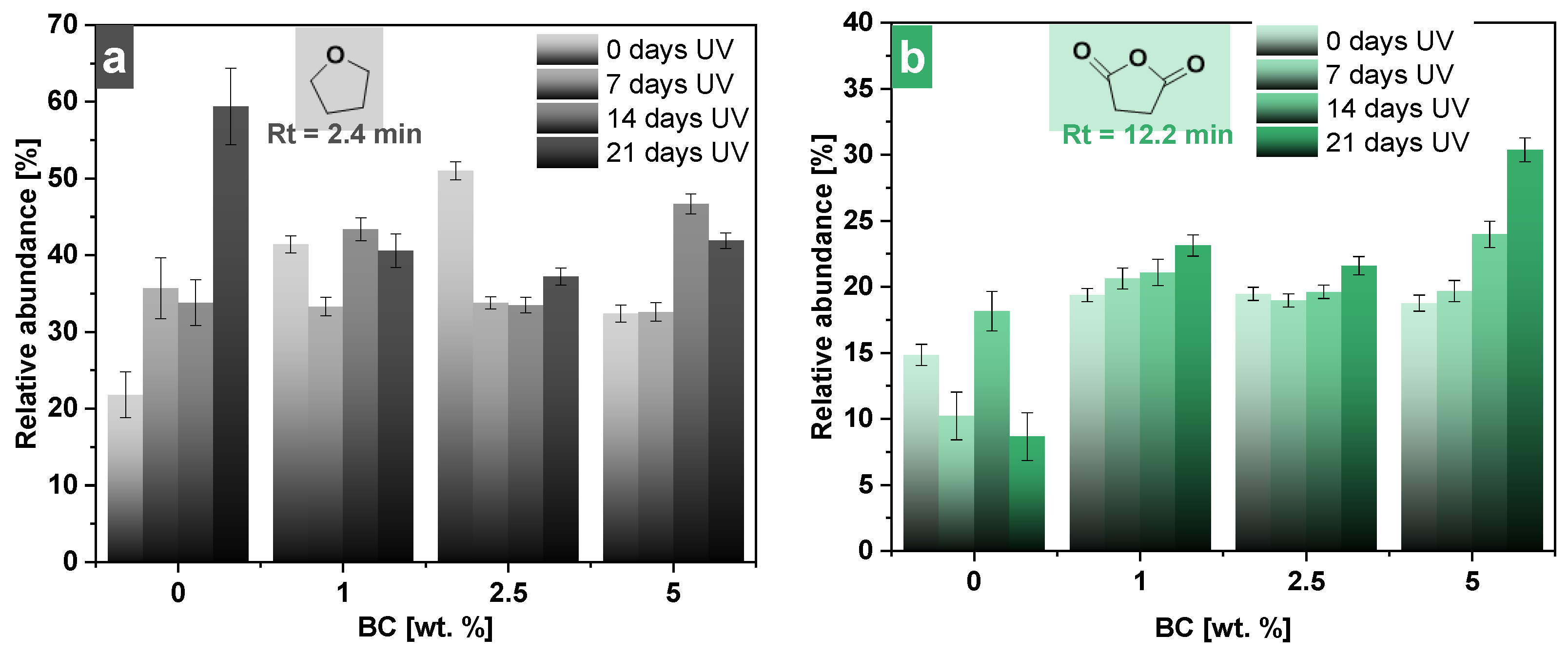
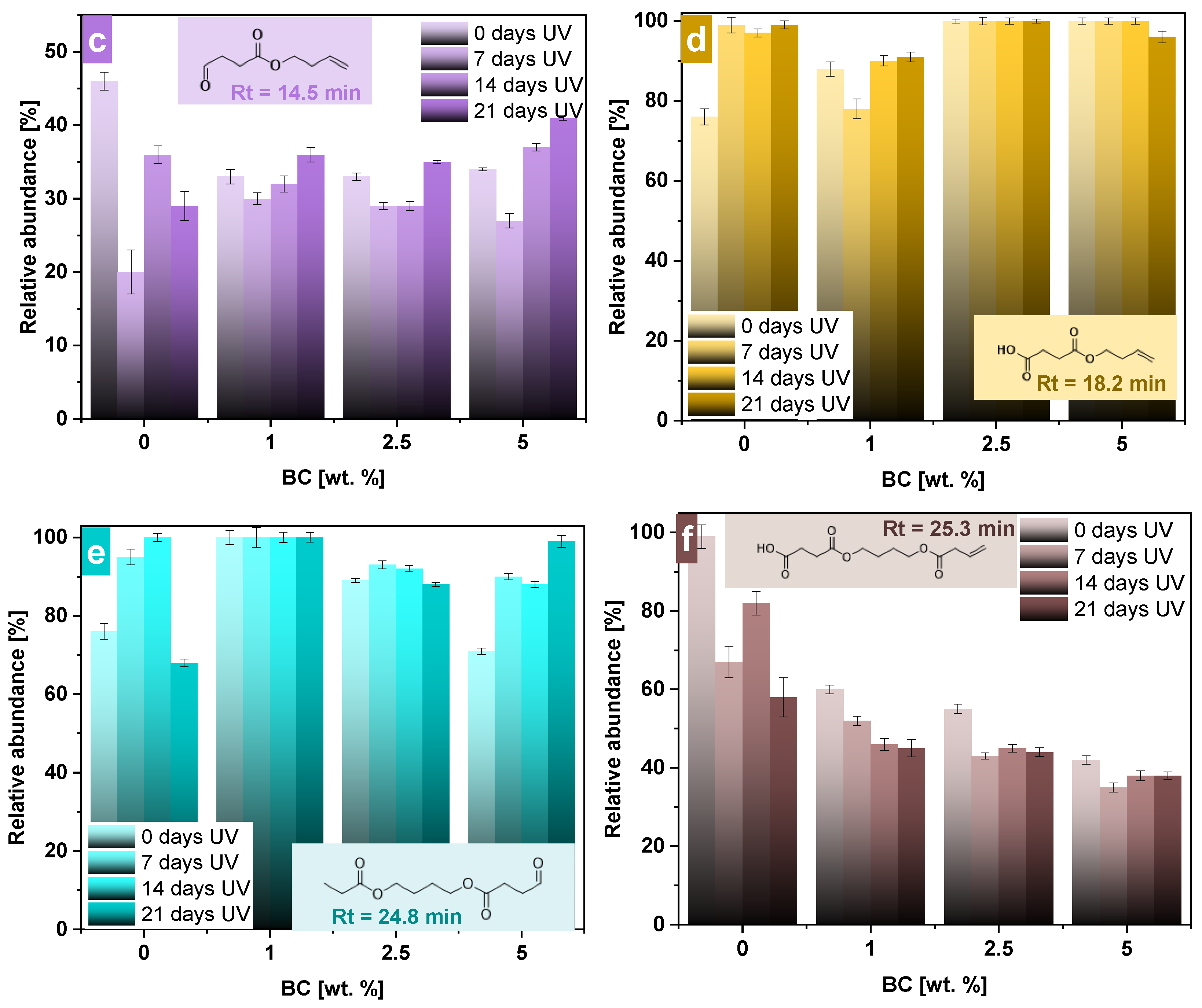

| Sample | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|
| PBSu 0 d | 54,100 | 120,000 | 2.22 |
| PBSu 7 d | 21,600 | 35,900 | 1.66 |
| PBSu 14 d | 20,000 | 42,400 | 2.13 |
| PBSu 21d | 17,500 | 30,000 | 1.71 |
| PBSu/BC 1% 0 d | 52,700 | 91,200 | 1.73 |
| PBSu/BC 1% 7 d | 33,400 | 75,300 | 2.25 |
| PBSu/BC 1% 14 d | 32,900 | 73,000 | 2.22 |
| PBSu/BC 1% 21 d | 30,600 | 66,700 | 2.18 |
| PBSu/BC 2.5% 0 d | 48,600 | 126,000 | 2.59 |
| PBSu/BC 2.5% 7 d | 45,100 | 115,900 | 2.57 |
| PBSu/BC 2.5% 14 d | 44,000 | 117,000 | 2.66 |
| PBSu/BC 2.5% 21 d | 38,700 | 100,800 | 2.61 |
| PBSu/BC 5% 0 d | 44,000 | 101,500 | 2.3 |
| PBSu/BC 5% 7 d | 41,700 | 104,600 | 2.41 |
| PBSu/BC 5% 14 d | 37,500 | 87,400 | 2.33 |
| PBSu/BC 5% 21 d | 37,000 | 86,000 | 2.33 |
| Samples | Tm (°C) | Tc (°C) | Xc (%) |
|---|---|---|---|
| PBSu 0 d | 113 | 71 | 27 |
| PBSu 7 d | 110 | 72 | 29 |
| PBSu 14 d | 115 | 67 | 32 |
| PBSu 21 d | 102 | 47 | 21 |
| PBSu/BC 1% 0 d | 116 | 77 | 28 |
| PBSu/BC 1% 7 d | 117 | 77 | 30 |
| PBSu/BC 1% 14 d | 114 | 74 | 26 |
| PBSu/BC 1% 21 d | 114 | 73 | 26 |
| PBSu/BC 2.5% 0 d | 114 | 78 | 29 |
| PBSu/BC 2.5% 7 d | 115 | 78 | 28 |
| PBSu/BC 2.5% 14 d | 114 | 77 | 28 |
| PBSu/BC 2.5% 21d | 114 | 76 | 26 |
| PBSu/BC 5% 0 d | 115 | 81 | 28 |
