Synthesis, Characterization and Catechol-Based Bioinspired Adhesive Properties in Wet Medium of Poly(2-Hydroxyethyl Methacrylate-co-Acrylamide) Hydrogels
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Measurement
2.2.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.2. Thermal Analysis
2.3. Catechol-Based Bioinspired Adhesives on Poly(HEMA-co-AAm) Hydrogels
2.4. Hydrogel Swelling Studies
Effect of Crosslinker MBA/DOPA Composition on Swelling Properties
2.5. Mechanical Property Studies
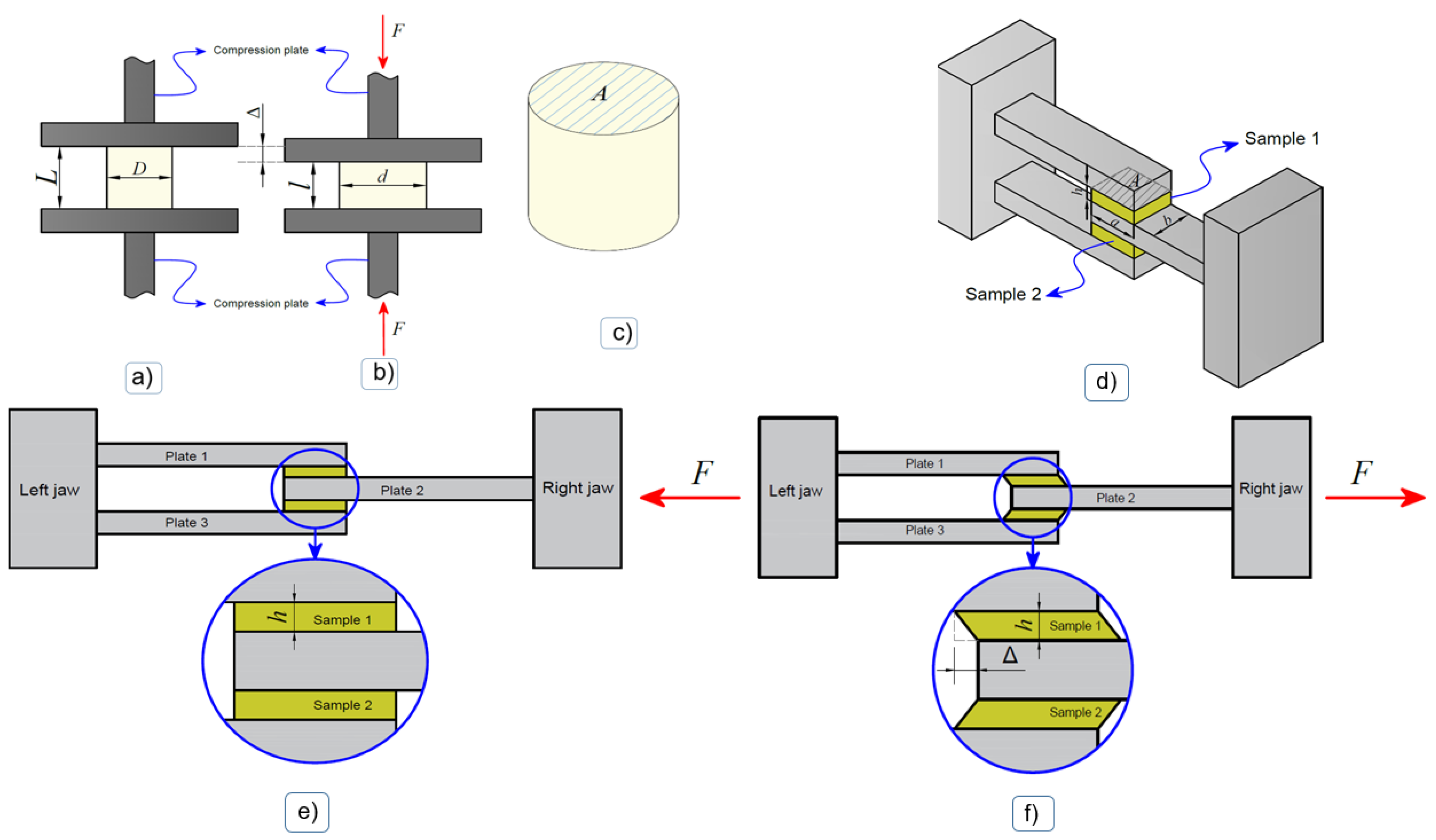
2.5.1. Compression Test
2.5.2. Shear Test
3. Results and Discussion
3.1. Synthesis and Characterization by FT-IR, TGA and DSC
3.2. FT-IR Spectroscopic Studies
3.3. Thermal Behavior by TGA and DSC
3.3.1. Thermal Analysis for Copolymer with MBA without/with DOPA by TGA
3.3.2. Thermal Analysis of Hydrogels by DSC
3.4. Mechanical Properties
3.4.1. Biomimetic Hydrogels as Adhesive Materials with Improved Mechanical Properties in Wet Conditions
3.4.2. Effect of MBA and DOPA Crosslinker Agents at 0.1, 0.3 and 0.5 mol.-% on the Mechanical Properties of the Biomimetic Hydrogels at 1:1 Feed Monomer Ratio
3.4.3. Shear Test between the Biomimetic Polymeric Hydrogel and a Rigid Substrate
3.5. Swelling Studies
3.5.1. Influence of the pH on Hydration Capacity of the Hydrogels
3.5.2. Influence of the Percentage of MBA with or without DOPA at 1:1 Feed Monomer Ratio on Hydration Capacity at Different pH
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lum, L.; Elisseeff, J. Injectable Hydrogels for Cartilage Tissue Engineering. Top. Tissue Eng. 2003, 3, 1–25. [Google Scholar]
- Elisseeff, J.; Puleo, C.; Yang, F.; Sharma, B. Advances in Skeletal Tissue Engineering with Hydrogels. Orthod. Craniofacial Res. 2005, 8, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-Linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical Properties of Hydrogels and Their Experimental Determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Ahearne, M.; Yang, Y.; Liu, K. Mechanical Characterisation of Hydrogels for Tissue Engineering Applications. Tissue Eng. 2008, 4, 1–16. [Google Scholar]
- Oyen, M.L. Mechanical Characterisation of Hydrogel Materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of Hydrogel Biomaterials with Controlled Mechanical Properties for Tissue Engineering Applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular Gels: Functions and Uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A. Study on Superabsorbent Composites. IX: Synthesis, Characterization and Swelling Behaviors of Polyacrylamide/Clay Composites Based on Various Clays. React. Funct. Polym. 2007, 67, 737–745. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M.; Liang, R. Preparation and Properties of a Double-Coated Slow-Release NPK Compound Fertilizer with Superabsorbent and Water-Retention. Bioresour. Technol. 2008, 99, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhang, X.N.; Song, Y.; Zhao, Y.; Chen, L.; Su, F.; Li, L.; Wu, Z.L.; Zheng, Q. Ultrastiff and Tough Supramolecular Hydrogels with a Dense and Robust Hydrogen Bond Network. Chem. Mater. 2019, 31, 1430–1440. [Google Scholar] [CrossRef]
