S/N/O-Enriched Carbons from Polyacrylonitrile-Based Block Copolymers for Selective Separation of Gas Streams
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymer and Carbon Synthesis
2.2. Polymer and Carbon Characterization
3. Results
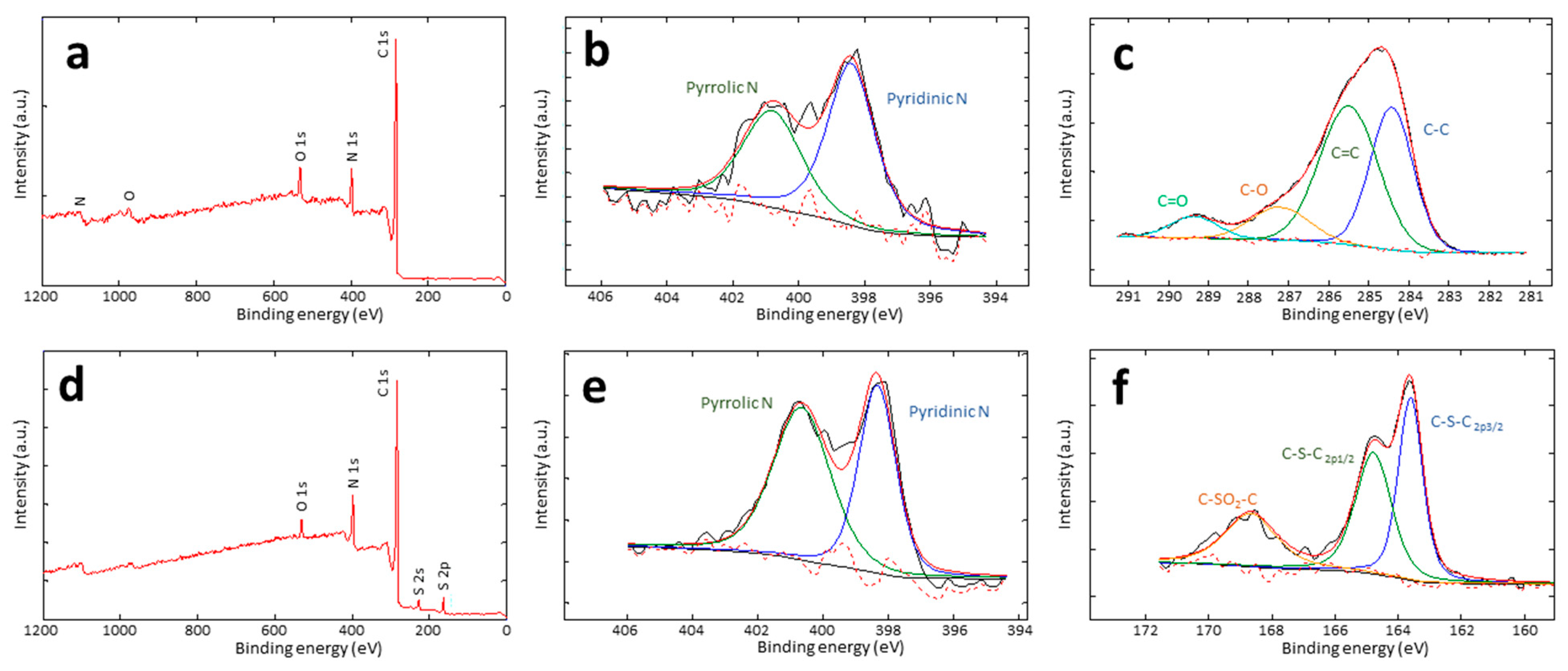
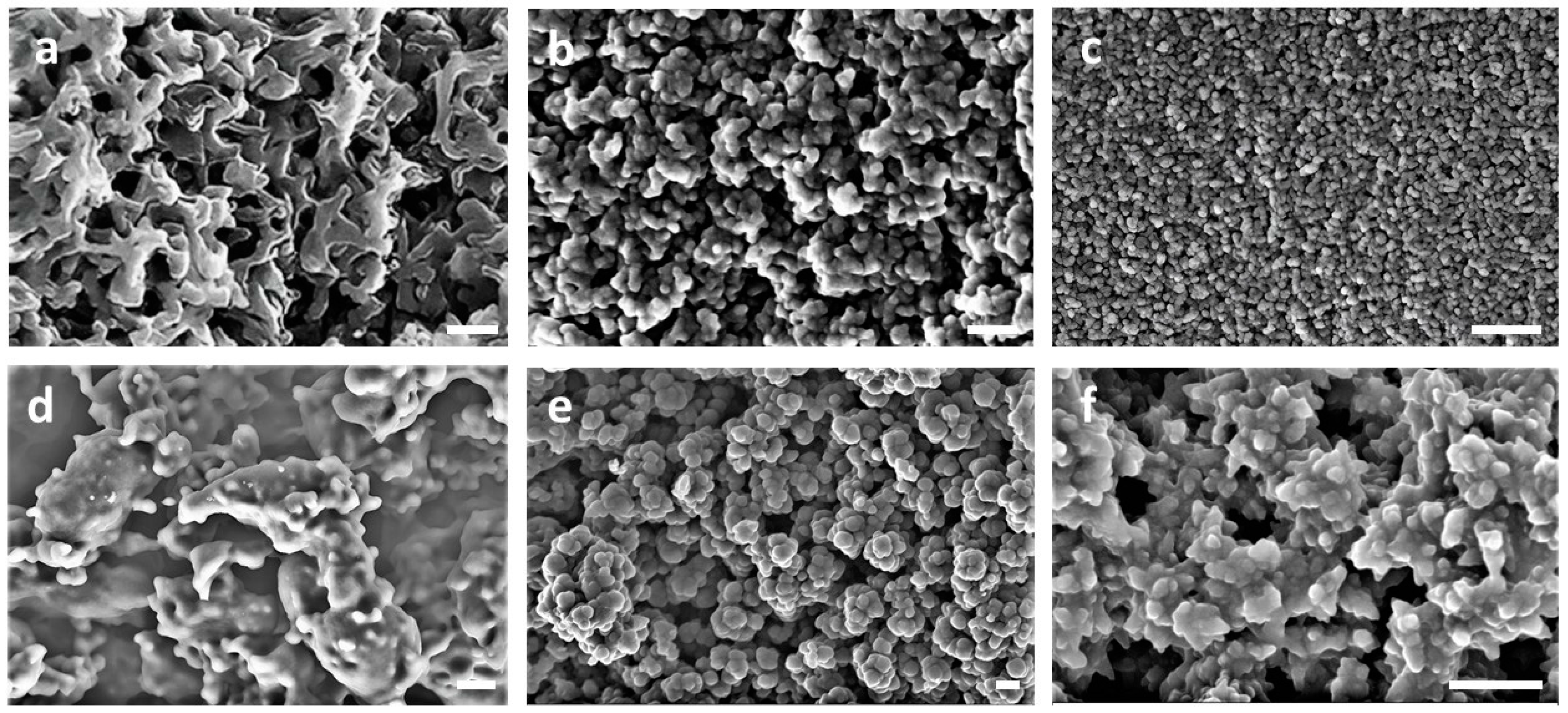
3.1. Synthesis and Structural Characterization
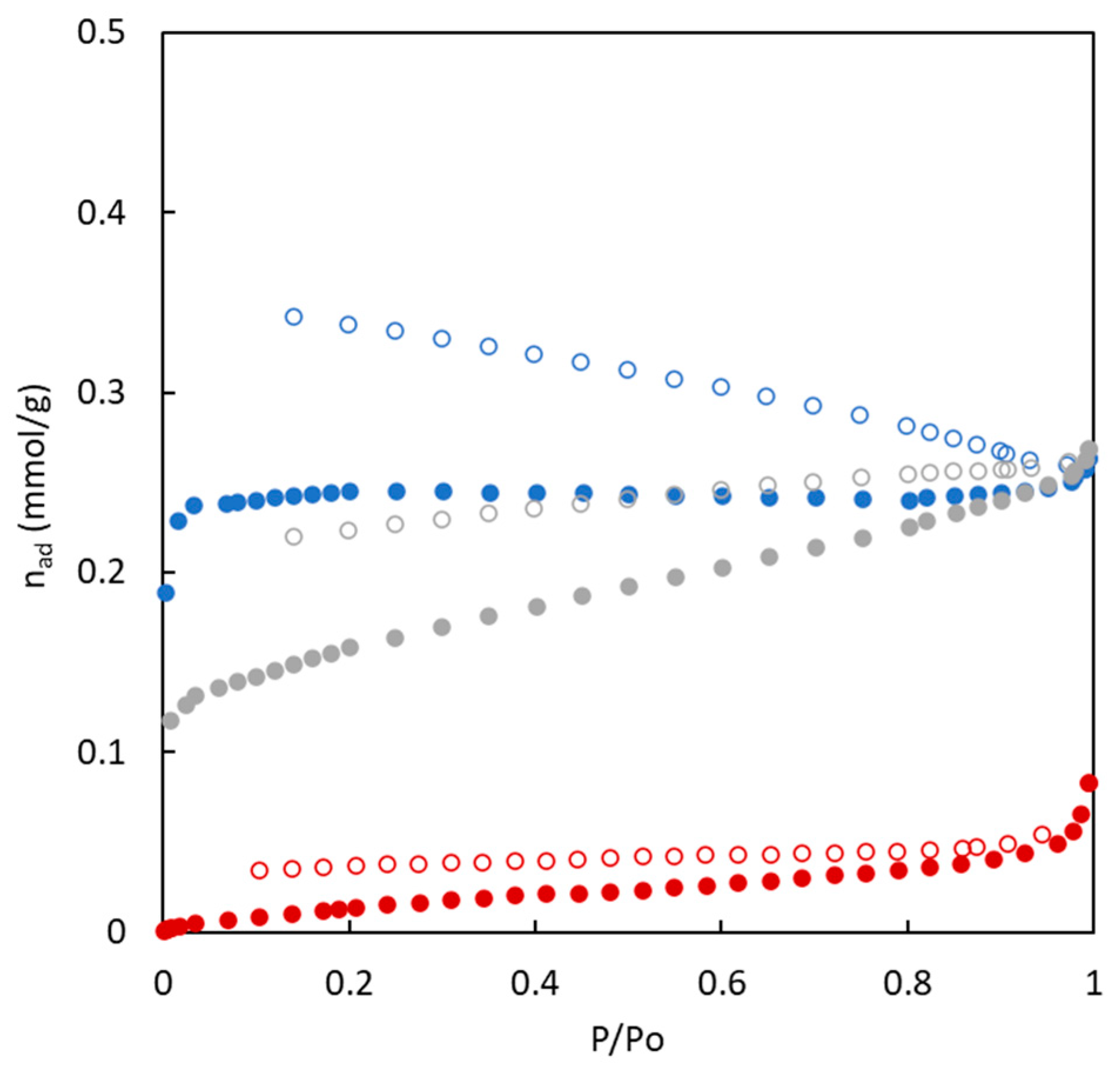
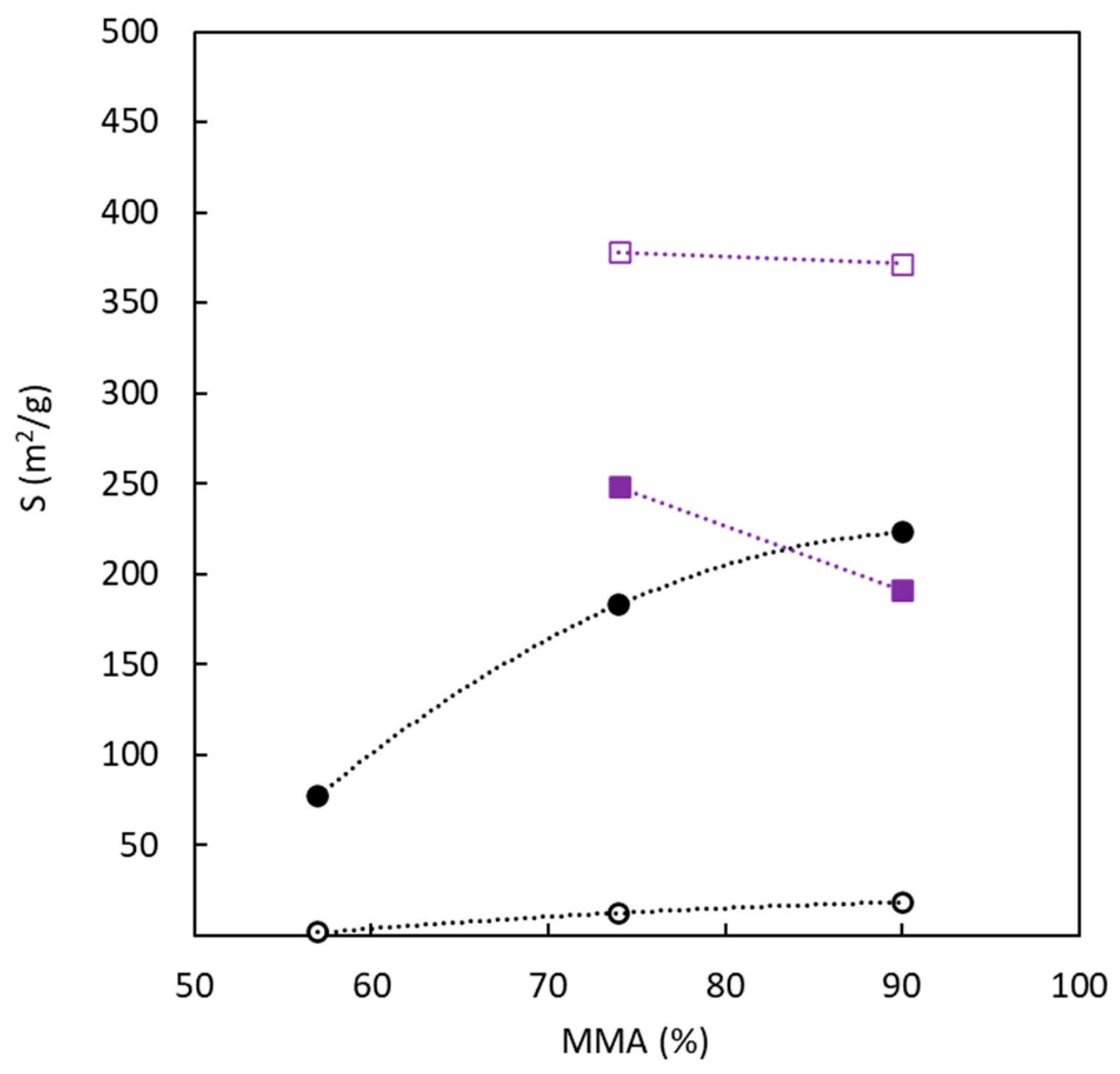
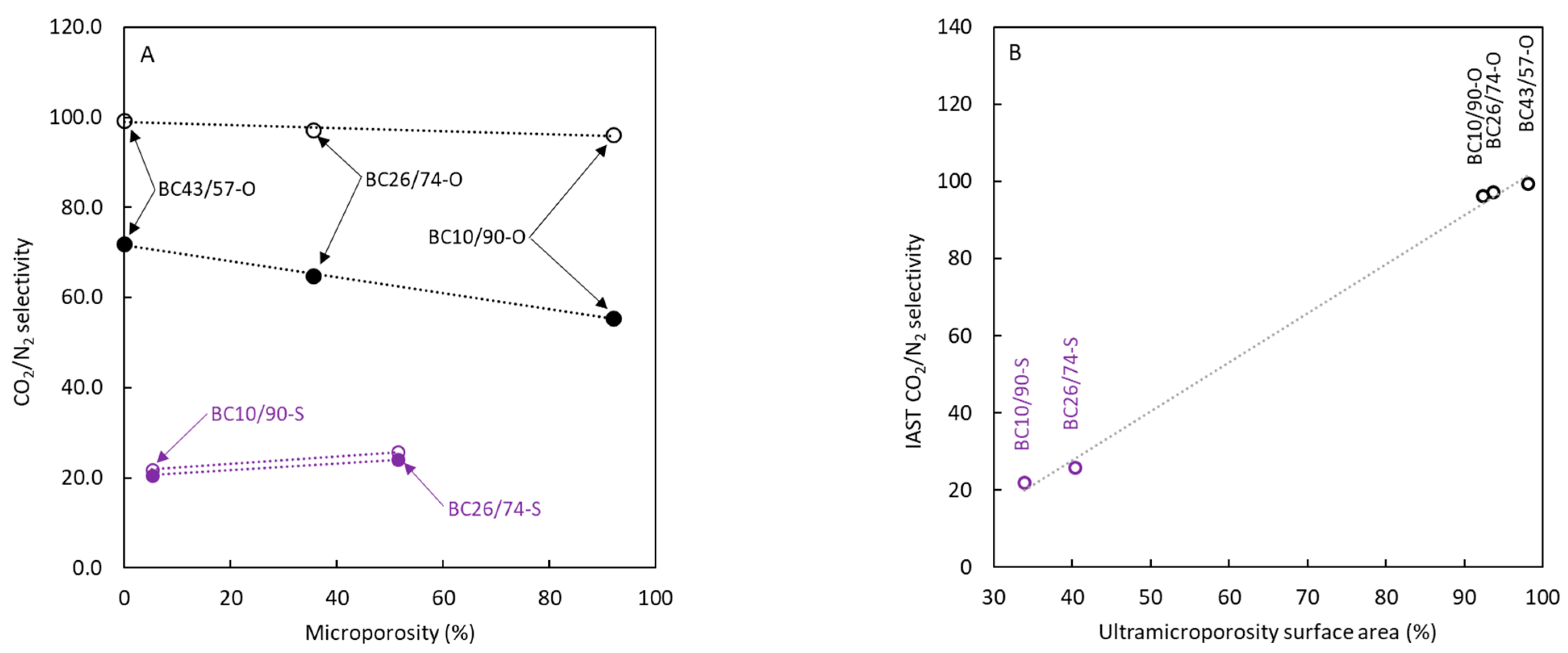
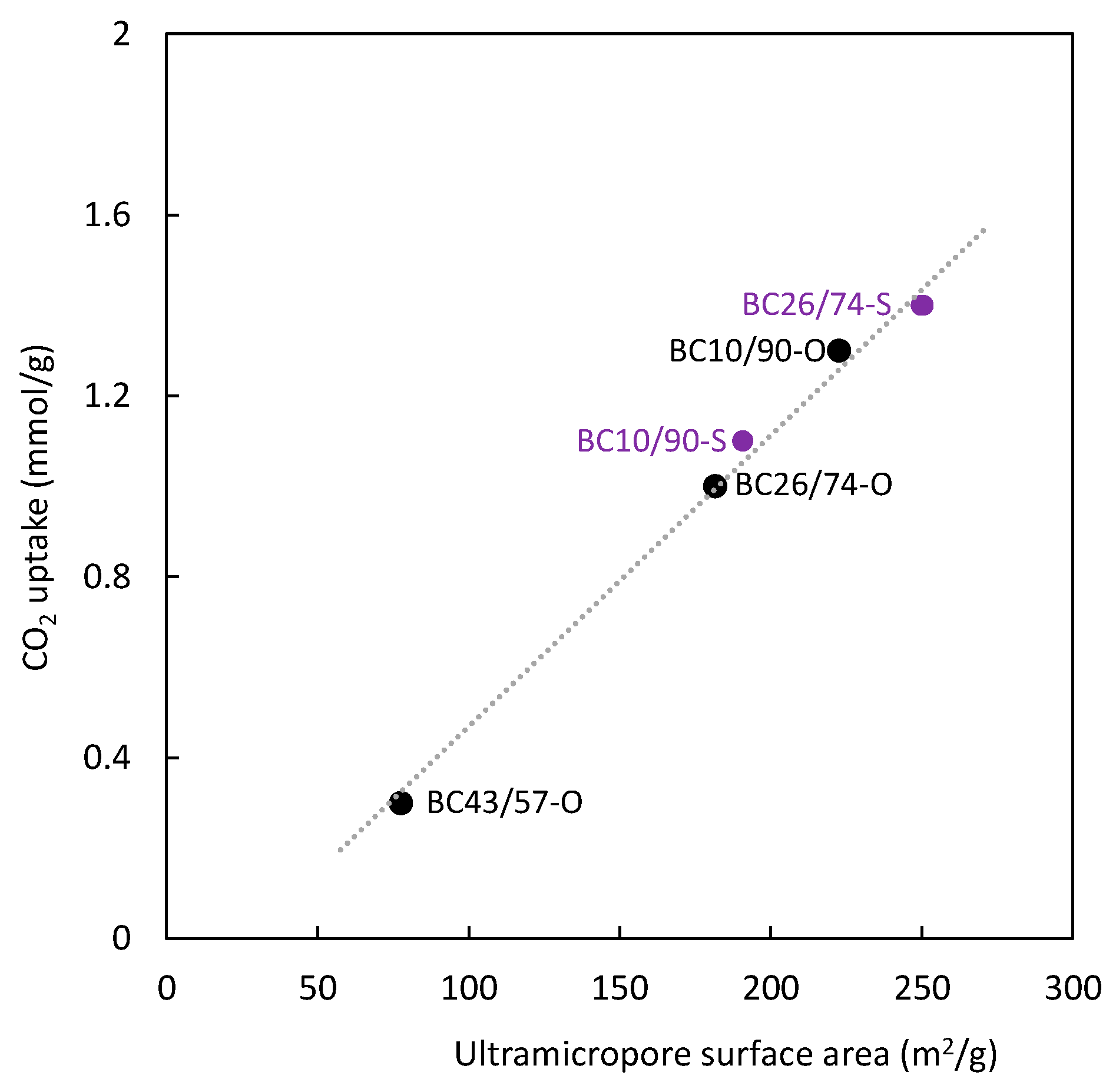
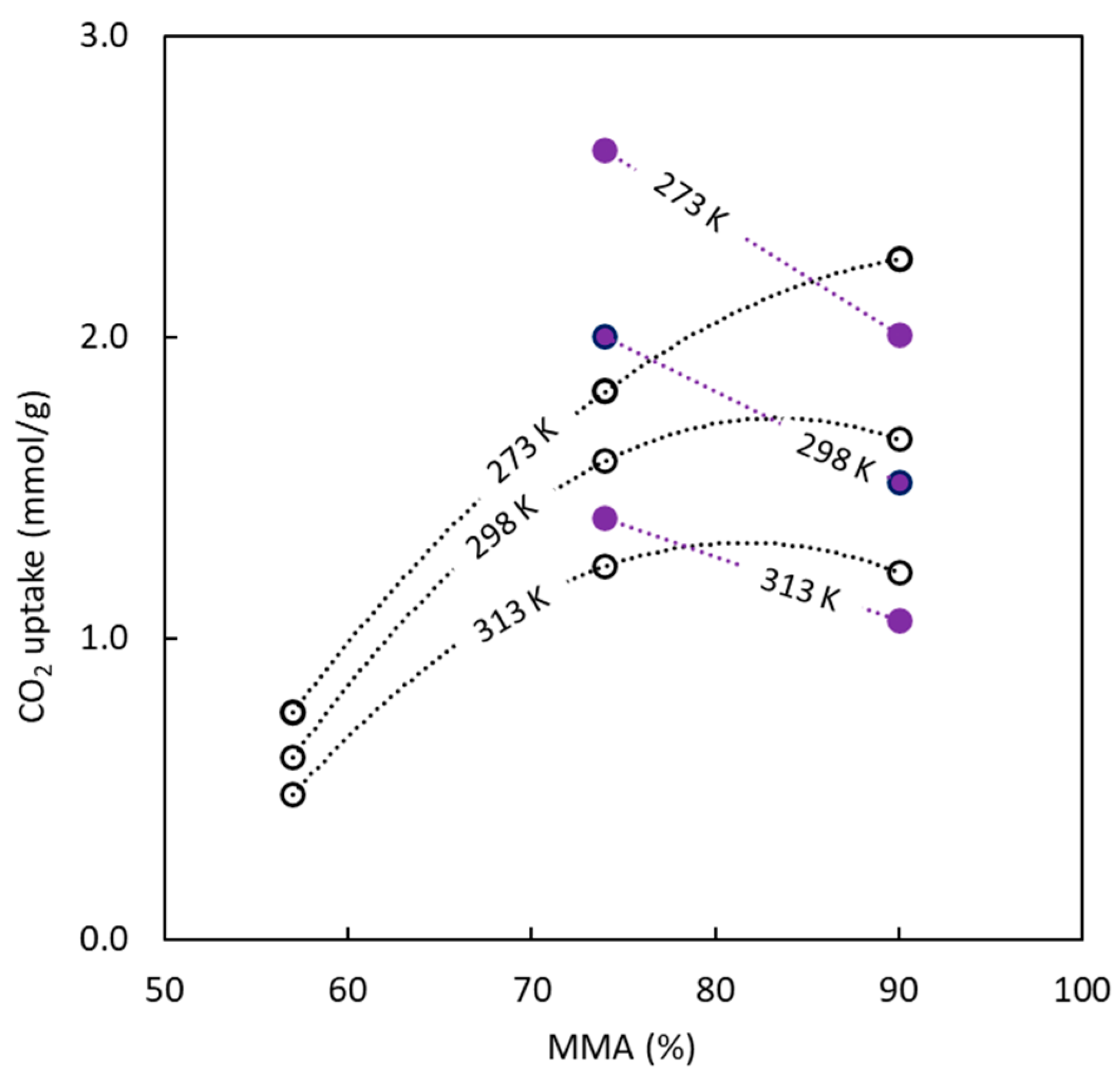
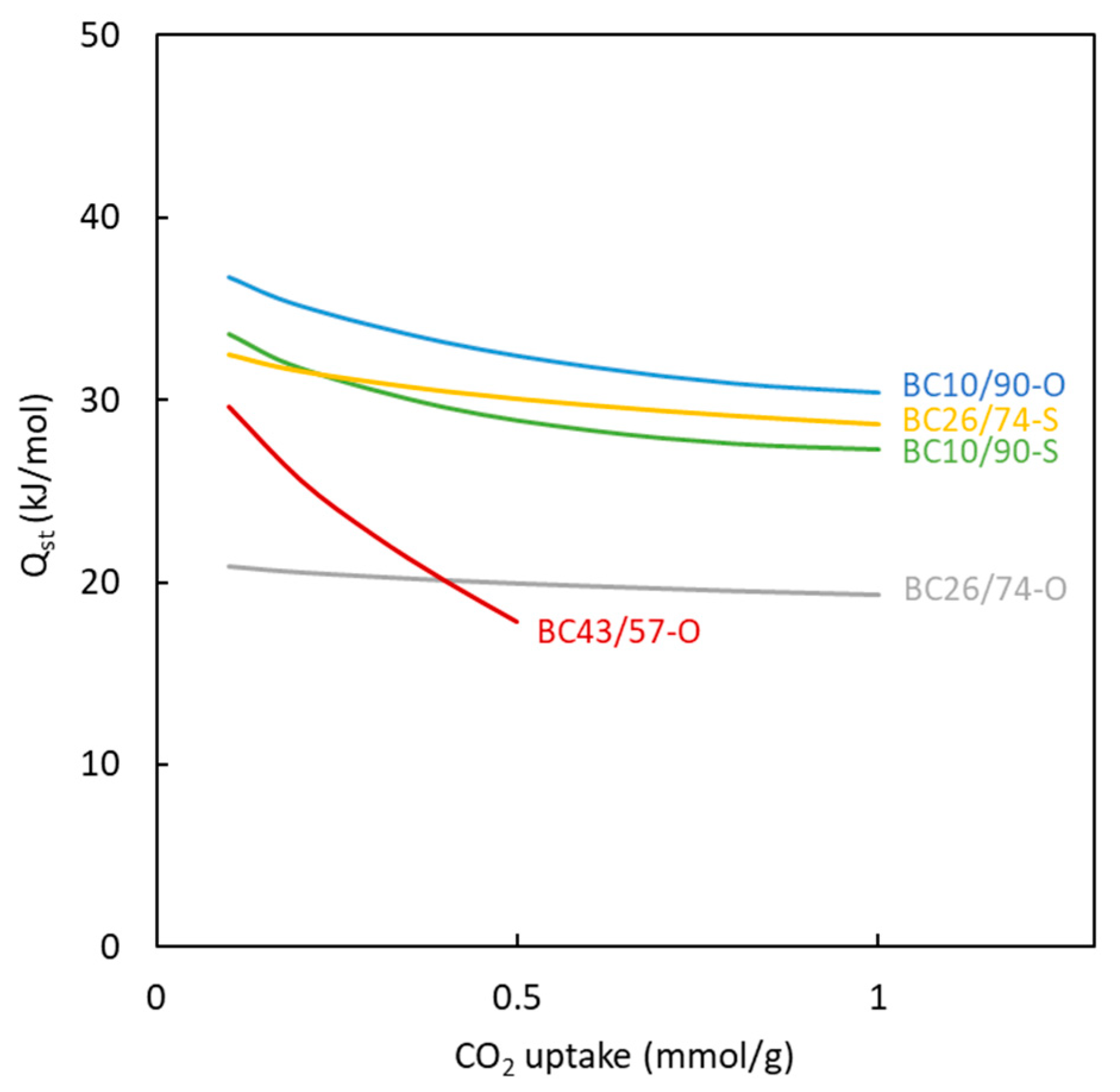
3.2. Surface Area and Pore Size
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lodge, T.P. Block Copolymers: Long-Term Growth with Added Value. Macromolecules 2020, 53, 2–4. [Google Scholar] [CrossRef]
- Lazzari, M.; Torneiro, M. A Global View on Block Copolymers. Polymers 2020, 12, 869. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic Block Copolymers in Drug Delivery: Advances in Formulation Structure and Performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [PubMed]
- Liu, T.; Liu, G. Block Copolymer-Based Porous Carbons for Supercapacitors. J. Mater. Chem. A 2019, 7, 23476–23488. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Lazzari, M.; Arturo López-Quintela, M. Block Copolymers as a Tool for Nanomaterial Fabrication. Adv. Mater. 2003, 15, 1583–1594. [Google Scholar] [CrossRef]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Zhong, M.; Kim, E.K.; McGann, J.P.; Chun, S.E.; Whitacre, J.F.; Jaroniec, M.; Matyjaszewski, K.; Kowalewski, T. Electrochemically Active Nitrogen-Enriched Nanocarbons with Well-Defined Morphology Synthesized by Pyrolysis of Self-Assembled Block Copolymer. J. Am. Chem. Soc. 2012, 134, 14846–14857. [Google Scholar] [CrossRef]
- Zhong, M.; Natesakhawat, S.; Baltrus, J.P.; Luebke, D.; Nulwala, H.; Matyjaszewski, K.; Kowalewski, T. Copolymer-Templated Nitrogen-Enriched Porous Nanocarbons for CO2 Capture. Chem. Commun. 2012, 48, 11516–11518. [Google Scholar] [CrossRef]
