Efficient Extraction and Analysis of Wheat Straw Lignin by Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemicals
2.2. Analysis of Wheat Straw Composition
2.3. Lignin Extraction Experiment
2.4. Experiment Design and Optimization
2.5. Characterization of Extracted Lignin
2.6. Statistical Analysis
3. Results and Discussion
3.1. Raw Material Composition Analysis
3.2. Effects of Four Experimental Influencing Factors
3.3. Response Surface Analysis
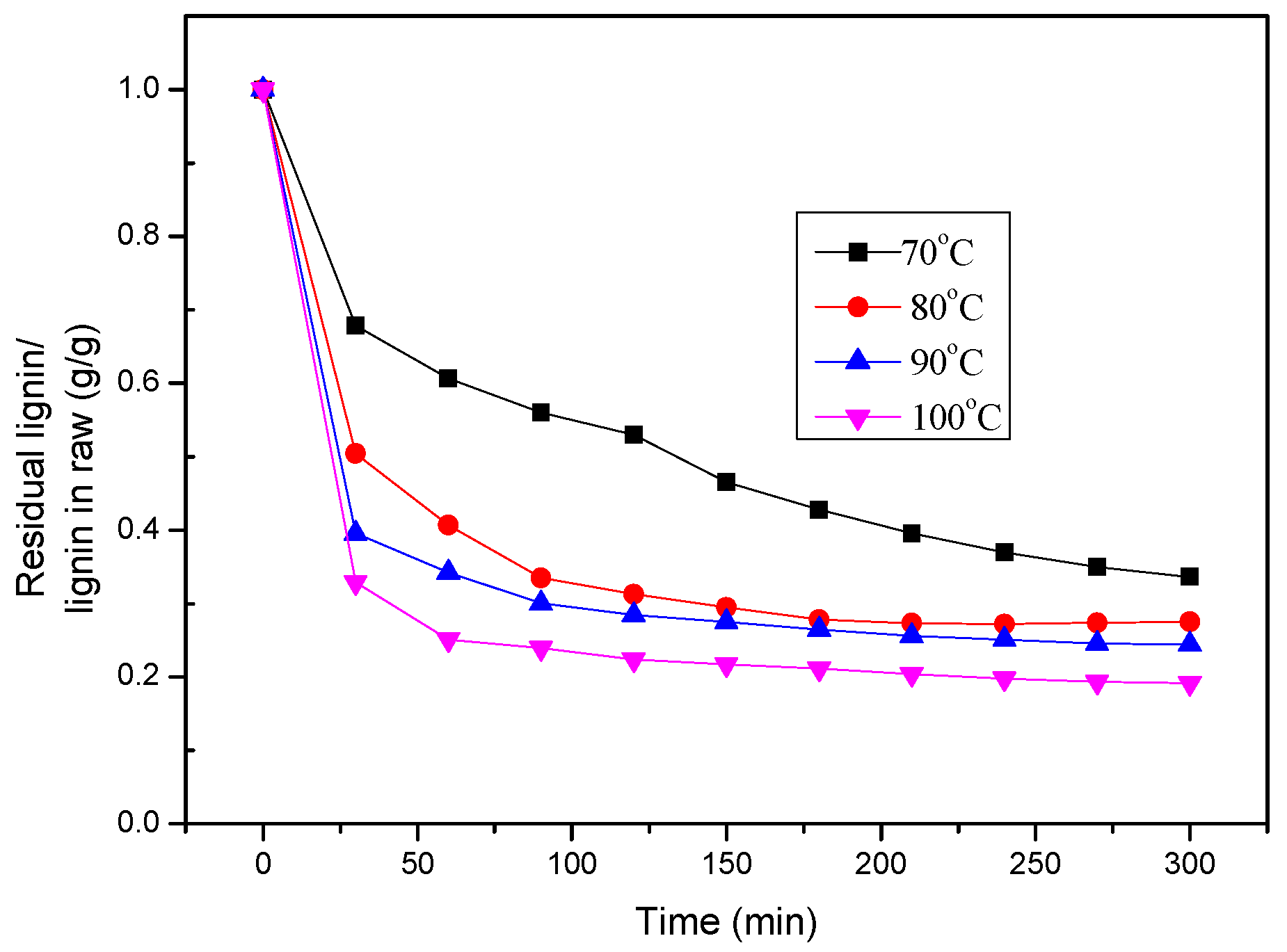
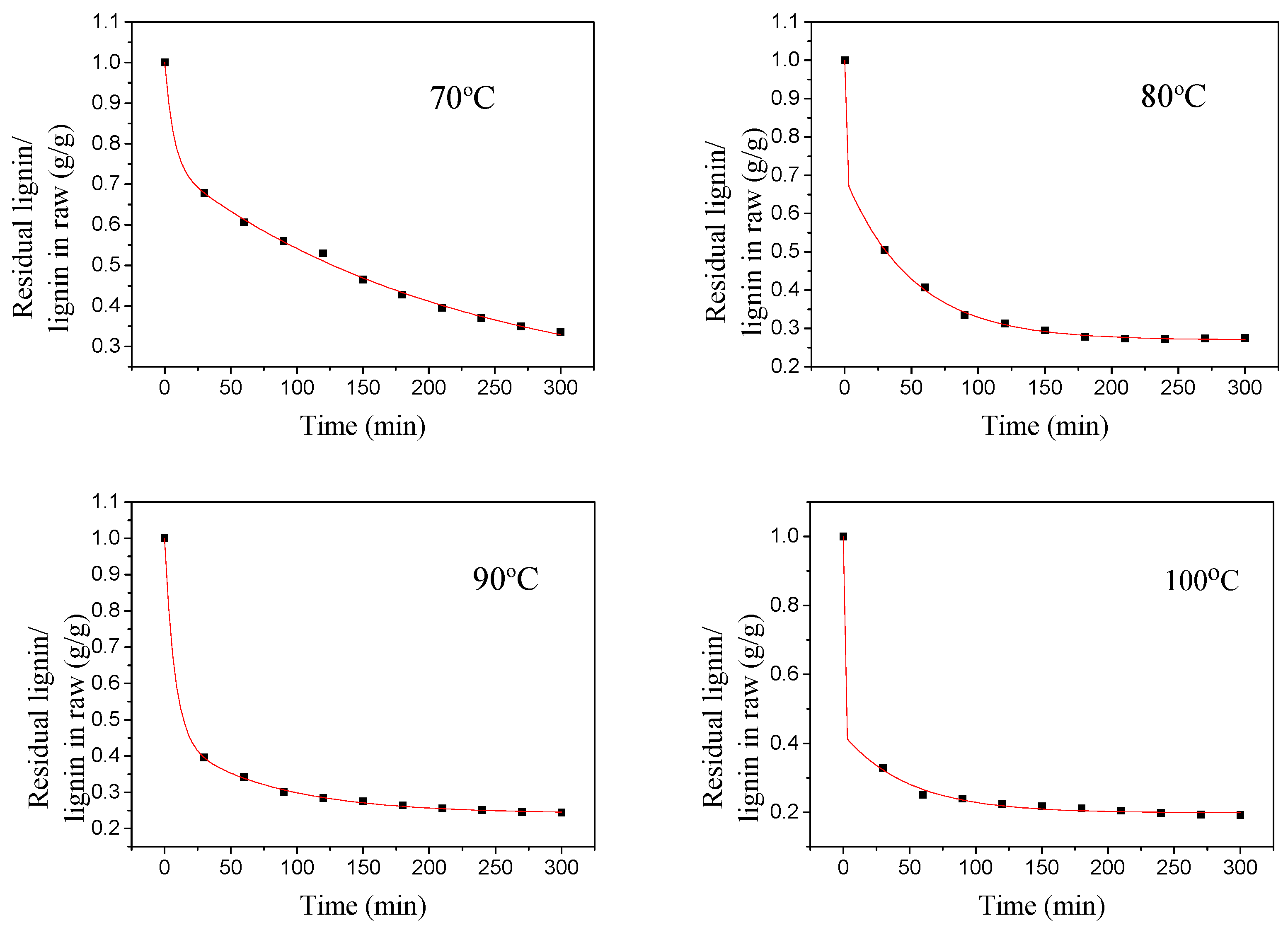
3.4. Lignin Extraction Kinetics
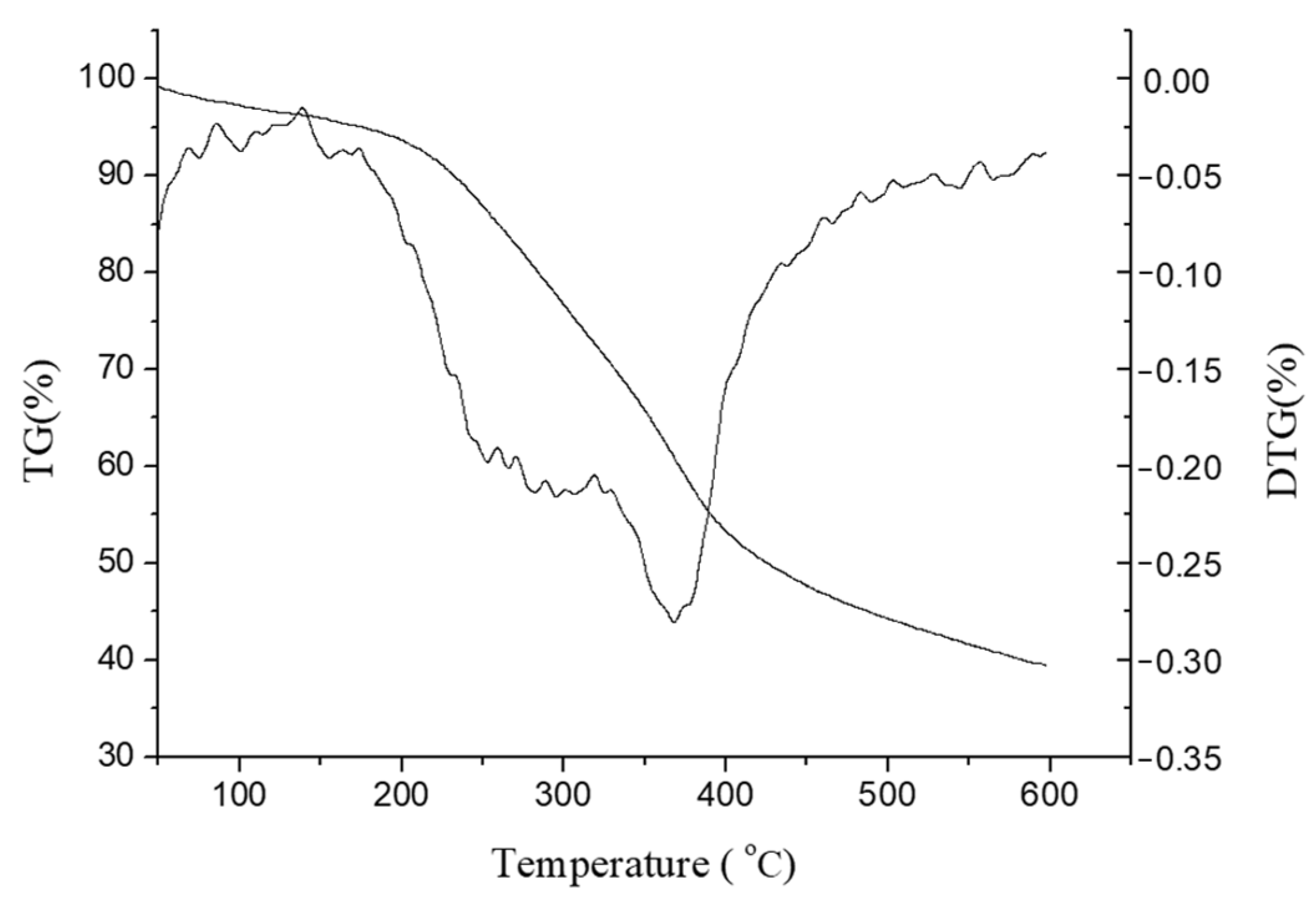
3.5. Structural Analysis of Extracted Lignin Fractions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Phan, D.-P.; Sarwar, A.; Tran, M.H.; Lee, O.K.; Lee, E.Y. Valorization of industrial lignin to value-added chemicals by chemical depolymerization and biological conversion. Ind. Crops Prod. 2021, 161, 113219. [Google Scholar] [CrossRef]
- Li, Z. Research on renewable biomass resource—Lignin. J. Nanjing For. Univ. 2012, 55, 1. [Google Scholar]
- Sadeghifar, H.; Ragauskas, A. Perspective on technical lignin fractionation. ACS Sustain. Chem. Eng. 2020, 8, 8086–8101. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Li, L.; Yu, S.; Xie, C.; Liu, F.; Song, Z. Application of dissociation extraction in oxidation degradation reaction of lignin. Ind. Eng. Chem. Res. 2014, 53, 19370–19374. [Google Scholar] [CrossRef]
