Preparation of ZnO Nanosheet Array and Research on ZnO/PANI/ZnO Ultraviolet Photodetector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
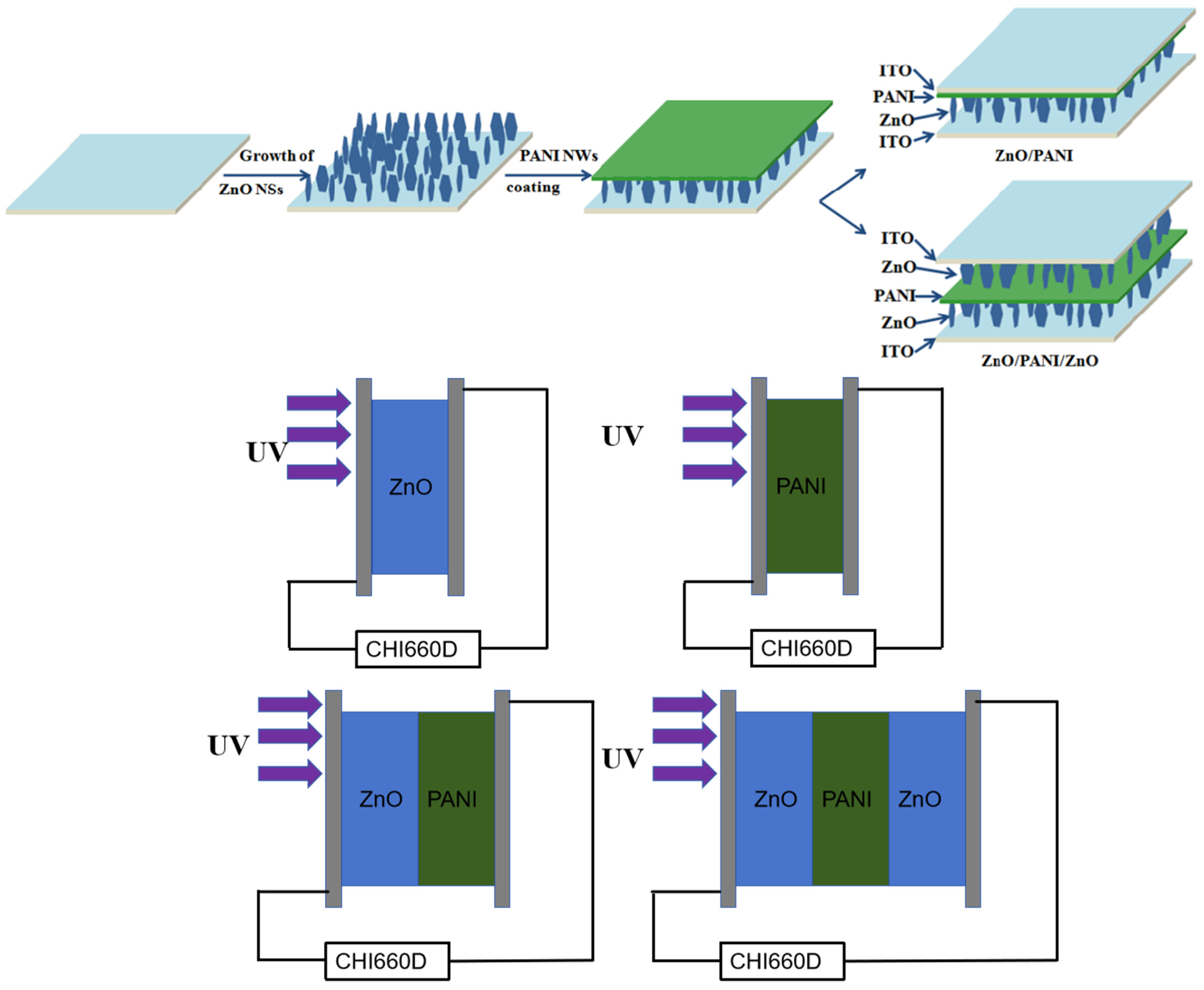
2.2. Preparation of ZnO NSs Array
2.3. Preparation of ZnO NSs/PANI Nano-Porous Film Heterostructure Arrays
2.4. Structural Characterizations of Materials
2.4.1. Field Emission Scanning Electron Microscope, FESEM
2.4.2. X-ray Powder Diffraction, XRD
2.4.3. UV–Vis Spectrophotometer
2.4.4. Current Time Curve
3. Results and Discussion
3.1. Control of Process Parameters
3.2. Characterization of ZnO Nanosheets and ZnO/PANI Heterostructures
3.3. Photoelectric Performance Testing of ZnO/PANI Heterostructures
3.4. Working Principle of ZnO/PANI/ZnO Sandwich-Structure Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, A.; Chaudhary, P.; Tripathi, K.; Singh, A.; Yadav, B.C. State of the Art Metallopolymer Based Functional Nanomaterial for Photodetector and Solar Cell Application. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2807–2826. [Google Scholar] [CrossRef]
- Wu, Y.T.; Zhang, K.; Li, G.W.; Wang, Q.S.; Fu, X.; Wang, L.; Luo, J.L.; Feng, S.L.; Tao, Z.Y.; Fan, Y.X.; et al. Ultrafast response solar-blind UV sensor based on ZnGa2O4 nanowire bridge arrays. Phys. E-Low-Dimens. Syst. Nanostruct. 2023, 146, 115505. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, F.; Zheng, R.S.; Wu, H.L. Low-Dimensional Structure Vacuum-Ultraviolet-Sensitive (lambda < 200 nm) Photodetector with Fast-Response Speed Based on High-Quality AlN Micro/Nanowire. Adv. Mater. 2015, 27, 3921–3927. [Google Scholar] [CrossRef] [PubMed]
- Song, W.D.; Chen, J.X.; Li, Z.L.; Fang, X.S. Self-Powered MXene/GaN van der Waals Heterojunction Ultraviolet Photodiodes with Superhigh Efficiency and Stable Current Outputs. Adv. Mater. 2021, 33, 2101059. [Google Scholar] [CrossRef]
- Zhou, S.R.; Zheng, Q.Q.; Yu, C.X.; Huang, Z.H.; Chen, L.R.; Zhang, H.; Li, H.L.; Xiong, Y.Q.; Kong, C.Y.; Ye, L.J.; et al. A High-Performance epsilon-Ga2O3-Based Deep-Ultraviolet Photodetector Array for Solar-Blind Imaging. Materials 2023, 16, 295. [Google Scholar] [CrossRef]
- Alaie, Z.; Nejad, S.M.; Yousefi, M.H. Recent advances in ultraviolet photodetectors. Mater. Sci. Semicond. Process. 2015, 29, 16–55. [Google Scholar] [CrossRef]
- Zhang, J.T.; Tang, K.; Wei, T.C.; Wan, P.; Shi, D.N.; Kan, C.X.; Jiang, M.M. High-photosensitive ultraviolet photodetector based on an n-ZnO microwire/p-InGaN heterojunction. Phys. E-Low-Dimens. Syst. Nanostruct. 2023, 146, 115562. [Google Scholar] [CrossRef]