| PBSu/BC 5% 7 d | 114 | 82 | 28 |
| PBSu/BC 5% 14 d | 114 | 82 | 28 |
| PBSu/BC 5% 21 d | 114 | 80 | 26 |
| Retention Time (min) | Mw (g/mol) | Assigned Compound | |||
|---|---|---|---|---|---|
| PBSu 0d | PBSu 7d | PBSu 14d | PBSu 21d | ||
| 1.88 | 1.92 | 1.80 | 1.77 | 44 | CO, CO2 |
| 2.43 | 2.51 | 2.52 | 2.40 | 72 | 2-Propenoic acid or tetrahydrofuran |
| 6.79 | 7.26 | 6.95 | 7.55 | 86 | Pent-4-en-1-ol |
| 8.75 | 9.60 | 8.89 | 9.93 | 90 | 1,4-Butanediol |
| n.d. | n.d. | 9.84 | n.d. | 110 | Not identified |
| 12.23 | 12.02 | 11.74 | 12.14 | 100 | Succinic anhydride |
| 12.64 | 12.41 | 12.30 | 12.46 | 118 | Succinic acid |
| n.d. | 12.82 | 12.66 | 13.01 | 142 | But-3-en-1-yl but-3-enoate |
| 14.43 | 14.49 | 14.44 | 14.56 | 155 | But-3-en-1-yl 4-oxobutanoate |
| 18.11 | 18.17 | 18.20 | 18.30 | 174 | 4-(but-3-en-1-yloxy)-4-oxobutanoic acid |
| n.d. | n.d. | n.d. | 18.50 | 190 | 4-hydroxybutyl 4-(propionyloxy)butyl succinate |
| 19.93 | 19.88 | 19.87 | 19.95 | 244 | But-3-en-1-yl (4-hydroxybutyl) succinate |
| 21.00 | 21.18 | 21.09 | 21.15 | 272 | 1,6,13-trioxacyclononadecane-7,12,14,19-tetraone |
| 24.84 | 24.91 | 24.82 | 24.99 | 230 | 4-(propionyloxy)butyl 4-oxobutanoate |
| 25.37 | 25.43 | 25.41 | 25.51 | 258 | 4-(4-(but-3-enoyloxy)butoxy)-4-oxobutanoic acid |