- Liang, Y.; Xue, J.; Du, B.; Nie, J. Ultrastiff, Tough, and Healable Ionic-Hydrogen Bond Cross-Linked Hydrogels and Their Uses as Building Blocks to Construct Complex Hydrogel Structures. ACS Appl. Mater. Interfaces 2019, 11, 5441–5454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Wang, Y.J.; Sun, S.; Hou, L.; Wu, P.; Wu, Z.L.; Zheng, Q. A Tough and Stiff Hydrogel with Tunable Water Content and Mechanical Properties Based on the Synergistic Effect of Hydrogen Bonding and Hydrophobic Interaction. Macromolecules 2018, 51, 8136–8146. [Google Scholar] [CrossRef]
- Chang, X.; Geng, Y.; Cao, H.; Zhou, J.; Tian, Y.; Shan, G.; Bao, Y.; Wu, Z.L.; Pan, P. Dual-Crosslink Physical Hydrogels with High Toughness Based on Synergistic Hydrogen Bonding and Hydrophobic Interactions. Macromol. Rapid Commun. 2018, 39, 1700806. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Pathak, S.K.; Tran, V.T.; Cui, J.; Jeon, I. Hydrogels with Superior Mechanical Properties from the Synergistic Effect in Hydrophobic–Hydrophilic Copolymers. Chem. Eng. J. 2019, 362, 325–338. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough and Processable Hydrogels Based on Lignin and Hydrophilic Polyurethane. ACS Appl. Bio Mater. 2018, 1, 2073–2081. [Google Scholar] [CrossRef]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable Scaffolds: Preparation and Application in Dental and Craniofacial Regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D.; Paul, A.; Cai, L.; Enejder, A.; Yang, F.; Heilshorn, S.C. Covalently Adaptable Elastin-Like Protein–Hyaluronic Acid (ELP–HA) Hybrid Hydrogels with Secondary Thermoresponsive Crosslinking for Injectable Stem Cell Delivery. Adv. Funct. Mater. 2017, 27, 1605609. [Google Scholar] [CrossRef]
- Shin, J.Y.; Yeo, Y.H.; Jeong, J.E.; Park, S.A.; Park, W.H. Dual-Crosslinked Methylcellulose Hydrogels for 3D Bioprinting Applications. Carbohydr. Polym. 2020, 238, 116192. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Carberry, B.J.; Worrell, B.T.; Dudaryeva, O.Y.; McBride, M.K.; Bowman, C.N.; Anseth, K.S. Photopolymerized Dynamic Hydrogels with Tunable Viscoelastic Properties through Thioester Exchange. Biomaterials 2018, 178, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; et al. Photopolymerized Maleilated Chitosan/Methacrylated Silk Fibroin Micro/Nanocomposite Hydrogels as Potential Scaffolds for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.; Kim, B.S.; An, Y.H.; Lee, U.J.; Lee, S.H.; Kim, S.L.; Kim, B.G.; Hwang, N.S. Fabrication of Polyphenol-Incorporated Anti-Inflammatory Hydrogel via High-Affinity Enzymatic Crosslinking for Wet Tissue Adhesion. Biomaterials 2020, 242, 119905. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Duan, J.; Ma, G.; Zhang, W.; Wang, Q.; Hu, Z. Enzymatic Crosslinking to Fabricate Antioxidant Peptide-Based Supramolecular Hydrogel for Improving Cutaneous Wound Healing. J. Mater. Chem. B 2019, 7, 2220–2225. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Reis, A.V.; Takahashi, S.H.; Rubira, A.F.; Feitosa, J.P.A.; Muniz, E.C. Synthesis of a Novel Superabsorbent Hydrogel by Copolymerization of Acrylamide and Cashew Gum Modified with Glycidyl Methacrylate. Carbohydr. Polym. 2005, 61, 464–471. [Google Scholar] [CrossRef]
