Production of Lignin-Derived Functional Material for Efficient Electromagnetic Wave Absorption with an Ultralow Filler Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
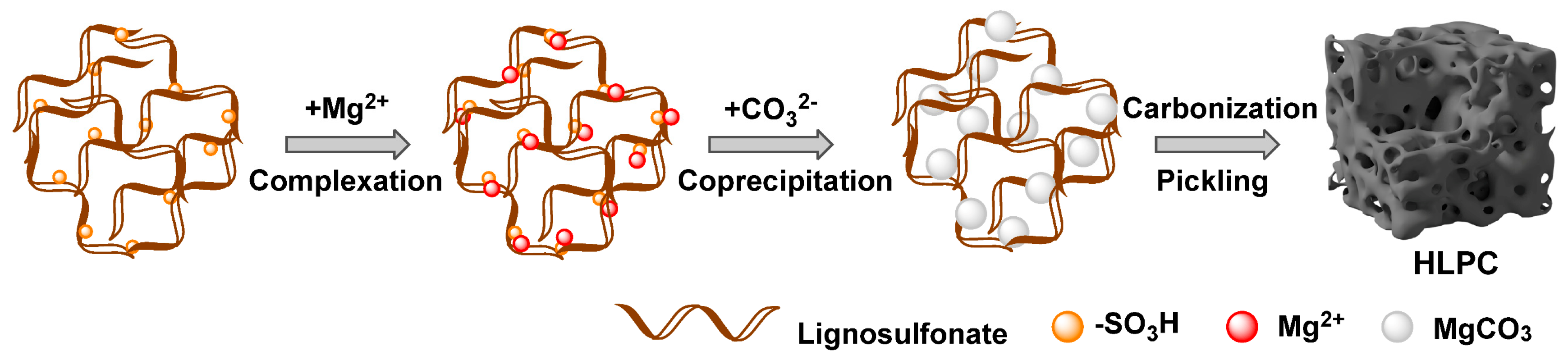
2.2. Preparation of Precursor
2.3. Preparation of LC, LPC and HLPC
2.4. Characterization
2.5. Electromagnetic Absorption Effectiveness
3. Results and Discussion
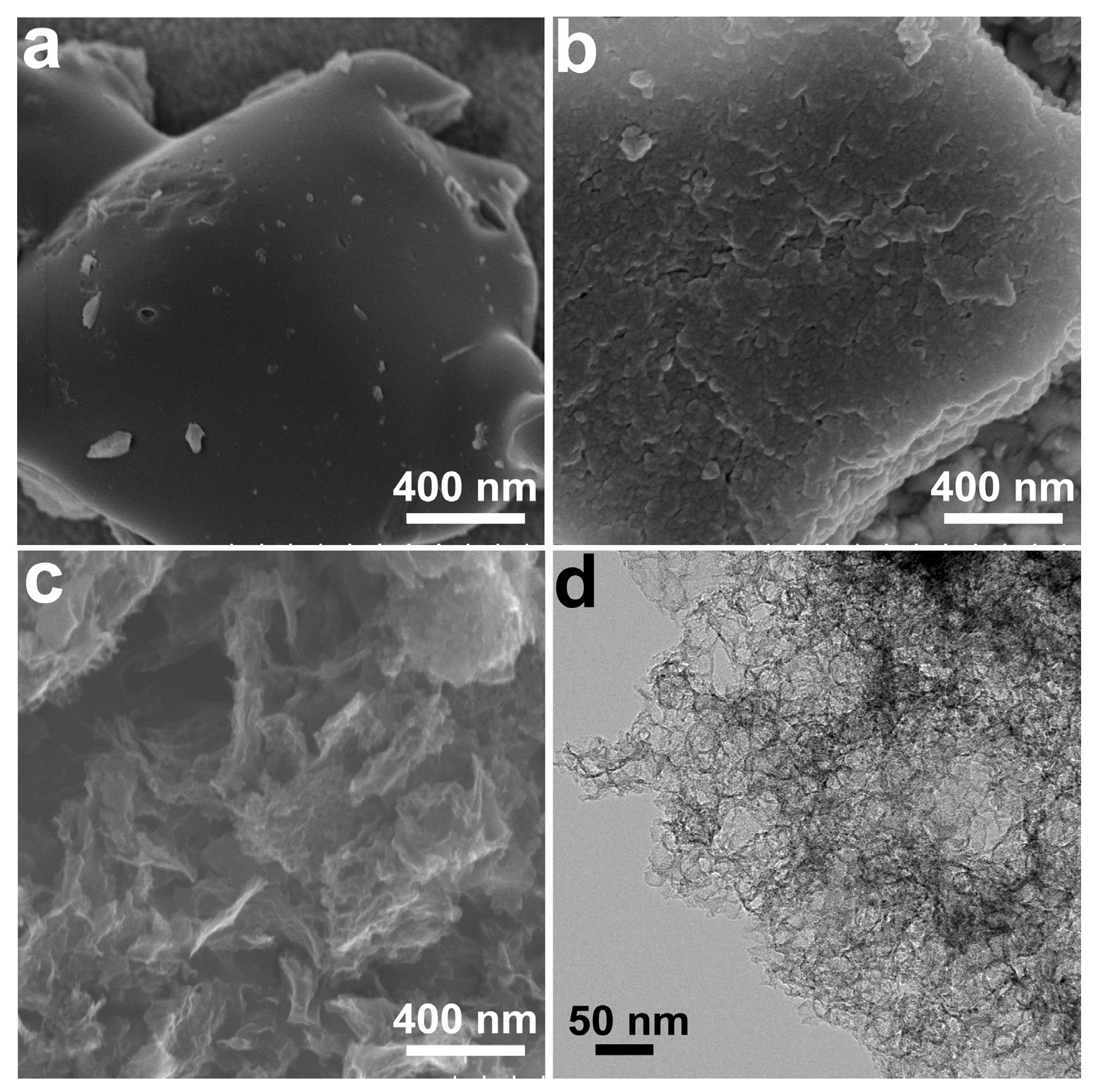
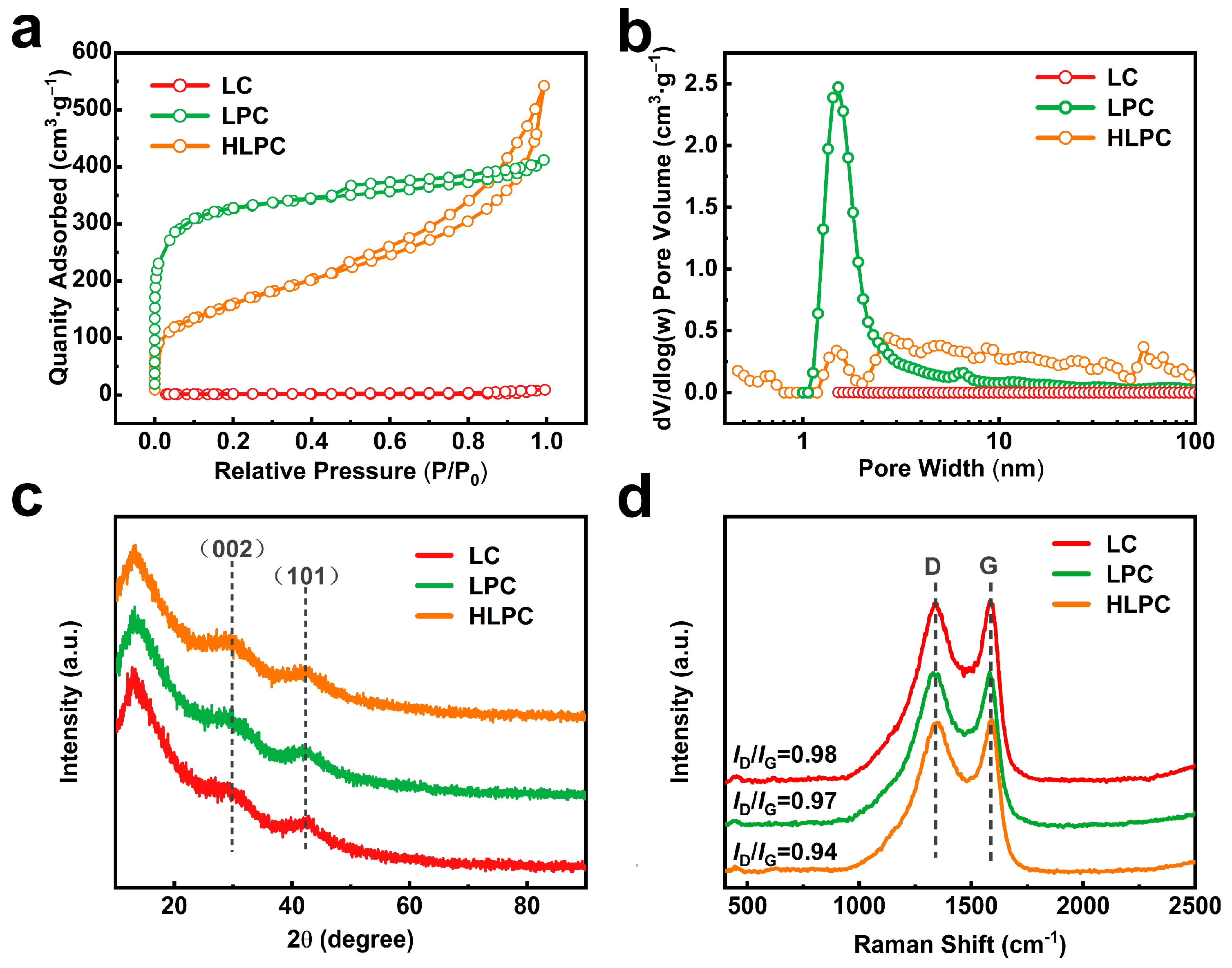
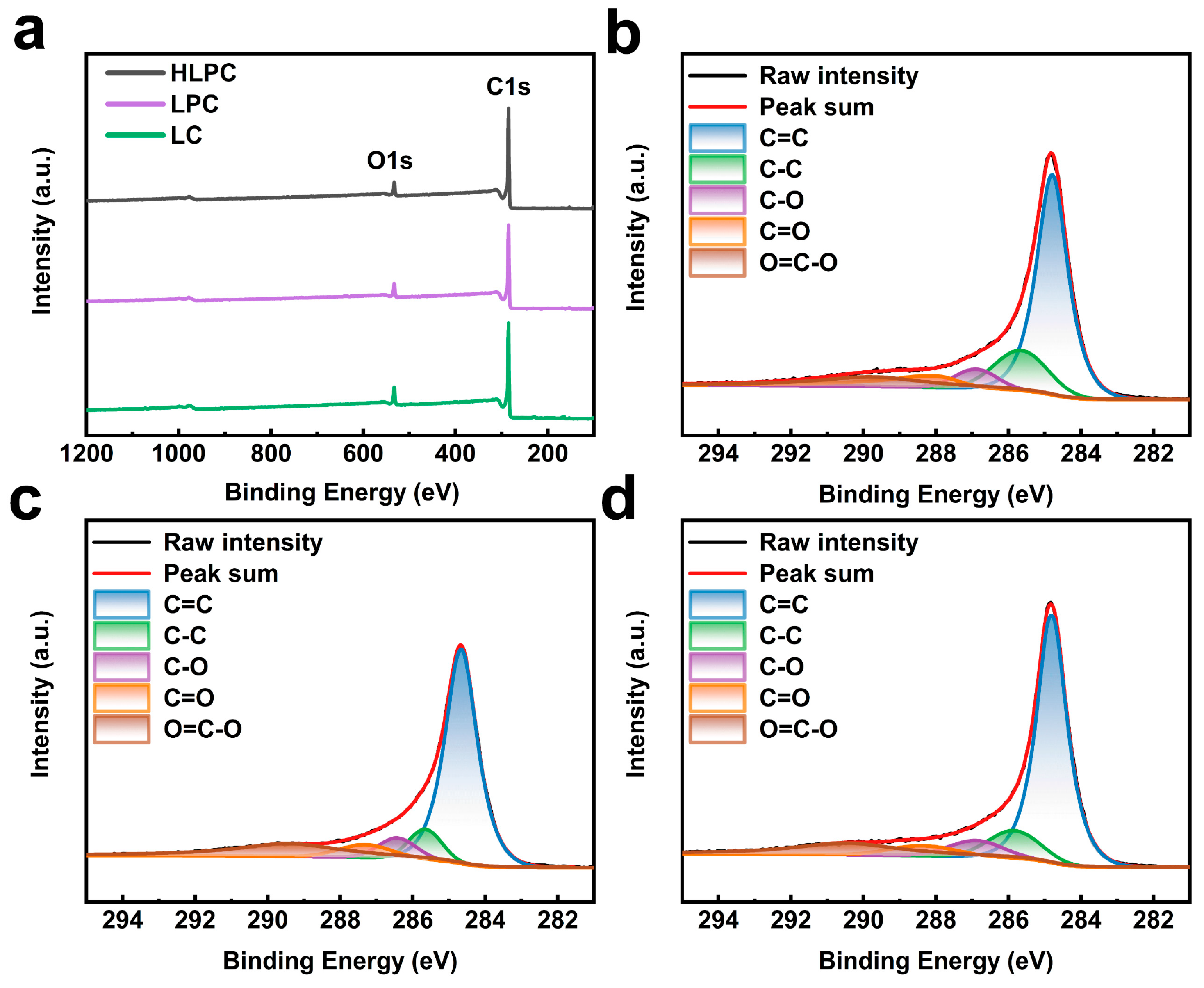
3.1. Synthesis and Characterization of HLPC
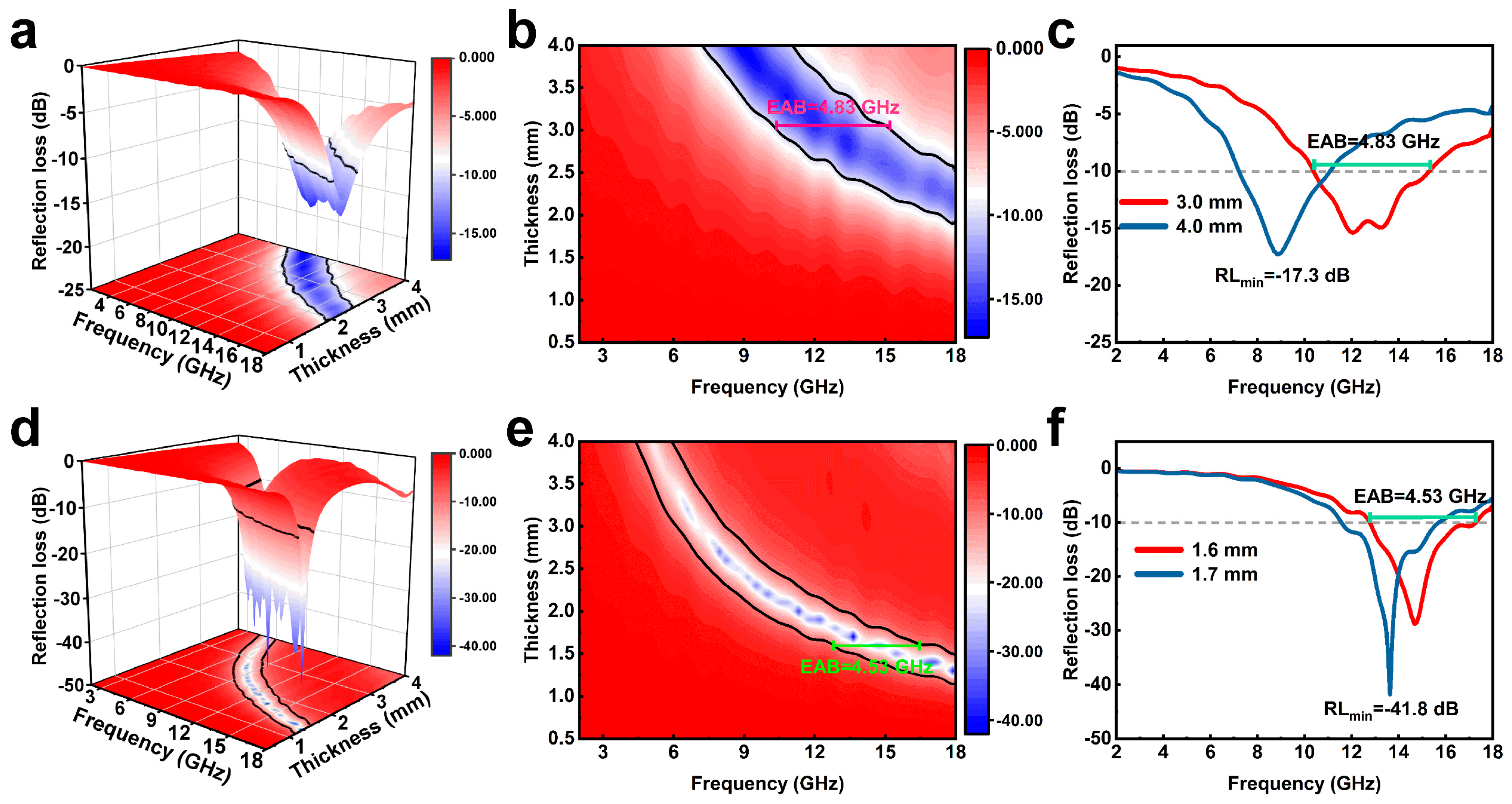
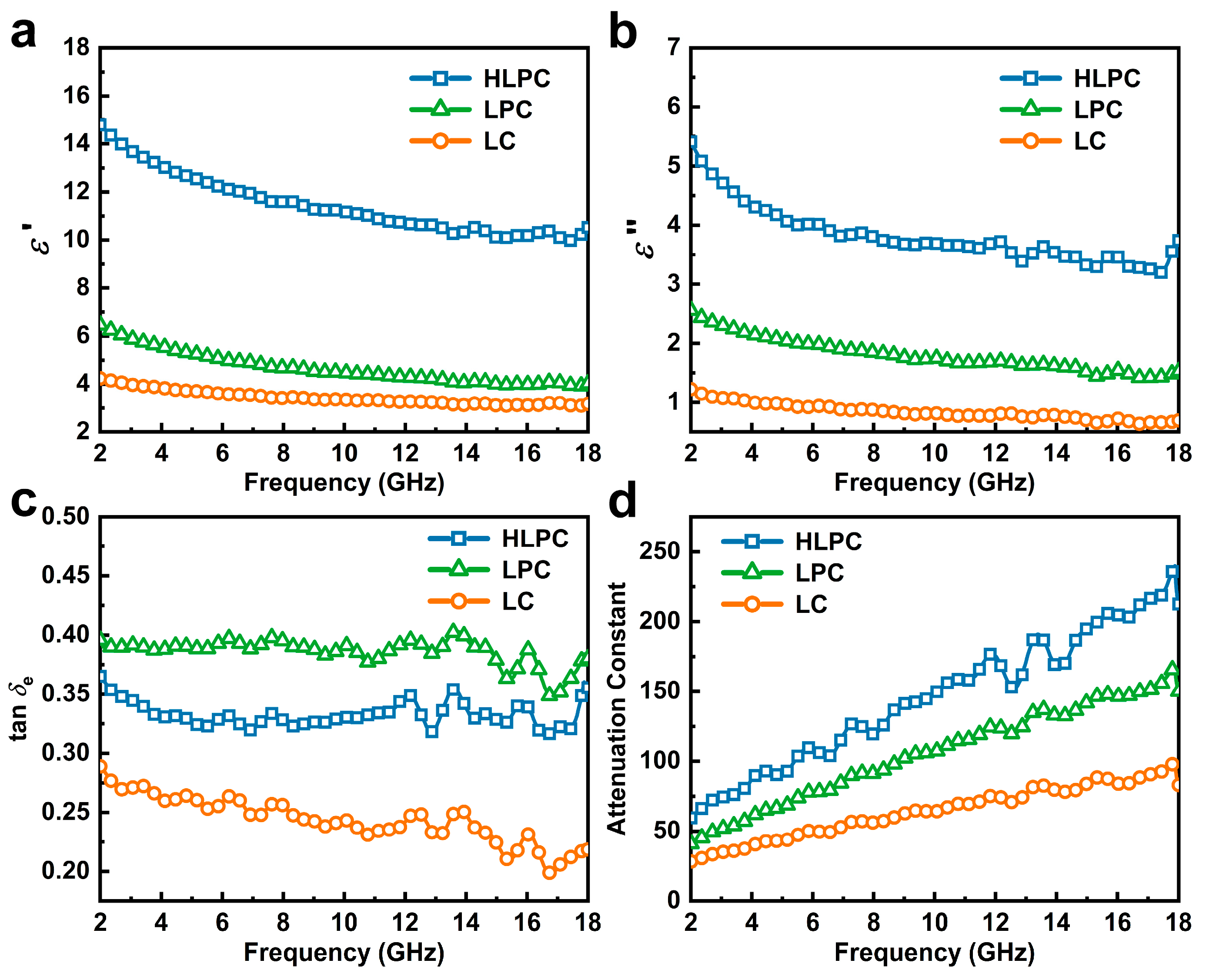
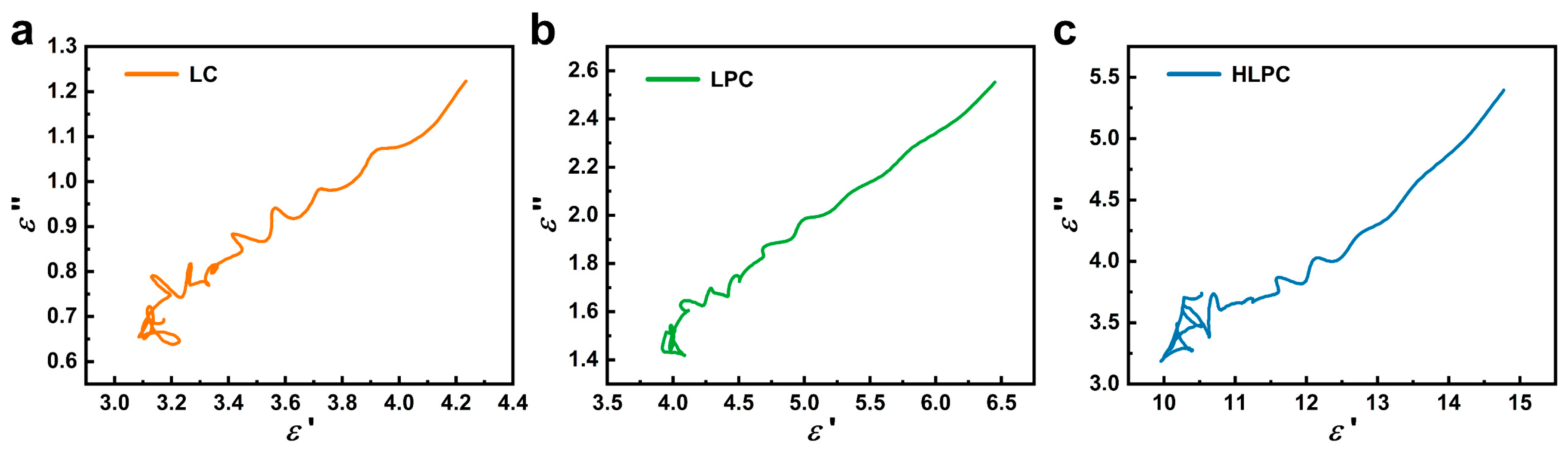
3.2. EMW Absorption Performance of LC, LPC and HLPC
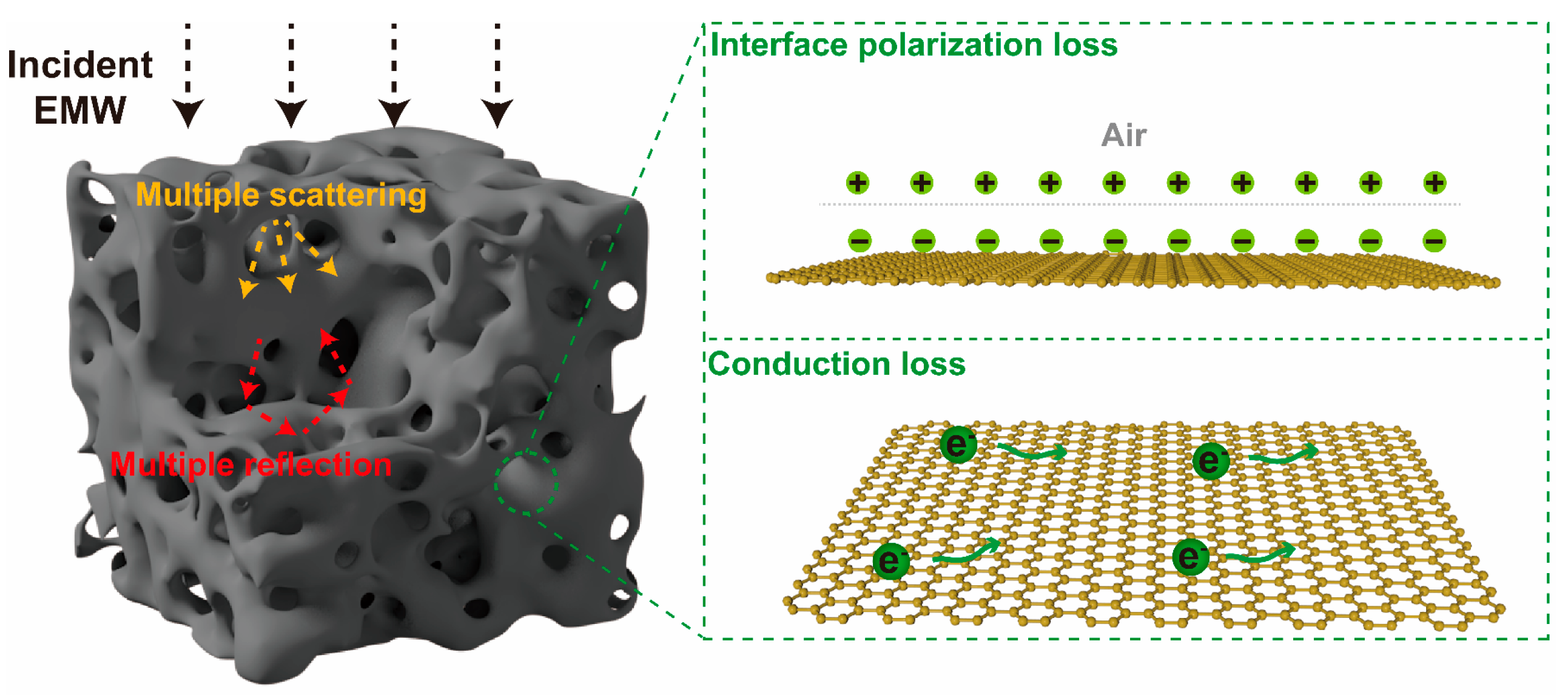
3.3. EMW Absorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, M.-S.; Wang, X.-X.; Zhang, M.; Shu, J.-C.; Cao, W.-Q.; Yang, H.-J.; Fang, X.-Y.; Yuan, J. Electromagnetic response and energy conversion for functions and devices in low-dimensional materials. Adv. Funct. Mater. 2019, 29, 1807398. [Google Scholar] [CrossRef]
- He, P.; Cao, M.-S.; Cao, W.-Q.; Yuan, J. Developing MXenes from wireless communication to electromagnetic attenuation. Nano-Micro Lett. 2021, 13, 115. [Google Scholar] [CrossRef]
- Guan, X.; Yang, Z.; Zhou, M.; Yang, L.; Peymanfar, R.; Aslibeiki, B.; Ji, G. 2D MXene nanomaterials: Synthesis, mechanism, and multifunctional applications in microwave absorption. Small Struct. 2022, 3, 2200102. [Google Scholar] [CrossRef]
