NH2-MIL-125-Derived N-Doped TiO2@C Visible Light Catalyst for Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NH2-MIL-125
2.3. Preparation of N-Doped TiO2@C Nanomaterials
2.4. Characterization
2.5. Photocatalytic Performance Measurements
3. Results
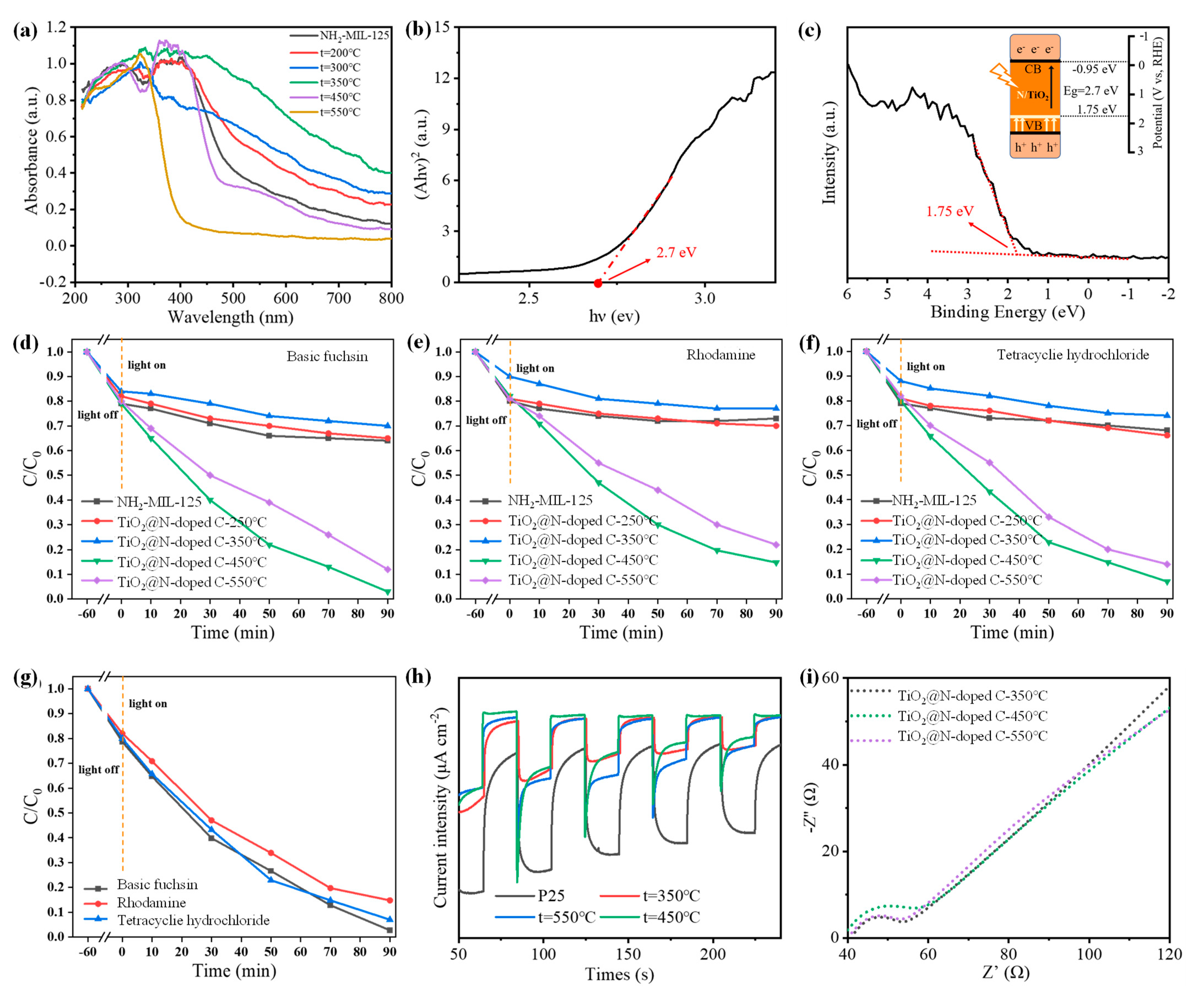
3.1. Synthesis and Characterization of N-Doped TiO2@C
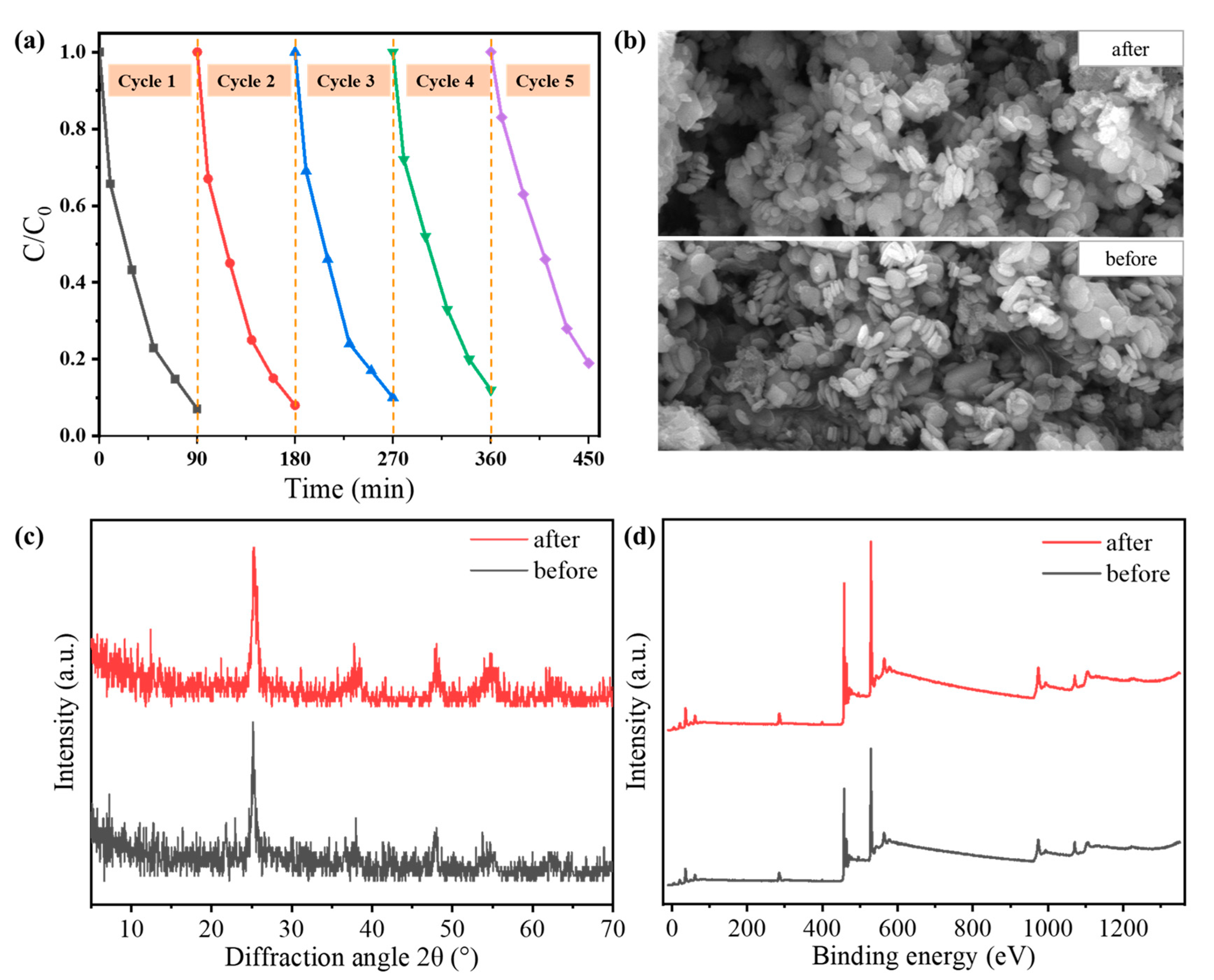
3.2. Photocatalytic Degradation of N-Doped TiO2@C
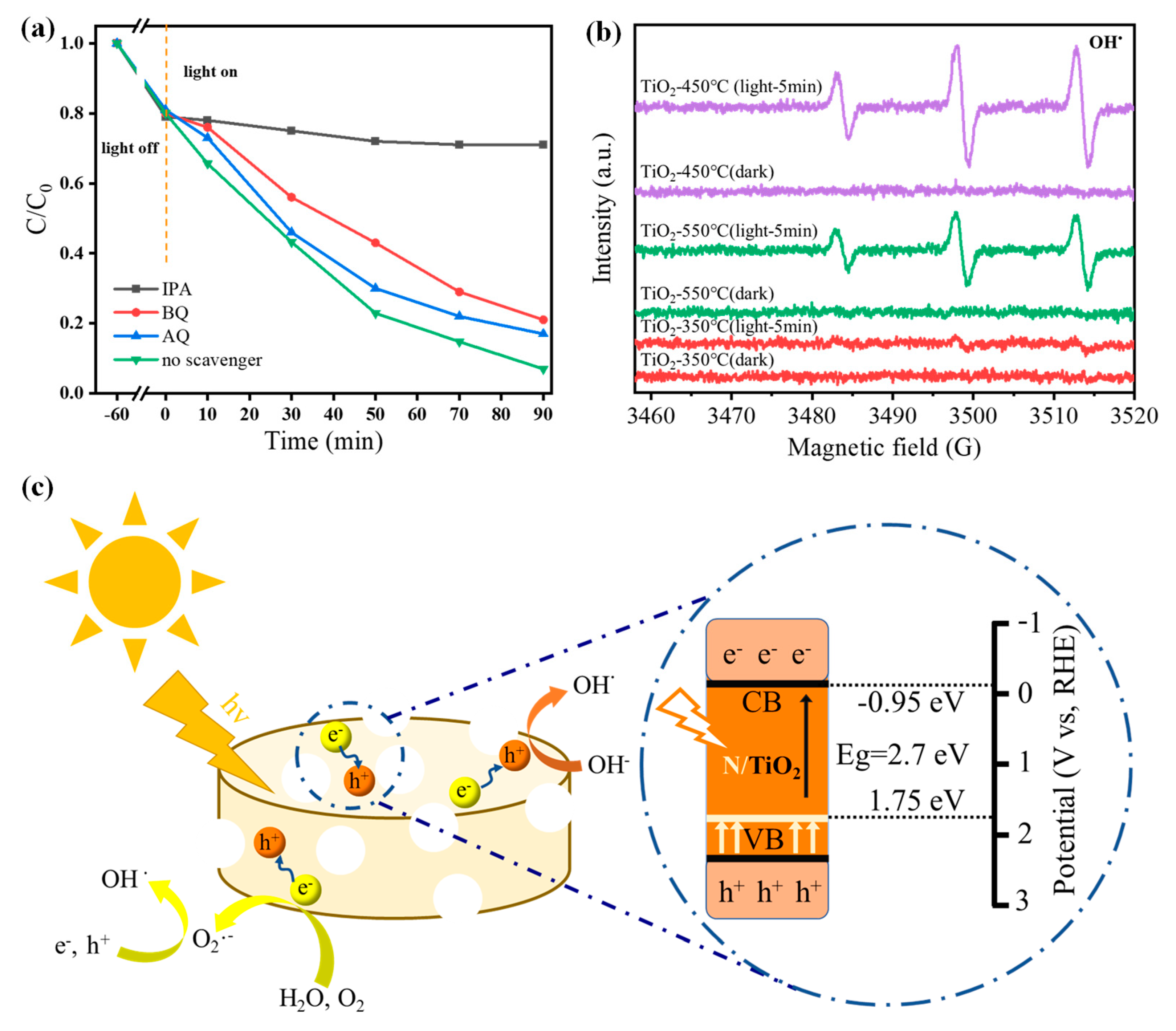
3.3. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hannah, D.M.; Abbott, B.W.; Khamis, K.; Kelleher, C.; Lynch, I.; Krause, S.; Ward, A.S. Illuminating the ‘invisible water crisis’ to address global water pollution challenges. Hydrol. Process. 2022, 36, e14525. [Google Scholar] [CrossRef]
- Sehar, S.; Rasool, T.; Syed, H.M.; Mir, M.A.; Naz, I.; Rehman, A.; Shah, M.S.; Akhter, M.S.; Mahmood, Q.; Younis, A. Recent advances in biodecolorization and biodegradation of environmental threatening textile finishing dyes. 3 Biotech 2022, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, T.H.; Kim, C.; Lee, S.A.; Choi, M.J.; Kim, H.; Yang, J.W.; Lim, J.; Jang, H.W. Hydrothermally obtained type-II heterojunction nanostructures of In2S3/TiO2 for remarkably enhanced photoelectrochemical water splitting. Appl. Catal. B Environ. 2021, 295, 120276. [Google Scholar] [CrossRef]
- Lu, Y.; Zang, Y.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Meaningful comparison of photocatalytic properties of {001} and {101} faceted anatase TiO2 nanocrystals. Sci. Bull. 2016, 61, 1003–1012. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wang, X.; Wu, Y.; Su, Y.; Tang, H. 3D/2D direct Z-scheme heterojunctions of hierarchical TiO2 microflowers/g-C3N4 nanosheets with enhanced charge carrier separation for photocatalytic H2 evolution. Carbon 2019, 149, 618–626. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of water pollution on human health and disease heterogeneity: A review. Surf. Interfaces 2022, 10, 1088. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Sultan, S.; Mathew, A.P. Binder-free Three-dimensional (3D) printing of Cellulose-ZIF8 (CelloZIF-8) for water treatment and carbon dioxide (CO2) adsorption. Chem. Eng. J. 2023, 468, 143567. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Cheng, Q.; Wang, X.; Wang, J.; Zhang, G. Sb-based photocatalysts for degradation of organic pollutants: A review. J. Clean. Prod. 2022, 367, 133060. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Lajaunie, L.; Rozman, N.; Caetano, A.P.F.; Seabra, M.P.; Škapin, A.S.; Arenal, R.; Labrincha, J.A. Impact of the absolute rutile fraction on TiO2 visible-light absorption and visible-light-promoted photocatalytic activity. J. Photochem. Photobiol. A Chem. 2019, 382, 111940. [Google Scholar] [CrossRef]
- Sun, H.; Lu, W.; Zhao, J. Structure and reactivity of aanatase TiO2(001)-(1 × 4) surface. J. Phys. Chem. C 2018, 122, 14528–14536. [Google Scholar] [CrossRef]
- Wei, C.; Lin, W.Y.; Zainal, Z.; Williams, N.E.; Zhu, K.; Kruzic, A.P.; Smith, R.L.; Rajeshwar, K. Bactericidal Activity of TiO2 Photocatalyst in Aqueous Media: Toward a Solar-Assisted Water Disinfection System. Environ. Sci. Technol. 1994, 28, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Playford, H.Y. Variations in the local structure of nano-sized anatase TiO2. J. Solid State Chem. 2020, 288, 121414. [Google Scholar] [CrossRef]
- Kong, X.; Li, J.; Yang, C.; Tang, Q.; Wang, D. Fabrication of Fe2O3/g-C3N4@N-TiO2 photocatalyst nanotube arrays that promote bisphenol A photodegradation under simulated sunlight irradiation. Sep. Purif. Technol. 2020, 248, 116924. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Jin, B.; Luo, J.; Xu, X.; Zhang, L.; Hong, Y. Heterojunctions in g-C3N4/B-TiO2 nanosheets with exposed {001} plane and enhanced visible-light photocatalytic activities. Int. J. Hydrogen Energy 2016, 41, 7292–7300. [Google Scholar] [CrossRef]