- Yan, K.; Kong, L.B.; Dai, Y.H.; Shi, M.; Shen, K.W.; Hu, B.; Luo, Y.C.; Kang, L. Design and Preparation of Highly Structure-Controllable Mesoporous Carbons at the Molecular Level and Their Application as Electrode Materials for Supercapacitors. J. Mater. Chem. A 2015, 3, 22781–22793. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, H.; Sun, M.; Damodaran, K.; Gottlieb, E.; Kopeć, M.; Eckhart, K.; Li, S.; Whitacre, J.; Matyjaszewski, K.; et al. Well-Defined N/S Co-Doped Nanocarbons from Sulfurized PAN- b-PBA Block Copolymers: Structure and Supercapacitor Performance. ACS Appl. Nano Mater. 2019, 2, 2467–2474. [Google Scholar] [CrossRef]
- Serrano, J.M.; Liu, T.; Khan, A.U.; Botset, B.; Stovall, B.J.; Xu, Z.; Guo, D.; Cao, K.; Hao, X.; Cheng, S.; et al. Composition Design of Block Copolymers for Porous Carbon Fibers. Chem. Mater. 2019, 31, 8898–8907. [Google Scholar] [CrossRef]
- Álvarez-Gómez, A.; Yuan, J.; Fernández-Blázquez, J.P.; San-Miguel, V.; Serrano, M.B. Polyacrylonitrile-b-Polystyrene Block Copolymer-Derived Hierarchical Porous Carbon Materials for Supercapacitor. Polymers 2022, 14, 5109. [Google Scholar] [CrossRef]
- Rodriguez-Ramos, I.; Rodriguez-Reinoso, F. The Adsorption of N2 and CO2 on PAN Carbons. Carbon 1988, 26, 905–906. [Google Scholar] [CrossRef]
- Manikandan, R.; Raj, C.J.; Moulton, S.E.; Todorov, T.S.; Yu, K.H.; Kim, B.C. High Energy Density Heteroatom (O, N and S) Enriched Activated Carbon for Rational Design of Symmetric Supercapacitors. Chem.—Eur. J. 2021, 27, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, X.; Jin, C.; Tian, J.; Yang, R. Ternary Doping of Phosphorus, Nitrogen, and Sulfur into Porous Carbon for Enhancing Electrocatalytic Oxygen Reduction. Carbon 2015, 92, 327–338. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface Functional Groups of Carbon-Based Adsorbents and Their Roles in the Removal of Heavy Metals from Aqueous Solutions: A Critical Review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, Y.; Tang, Y. Preparation of Sulfur-Doped Microporous Carbons for the Storage of Hydrogen and Carbon Dioxide. Carbon 2012, 50, 5543–5553. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O.; Mendichi, R.; Arturo López-Quintela, M. Synthesis of Polyacrylonitrile-Block-Polystyrene Copolymers by Atom Transfer Radical Polymerization. Macromol. Chem. Phys. 2005, 206, 1382–1388. [Google Scholar] [CrossRef]