- Lv, H.; Yang, Z.; Ong, S.J.H.; Wei, C.; Liao, H.; Xi, S.; Du, Y.; Ji, G.; Xu, Z.J. A flexible microwave shield with tunable frequency-transmission and electromagnetic compatibility. Adv. Funct. Mater. 2019, 29, 1900163. [Google Scholar] [CrossRef]
- Green, M.; Chen, X. Recent progress of nanomaterials for microwave absorption. J. Mater. 2019, 5, 503–541. [Google Scholar] [CrossRef]
- Zhao, T.; Jia, Z.; Zhang, Y.; Wu, G. Multiphase molybdenum carbide doped carbon hollow sphere engineering: The superiority of unique double-shell structure in microwave absorption. Small 2023, 19, 2206323. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yang, Z.; Wang, P.L.; Ji, G.; Song, J.; Zheng, L.; Zeng, H.; Xu, Z.J. A voltage-boosting strategy enabling a low-frequency, flexible electromagnetic wave absorption device. Adv. Mater. 2018, 30, 1706343. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.; Lu, M.; Cao, W.; Shi, H.; Liu, J.; Wang, X.; Jin, H.; Fang, X.; Wang, W.; et al. Reduced graphene oxides: Light-weight and high-efficiency electromagnetic interference shielding at elevated temperatures. Adv. Mater. 2014, 26, 3484–3489. [Google Scholar] [CrossRef]
- Fu, L.S.; Jiang, J.T.; Xu, C.Y.; Zhen, L. Synthesis of hexagonal Fe microflakes with excellent microwave absorption performance. Crystengcomm 2012, 14, 6827–6832. [Google Scholar] [CrossRef]
- Wen, S.L.; Liu, Y.; Zhao, X.C. Facile chemical synthesis, electromagnetic response, and enhanced microwave absorption of cobalt powders with controllable morphologies. J. Chem. Phys. 2015, 143, 084707. [Google Scholar] [CrossRef]