- Capecchi, E.; Piccinino, D.; Tomaino, E.; Bizzarri, B.M.; Polli, F.; Antiochia, R.; Mazzei, F.; Saladino, R. Lignin nanoparticles are renewable and functional platforms for the concanavalin an oriented immobilization of glucose oxidase–peroxidase in cas-cade bio-sensing. RSC Adv. 2020, 10, 29031–29042. [Google Scholar] [CrossRef]
- Morena, A.G.; Tzanov, T. Antibacterial lignin-based nanoparticles and their use in composite materials. Nanoscale Adv. 2022, 4, 4447–4469. [Google Scholar] [CrossRef]
- Hussin, M.H.; Appaturi, J.N.; Poh, N.E.; Latif, N.H.A.; Brosse, N.; Ziegler-Devin, I.; Vahabi, H.; Syamani, F.A.; Fatriasari, W.; Solihat, N.N.; et al. A recent advancement on preparation, characteri-zation and application of nanolignin. Int. J. Biol. Macromol. 2022, 200, 303–326. [Google Scholar] [CrossRef]
- Yao, S.; Nie, S.; Zhu, H.; Wang, S.; Song, X.; Qin, C. Extraction of hemicellulose by hot water to reduce adsorbable organic halogen formation in chlorine dioxide bleaching of bagasse pulp. Ind. Crops Prod. 2017, 96, 178–185. [Google Scholar] [CrossRef]
- Nie, S.; Liu, X.; Wu, Z.; Zhan, L.; Yin, G.; Yao, S.; Song, H.; Wang, S. Kinetics study of oxidation of the lignin model compounds by chlorine dioxide. Chem. Eng. J. 2014, 241, 410–417. [Google Scholar] [CrossRef]
- Kruger, J.S.; Dreiling, R.J.; Wilcox, D.G.; Ringsby, A.J.; Noon, K.L.; Amador, C.K.; Brandner, D.G.; Ramirez, K.J.; Haugen, S.J.; Klein, B.C. Lignin alkaline oxidation using reversibly-soluble bases. Green Chem. 2022, 24, 8733–8741. [Google Scholar] [CrossRef]