- Lazzari, M.; Scalarone, D.; Hoppe, C.E.; Vazquez-Vazquez, C.; Lòpez-Quintela, M.A. Tunable Polyacrylonitrile-Based Micellar Aggregates as a Potential Tool for the Fabrication of Carbon Nanofibers. Chem. Mater. 2007, 19, 5818–5820. [Google Scholar] [CrossRef]
- Lazzari, M.; Scalarone, D.; Vazquez-Vazquez, C.; López-Quintela, M.A. Cylindrical Micelles from the Self-Assembly of Polyacrylonitrile-Based Diblock Copolymers in Nonpolar Selective Solvents. Macromol. Rapid Commun. 2008, 29, 352–357. [Google Scholar] [CrossRef]
- Lazzari, M.; Liu, G.; Lecommandoux, S. (Eds.) Block Copolymers in Nanoscience; Wiley-VCH Verlag: Weinheim, Germany, 2006. [Google Scholar]
- Fitzer, E. Pan-Based Carbon Fibers-Present State and Trend of the Technology from the Viewpoint of Possibilities and Limits to Influence and to Control the Fiber Properties by the Process Parameters. Carbon 1989, 27, 621–645. [Google Scholar] [CrossRef]
- Manring, L.E. Thermal Degradation of Poly(Methyl Methacrylate). 2. Vinyl-Terminated Polymer. Macromolecules 1989, 22, 2673–2677. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-Doped Porous Carbons: Synthesis and Applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Domínguez-Ramos, L.; Prieto-Estalrich, A.; Malucelli, G.; Gómez-Díaz, D.; Freire, M.S.; Lazzari, M.; González-Álvarez, J. N- and S-Doped Carbons Derived from Polyacrylonitrile for Gases Separation. Sustainability 2022, 14, 3760. [Google Scholar] [CrossRef]
- Zavidovskiy, I.A.; Streletskiy, O.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Savchenko, N.F.; Tatarintsev, A.A.; Pavlikov, A.V. Highly Selective Polyene-Polyyne Resistive Gas Sensors: Response Tuning by Low-Energy Ion Irradiation. J. Compos. Sci. 2023, 7, 156. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Desimoni, E.; Casella, G.I.; Morone, A.; Salvi, A.M. XPS Determination of Oxygen-containing Functional Groups on Carbon-fibre Surfaces and the Cleaning of These Surfaces. Surf. Interface Anal. 1990, 15, 627–634. [Google Scholar] [CrossRef]
- Desimoni, E.; Casella, G.I.; Cataldi, T.R.I.; Salvi, A.M.; Rotunno, T.; Di Croce, E. Remarks on the Surface Characterization of Carbon Fibres. Surf. Interface Anal. 1992, 18, 623–630. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Highly Porous S-Doped Carbons. Microporous Mesoporous Mater. 2012, 158, 318–323. [Google Scholar] [CrossRef]