- Ye, F.; Song, Q.; Zhang, Z.; Li, W.; Zhang, S.; Yin, X.; Zhou, Y.; Tao, H.; Liu, Y.; Cheng, L.; et al. Direct growth of edge-rich graphene with tunable dielectric properties in porous Si3N4 ceramic for broadband high-performance microwave absorption. Adv. Funct. Mater. 2018, 28, 1707205. [Google Scholar] [CrossRef]
- Xie, A.; Wang, J.; Zhang, C.; Cheng, S. Single crystal to polycrystal: Enhanced dielectric loss and electromagnetic wave absorption of MoO2 ceramic at Gigahertz. Ceram. Int. 2022, 48, 29715–29721. [Google Scholar] [CrossRef]
- Xu, H.; Yin, X.; Zhu, M.; Li, M.; Zhang, H.; Wei, H.; Zhang, L.; Cheng, L. Constructing hollow graphene nano-spheres confined in porous amorphous carbon particles for achieving full X band microwave absorption. Carbon 2019, 142, 346–353. [Google Scholar] [CrossRef]
- Qiang, R.; Du, Y.; Wang, Y.; Wang, N.; Tian, C.; Ma, J.; Xu, P.; Han, X. Rational design of yolk-shell C@C microspheres for the effective enhancement in microwave absorption. Carbon 2016, 98, 599–606. [Google Scholar] [CrossRef]
- Tian, C.; Du, Y.; Xu, P.; Qiang, R.; Wang, Y.; Ding, D.; Xue, J.; Ma, J.; Zhao, H.; Han, X. Constructing uniform core–shell PPy@PANI composites with tunable shell thickness toward enhancement in microwave absorption. ACS Appl. Mater. Inter. 2015, 7, 20090–20099. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, Z.; Wang, Z.; Peng, Y.; Xia, L.; Ma, S.; Yin, Z.; Huang, Y. A review on metal–organic framework-derived porous carbon-based novel microwave absorption materials. Nano-Micro Lett. 2021, 13, 56. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Huang, Y.; Cheng, Y.; Wang, S.; Yin, W. Achieving super broadband electromagnetic absorption by optimizing impedance match of rGO sponge metamaterials. Adv. Funct. Mater. 2022, 32, 2107508. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Qi, L.P.; Liao, Q.L.; Kang, Z.; Zhang, Y. Toward the application of high frequency electromagnetic wave absorption by carbon nanostructures. Adv. Sci. 2019, 6, 1801057. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, B.; Che, S.; Yan, L.; Li, Z.-x.; Li, Y.-f. Research progress on carbon-based materials for electromagnetic wave absorption and the related mechanisms. New Carbon Mater. 2021, 36, 1016–1030. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Liu, W.; Yang, L.; Zhang, B.; Wang, L.P.; Ji, G.; Xu, Z.J. Biomass-derived porous carbon-based nanostructures for microwave absorption. Nano-Micro Lett. 2019, 11, 24. [Google Scholar] [CrossRef]
- Cheng, J.B.; Shi, H.G.; Cao, M.; Wang, T.; Wang, Y.Z. Porous carbon materials for microwave absorption. Mater. Adv. 2020, 1, 2631–2645. [Google Scholar] [CrossRef]
- Feng, P.; Wang, H.; Huang, P.; Zhong, L.; Gan, S.; Wang, W.; Niu, L. Nitrogen-doped lignin-derived porous carbons for supercapacitors: Effect of nanoporous structure. Chem. Eng. J. 2023, 471, 144817. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Q.; Kara, U.I.; Mamtani, R.S.; Zhou, X.; Bian, H.; Yang, Z.; Li, Y.; Lv, H.; Adera, S.; et al. Biomass-Derived Carbon Heterostructures Enable Environmentally Adaptive Wideband Electromagnetic Wave Absorbers. Nano-Micro Lett. 2021, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Prakash, H.; Akhtar, M.J.; Kar, K.K. Lightweight and high-performance microwave absorbing heteroatom-doped carbon derived from chicken feather fibers. ACS Sustain. Chem. Eng. 2018, 6, 5381–5393. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, B.; Wang, Q.; Zhao, X.; Wu, D.; Wu, H.; Sun, X.; Hou, C.; Yang, X.; Yu, R.; et al. Efficient microwave absorber and supercapacitors derived from puffed-rice-based biomass carbon: Effects of activating temperature. J. Colloid Interface Sci. 2021, 594, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ren, P.; Zhang, F.; Duan, H.; Chen, Z.; Jin, Y.; Ren, F.; Li, Z. Magnetic coupling N self-doped porous carbon derived from biomass with broad absorption bandwidth and high-efficiency microwave absorption. J. Colloid Interface Sci. 2022, 610, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Dai, Z.; Feng, M.; Chen, X.; Sun, C.; Xu, Y. Hierarchically porous carbon derived from natural Porphyra for excellent electromagnetic wave absorption. J. Mater. Sci. Technol. 2022, 129, 206–214. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Hu, H.; Zhang, T.; Yuan, Y.; Li, Y.; He, X. Lightweight and efficient microwave-absorbing materials based on loofah-sponge-derived hierarchically porous carbons. ACS Sustain. Chem. Eng. 2019, 7, 1228–1238. [Google Scholar] [CrossRef]
- Wang, H.; Feng, P.; Fu, F.; Yu, X.; Yang, D.; Zhang, W.; Niu, L.; Qiu, X. Lignin-derived carbon materials for catalysis and electrochemical energy storage. Carbon Neutralization 2022, 1, 277–297. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Feng, P.; Huang, P.; Huang, M.; Lin, X.; Feng, Y.; Gan, S.; Han, D.; Wang, W.; et al. Solvent-induced molecular structure engineering of lignin for hierarchically porous carbon: Mechanisms and supercapacitive properties. Ind. Crops Prod. 2022, 189, 115831. [Google Scholar] [CrossRef]
- Xu, H.; Li, B.; Jiang, X.; Shi, Y.; Zhang, X.; Zhu, C.; Zhang, X.; Chen, Y. Fabrication of N−doped carbon nanotube/carbon fiber dendritic composites with abundant interfaces for electromagnetic wave absorption. Carbon 2023, 201, 234–243. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Guo, Z.; Liu, H.; Zhao, L.; Qian, L. A synergistic route of heterointerface and metal single-atom configurations towards enhancing microwave absorption. Chem. Eng. J. 2023, 452, 139430. [Google Scholar] [CrossRef]
- Yang, B.T.; Fang, J.F.; Xu, C.Y.; Cao, H.; Zhang, R.X.; Zhao, B.A.; Huang, M.Q.; Wang, X.Y.; Lv, H.L.; Che, R.C. One-dimensional magnetic FeCoNi alloy toward low-frequency electromagnetic wave absorption. Nano-Micro Lett. 2022, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.C.; Cheng, H.W.; Jin, C.; Yang, B.T.; Xu, C.Y.; Pei, K.; Zhang, H.B.; Yang, Z.Q.; Che, R.C. Dimensional design and core-shell engineering of nanomaterials for electromagnetic wave absorption. Adv. Mater. 2022, 34, 2107538. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cao, Q.; Bi, H.; Liang, C.; Yuan, K.; She, W.; Yang, Y.; Che, R. CoNi@SiO2@TiO2 and CoNi@Air@TiO2 microspheres with strong wideband microwave absorption. Adv. Mater. 