- Dai, Y.J.; Wang, X.F.; Peng, W.B.; Xu, C.; Wu, C.S.; Dong, K.; Liu, R.Y.; Wang, Z.L. Self-Powered Si/CdS Flexible Photodetector with Broadband Response from 325 to 1550 nm Based on Pyro-phototronic Effect: An Approach for Photosensing below Bandgap Energy. Adv. Mater. 2018, 30, 1705893. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Jin, D.Y.; Tricoli, A. Nanoarchitechtonics of Visible-Blind Ultraviolet Photodetector Materials: Critical Features and Nano-Microfabrication. Adv. Opt. Mater. 2019, 7, 180058. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Hsiao, I.P.; Chang, F.Y.; Hsu, C.L. Improving the photoelectrical characteristics of self-powered p-GaN film/n-ZnO nanowires heterojunction ultraviolet photodetectors through gallium and indium co-doping. Mater. Sci. Semicond. Process. 2021, 121, 105295. [Google Scholar] [CrossRef]
- Mohamed, M.; Jayiz, M.; Alshammari, A.S.; Sedky, A.; Khan, Z.R. Comparative study on structural, morphological, optical and photocatalytic properties of Mn3O4/ZnO, CuO/ZnO and Fe2O3/ZnO nanocomposites. Opt. Quantum Electron. 2023, 55, 562. [Google Scholar] [CrossRef]
- Wang, J.F.; Wang, J.D.; Ding, J.; Wei, Y.J.; Zhang, J.B. Preparation of ZnO compact layer using vacuum ultraviolet for dye-sensitized solar cells. Solid State Sci. 2022, 127, 106860. [Google Scholar] [CrossRef]
- Wang, D.L.; Liu, M.S.; Shang, S.A.; Wan, P.; Shi, D.N.; Kan, C.X.; Li, B.H.; Jiang, M.M. An individual ZnO microwire homojunction LED with ultraviolet electroluminescence spectrally purified using Pt nanoparticles cladding. Opt. Laser Technol. 2023, 160, 109052. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Kheirabadi, M.; Ebrahimi, M.; Moshfegh, A.Z. Design and tailoring of one-dimensional ZnO nanomaterials for photocatalytic degradation of organic dyes: A review. Res. Chem. Intermed. 2019, 45, 2197–2254. [Google Scholar] [CrossRef]
- Krishna, M.S.; Singh, S. Selective edge hydrogenated armchair ZnO nanoribbons for negative differential resistance based nanoelectronic devices. Phys. E-Low-Dimens. Syst. Nanostruct. 2023, 147, 115570. [Google Scholar] [CrossRef]
- Ramos, P.G.; Sanchez, L.A.; Rodriguez, J.M. A review on improving the efficiency of photocatalytic water decontamination using ZnO nanorods. J. Sol-Gel Sci. Technol. 2022, 102, 105–124. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kawai, Y.; Arimoto, T.; Sasaki, T.; Ohashi, A.; Hara, K.; Kouno, T. Selectively enhanced crystal growth of periodically arrayed ZnO nanowires by mist chemical vapor deposition and electron beam lithography. J. Cryst. Growth 2023, 618, 127309. [Google Scholar] [CrossRef]
- Kem, A.; Pasupuleti, K.S.; Jayasimhadri, M.; Kim, M.D.; Peta, K.R. Core shell heterojunction interface in green synthesized Sm3+ ions doped ZnO nano-particles to promote the charge separation for efficient photocatalytic applications. J. Alloys Compd. 2023, 960, 170841. [Google Scholar] [CrossRef]