- Lazzari, M.; De Rosa, C. Methods for the Alignment and the Large-Scale Ordering of Block Copolymer Morphologies. In Block Copolymers in Nanoscience; Lazzari, M., Liu, G., Lecommandoux, S., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2006; ISBN 9783527313099. [Google Scholar]
- Aoyagi, T. Deep Learning Model for Predicting Phase Diagrams of Block Copolymers. Comput. Mater. Sci. 2021, 188, 110224. [Google Scholar] [CrossRef]
- Boehm, H.P. Some Aspects of the Surface Chemistry of Carbon Blacks and Other Carbons. Carbon 1994, 32, 759–769. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Shi, J.; Yan, N.; Cui, H.; Liu, Y.; Weng, Y. Sulfur Doped Microporous Carbons for CO2 Adsorption. J. Environ. Chem. Eng. 2017, 5, 4605–4611. [Google Scholar] [CrossRef]
- Manyà, J.J.; González, B.; Azuara, M.; Arner, G. Ultra-Microporous Adsorbents Prepared from Vine Shoots-Derived Biochar with High CO2 Uptake and CO2/N2 Selectivity. Chem. Eng. J. 2018, 345, 631–639. [Google Scholar] [CrossRef]
- Sethia, G.; Sayari, A. Comprehensive Study of Ultra-Microporous Nitrogen-Doped Activated Carbon for CO2 Capture. Carbon 2015, 93, 68–80. [Google Scholar] [CrossRef]
- Fechler, N.; Fellingera, T.-P.; Antonietti, M. One-pot synthesis of nitrogen–sulfur-co-doped carbons with tunable composition using a simple isothiocyanate ionic liquid. J. Mater. Chem. A 2013, 1, 14097. [Google Scholar] [CrossRef]
- Deng, S.; Wei, H.; Chen, T.; Wang, B.; Huang, J.; Yu, G. Superior CO2 Adsorption on Pine Nut Shell-Derived Activated Carbons and the Effective Micropores at Different Temperatures. Chem. Eng. J. 2014, 253, 46–54. [Google Scholar] [CrossRef]
- Durán, I.; Rubiera, F.; Pevida, C.; Weihs, G.F. Separation of CO2 in a Solid Waste Management Incineration Facility Using Activated Carbon Derived from Pine Sawdust. Energies 2017, 10, 827. [Google Scholar] [CrossRef]