- Yue, X.; Suopajarvi, T.; Mankinen, O.; Mikola, M.; Mikkelson, A.; Ahola, J.; Hiltunen, S.; Komulainen, S.; Kantola, A.M.; Telkki, V.-V. Comparison of lignin fractions isolated from wheat straw using alkaline and acidic deep eutectic solvents. J. Agric. Food Chem. 2020, 68, 15074–15084. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Budarin, V.; Fan, J.; Sloan, R.; Macquarrie, D. Efficient method of lignin isolation using microwave-assisted acidolysis and characterization of the residual lignin. ACS Sustain. Chem. Eng. 2017, 5, 3768–3774. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Zhai, H.; Ren, H. Lignin dissolution model in formic acid–acetic acid–water systems based on lignin chemical structure. Int. J. Biol. Macromol. 2021, 182, 51–58. [Google Scholar] [CrossRef]
- Vanderghem, C.; Brostaux, Y.; Jacquet, N.; Blecker, C.; Paquot, M. Optimization of formic/acetic acid delignification of Miscanthus× giganteus for enzymatic hydrolysis using response surface methodology. Ind. Crops Prod. 2012, 35, 280–286. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. I. Preparation of fiber residues of low nitrogen content. Assoc. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- GB/T2677.6; Fibrous Raw Material–Determination of Solvent Extractives. China Standard Press: Beijing, China, 1994.