| n.d. | n.d. | 27.39 | 27.48 | 344 | 1,6,11,16-tetraoxacycloicosane-2,5,12,15-tetraone or 4-(4-((4-(but-3-en-1-yloxy)-4- oxobutanoyl)oxy)butoxy)-4-oxobutanoic acid |
| 27.91 | n.d. | 27.91 | n.d. | 399 | (4-(propionyloxy)butyl) succinate bis(4-((4-oxobutanoyl)oxy)butyl) |
| 29.45 | 29.49 | 29.45 | 29.54 | 429 | bis(4-((4-oxobutanoyl)oxy)butyl) succinate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, K.; Ainali, N.M.; Mašek, O.; Bikiaris, D.N. Biochar as a UV Stabilizer: Its Impact on the Photostability of Poly(butylene succinate) Biocomposites. Polymers 2024, 16, 3080. https://doi.org/10.3390/polym16213080
Papadopoulou K, Ainali NM, Mašek O, Bikiaris DN. Biochar as a UV Stabilizer: Its Impact on the Photostability of Poly(butylene succinate) Biocomposites. Polymers. 2024; 16(21):3080. https://doi.org/10.3390/polym16213080
Chicago/Turabian StylePapadopoulou, Katerina, Nina Maria Ainali, Ondřej Mašek, and Dimitrios N. Bikiaris. 2024. "Biochar as a UV Stabilizer: Its Impact on the Photostability of Poly(butylene succinate) Biocomposites" Polymers 16, no. 21: 3080. https://doi.org/10.3390/polym16213080
APA StylePapadopoulou, K., Ainali, N. M., Mašek, O., & Bikiaris, D. N. (2024). Biochar as a UV Stabilizer: Its Impact on the Photostability of Poly(butylene succinate) Biocomposites. Polymers, 16(21), 3080. https://doi.org/10.3390/polym16213080