- González, A.S.; Plaza, M.G.; Rubiera, F.; Pevida, C. Sustainable Biomass-Based Carbon Adsorbents for Post-Combustion CO2 Capture. Chem. Eng. J. 2013, 230, 456–465. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of Mixed-Gas Adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Cessford, N.F.; Seaton, N.A.; Düren, T. Evaluation of Ideal Adsorbed Solution Theory as a Tool for the Design of Metal−Organic Framework Materials. Ind. Eng. Chem. Res. 2012, 51, 4911–4921. [Google Scholar] [CrossRef]
- Shao, L.; Wan, H.; Wang, L.; Wang, J.; Liu, Z.; Wu, Z.; Zhan, P.; Zhang, L.; Ma, X.; Huang, J. N-Doped Highly Microporous Carbon Derived from the Self-Assembled Lignin/Chitosan Composites Beads for Selective CO2 Capture and Efficient p-Nitrophenol Adsorption. Sep. Purif. Technol. 2023, 313, 123440. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Kilic, M.; Altay, B.N.; Hu, X.; Wang, L. N-Doped Porous Carbon Derived from Macadamia Nut Shell for CO2 Adsorption. Fuel Process. Technol. 2023, 249, 107854. [Google Scholar] [CrossRef]
- Yang, L.; Guo, M.; Qian, Y.; Xu, D.; Gholizadeh, M.; Karnowo; Zhang, H.; Hu, X.; Zhang, S. The Effects of Interactions between Fiberboard-Derived Volatiles and Glucose-Derived Biochar on N Retention and Char Structure during the Decoupled Pyrolysis of Fiberboard and Glucose Using a Double-Bed Reactor. Renew. Energy 2022, 191, 134–140. [Google Scholar] [CrossRef]
- Li, D.; Tian, Y.; Li, L.; Li, J.; Zhang, H. Production of Highly Microporous Carbons with Large CO2 Uptakes at Atmospheric Pressure by KOH Activation of Peanut Shell Char. J. Porous Mater. 2015, 22, 1581–1588. [Google Scholar] [CrossRef]
- Li, D.; Ma, T.; Zhang, R.; Tian, Y.; Qiao, Y. Preparation of Porous Carbons with High Low-Pressure CO2 Uptake by KOH Activation of Rice Husk Char. Fuel 2015, 139, 68–70. [Google Scholar]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular Bamboo-Derived Activated Carbon for High CO2 Adsorption: The Dominant Role of Narrow Micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, X.; Chen, Y.; Huang, J.; Luo, H.; Deng, S. Adsorption of CO2, CH4, and N2 on Ordered Mesoporous Carbon: Approach for Greenhouse Gases Capture and Biogas Upgrading. Environ. Sci. Technol. 2013, 47, 5474–5480. [Google Scholar] [CrossRef]
- Mahurin, S.M.; Górka, J.; Nelson, K.M.; Mayes, R.T.; Dai, S. Enhanced CO2/N2 Selectivity in Amidoxime-Modified Porous Carbon. Carbon 2014, 67, 457–464. [Google Scholar] [CrossRef]