- Xu, F.; Sun, J.; Sun, R.; Fowler, P.; Baird, M.S. Comparative study of organosolv lignins from wheat straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 2013, 57, 264–279. [Google Scholar] [CrossRef]
- Yang, L.; Seshan, K.; Li, Y. A review on thermal chemical reactions of lignin model compounds. Catal. Today 2017, 298, 276–297. [Google Scholar] [CrossRef]
- Tian, D.; Chandra, R.P.; Lee, J.-S.; Lu, C.; Saddler, J.N. A comparison of various lignin-extraction methods to enhance the accessibility and ease of enzymatic hydrolysis of the cellulosic component of steam-pretreated poplar. Biotechnol. Biofuels 2017, 10, 157. [Google Scholar] [CrossRef]
- Azelee, N.I.W.; Jahim, J.M.; Rabu, A.; Murad, A.M.A.; Bakar, F.D.A.; Illias, R.M. Efficient removal of lignin with the maintenance of hemicellulose from kenaf by two-stage pretreatment process. Carbohydr. Polym. 2014, 99, 447–453. [Google Scholar] [CrossRef] [PubMed]
- He, M.-K.; He, Y.-L.; Li, Z.-Q.; Zhao, L.-N.; Zhang, S.-Q.; Liu, H.-M.; Qin, Z. Structural characterization of lignin and lignin-carbohydrate complex (LCC) of sesame hull. Int. J. Biol. Macromol. 2022, 209, 258–267. [Google Scholar] [CrossRef]
- You, T.-T.; Zhang, L.-M.; Zhou, S.-K.; Xu, F. Structural elucidation of lignin–carbohydrate complex (LCC) preparations and lignin from Arundo donax Linn. Ind. Crops Prod. 2015, 71, 65–74. [Google Scholar] [CrossRef]
- Zwilling, J.D.; Jiang, X.; Zambrano, F.; Venditti, R.A.; Jameel, H.; Velev, O.D.; Rojas, O.J.; Gonzalez, R. Understanding lignin micro-and nanoparticle nucleation and growth in aqueous suspensions by solvent fractionation. Green Chem. 2021, 23, 1001–1012. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Shui, T.; Feng, S.; Yuan, Z.; Kuboki, T.; Xu, C.C. Highly efficient organosolv fractionation of cornstalk into cellulose and lignin in organic acids. Bioresour. Technol. 2016, 218, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, L.; Deng, B.; Huang, C.; Zhu, J.; Liang, L.; He, X.; Wei, Y.; Qin, C.; Liang, C. Application and prospect of organic acid pretreatment in lignocellulosic biomass separation: A review. Int. J. Biol. Macromol. 