- Akbari, M.; Ghasemzadeh, M.A.; Fadaeian, M. Synthesis and application of ZIF-8 MOF incorporated in a TiO2@Chitosan nanocomposite as a strong nanocarrier for the drug delivery of acyclovir. ChemistrySelect 2020, 5, 14564–14571. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Hu, Y.; Zhao, H.; Sun, Y.; Hua, K.; Chen, G. Interior supported hierarchical TiO2@Co3O4 derived from MOF-on-MOF architecture with enhanced electrochemical properties for lithium storage. ChemElectroChem 2019, 6, 3657–3666. [Google Scholar] [CrossRef]
- Chen, K.; Guo, H.-N.; Li, W.-Q.; Wang, Y.J. MOF-derived core-shell CoP@NC@TiO2 composite as a high-performance anode material for Li-ion batteries. Chem. Asian J. 2021, 16, 322–328. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Geng, F.; Zhao, L.; Wang, Y. TiO2@MOF photocatalyst for the synergetic oxidation of microcystin-LR and reduction of Cr(VI) in a media. Catalysts 2021, 11, 1186. [Google Scholar] [CrossRef]
- He, X.; Fang, H.; Gosztola, D.J.; Jiang, Z.; Jena, P.; Wang, W.N. Mechanistic insight into photocatalytic pathways of MIL-100(Fe)/TiO2 composites. ACS Appl. Mater. Interfaces 2019, 11, 12516–12524. [Google Scholar] [CrossRef]
- Lee, D.T.; Zhao, J.; Oldham, C.J.; Peterson, G.W.; Parsons, G.N. UiO-66-NH2 Metal–Organic Framework (MOF) nucleation on TiO2, ZnO, and Al2O3 atomic layer deposition-treated polymer fibers: Role of metal oxide on MOF growth and catalytic hydrolysis of chemical warfare agent simulants. ACS Appl. Mater. Interfaces 2017, 9, 44847–44855. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wen, Q.; Wang, C.; Wang, B.; Yu, S.; Hao, C.; Chen, K. Porous TiO2 nanoparticles derived from titanium Metal– Organic Framework and its improved electrorheological performance. Ind. Eng. Chem. Res. 2018, 57, 6888–6896. [Google Scholar] [CrossRef]
