Highly Porous 3D Nanofibrous Scaffold of Polylactic Acid/Polyethylene Glycol/Calcium Phosphate for Bone Regeneration by a Two-Step Solution Blow Spinning (SBS) Facile Route
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Synthesis of Calcium Phosphate Fibers and Scaffold Manufacture
2.3. Characterization
2.3.1. Microstructure Characterization
2.3.2. In Vitro Apatite Formation
2.3.3. Cell Viability Assay
2.3.4. Cell Proliferation Assay
2.3.5. Total Protein Dosage
2.3.6. Quantification of Alkaline Phosphatase (ALP)
2.3.7. Statistical Analysis
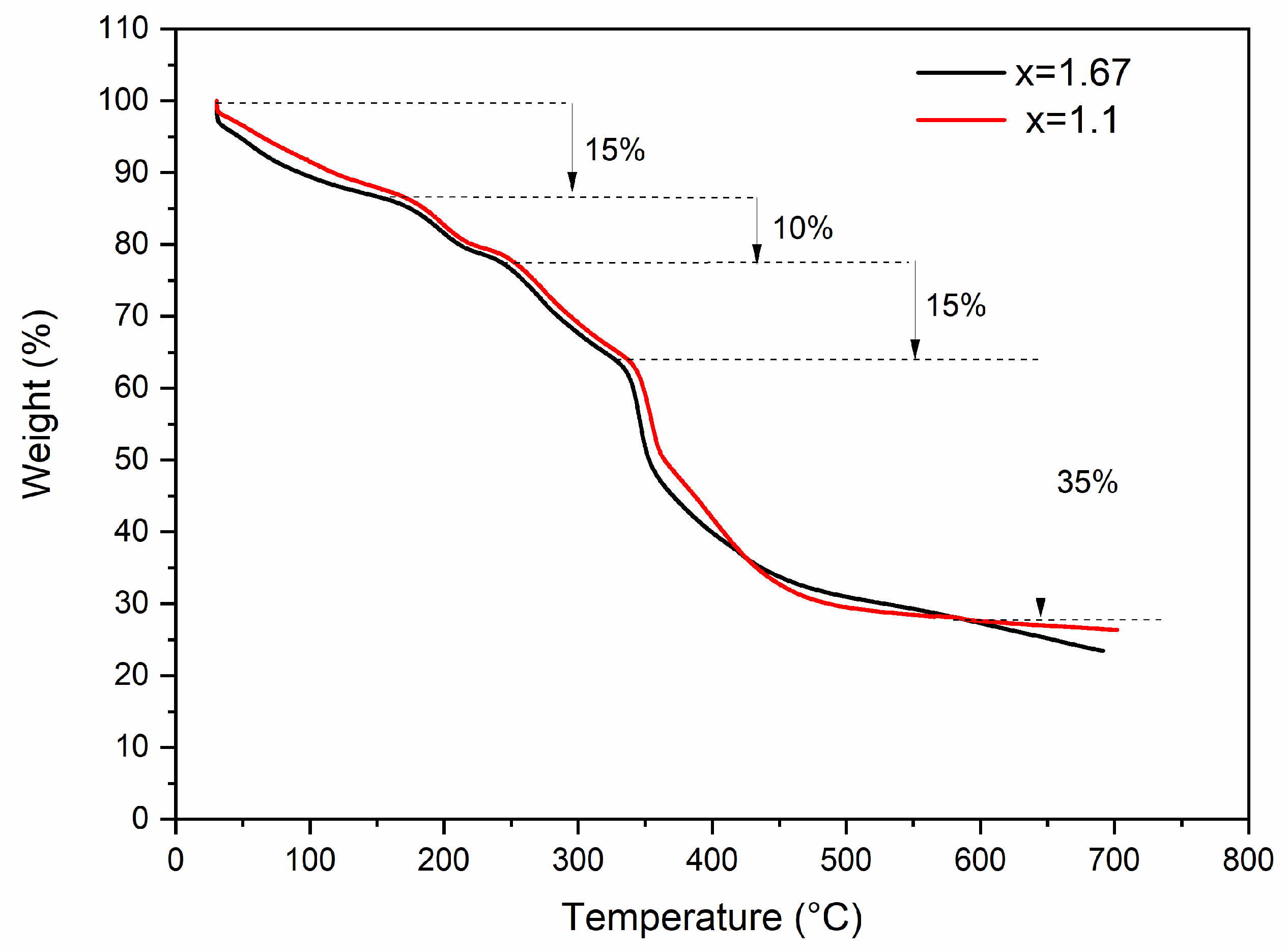
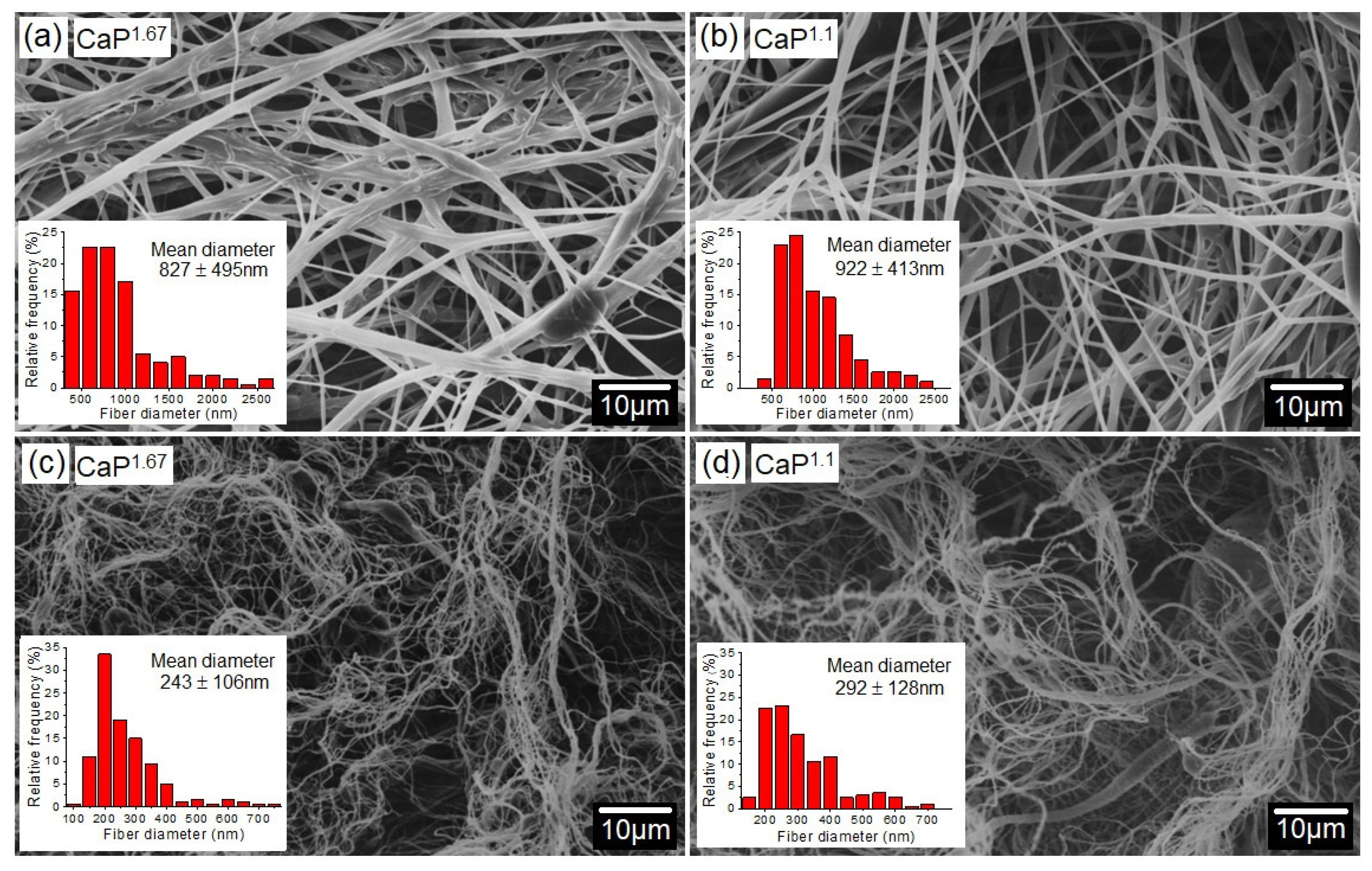
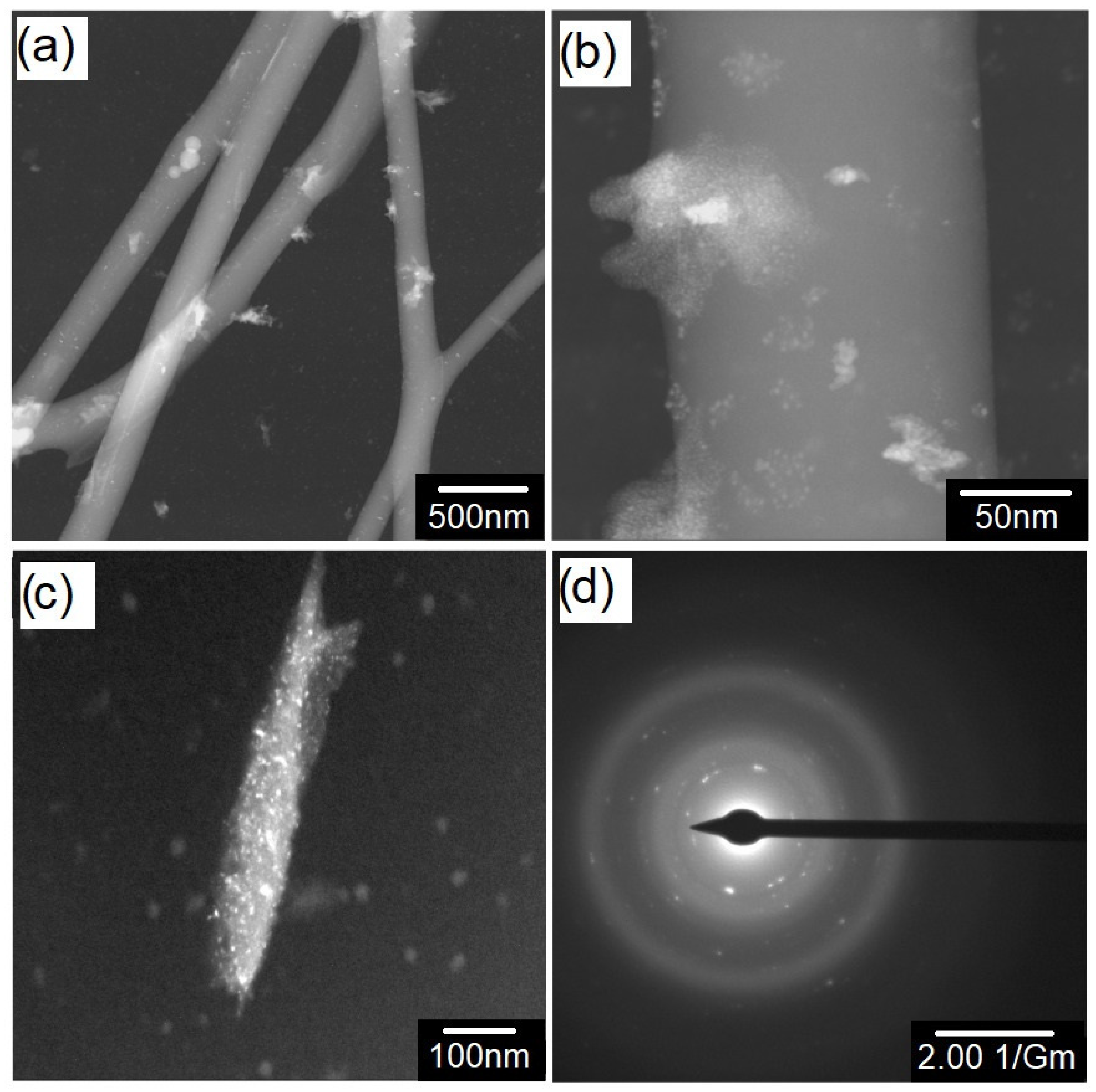
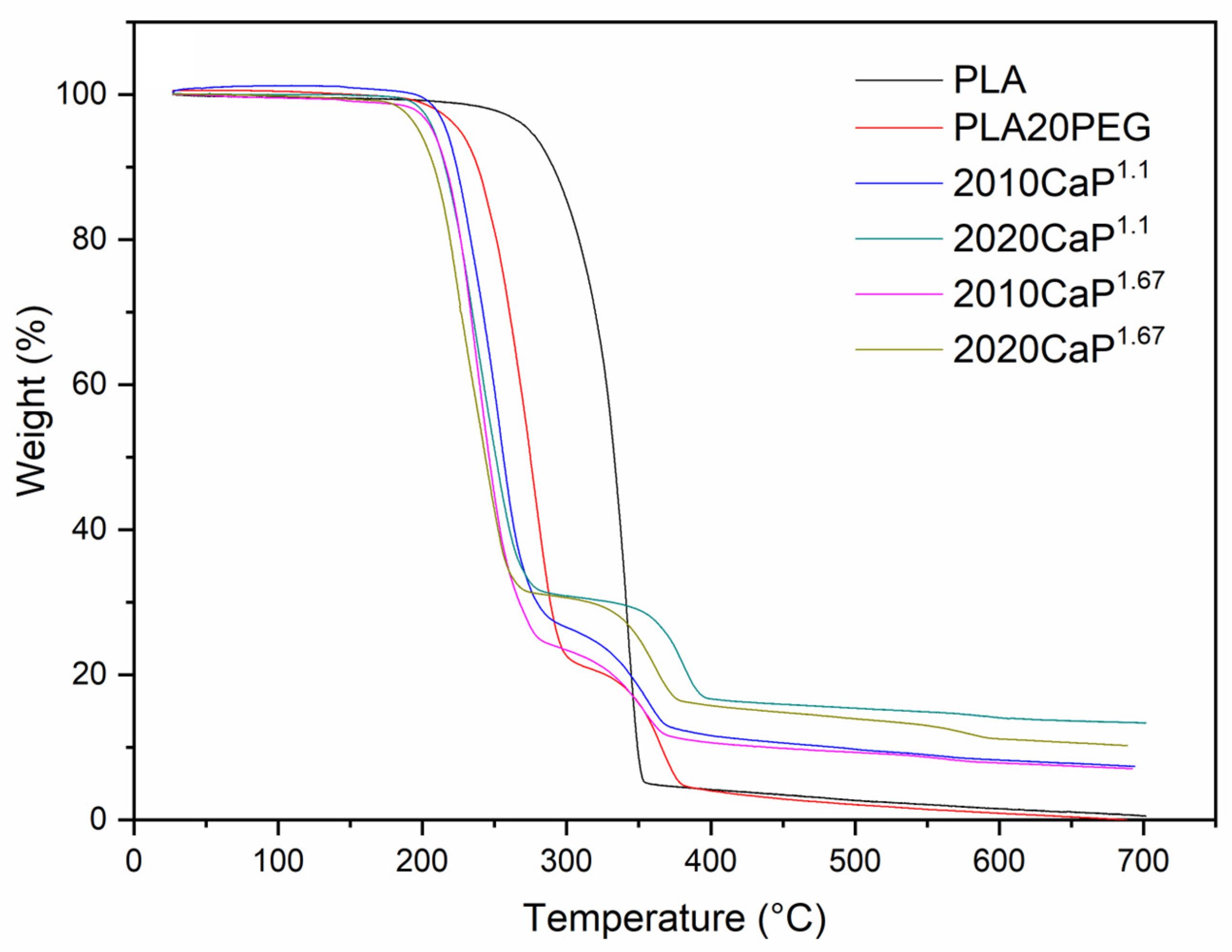
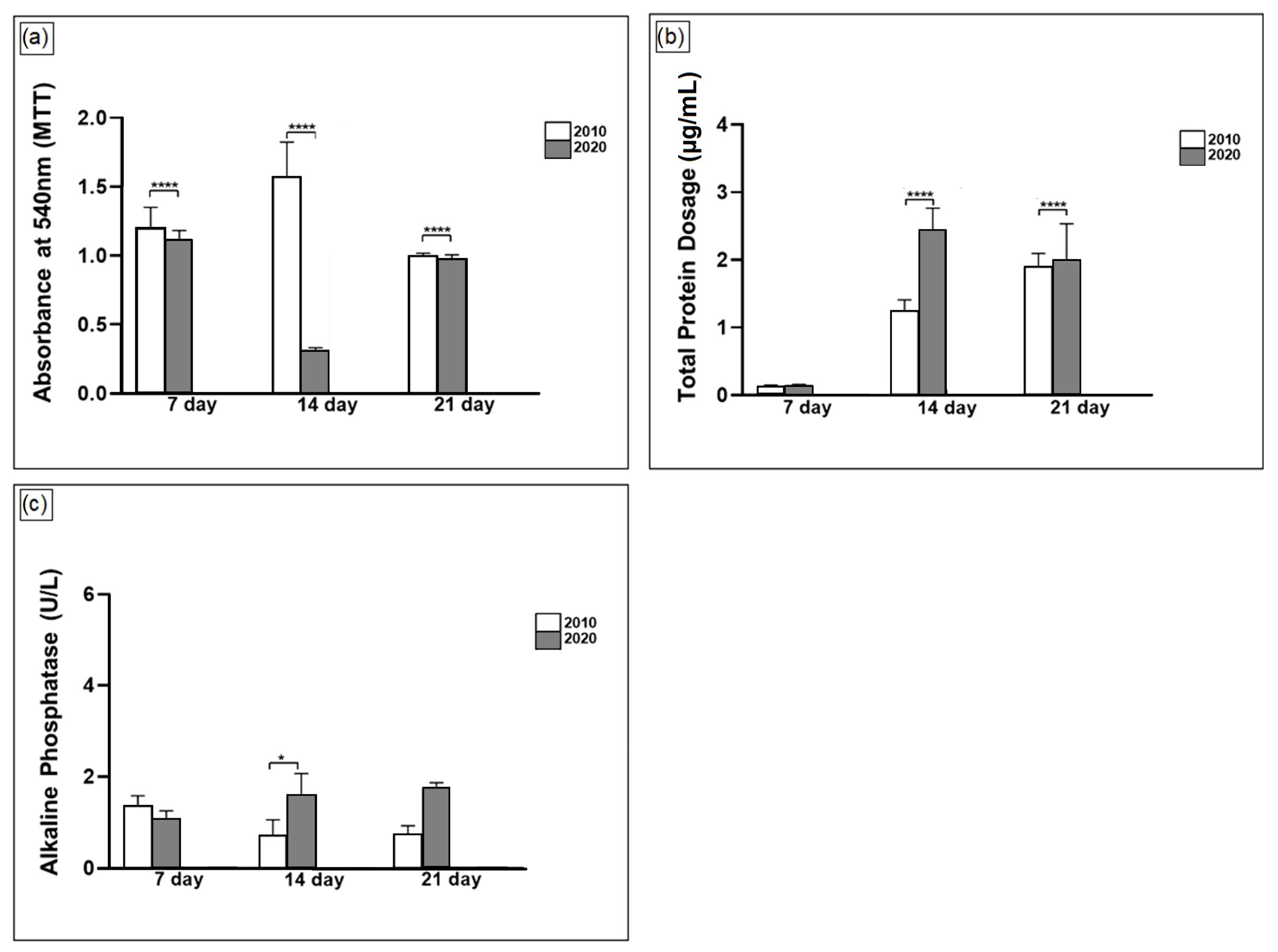
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Karimi, M.; Asadi-Eydivand, M.; Abolfathi, N.; Chehrehsaz, Y.; Solati-Hashjin, M. The effect of pore size and layout on mechanical and biological properties of 3D-printed bone scaffolds with gradient porosity. Polym. Compos. 2023, 44, 1343–1359. [Google Scholar] [CrossRef]
- Kun, M.; Chan, C.; Ramakrishna, S.; Kulkarni, A.; Vadodaria, K. Textile-based scaffolds for tissue engineering. In Advanced Textiles for Wound Care; Elsevier: Amsterdam, The Netherlands, 2019; pp. 329–362. [Google Scholar]
- Ghomi, E.R.; Lakshminarayanan, R.; Chellappan, V.; Verma, N.K.; Chinnappan, A.; Neisiany, R.E.; Amuthavalli, K.; Poh, Z.S.; Wong, B.H.S.; Dubey, N. Electrospun aligned PCL/gelatin scaffolds mimicking the skin ECM for effective antimicrobial wound dressings. Adv. Fiber Mater. 2023, 5, 235–251. [Google Scholar] [CrossRef]
- Cheng, G.; Dai, J.; Dai, J.; Wang, H.; Chen, S.; Liu, X.; Li, X.; Zhou, X.; Deng, H.; Li, Z. Extracellular matrix imitation utilizing nanofibers-embedded biomimetic scaffolds for facilitating cartilage regeneration. Chem. Eng. J. 2021, 410, 128379. [Google Scholar] [CrossRef]
- Jing, X.; Hu, X.; Feng, P.; Liu, Y.; Yang, J. Modification of nanofibrous scaffolds to mimic extracellular matrix in physical and chemical structuring. Polym. Eng. Sci. 2023, 63, 467–477. [Google Scholar] [CrossRef]
- Feng, P.-Y.; Jing, X. Novel shish-kebab structured nanofibrous decorating chitosan unidirectional scaffolds to mimic extracellular matrix for tissue engineering. J. Mech. Behav. Biomed. Mater. 2024, 158, 106677. [Google Scholar] [CrossRef] [PubMed]
- Hameed, P.; Manivasagam, V.K.; Sankar, M.; Popat, K.C.; Manivasagam, G. Nanofibers and nanosurfaces. In Nanomaterials and Their Biomedical Applications; Springer: Singapore, 2021; pp. 107–130. [Google Scholar]
- Bernardo, M.P.; da Silva, B.C.; Hamouda, A.E.; de Toledo, M.A.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Roberta, P.; Carlo, M.; Giovanna, I. Hydroxyapatite/tricalcium phosphate (HA/beta-TCP) scaffold combined with bone and endothelial cells as a potential candidate for oral and maxillofacial bone regeneration. J. Dent. 2022, 121, 103960. [Google Scholar]