2022, 222, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cen, K.; Wang, L.; Jia, D.; Zhu, X.; Chen, D. Co-pyrolysis of cellulose and lignin: Effects of pyrolysis temperature, residence time, and lignin percentage on the properties of biochar using response surface methodology. Ind. Crops Prod. 2024, 219, 119071. [Google Scholar] [CrossRef]
- Gunasekaran, V.; Ramesh, S.; Sathiasivan, K.; Shankar, M.; Rajesh, M.; Tamilarasan, K. Simultaneous organosolv pretreatment and detoxification of agro-biomass for efficient lignin extraction and characterization. Chem. Pap. 2020, 74, 273–283. [Google Scholar] [CrossRef]
- Dhara, S.; Samanta, N.S.; Uppaluri, R.; Purkait, M. High-purity alkaline lignin extraction from Saccharum ravannae and optimization of lignin recovery through response surface methodology. Int. J. Biol. Macromol. 2023, 234, 123594. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, L.; Jiang, Y.; Wang, X.; Xu, J.; Wang, Q.; Jiang, S. Recent advances in the acid-catalyzed conversion of lignin. Biomass Convers. Biorefinery 2023, 13, 519–539. [Google Scholar] [CrossRef]
- Jing, Y.; Li, F.; Li, Y.; Jiang, D.; Lu, C.; Zhang, Z.; Zhang, Q. Biohydrogen production by deep eutectic solvent delignification-driven enzymatic hydrolysis and photo-fermentation: Effect of liquid–solid ratio. Bioresour. Technol. 2022, 349, 126867. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.G.; Gopinath, K.P.; Vinatha, V.; Shreya, S.; Sivaramakrishnan, R.; Chi, N.T.L. Enhanced production of hydrocarbons from lignin isolated from sugarcane bagasse using formic acid induced supercritical ethanol liquefaction followed by hydrodeoxygenation. Chemosphere 2021, 285, 131491. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Lee, J.H.; Choi, J.W. Optimization of lignin extraction variables by response surface methodology from pine saw dust, and quantification of major structural units in isolated lignin fraction. Appl. Sci. 2021, 11, 1739. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Z.; Guo, L.; Zhai, H.; Ren, H. Formation of high carbohydrate and acylation condensed lignin from formic acid-acetic acid-H2O biorefinery of corn stalk rind. Ind. Crops Prod. 2021, 161, 113165. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, X. Development of different pretreatments and related technologies for efficient biomass conversion of lignocellulose. Int. J. Biol. Macromol. 2022, 202, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Gahlot, P.; Moustakas, K.; Kazmi, A.; Ojha, C.S.P.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [Google Scholar] [CrossRef]
- Vermaas, J.V.; Crowley, M.F.; Beckham, G.T. Molecular lignin solubility and structure in organic solvents. ACS Sustain. Chem. Eng. 2020, 8, 17839–17850. [Google Scholar] [CrossRef]
- Nazimudheen, G.; Sekhar, N.C.; Sunny, A.; Kallingal, A.; Hasanath, B. Physiochemical characterization and thermal kinetics of lignin recovered from sustainable agrowaste for bioenergy applications. Int. J. Hydrogen Energy 2021, 46, 4798–4807. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, X. Pyrolysis characteristics and kinetics of lignin: Effect of starting lignins. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 8096–8108. [Google Scholar] [CrossRef]
- Hladnik, L.; Vicente, F.A.; Novak, U.; Grilc, M.; Likozar, B. Solubility assessment of lignin monomeric compounds and organosolv lignin in deep eutectic solvents using in situ Fourier-transform infrared spectroscopy. Ind. Crops Prod. 2021, 164, 113359. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of hemicellulose, cellulose, and lignin content in different types of biomasses by thermogravimetric analysis and pseudocomponent kinetic model (TGA-PKM method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Tanase-Opedal, M.; Syverud, K. Ultraviolet spectrophotometry of lignin revisited: Exploring solvents with low harmfulness, lignin purity, hansen solubility parameter, and determination of phenolic hydroxyl groups. ACS Omega 2022, 7, 46371–46383. [Google Scholar] [CrossRef] [PubMed]