- Sheng, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Urchin-inspired TiO2@MIL-101 double-shell hollow particles: Adsorption and highly efficient photocatalytic degradation of hydrogen sulfide. Chem. Mater. 2017, 29, 5612–5616. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, J.; Dai, Z.; Liu, C.; Zhao, J.; Gao, Z.; Song, Y.-Y. Engineering homochiral MOFs in TiO2 nanotubes as enantioselective photoelectrochemical electrode for chiral recognition. Anal. Chem. 2021, 93, 12067–12074. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Ren, X.-C. Synthesis of double-shell hollow TiO2@ZIF-8 nanoparticles with enhanced photocatalytic activities. Front. Chem. 2020, 8, 578847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, B.; Zhao, Y.; Hou, M.; Xin, C.; Li, Q.; Yu, X. High-performance visible-light photocatalysis induced by dye-sensitized Ti3+-TiO2 microspheres. J. Phys. Chem. Solids 2023, 179, 111374. [Google Scholar] [CrossRef]
- Bi, R.; Liu, J.; Zhou, C.; Shen, Y.; Liu, Z.; Wang, Z. In situ synthesis of g-C3N4/TiO2 heterojunction by a concentrated absorption process for efficient photocatalytic degradation of tetracycline hydrochloride. Environ. Sci. Pollut. Res. 2023, 30, 55044–55056. [Google Scholar] [CrossRef] [PubMed]
- Feizpoor, S.; Habibi-Yangjeh, A.; Luque, R. Preparation of TiO2/Fe-MOF n–n heterojunction photocatalysts for visible-light degradation of tetracycline hydrochloride. Chemosphere 2023, 336, 139101. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Hou, C.; Xie, Y.; Wang, L.; Zhang, M. Transformation of titanium-based photocatalyst and its degradation of tetracycline hydrochloride. J. Alloys Compd. 