- Vallmajo-Martin, Q.; Broguiere, N.; Millan, C.; Zenobi-Wong, M.; Ehrbar, M. PEG/HA hybrid hydrogels for biologically and mechanically tailorable bone marrow organoids. Adv. Funct. Mater. 2020, 30, 1910282. [Google Scholar] [CrossRef]
- Lai, W.-C.; Liu, L.-J. Poly (lactic acid)/poly (ethylene glycol) green nanocomposites assisted by sorbitol-derivative nanofibrillar scaffolds with improved crystallization rates and mechanical properties. J. Therm. Anal. Calorim. 2023, 148, 13337–13348. [Google Scholar] [CrossRef]
- Singhawannurat, S.; Lawtae, P.; Rojviriya, C.; Phoovasawat, C. Development of PLA/HA porous scaffolds with controlled pore sizes using the combined freeze drying and sucrose leaching technique for bone tissue engineering. J. Met. Mater. Miner. 2024, 34, 1928. [Google Scholar] [CrossRef]
- Pudełko-Prażuch, I.; Balasubramanian, M.; Ganesan, S.M.; Marecik, S.; Walczak, K.; Pielichowska, K.; Chatterjee, S.; Kandaswamy, R.; Pamuła, E. Characterization and In Vitro Evaluation of Porous Polymer-Blended Scaffolds Functionalized with Tricalcium Phosphate. J. Funct. Biomater. 2024, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Monia, T.; Ridha, B.C. Polymer-ceramic composites for bone challenging applications: Materials and manufacturing processes. J. Thermoplast. Compos. Mater. 2024, 37, 1540–1557. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.H.; Hendawy, H.D.; Gaber, R.A.; Helal, N.R.; Aboushelib, M.N. Osteogenesis ability of CAD-CAM biodegradable polylactic acid scaffolds for reconstruction of jaw defects. J. Prosthet. Dent. 2019, 121, 118–123. [Google Scholar] [CrossRef]
- Behera, K.; Sivanjineyulu, V.; Chang, Y.-H.; Chiu, F.-C. Thermal properties, phase morphology and stability of biodegradable PLA/PBSL/HAp composites. Polym. Degrad. Stab. 2018, 154, 248–260. [Google Scholar] [CrossRef]
- Kutikov, A.B.; Song, J. An amphiphilic degradable polymer/hydroxyapatite composite with enhanced handling characteristics promotes osteogenic gene expression in bone marrow stromal cells. Acta Biomater. 2013, 9, 8354–8364. [Google Scholar] [CrossRef]
- Nazari, T.; Moghaddam, A.B.; Davoodi, Z. Optimized polylactic acid/polyethylene glycol (PLA/PEG) electrospun fibrous scaffold for drug delivery: Effect of graphene oxide on the cefixime release mechanism. Mater. Res. Express 2019, 6, 115351. [Google Scholar] [CrossRef]
- Meyhami, T.; Hassanajili, S.; Tanideh, N.; Taheri, E. Three dimensional scaffolds of hybrid PLA/PCL/HA/silica nanocomposites for bone tissue engineering. Polym. Bull. 2024, 81, 6025–6053. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Qiu, S.; Wang, C.; Zhang, H.; Guo, J.; Wang, S.; Ma, H. 3D-printed filters for efficient heavy metal removal from water using PLA@ CS/HAP composites. Polymers 2023, 15, 4144. [Google Scholar] [CrossRef]