- Snelders, J.; Dornez, E.; Benjelloun-Mlayah, B.; Huijgen, W.J.; de Wild, P.J.; Gosselink, R.J.; Gerritsma, J.; Courtin, C.M. Biorefining of wheat straw using an acetic and formic acid based organosolv fractionation process. Bioresour. Technol. 2014, 156, 275–282. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, H.; Lin, L.; Sun, Y.; Pan, C.; Liu, S. Isolation and characterization of wheat straw lignin with a formic acid process. Bioresour. Technol. 2010, 101, 2311–2316. [Google Scholar] [CrossRef]
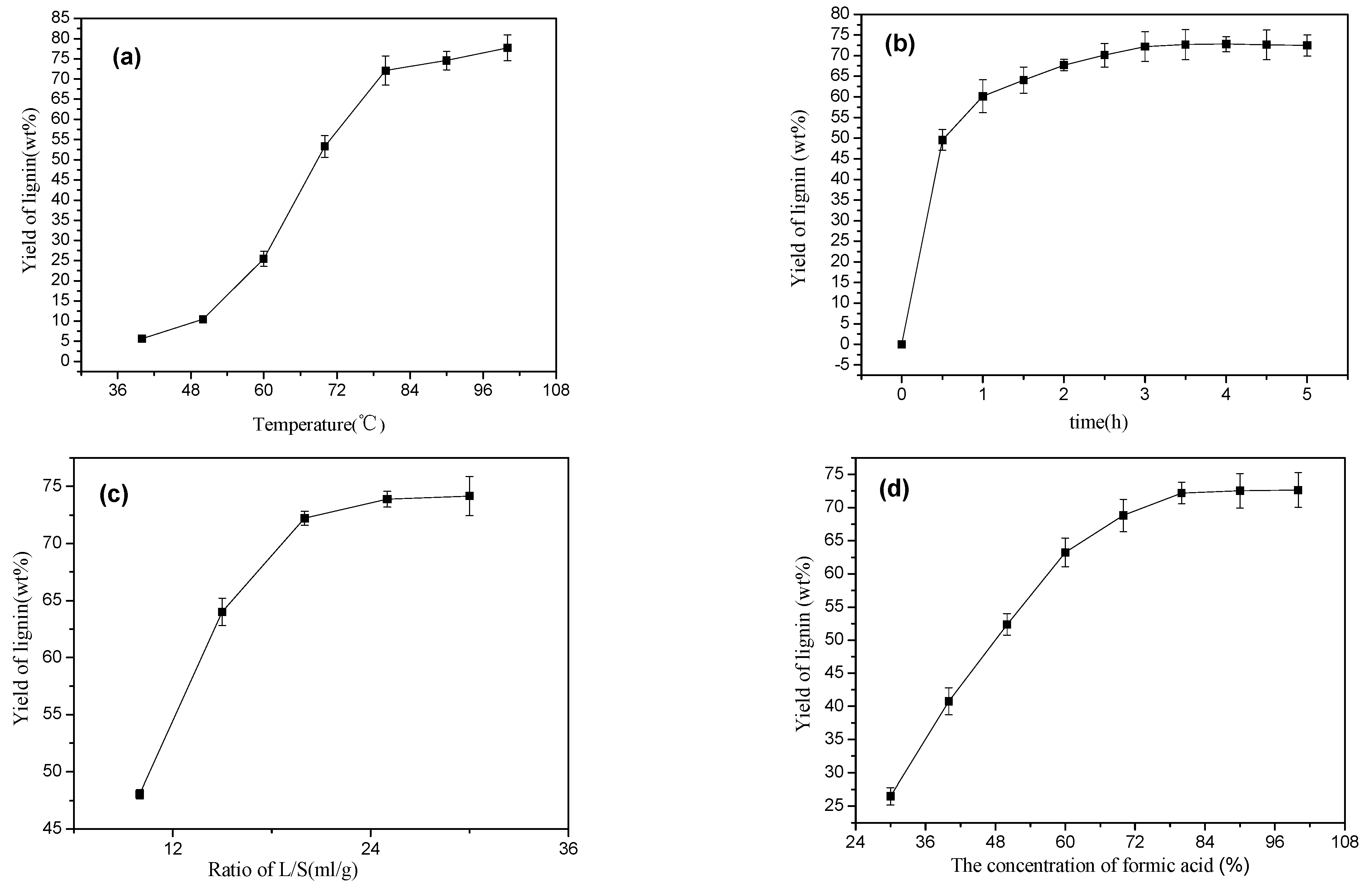
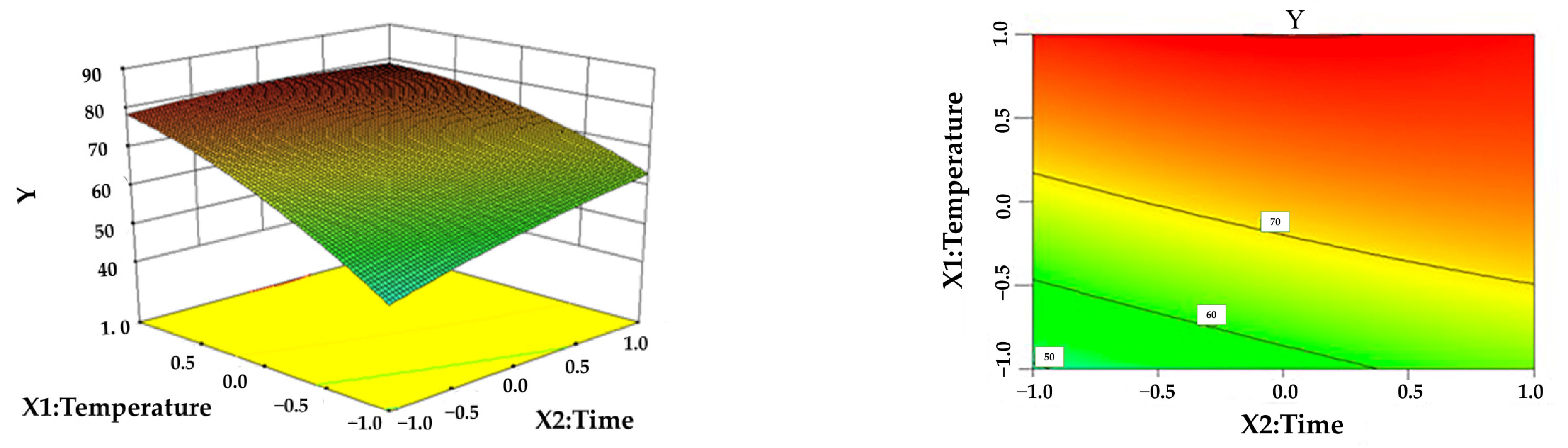
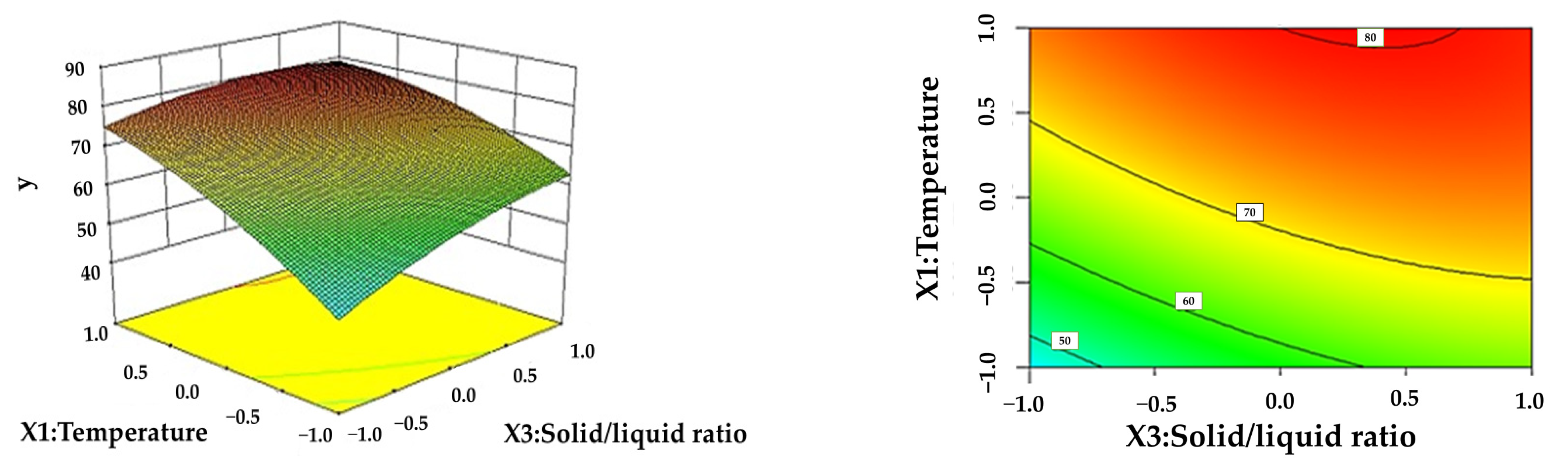
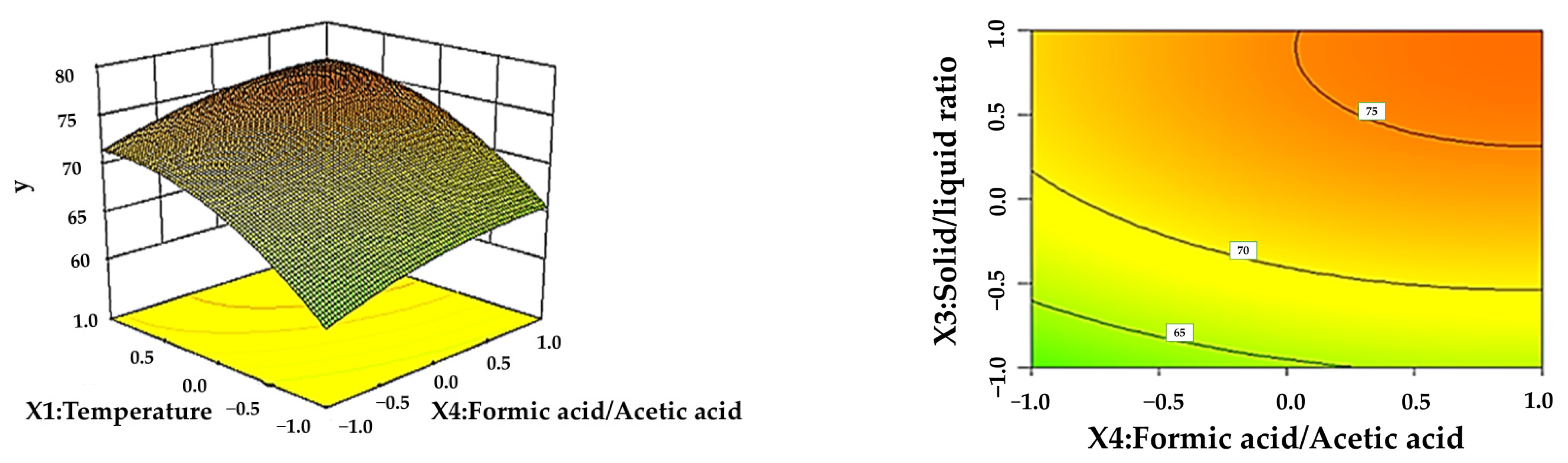


| Level | X1 Temperature (°C) | X2 Time (h) | X3 Solid–Liquid Ratio (g/mL) | X4 Formic Acid Content (%) |
|---|---|---|---|---|
| −2 | 60 | 1 | 1:10 | 60 |
| −1 | 70 | 2 | 1:15 | 70 |
| 0 | 80 | 3 | 1:20 | 80 |
| 1 | 90 | 4 | 1:25 | 90 |
| 2 | 100 | 5 | 1:30 | 100 |
| Assay | X1 | X2 | X3 | X4 | Y/% |
|---|---|---|---|---|---|
| 1 | 0 | 2 | 0 | 0 | 75.18 |
| 2 | −2 | 0 | 0 | 0 | 35.77 |
| 3 | 1 | 1 | −1 | −1 | 69.56 |
| 4 | 0 | 0 | −2 | 0 | 47.91 |
| 5 | 0 | 0 | 0 | 0 | 72.71 |
| 6 | 0 | −2 | 0 | 0 | 60.21 |
| 7 | 0 | 0 | 0 | 0 | 70.45 |
| 8 | 1 | −1 | 1 | −1 | 72.46 |
| 9 | 0 | 0 | 0 | −2 | 63.22 |
| 10 | 1 | 1 | 1 | −1 | 75.61 |
| 11 | 0 | 0 | 0 | 0 | 73.45 |
| 12 | −1 | 1 | −1 | 1 | 52.37 |
| 13 | −1 | 1 | 1 | −1 | 64.54 |
| 14 | 1 | −1 | 1 | 1 | 76.86 |
| 15 | −1 | −1 | −1 | −1 | 34.47 |
| 16 | 1 | −1 | −1 | −1 | 73.56 |
| 17 | −1 | −1 | 1 | −1 | 48.92 |
| 18 | 0 | 0 | 0 | 2 | 72.56 |
| 19 | 0 | 0 | 2 | 0 | 74.06 |
| 20 | −1 | −1 | 1 | 1 | 56.12 |
| 21 | 1 | −1 | −1 | 1 | 75.06 |
| 22 | −1 | 1 | −1 | −1 | 48.83 |
| 23 | −1 | −1 | −1 | 1 | 39.78 |
| 24 | 1 | 1 | −1 | 1 | 74.37 |
| 25 | 1 | 1 | 1 | 1 | 79.01 |
| 26 | 2 | 0 | 0 | 0 | 80.64 |
| 27 | −1 | 1 | 1 | 1 | 68.82 |
| Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) |
|---|---|---|---|
| 48.34 | 22.64 | 18.86 | 2.56 |
| Source | Sum of Square | Degree of Freedom | Mean Square | F Value | Significant Level (p) |