- Astuti; Arief, S.; Muldarisnur, M.; Zulhadjri; Usna, S.R.A. Enhancement in photoluminescence performance of carbon-based Fe3O4@ZnO-C nanocomposites. Vacuum 2023, 211, 111935. [Google Scholar] [CrossRef]
- Irtiqa, S.; Rahman, A. Enhanced Photocatalytic and Photoluminescence Properties of Ce and Dy Co-Doped ZnO Nanoparticles. Russ. J. Phys. Chem. A 2021, 95, 1900–1910. [Google Scholar] [CrossRef]
- Wang, J.P.; Yang, H.Y.; Yang, P. Photoelectric properties of 2D ZnO, graphene, silicene materials and their heterostructures. Compos. Part B-Eng. 2022, 233, 109645. [Google Scholar] [CrossRef]
- Guan, H.Y.; Mao, G.J.; Zhong, T.Y.; Zhao, T.M.; Liang, S.; Xing, L.L.; Xue, X.Y. A self-powered UV photodetector based on the hydrovoltaic and photoelectric coupling properties of ZnO nanowire arrays. J. Alloys Compd. 2021, 867, 159073. [Google Scholar] [CrossRef]
- Bhavsar, K.; Prabhu, R.; Pollard, P. Investigations on surface wettability of ZnO nanowires using UV LEDs for biosensing applications. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012033. [Google Scholar] [CrossRef]
- Yao, C.J.; Lin, J.M.; Qu, Y.P.; Jiang, K.; Hu, Z.G.; Li, L.Q.; Xu, N.; Sun, J.; Wu, J.D. WS2-decorated ZnO nanorods and enhanced ultraviolet emission. Mater. Lett. 2022, 306, 130880. [Google Scholar] [CrossRef]
- Sun, Q.Z.; Tang, J.L.; Zhang, C.X.; Li, Y.L.; Xie, W.H.; Deng, H.; Zheng, Q.; Wu, J.H.; Cheng, S.Y. Efficient Environmentally Friendly Flexible CZTSSe/ZnO Solar Cells by Optimizing ZnO Buffer Layers. Materials 2023, 16, 2869. [Google Scholar] [CrossRef]
- He, Y.Y.; Zhang, L.L.; Chen, G.T.; Liu, Y.H.; Shi, S.S.; Jiang, P.P.; Ding, J.; Xu, S.; Geng, C. ZnO/SiO2 encapsulation of perovskite nanocrystals for efficient and stable light-emitting diodes. Appl. Surf. Sci. 2023, 611, 155724. [Google Scholar] [CrossRef]
- Sun, F.Z.; Qiao, X.L.; Tan, F.T.; Wang, W.; Qiu, X.L. One-step microwave synthesis of Ag/ZnO nanocomposites with enhanced photocatalytic performance. J. Mater. Sci. 2012, 47, 7262–7268. [Google Scholar] [CrossRef]
- Kochnev, N.D.; Tkachenko, D.S.; Kirsanov, D.O.; Bobrysheva, N.P.; Osmolowsky, M.G.; Voznesenskiy, M.A.; Osmolovskaya, O.M. Regulation and prediction of defect-related properties in ZnO nanosheets: Synthesis, morphological and structural parameters, DFT study and QSPR modelling. Appl. Surf. Sci. 2023, 621, 156828. [Google Scholar] [CrossRef]
- George, J.; Aiswarya, M.; Mythri, V.K.; Sathiyamoorthy, S.; Paulraj, S.; Kathirvel, V.; Maaza, M.; Majumdar, A.; Veluswamy, P. Ultra-high thermopower of 3D network architectures of ZnO nanosheet and porous ZnO nanosheet coated carbon fabric for wearable multi-applications. Ceram. Int. 2022, 48, 28874–28880. [Google Scholar] [CrossRef]