- Guo, L.P.; Hu, Q.T.; Zhang, P.; Li, W.C.; Lu, A.H. Polyacrylonitrile-Derived Sponge-Like Micro/Macroporous Carbon for Selective CO2 Separation. Chem.—Eur. J. 2018, 24, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- Selmert, V.; Kretzschmar, A.; Weinrich, H.; Tempel, H.; Kungl, H.; Eichel, R.A. CO2/N2 Separation on Highly Selective Carbon Nanofibers Investigated by Dynamic Gas Adsorption. ChemSusChem 2022, 15, e202200761. [Google Scholar] [CrossRef]
- Ojeda-López, R.; Vilarrasa-García, E.; Azevedo, D.C.S.; Felipe, C.; Cecilia, J.A.; Rodríguez-Castellón, E. CO2 Selectivity in CO2:CH4 and CO2:N2 Mixtures on Carbon Microfibers (CMFs) and Carbon Microspheres (CMSs). Fuel 2022, 324, 124242. [Google Scholar] [CrossRef]
- Hong, L.; Ju, S.; Liu, X.; Zhuang, Q.; Zhan, G.; Yu, X. Highly Selective CO2 Uptake in Novel Fishnet-like Polybenzoxazine-Based Porous Carbon. Energy Fuels 2019, 33, 11454–11464. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Three-Dimensional Graphene-Based Porous Adsorbents for Postcombustion CO2 Capture. Ind. Eng. Chem. Res. 2016, 55, 7906–7916. [Google Scholar] [CrossRef]
- Yang, M.; Guo, L.; Hu, G.; Hu, X.; Chen, J.; Shen, S.; Dai, W.; Fan, M. Adsorption of CO2 by Petroleum Coke Nitrogen-Doped Porous Carbons Synthesized by Combining Ammoxidation with KOH Activation. Ind. Eng. Chem. Res. 2016, 55, 757–765. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Rashidi, A.; Ghoreyshi, A.A. Evaluation of CO2 Adsorption with Eucalyptus Wood Based Activated Carbon Modified by Ammonia Solution through Heat Treatment. Chem. Eng. J. 2014, 254, 503–513. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated Carbons Prepared from Hydrothermally Carbonized Waste Biomass Used as Adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]




| Designation | PAN Mn (kDa) a | PMMA Mn (kDa) a | Molar Composition (AN/MMA) a | φPAN b |
|---|---|---|---|---|
| PAN | 1.3 | - | 100/0 | 1 |
| BC10/90 | 1.3 | 23.0 | 10/90 | 0.10 |
| BC26/74 | 1.3 | 7.2 | 26/74 | 0.26 |
| BC43/57 | 1.3 | 3.3 | 43/57 | 0.44 |
| Carbon | Char Yield | SN2 a | VT b | VMES c | VMIC d | Microporosity | DP e | SCO2 a | Uptake f |