2016, 28, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zuo, X.; Guo, Y.; Huang, H.; Zhang, H.; Wang, T.; Wen, N.; Chen, H.; Cong, T.; Muhammad, J.; et al. Structural engineering of hierarchical aerogels comprised of multi-dimensional gradient carbon nanoarchitectures for highly efficient microwave absorption. Nano-Micro Lett. 2021, 13, 144. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of lignocellulosic fibers and lignin in bioplastics: A review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Wang, J.; Fan, D.; Zhang, L.; Yang, D.; Qiu, X.; Lin, X. Lignin-derived hierarchical porous carbon with high surface area and interconnected pores for efficient antibiotics adsorption. Chem. Eng. J. 2023, 454, 139789. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Wu, H.; Zhou, M.S.; Yang, D.J. Preparation of a novel high-performance lignin-based anionic adsorption resin for efficient removal of Cr(VI) in aqueous solutions. Ind. Crop. Prod. 2023, 199, 116720. [Google Scholar] [CrossRef]
- Liao, B.; Quan, R.; Feng, P.; Wang, H.; Wang, W.; Niu, L. Carbon steel anticorrosion performance and mechanism of sodium lignosulfonate. Rare Met. 2023, 43, 356–365. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, J.; Li, X.; Lin, Q.; Xiao, R.; Zhang, M.; Yin, G.; Zhou, Y. Effect of lignosulfonate on the adsorption performance of hematite for Cd(II). Sci. Total Environ. 2020, 738, 139952. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, W.; Qiu, X.; Fu, F.; Zhong, R.; Liu, W.; Yang, D. In situ synthesis of flowerlike lignin/ZnO composite with excellent UV-absorption properties and its application in polyurethane. ACS Sustain. Chem. Eng. 2018, 6, 3696–3705. [Google Scholar] [CrossRef]