- Kaniuk, E.; Lechowska-Liszka, A.; Gajek, M.; Nikodem, A.; Ścisłowska-Czarnecka, A.; Rapacz-Kmita, A.; Stodolak-Zych, E. Correlation between porosity and physicochemical and biological properties of electrospinning PLA/PVA membranes for skin regeneration. Biomater. Adv. 2023, 152, 213506. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Kalva, S.N.; Mroue, K.H.; Keyan, K.S.; Tong, Y.; Khan, O.M.; Koç, M. Degradation assessment of Mg-Incorporated 3D printed PLA scaffolds for biomedical applications. Bioprinting 2023, 35, e00302. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J. Drug Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Sirc, J.; Hampejsova, Z.; Trnovska, J.; Kozlik, P.; Hrib, J.; Hobzova, R.; Zajicova, A.; Holan, V.; Bosakova, Z. Cyclosporine a loaded electrospun poly (D, L-lactic acid)/poly (ethylene glycol) nanofibers: Drug carriers utilizable in local immunosuppression. Pharm. Res. 2017, 34, 1391–1401. [Google Scholar] [CrossRef]
- Kumar, R.; Alex, Y.; Nayak, B.; Mohanty, S. Effect of poly (ethylene glycol) on 3D printed PLA/PEG blend: A study of physical, mechanical characterization and printability assessment. J. Mech. Behav. Biomed. Mater. 2023, 141, 105813. [Google Scholar] [CrossRef]
- Hobzova, R.; Hampejsova, Z.; Cerna, T.; Hrabeta, J.; Venclikova, K.; Jedelska, J.; Bakowsky, U.; Bosakova, Z.; Lhotka, M.; Vaculin, S. Poly (d, l-lactide)/polyethylene glycol micro/nanofiber mats as paclitaxel-eluting carriers: Preparation and characterization of fibers, in vitro drug release, antiangiogenic activity and tumor recurrence prevention. Mater. Sci.Eng. C 2019, 98, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Brosse, N.; Hoppe, S.; Wang, Z.; Ziegler-Devin, I.; Zhang, H.; Shu, B. Thermal and mechanical properties of polyethylene glycol (PEG)-modified lignin/polylactic acid (PLA) biocomposites. Int. J. Biol. Macromol. 2024, 262, 129997. [Google Scholar] [CrossRef]
- Hampejsova, Z.; Batek, J.; Sirc, J.; Hobzova, R.; Bosakova, Z. Polylactide/polyethylene glycol fibrous mats for local paclitaxel delivery: Comparison of drug release into liquid medium and to HEMA-based hydrogel model. Chem. Mon. 2019, 150, 1691–1696. [Google Scholar] [CrossRef]
- Gomes, D.; Santos, A.; Neves, G.; Menezes, R. A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Tang, X.; Yu, Y. Electrospinning Preparation And Characterization Of Alumina Nanofibers With High Aspect Ratio. Ceram. Int. 2015, 41, 9232–9238. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oh, T.H. Encapsulation of calcium phosphates on electrospun nanofibers for tissue engineering applications. Crystals 2021, 11, 199. [Google Scholar] [CrossRef]
- Farkas, N.-I.; Marincaș, L.; Barabás, R.; Bizo, L.; Ilea, A.; Turdean, G.L.; Toșa, M.; Cadar, O.; Barbu-Tudoran, L. Preparation and characterization of doxycycline-loaded electrospun PLA/HAP nanofibers as a drug delivery system. Materials 2022, 15, 2105. [Google Scholar] [CrossRef] [PubMed]