2024, 970, 172644. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, H.; Wong, P.K.; Wu, Y.; Rittmann, B. Biodegradation of tetracycline using hybrid material (UCPs-TiO2) coupled with biofilms under visible light. Bioresour. Technol. 2021, 323, 124638. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Tian, Y.; Lin, Y.; Hu, Y.H. Excellent photocatalytic degradation of tetracycline over black anatase-TiO2 under visible light. Chem. Eng. J. 2021, 406, 126747. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, Z.; Xu, Y.; Tian, T.; Zhang, J.; Pang, J.; Peng, X.; Zhang, Q.; Shao, M.; Tan, W.; et al. Visible-light-driven AgBreTiO2-Palygorskite photocatalyst with excellent photocatalytic activity for tetracycline hydrochloride. J. Clean. Prod. 2020, 277, 124021. [Google Scholar] [CrossRef]
- Ning, P.; Chen, H.; Pan, J.; Liang, J.; Qin, L.; Chen, D.; Huang, Y. Surface defect-rich g-C3N4/TiO2 Z-scheme heterojunction for efficient photocatalytic antibiotic removal: Rational regulation of free radicals and photocatalytic mechanism. Catal. Sci. Technol. 2020, 10, 8295–8304. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, R.B.; Pw, A.; Zs, A.; Lz, A. Photocatalytic activity of N-TiO2/O-doped N vacancy g-C3N4 and the intermediates toxicity evaluation under tetracycline hydrochloride and Cr(VI) coexistence environment. Appl. Catal. B Environ. 2020, 262, 118308. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, L.; Wang, H.; Yang, J.; Yuan, X.; Wang, H.; Liang, J.; Li, X.; Li, H.; Bu, Y. Oxygen Vacancies Modified TiO2/O-Terminated Ti3C2 Composites: Unravelling the Dual Effects between Oxygen Vacancy and High-Work-Function Titanium Carbide. Adv. Funct. Mater. 2023, 33, 2307702. [Google Scholar] [CrossRef]