| Sample | Purity (%) | Mw (g/mol) | Phenolic OH (mmol/g) | Carboxy (mmol/g) | Sulfonic Group (mmol/g) |
|---|---|---|---|---|---|
| LS | 95.1 | 19,400 | 1.87 | 1.52 | 1.57 |
| Sample | SBET (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Cmicro (%) | Vmeso (cm3/g) | Cmeso (%) |
|---|---|---|---|---|---|---|
| LC | 5.1 | 0.0140 | 0.0007 | 4.9 | 0.0040 | 31.6 |
| LPC | 1224.4 | 0.6362 | 0.3200 | 50.2 | 0.1751 | 28.9 |
| HLPC | 586.5 | 0.8145 | 0.0064 | 0.8 | 0.4006 | 71.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Y.; Ji, X.; Kong, F.; Li, T.; Zhang, B. Production of Lignin-Derived Functional Material for Efficient Electromagnetic Wave Absorption with an Ultralow Filler Ratio. Polymers 2024, 16, 201. https://doi.org/10.3390/polym16020201
Xi Y, Ji X, Kong F, Li T, Zhang B. Production of Lignin-Derived Functional Material for Efficient Electromagnetic Wave Absorption with an Ultralow Filler Ratio. Polymers. 2024; 16(2):201. https://doi.org/10.3390/polym16020201
Chicago/Turabian StyleXi, Yuebin, Xingxiang Ji, Fangong Kong, Tianjin Li, and Binpeng Zhang. 2024. "Production of Lignin-Derived Functional Material for Efficient Electromagnetic Wave Absorption with an Ultralow Filler Ratio" Polymers 16, no. 2: 201. https://doi.org/10.3390/polym16020201
APA StyleXi, Y., Ji, X., Kong, F., Li, T., & Zhang, B. (2024). Production of Lignin-Derived Functional Material for Efficient Electromagnetic Wave Absorption with an Ultralow Filler Ratio. Polymers, 16(2), 201. https://doi.org/10.3390/polym16020201