- Popkov, A.; Kulbakin, D.; Popkov, D.; Gorbach, E.; Kononovich, N.; Danilenko, N.; Stankevich, K.; Choynzonov, E.; Zheravin, A.A.; Khlusov, I. Solution blow spinning of PLLA/hydroxyapatite composite scaffolds for bone tissue engineering. Biomed. Mater. 2021, 16, 055005. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.J.C.d.; Bonan, R.F.; Campos, S.G.; Neves, G.d.A.; Menezes, R.R. Calcium phosphate submicrometric fibers produced by solution blow spinning. Mater. Res. 2019, 22, e20180753. [Google Scholar] [CrossRef]
- Silva, V.; Farias, R.; Bonan, R.; Cartaxo, J.; Medeiros, E.; Figueiredo, L.; Neves, G.; Menezes, R. Novel synthesis of BCP cotton-wool-like nanofibrous scaffolds by air-heated solution blow spinning (A-HSBS) technique. Ceram. Int. 2023, 49, 24084–24092. [Google Scholar] [CrossRef]
- Saed, A.B.; Behravesh, A.H.; Hasannia, S.; Ardebili, S.A.A.; Akhoundi, B.; Pourghayoumi, M. Functionalized poly l-lactic acid synthesis and optimization of process parameters for 3D printing of porous scaffolds via digital light processing (DLP) method. J. Manuf. Process. 2020, 56, 550–561. [Google Scholar] [CrossRef]
- Fong, M.K. Polycaprolactone (PCL)/polylactice acid (PLA) reinforced with polyethlene gylcol (PEG) and nano-hydroxyapatite (n-HA) for fused deposition modeling (FDM) composite filament. Ph.D. Thesis, Universiti Tun Hussein Malaysia, Parit Raja, Malaysia, 2021. [Google Scholar]
- Akbarzadeh, R.; Yousefi, A.M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef]
- Majchrowicz, A.; Roguska, A.; Krawczyńska, A.; Lewandowska, M.; Marti-Munoz, J.; Engel, E.; Castano, O. In vitro evaluation of degradable electrospun polylactic acid/bioactive calcium phosphate ormoglass scaffolds. Arch. Civ. Mech. Eng. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Cerqueira, G.R.; Gomes, D.S.; Victor, R.S.; Figueiredo, L.R.; Medeiros, E.S.; Neves, G.A.; Menezes, R.R.; Silva, S.M. Development of PVA/chitosan nanofibers by a green route using solution blow spinning. J. Polym. Environ. 2024, 32, 1489–1499. [Google Scholar] [CrossRef]
- de S. Victor, R.; dos S. Gomes, D.; Santos, A.M.d.C.; Torres, S.M.; Neves, G.d.A.; Menezes, R.R. 3D nanofibrous structures formed of high content chitosan/PVA and chitosan/PLA blends using air-heated solution blow spinning (A-HSBS). J. Mater. Sci. 2024, 59, 16768–16788. [Google Scholar] [CrossRef]
- Pimenta, F.; Carbonari, R.; Malmonge, S. Nanofibrous tubular scaffolds for tissue engineering of small-diameter vascular grafts—Development using SBS fabrication technique and mechanical performance. Res. Biomed. Eng. 2022, 38, 797–811. [Google Scholar] [CrossRef]