- Li, J.; Jin, Z.; Chao, Y.; Wang, A.J.; Wang, D.C.; Chen, S.H.; Qian, Q. Synthesis of Graphene-Oxide-Decorated Porous ZnO Nanosheet Composites and Their Gas Sensing Properties. Chemosensors 2023, 11, 11010065. [Google Scholar] [CrossRef]
- Huang, H.K.; Lai, J.C.; Lu, J.; Li, Z.H. Performance enhancement of ZnO ultraviolet detector by localized surface plasmon resonance of Al nanoparticles. Appl. Phys. A 2021, 127, 679. [Google Scholar] [CrossRef]
- Grigoryev, L.V.; Morozov, I.S.; Zhuravlev, N.V.; Semenov, A.A.; Nikitin, A.A. Photoluminescence and Photoelectric Properties of the ZnO–LiNbO3 Thin-Film Structure in the Ultraviolet and Visible Spectral Regions. Semiconductors 2020, 54, 285–290. [Google Scholar] [CrossRef]
- Yin, J.Q.; Yu, C.Y.; Dong, H.L.; Li, T.B.; Jia, W.; Mei, F.H.; Zhai, G.M.; Zhang, Z.X. Effect of copper and silver co-doping on growth behaviour and photoelectric properties of n-ZnO nanorods/p-GaN heterojunction light-emitting diodes. Philos. Mag. 2022, 102, 1247–1260. [Google Scholar] [CrossRef]
- Feng, T.; Mo, Y.; Lv, S.; Liu, G. Heterolayered 2D Nanohybrids of Graphene-WS2 Nanosheets: Enabling Enhanced Supercapacitive Performance of Polyaniline. Energy Fuels 2023, 37, 6266–6275. [Google Scholar] [CrossRef]
- Mobin, M.; Ansar, F.; Shoeb, M. Chitosan-polyaniline-TiO2 ternary nanocomposite coating as effective anti-corrosion material for low carbon steel in 3.5 wt% NaCl solution. J. Adhes. Sci. Technol. 2023, 32, 46717–46730. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shawon, M.R.; Rahman, M.H.; Alam, I.; Faruk, M.O.; Khan, M.M.R.; Okoli, O. Synthesis of polyaniline-graphene oxide based ternary nanocomposite for supercapacitor application. J. Energy Storage 2023, 67, 107615. [Google Scholar] [CrossRef]
- Rehman, M.N.U.; Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Akbar, U.A.; Manzoor, S.; Hakeem, A.S.; Ashiq, M.N.; Iqbal, F. Facile synthesis of novel PANI covered Y2O3–ZnO nanocomposite: A promising electrode material for supercapacitor. Solid State Sci. 2022, 128, 106883. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Y.; Qi, Y.; Zhang, A.; Du, J.; Li, J.; Guo, T. Fabrication of ZnO nanosheets self-assembled by nanoparticles for accelerated electrocatalytic reduction of CO2 to CO. Fuel 2023, 333, 126431. [Google Scholar] [CrossRef]
- Lu, H.; Sha, S.M.; Li, T.; Wen, Q.; Yang, S.L.; Wu, J.D.; Wang, K.; Sheng, Z.L.; Ma, J.F. One-step electrodeposition of ZnO/graphene composites with enhanced capability for photocatalytic degradation of organic dyes. Front. Chem. 2022, 10, 1061129. [Google Scholar] [CrossRef]