|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | (m2 g−1) | (cm3 g−1) | (cm3 g−1) | (cm3 g−1) | (%) | (nm) | (m2 g−1) | (mmol g−1) | |
| BC10/90-O | 5.9 | 18.3 ± 0.3 | 0.0089 | 0.0007 | 0.0082 | 92.1 | 2.0 | 222.7 ± 2.3 | 1.3 |
| BC26/74-O | 16 | 12.3 ± 0.1 | 0.0091 | 0.0059 | 0.0032 | 35.2 | 3.0 | 181.7 ± 2.2 | 1.0 |
| BC43/57-O | 24 | 1.5 ± 0.1 | 0.0029 | 0.0029 | 0.0000 | 0.0 | 7.9 | 77.6 ± 1.1 | 0.3 |
| BC10/90-S | 6.2 | 371.6 ± 2.2 | 0.7411 | 0.7023 | 0.0388 | 5.2 | 8.0 | 190.8 ± 3.2 | 1.1 |
| BC26/74-S | 15 | 368.6 ± 4.8 | 0.2527 | 0.1227 | 0.1300 | 51.4 | 2.7 | 249.9 ± 4.4 | 1.4 |
| Carbon Series | C (wt.%; Bond Types) | N (wt.%; Bond Types) | O (wt.%) | S (wt.%; Bond Types) |
|---|---|---|---|---|
| BC-O | 81; C=C, C-H, C-O, C=O | 13; pyridinic, pyrrolic | 6 | - |
| BC-S | 80; C=C, C-H, C-S | 12; pyridinic, pyrrolic | 5 | 3; C-S-C, C-S(O)2-C |
| Adsorbent (Precursor) a | Conditions b T(K)/t(min) | CO2 Uptake (mmol g−1) | CO2/N2 Selectivity | Refs. |
|---|---|---|---|---|
| AC (peanut shell) | C-823/30 A-953/90/KOH | 7.3 | 7.9 | [48] |
| AC (rice husk) | C-793/20 A-1053/60/KOH | 6.2 | 19.9 | [49] |
| AC (bamboo) | C-773/90 A-873/90/KOH | 7.0 | 10.2 | [50] |
| OMC (Pluronic F127) | C-623/300 | 3.0 | 12.8 | [51] |
| OMC (N-dopped Pluronic F127) | C-673/120 A-1123/120/KOH | 4.9 | 14.4 | [52] |
| GO (N-dopped) | C-1073/120 | 6.5 | 454 | [53] |
| CNF (PAN) | C-873/180 | 2.3 | 95 | [54] |
| CMF (PAN) | C-1173/90 | 3.4 | 18 | [55] |
| AC (PBZC) | C-773/60 A-873/60/KOH | 6.67 | 35 | [56] |
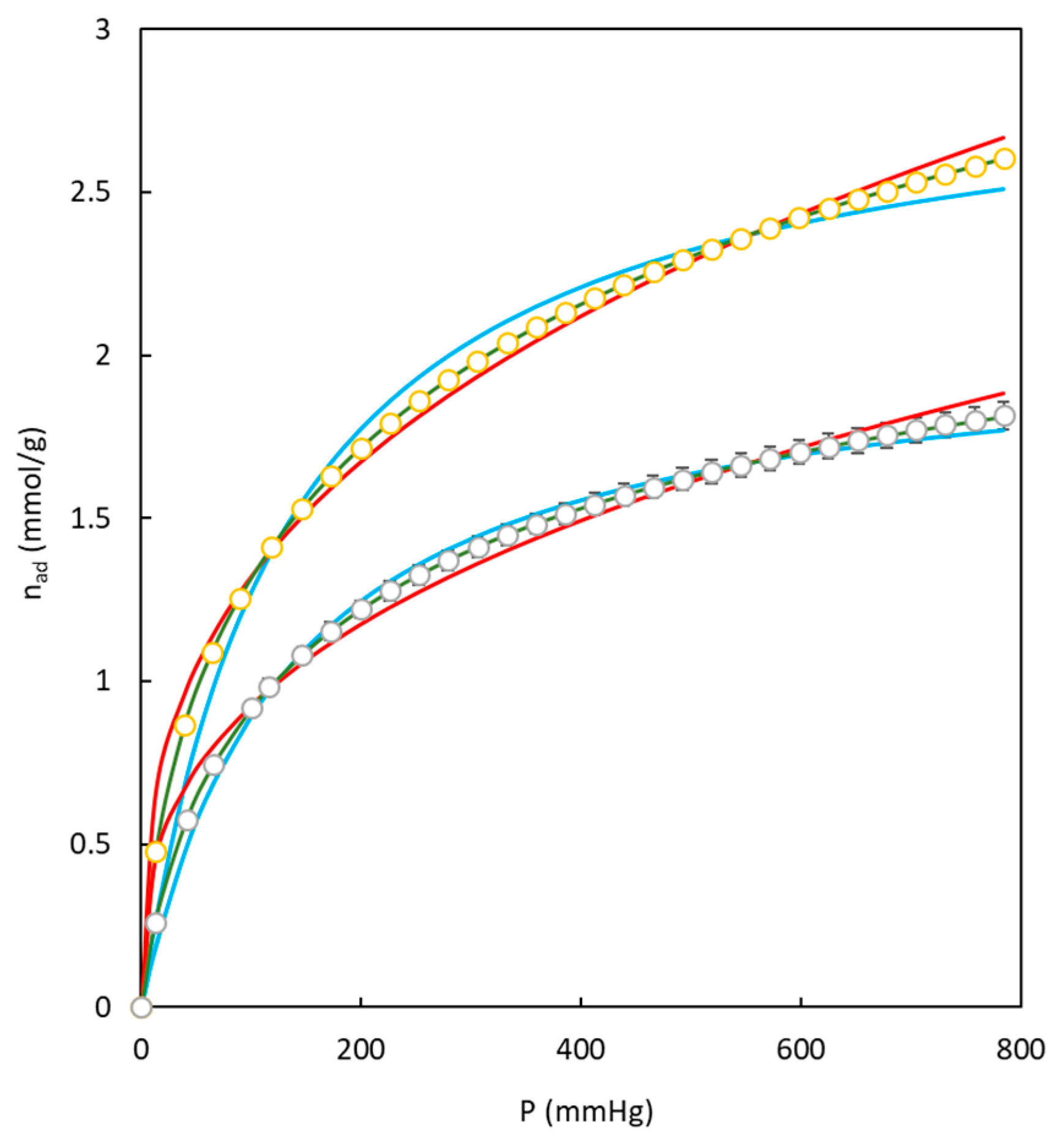
| Langmuir | Freundlich | Toth | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbon | T (K) | nm,L | KL | SSEL | nm,F | KF | SSEF | nm,T | KT | t | SSEL |
| BC10/90-O | 273 | 2.485 | 0.0095 | 0.081 | 3.211 | 0.2926 | 0.123 | 3.586 | 0.0228 | 0.48 | 0.0002 |
| BC10/90-O | 298 | 2.078 | 0.0047 | 0.022 | 2.328 | 0.0992 | 0.041 | 3.306 | 0.0063 | 0.53 | 0.0003 |
| BC10/90-O | 323 | 1.899 | 0.0023 | 0.003 | 1.762 | 0.0291 | 0.018 | 2.791 | 0.0021 | 0.67 | 0.0002 |
| BC26/74-O | 273 | 2.067 | 0.0761 | 0.033 | 2.903 | 0.1895 | 0.093 | 2.810 | 0.0132 | 0.55 | 0.0002 |
| BC26/74-O | 298 | 1.971 | 0.0049 | 0.017 | 2.387 | 0.1018 | 0.044 | 2.891 | 0.0065 | 0.57 | 0.0003 |