- Medeiros, E.L.; Gomes, D.S.; Santos, A.M.; Vieira, R.H.; de Lima, I.L.; Rocha, F.S.; Castro-Filice, L.d.S.; Medeiros, E.S.; Neves, G.A.; Menezes, R.R. 3D nanofibrous bioactive glass scaffolds produced by one-step spinning process. Ceram. Int. 2021, 47, 102–110. [Google Scholar] [CrossRef]
- Araújo, M.E.; Farias, R.M.; Araujo, R.N.; Maciel, P.P.; Bonan, P.R.F.; Barboza, C.A.G.; Melo, J.C.; Menezes, R.R.; Neves, G.A. Cu-doped 70S bioactive glass fibrous membranes produced using solution blow spinning (SBS). Ceram. Int. 2024, 50, 41257–41267. [Google Scholar] [CrossRef]
- dos Reis, D.C.S.; Linhares, C.R.B.; da Costa Farias, R.M.; Gomes, D.S.; de Araújo Neves, G.; Batista, J.D.; Dechichi, P.; de Souza Castro Filice, L.; Menezes, R.R.; Rocha, F.S. Physicochemical characterization and biological effect of 3D-nanofibrous alumina scaffolds produced by solution blow spinning. J. Nanopart. Res. 2024, 26, 26. [Google Scholar] [CrossRef]
- Costa, D.L.; Leite, R.S.; Neves, G.A.; de Lima Santana, L.N.; Medeiros, E.S.; Menezes, R.R. Synthesis of TiO2 and ZnO nano and submicrometric fibers by solution blow spinning. Mater. Lett. 2016, 183, 109–113. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Garibay-Alvarado, J.A.; Espinosa Cristóbal, L.F.; Reyes-López, S.Y. Fibrous silica-hydroxyapatite composite by electrospinning. Int. J. Res.—GRANTHAALAYAH 2017, 5, 39–47. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Rajendran, A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O.; Pattanayak, D.K. Synthesis of organic derived hydroxyapatite scaffold from pig bone waste for tissue engineering applications. Adv. Powder Technol. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Millán-Malo, B.M.; del Real-López, A.; Rodriguez-García, M.E. In situ study of hydroxyapatite from cattle during a controlled calcination process using HT-XRD. Mater. Sci.Eng. C 2019, 105, 110020. [Google Scholar] [CrossRef]
- Ion, R.-M.; Iancu, L.; Vasilievici, G.; Grigore, M.E.; Andrei, R.E.; Radu, G.-I.; Grigorescu, R.M.; Teodorescu, S.; Bucurica, I.A.; Ion, M.-L. Ion-substituted carbonated hydroxyapatite coatings for model stone samples. Coatings 2019, 9, 231. [Google Scholar] [CrossRef]
- Rusu, V.M.; Ng, C.-H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size-controlled hydroxyapatite nanoparticles as self-organized organic–inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Brazete, D.; Abrantes, J.; Ferreira, J. Influence of the Ca/P ratio and cooling rate on the allotropic α↔ β-tricalcium phosphate phase transformations. Ceram. Int. 2018, 44, 8249–8256. [Google Scholar] [CrossRef]
- Natarajan, U.V.; Rajeswari, S. Influence of calcium precursors on the morphology and crystallinity of sol–gel-derived hydroxyapatite nanoparticles. J. Cryst. Growth 2008, 310, 4601–4611. [Google Scholar] [CrossRef]
- Eshtiagh-Hosseini, H.; Housaindokht, M.R.; Chahkandi, M. Effects of parameters of sol–gel process on the phase evolution of sol–gel-derived hydroxyapatite. Mater. Chem. Phys. 2007, 106, 310–316. [Google Scholar] [CrossRef]