- Somvanshi, D.; Jit, S. Fabrication and Characterization of ZnO Nanowires by Thermal Oxidation Method. International Conference on Advanced Materials Processing—Challenges and Opportunities. Adv. Mater. Res. 2012, 585, 124–128. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, T.Y.; Li, J.; Meng, F.L. Design and sensing characteristics of ethanol fiber probe elaborated by ZnO nanosheets. J. Phys. Chem. Solids 2022, 162, 110495. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.Z.; Liu, H.H.; Zhao, C.F.; Zhao, X.J.; Xie, H. Intensitive UV-Vis-IR driven catalytic activity of Pt supported on hierarchical ZnO porous nanosheets for benzene degradation via novel photothermocatalytic synergetic effect. J. Environ. Chem. Eng. 2022, 10, 107694. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Hao, F.; Kang, C.; Cui, B.; Wei, D.; Meng, F. P-n junctions based on CuO-decorated ZnO nanowires for ethanol sensing application. Appl. Surf. Sci. 2021, 538, 148140. [Google Scholar] [CrossRef]
- Yan, G.; Wang, K.; Jiang, Z.; Jiang, K.; Peng, J.; Xu, J. Flower-like ZnO/BiOI p-n heterojunction composites for enhanced photodegradation of formaldehyde and dyes. J. Mater. Sci. Mater. Electron. 2022, 33, 23064–23074. [Google Scholar] [CrossRef]
- Wannapop, S.; Khawsaad, A.; Supanpong, A.; Janorat, Y.; Chuminjak, Y.; Tuantranont, A.; Phuruangrat, A.; Thongtem, T.; Thongtem, S.; Somdee, A. Photocatalytic study of metal oxide enhanced ZnO synthesized by a one-step cyclic-microwave method: The role of the p-n heterostructure. Inorg. Chem. Commun. 2022, 138, 109210. [Google Scholar] [CrossRef]
- Gong, F.L.; Peng, M.X.; Yue, L.J.; Chen, J.L.; Xie, K.F.; Zhang, Y.H. Design of p-n heterojunction on mesoporous ZnO/Co3O4 nanosheets for triethylamine sensor. Chem. Phys. Lett. 2021, 779, 138891. [Google Scholar] [CrossRef]






| Sample | On–Off Ratio | Responsiveness S(A/W) | Rise Time (80%)/s | Fall Time (80%)/s |
|---|---|---|---|---|
| ZnO | 9.27 | 0.000465 | 20.4 | 28.5 |
| ZnO/PANI | 33.46 | 0.00622 | 13.0 | 22.9 |
| ZnO/PANI/ZnO | 60.34 | 0.00968 | 5.4 | 20.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Feng, Y.; Fu, F.; Wang, H. Preparation of ZnO Nanosheet Array and Research on ZnO/PANI/ZnO Ultraviolet Photodetector. Polymers 2023, 15, 4399. https://doi.org/10.3390/polym15224399
Zhang X, Feng Y, Fu F, Wang H. Preparation of ZnO Nanosheet Array and Research on ZnO/PANI/ZnO Ultraviolet Photodetector. Polymers. 2023; 15(22):4399. https://doi.org/10.3390/polym15224399
Chicago/Turabian StyleZhang, Xuanzhen, Yunhui Feng, Fangbao Fu, and Huan Wang. 2023. "Preparation of ZnO Nanosheet Array and Research on ZnO/PANI/ZnO Ultraviolet Photodetector" Polymers 15, no. 22: 4399. https://doi.org/10.3390/polym15224399
APA StyleZhang, X., Feng, Y., Fu, F., & Wang, H. (2023). Preparation of ZnO Nanosheet Array and Research on ZnO/PANI/ZnO Ultraviolet Photodetector. Polymers, 15(22), 4399. https://doi.org/10.3390/polym15224399