|---|---|---|---|---|---|
| model | 4902.32 | 14 | 350.17 | 114.60 | <0.0001 |
| X1 | 3091.29 | 1 | 3091.29 | 1011.69 | <0.0001 |
| X2 | 306.88 | 1 | 306.88 | 100.43 | <0.0001 |
| X3 | 668.24 | 1 | 668.24 | 218.69 | <0.0001 |
| X4 | 118.37 | 1 | 118.37 | 37.84 | <0.0001 |
| X1X2 | 186.73 | 1 | 186.73 | 61.11 | <0.0001 |
| X1X3 | 166.15 | 1 | 166.15 | 54.38 | <0.0001 |
| X1X4 | 2.42 | 1 | 2.42 | 0.79 | 0.3912 |
| X2X3 | 8.07 | 1 | 8.07 | 2.64 | 0.1302 |
| X2X4 | 0.35 | 1 | 0.35 | 0.12 | 0.7395 |
| X3X4 | 1.06 | 1 | 1.06 | 0.35 | 0.5666 |
| X12 | 275.36 | 1 | 275.36 | 90.12 | <0.0001 |
| X22 | 31.76 | 1 | 31.76 | 10.40 | 0.0073 |
| X32 | 179.13 | 1 | 179.13 | 58.62 | <0.0001 |
| X42 | 28.72 | 1 | 28.72 | 9.40 | 0.0098 |
| Residual | 36.67 | 12 | 3.06 | — | — |
| Misfit error | 31.78 | 10 | 3.18 | 1.30 | 0.5108 |
| Pure error | 4.89 | 2 | 2.44 | — | — |
| sum | 4938.99 | 26 | — | — | — |
| Temperature Reflex/°C | k1/min−1 | k2/min−1 | k3/min−1 | R2 |
|---|---|---|---|---|
| 70 | — | 0.1536 | 0.0052 | 0.9874 |
| 80 | — | 0.2840 | 0.0097 | 0.9943 |
| 90 | — | 0.5342 | 0.0163 | 0.9974 |
| 100 | — | 1.2391 | 0.0275 | 0.9945 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sun, X.-F.; Chen, J.; Hu, S.; Sun, R. Efficient Extraction and Analysis of Wheat Straw Lignin by Response Surface Methodology. Polymers 2024, 16, 2935. https://doi.org/10.3390/polym16202935
Wang Y, Sun X-F, Chen J, Hu S, Sun R. Efficient Extraction and Analysis of Wheat Straw Lignin by Response Surface Methodology. Polymers. 2024; 16(20):2935. https://doi.org/10.3390/polym16202935
Chicago/Turabian StyleWang, Yongke, Xiao-Feng Sun, Jiayi Chen, Sihai Hu, and Ran Sun. 2024. "Efficient Extraction and Analysis of Wheat Straw Lignin by Response Surface Methodology" Polymers 16, no. 20: 2935. https://doi.org/10.3390/polym16202935
APA StyleWang, Y., Sun, X.-F., Chen, J., Hu, S., & Sun, R. (2024). Efficient Extraction and Analysis of Wheat Straw Lignin by Response Surface Methodology. Polymers, 16(20), 2935. https://doi.org/10.3390/polym16202935