- Santos, A.; Mota, M.; Leite, R.; Neves, G.; Medeiros, E.; Menezes, R. Solution blow spun titania nanofibers from solutions of high inorganic/organic precursor ratio. Ceram. Int. 2018, 44, 1681–1689. [Google Scholar] [CrossRef]
- da Costa Farias, R.M.; Menezes, R.R.; Oliveira, J.E.; de Medeiros, E.S. Production of submicrometric fibers of mullite by solution blow spinning (SBS). Mater. Lett. 2015, 149, 47–49. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Lao, L.; Wang, Y.; Zhu, Y.; Zhang, Y.; Gao, C. Poly (lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 1873–1884. [Google Scholar] [CrossRef]
- González-Benito, J.; Zuñiga-Prado, S.; Najera, J.; Olmos, D. Non-Woven Fibrous Polylactic Acid/Hydroxyapatite Nanocomposites Obtained via Solution Blow Spinning: Morphology, Thermal and Mechanical Behavior. Nanomaterials 2024, 14, 196. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Zhao, C.; Zi, Q.; He, F.; Wang, W. VEGF-modified PLA/HA nanocomposite fibrous membrane for cranial defect repair in rats. J. Biomater. Appl. 2023, 38, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Xiao, J.; Zhang, T.; Zhang, Y.; Xiong, C. Investigation of the nano-hydroxyapatite with different surface modifications on the properties of poly (lactide-co-glycolide acid)/poly (trimethylene carbonate)/nano-hydroxyapatite composites. Colloid Polym. Sci. 2021, 299, 623–635. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res. Lett. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Yan, C. 3D-printed cellular structures for bone biomimetic implants. Addit. Manuf. 2017, 15, 93–101. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S. Effect of PEG on PLA/PEG blend and its nanocomposites: A study of thermo-mechanical and morphological characterization. Polym. Compos. 2014, 35, 283–293. [Google Scholar] [CrossRef]
- Ozdemir, E.; Tinçer, T.; Hacaloglu, J. Characterization of polylactide/poly (ethylene glycol) blends via direct pyrolysis mass spectrometry. J. Anal. Appl. Pyrolysis 2016, 122, 315–322. [Google Scholar] [CrossRef]
- Salimi, A.; Ahmadi, S.; Faramarzi, M.; Faghihi, J. Reactive blending of polylactic acid/polyethylene glycol toward biodegradable film. Macromol. Res. 2023, 31, 873–881. [Google Scholar] [CrossRef]
- Li, D.; Jiang, Y.; Lv, S.; Liu, X.; Gu, J.; Chen, Q.; Zhang, Y. Preparation of plasticized poly (lactic acid) and its influence on the properties of composite materials. PLoS ONE 2018, 13, e0193520. [Google Scholar] [CrossRef] [PubMed]
- Ftiti, S.; Cifuentes, S.C.; Guidara, A.; Rams, J.; Tounsi, H.; Fernández-Blázquez, J.P. The Structural, Thermal and Morphological Characterization of Polylactic Acid/Β-Tricalcium Phosphate (PLA/Β-TCP) Composites upon Immersion in SBF: A Comprehensive Analysis. Polymers 2024, 16, 719. [Google Scholar] [CrossRef]