| BC26/74-O | 323 | 1.737 | 0.0030 | 0.006 | 1.963 | 0.0433 | 0.020 | 2.754 | 0.0031 | 0.59 | 0.0001 |
| BC43/57-O | 273 | 0.852 | 0.0074 | 0.011 | 2.882 | 0.0765 | 0.009 | 1.505 | 0.0193 | 0.41 | 0.0001 |
| BC43/57-O | 298 | 0.779 | 0.0059 | 0.014 | 2.579 | 0.0514 | 0.007 | 2.110 | 0.0175 | 0.32 | 0.0019 |
| BC43/57-O | 323 | 0.756 | 0.0025 | 0.004 | 1.822 | 0.0136 | 0.002 | 4.742 | 0.0018 | 0.29 | 0.0007 |
| BC10/90-S | 273 | 2.276 | 0.0071 | 0.099 | 2.839 | 0.1958 | 0.042 | 5.262 | 0.0262 | 0.33 | 0.0004 |
| BC10/90-S | 298 | 1.927 | 0.0073 | 0.029 | 2.272 | 0.0838 | 0.018 | 4.622 | 0.0066 | 0.39 | 0.0001 |
| BC10/90-S | 323 | 1.569 | 0.0026 | 0.006 | 1.847 | 0.0299 | 0.010 | 3.187 | 0.0023 | 0.51 | 4 × 10−5 |
| BC26/74-S | 273 | 2.922 | 0.0077 | 0.170 | 2.935 | 0.2754 | 0.080 | 6.194 | 0.0289 | 0.34 | 8 × 10−6 |
| BC26/74-S | 298 | 2.95 | 0.0075 | 0.147 | 2.878 | 0.266 | 0.077 | 6.161 | 0.0252 | 0.35 | 7 × 10−6 |
| BC26/74-S | 323 | 2.005 | 0.0027 | 0.014 | 1.891 | 0.0423 | 0.012 | 5.253 | 0.0025 | 0.43 | 4 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Díaz, D.; Domínguez-Ramos, L.; Malucelli, G.; Freire, M.S.; González-Álvarez, J.; Lazzari, M. S/N/O-Enriched Carbons from Polyacrylonitrile-Based Block Copolymers for Selective Separation of Gas Streams. Polymers 2024, 16, 269. https://doi.org/10.3390/polym16020269
Gómez-Díaz D, Domínguez-Ramos L, Malucelli G, Freire MS, González-Álvarez J, Lazzari M. S/N/O-Enriched Carbons from Polyacrylonitrile-Based Block Copolymers for Selective Separation of Gas Streams. Polymers. 2024; 16(2):269. https://doi.org/10.3390/polym16020269
Chicago/Turabian StyleGómez-Díaz, Diego, Lidia Domínguez-Ramos, Giulio Malucelli, María Sonia Freire, Julia González-Álvarez, and Massimo Lazzari. 2024. "S/N/O-Enriched Carbons from Polyacrylonitrile-Based Block Copolymers for Selective Separation of Gas Streams" Polymers 16, no. 2: 269. https://doi.org/10.3390/polym16020269
APA StyleGómez-Díaz, D., Domínguez-Ramos, L., Malucelli, G., Freire, M. S., González-Álvarez, J., & Lazzari, M. (2024). S/N/O-Enriched Carbons from Polyacrylonitrile-Based Block Copolymers for Selective Separation of Gas Streams. Polymers, 16(2), 269. https://doi.org/10.3390/polym16020269