- Biswal, L.; Mishra, B.P.; Das, S.; Acharya, L.; Nayak, S.; Parida, K. Nanoarchitecture of a Ti3C2@TiO2 Hybrid for Photocatalytic Antibiotic Degradation and Hydrogen Evolution: Stability, Kinetics, and Mechanistic Insights. Inorg. Chem. 2023, 62, 7584–7597. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Lin, J.; Qiang, W.; Chen, C.; Sun, D. Self-doped defect-mediated TiO2 with disordered surface for high-efficiency photodegradation of various pollutants. Chemosphere 2022, 308, 136239. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, L.; An, N.; Xing, L.; Ma, H.; Cheng, L.; Yang, J.; Zhang, Q. Enhanced visible-light photocatalytic activity of carbonate-doped anatase TiO2 based on the electron-withdrawing bidentate carboxylate linkage. Appl. Catal. B Environ. 2017, 202, 642–652. [Google Scholar] [CrossRef]
- Imparato, C.; Iervolino, G.; Fantauzzi, M.; Koral, C.; Macyk, W.; Kobielusz, M.; D’Errico, G.; Rea, I.; Di Girolamo, R.; De Stefano, L.; et al. Photocatalytic hydrogen evolution by co-catalyst-free TiO2/C bulk heterostructures synthesized under mild conditions. RSC Adv. 2020, 10, 12519–12534. [Google Scholar] [CrossRef]
- Pukdeejorhor, L.; Wannapaiboon, S.; Berger, J.; Rodewald, K.; Thongratkaew, S.; Impeng, S.; Warnan, J.; Bureekaew, S.; Fischer, R.A. Defect engineering in MIL-125-(Ti)-NH2 for enhanced photocatalytic H2 generation. J. Mater. Chem. A 2023, 11, 9143–9151. [Google Scholar] [CrossRef]


| Sample | SBET (m2g−1) | Vtotal (cm3g−1) | Average Pore Diameter (nm) | N/C * |
|---|---|---|---|---|
| NH2-MIL-125 | 539 | 0.087 | 5.32 | 0.146 |
| N-doped TiO2@C-200 °C | 763 | 0.168 | 6.54 | 0.0035 |
| N-doped TiO2@C-350 °C | 89 | 0.157 | 9.30 | 0.0022 |
| N-doped TiO2@C-450 °C | 63 | 0.141 | 10.89 | 0.0012 |
| N-doped TiO2@C-550 °C | 13 | 0.046 | 12.26 | 0.0007 |