- Liu, C.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Novel electrospun polylactic acid nanocomposite fiber mats with hybrid graphene oxide and nanohydroxyapatite reinforcements having enhanced biocompatibility. Polymers 2016, 8, 287. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Baran, E.H.; Erbil, H.Y. Surface modification of 3D printed PLA objects by fused deposition modeling: A review. Colloids Interfaces 2019, 3, 43. [Google Scholar] [CrossRef]
- Asadollahi, M.; Gerashi, E.; Zohrevand, M.; Zarei, M.; Sayedain, S.S.; Alizadeh, R.; Labbaf, S.; Atari, M. Improving mechanical properties and biocompatibility of 3D printed PLA by the addition of PEG and titanium particles, using a novel incorporation method. Bioprinting 2022, 27, e00228. [Google Scholar] [CrossRef]
- Nedaipour, F.; Bagheri, H.; Mohammadi, S. “Polylactic acid-polyethylene glycol-hydroxyapatite composite” an efficient composition for interference screws. Nanocomposites 2020, 6, 99–110. [Google Scholar] [CrossRef]
- Bhaskar, B.; Owen, R.; Bahmaee, H.; Wally, Z.; Sreenivasa Rao, P.; Reilly, G.C. Composite porous scaffold of PEG/PLA support improved bone matrix deposition in vitro compared to PLA-only scaffolds. J. Biomed. Mater. Res. Part A 2018, 106, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Kucko, S.K.; Raeman, S.M.; Keenan, T.J. Current advances in hydroxyapatite-and β-tricalcium phosphate-based composites for biomedical applications: A review. Biomed. Mater. Devices 2023, 1, 49–65. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, Y.; Ma, Y.; Xiao, L.; Ji, W.; Zhang, Y.; Wang, X. Current application of beta-tricalcium phosphate in bone repair and its mechanism to regulate osteogenesis. Front. Mater. 2021, 8, 698915. [Google Scholar] [CrossRef]
- Bohner, M.; Gbureck, U.; Barralet, J. Technological issues for the development of more efficient calcium phosphate bone cements: A critical assessment. Biomaterials 2005, 26, 6423–6429. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Bongio, M.; Bohner, M.; Cuijpers, V.; Winnubst, L.A.; Van Dijk, N.; Wolke, J.G.; Van Den Beucken, J.J.; Jansen, J.A. Processing and in vivo evaluation of multiphasic calcium phosphate cements with dual tricalcium phosphate phases. Acta Biomater. 2012, 8, 3500–3508. [Google Scholar] [CrossRef]
- Carson, J.S.; Bostrom, M.P. Synthetic bone scaffolds and fracture repair. Injury 2007, 38, S33–S37. [Google Scholar] [CrossRef]
- Rh. Owen, G.; Dard, M.; Larjava, H. Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2493–2512. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Hench, L.L. Biomaterials: A forecast for the future. Biomaterials 1998, 19, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/hydroxyapatite composite scaffolds: Preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J. Biomed. Mater. Res. A 2010, 94, 241–251. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, J.E.; Balikov, D.A.; Bae, M.S.; Heo, D.N.; Lee, D.; Rim, H.J.; Lee, D.W.; Sung, H.J.; Kwon, I.K. Poly (l-lactic acid)/gelatin fibrous scaffold loaded with simvastatin/beta-cyclodextrin-modified hydroxyapatite inclusion complex for bone tissue regeneration. Macromol. Biosci. 2016, 16, 1027–1038. [Google Scholar] [CrossRef]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Tsuchiya, A.; Hayashi, K.; Tsuru, K.; Ohe, G. Physical and histological comparison of hydroxyapatite, carbonate apatite, and β-tricalcium phosphate bone substitutes. Materials 2018, 11, 1993. [Google Scholar] [CrossRef]




| Nomenclature a | PEG (wt%) b | CaP (wt%) b |
|---|---|---|
| PLA20PEG | 20 | - |
| 2010CaP1.1 | 20 | 10 |
| 2020CaP1.1 | 20 | 20 |
| 2010CaP1.67 | 20 | 10 |
| 2020CaP1.67 | 20 | 20 |
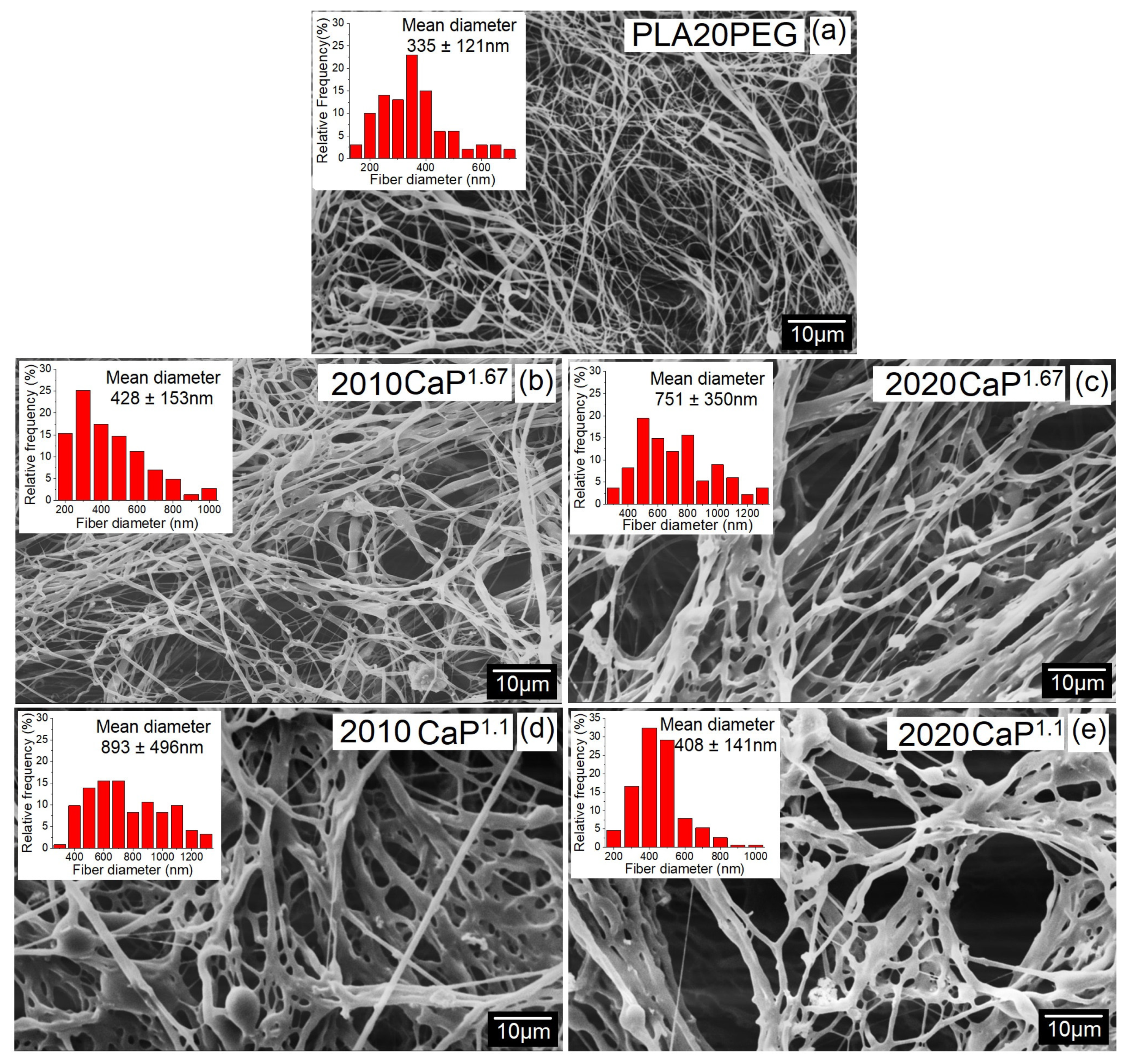
| Nomenclature | Mean Diameter ± Standard Deviation (nm) |
|---|---|
| PLA20PEG | 335 ± 121 |
| 2010CaP1.1 | 484 ± 291 |
| 2020CaP1.1 | 751 ± 350 |
| 2010CaP1.67 | 893 ± 496 |
| 2020CaP1.67 | 408 ± 141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, V.C.; Gomes, D.d.S.; de Medeiros, E.L.G.; Santos, A.M.d.C.; de Lima, I.L.; Rosa, T.P.; Rocha, F.S.; Filice, L.d.S.C.; Neves, G.d.A.; Menezes, R.R. Highly Porous 3D Nanofibrous Scaffold of Polylactic Acid/Polyethylene Glycol/Calcium Phosphate for Bone Regeneration by a Two-Step Solution Blow Spinning (SBS) Facile Route. Polymers 2024, 16, 3041. https://doi.org/10.3390/polym16213041
da Silva VC, Gomes DdS, de Medeiros ELG, Santos AMdC, de Lima IL, Rosa TP, Rocha FS, Filice LdSC, Neves GdA, Menezes RR. Highly Porous 3D Nanofibrous Scaffold of Polylactic Acid/Polyethylene Glycol/Calcium Phosphate for Bone Regeneration by a Two-Step Solution Blow Spinning (SBS) Facile Route. Polymers. 2024; 16(21):3041. https://doi.org/10.3390/polym16213041
Chicago/Turabian Styleda Silva, Vanderlane Cavalcanti, Déborah dos Santos Gomes, Eudes Leonan Gomes de Medeiros, Adillys Marcelo da Cunha Santos, Isabela Lemos de Lima, Taciane Pedrosa Rosa, Flaviana Soares Rocha, Leticia de Souza Castro Filice, Gelmires de Araújo Neves, and Romualdo Rodrigues Menezes. 2024. "Highly Porous 3D Nanofibrous Scaffold of Polylactic Acid/Polyethylene Glycol/Calcium Phosphate for Bone Regeneration by a Two-Step Solution Blow Spinning (SBS) Facile Route" Polymers 16, no. 21: 3041. https://doi.org/10.3390/polym16213041
APA Styleda Silva, V. C., Gomes, D. d. S., de Medeiros, E. L. G., Santos, A. M. d. C., de Lima, I. L., Rosa, T. P., Rocha, F. S., Filice, L. d. S. C., Neves, G. d. A., & Menezes, R. R. (2024). Highly Porous 3D Nanofibrous Scaffold of Polylactic Acid/Polyethylene Glycol/Calcium Phosphate for Bone Regeneration by a Two-Step Solution Blow Spinning (SBS) Facile Route. Polymers, 16(21), 3041. https://doi.org/10.3390/polym16213041