| Order | Catalyst | Light Source | Concentrations of Catalysts (g/L) | Concentrations of Pollutants (mg/L) | Degradation Efficiency | Time (h) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | g-C3N4/TiO2 | 300 Xe (UV–Vis) | 0.4 | 20 | 90.1% | 1.0 | [26] |
| 2 | TiO2/Fe-MOF (15%) | 300 Xe (UV–Vis, λ = 370 nm) | 1 | 96 | 97% | 4 | [27] |
| 3 | TiO2, H2Ti3O7 | Xe lamp | 0.02 | 20 | 89%, 94% | 1 | [28] |
| 4 | Biofilm-UCPs-TiO2 | lamp (20 W) of 1800 Lux | 1 | 40 | 82.1% | 24 | [29] |
| 5 | Black-TiO2 | SPD-16 UV–vis detector at 357 nm | 0.5 | 10 | 66.2% | 4.5 | [30] |
| 6 | AgBreTiO2-Pal (50%) | 200–800 nm by UV-Vis DRS | 0.5 | 10 | 89.6% | 1.5 | [31] |
| 7 | Defect-rich hydrogenated g-C3N4/TiO2 | 300 Xe (λ > 400 nm) | 0.6 | 30 | 60% | 1.5 | [32] |
| 8 | N-TiO2/Ov carbon nitride doped with oxygen | 300 Xe (λ > 420 nm) | 0.4 | 30 | 79.9% | 1.0 | [33] |
| 9 | Oxygen vacancies modified TiO2/O-terminated Ti3C2 composites | Vis (300 W) | 0.4 | 20 | 88.5% | 1.5 | [34] |
| 10 | Ti3C2@TiO2 | 125 W Xe (λ > 400 nm) | 1 | 20 | 90% | 1.5 | [35] |
| 11 | N-doped TiO2@C | 300 W Xe (λ > 420 nm) | 0.67 | 30 | 93% | 1.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Qiang, W.; Chen, C.; Sun, D. NH2-MIL-125-Derived N-Doped TiO2@C Visible Light Catalyst for Wastewater Treatment. Polymers 2024, 16, 186. https://doi.org/10.3390/polym16020186
Wang W, Qiang W, Chen C, Sun D. NH2-MIL-125-Derived N-Doped TiO2@C Visible Light Catalyst for Wastewater Treatment. Polymers. 2024; 16(2):186. https://doi.org/10.3390/polym16020186
Chicago/Turabian StyleWang, Wenbin, Wei Qiang, Chuntao Chen, and Dongping Sun. 2024. "NH2-MIL-125-Derived N-Doped TiO2@C Visible Light Catalyst for Wastewater Treatment" Polymers 16, no. 2: 186. https://doi.org/10.3390/polym16020186
APA StyleWang, W., Qiang, W., Chen, C., & Sun, D. (2024). NH2-MIL-125-Derived N-Doped TiO2@C Visible Light Catalyst for Wastewater Treatment. Polymers, 16(2), 186. https://doi.org/10.3390/polym16020186