- Patachia, S.; Valente, A.J.M.; Baciu, C. Effect of Non-Associated Electrolyte Solutions on the Behaviour of Poly(Vinyl Alcohol)-Based Hydrogels. Eur. Polym. J. 2007, 43, 460–467. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Xiong, Z.C.; He, C.C.; Huang, X.; Xu, L.; Zhang, L.F.; Xiong, C. Preparation and Properties of Thermo-Sensitive Hydrogels of Konjac Glucomannan Grafted n-Isopropylacrylamide for Controlled Drug Delivery. Iran. J. Polym. Sci. Technol. 2007. Available online: https://www.researchgate.net/publication/239589699_Preparation_and_Properties_of_Thermosensitive_Hydrogels_of_Konjac_Glucomannan_Grafted_N-Isopropylacrylamide_for_Controlled_Drug_Delivery (accessed on 9 December 2023).
- Alvarez-Lorenzo, C.; Concheiro, A. Reversible Adsorption by a PH- and Temperature-Sensitive Acrylic Hydrogel. J. Control. Release 2002, 80, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Shymborska, Y.; Stetsyshyn, Y.; Awsiuk, K.; Raczkowska, J.; Bernasik, A.; Janiszewska, N.; Da̧bczyński, P.; Kostruba, A.; Budkowski, A. Temperature- and pH-Responsive Schizophrenic Copolymer Brush Coatings with Enhanced Temperature Response in Pure Water. ACS Appl. Mater. Interfaces 2023, 15, 8676–8690. [Google Scholar] [CrossRef] [PubMed]
- Westwood, G.; Horton, T.N.; Wilker, J.J. Simplified Polymer Mimics of Cross-Linking Adhesive Proteins. Macromolecules 2007, 40, 3960–3964. [Google Scholar] [CrossRef]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and Gelation of DOPA-Modified Poly(Ethylene Glycol) Hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Chao, C.Y.; Nelson Nunalee, F.; Motan, E.; Shull, K.R.; Messersmith, P.B. Rapid Gel Formation and Adhesion in Photocurable and Biodegradable Block Copolymers with High DOPA Content. Macromolecules 2006, 39, 1740–1748. [Google Scholar] [CrossRef]
- Anderson, T.H.; Yu, J.; Estrada, A.; Hammer, M.U.; Waite, J.H.; Israelachvili, J.N. The Contribution of DOPA to Substrate-Peptide Adhesion and Internal Cohesion of Mussel-Inspired Synthetic Peptide Films. Adv. Funct. Mater. 2010, 20, 4196–4205. [Google Scholar] [CrossRef] [PubMed]
- Núñez, C.; Celentano, D. Caracterización Experimental y Numérica de Compuestos Elastoméricos Utilizados En Disipadores de Energía. Mecánica Comput. 2005, XXIV, 391–412. [Google Scholar]
- Morita, S.; Kitagawa, K.; Ozaki, Y. Hydrogen-Bond Structures in Poly(2-Hydroxyethyl Methacrylate): Infrared Spectroscopy and Quantum Chemical Calculations with Model Compounds. Vib. Spectrosc. 2009, 51, 28–33. [Google Scholar] [CrossRef]
- Moses, D.N.; Harreld, J.H.; Stucky, G.D.; Waite, J.H. Melanin and Glycera Jaws: Emerging Dark Side of a Robust Biocomposite Structure. J. Biol. Chem. 2006, 281, 34826–34832. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 8. [Google Scholar] [CrossRef]
- White, J.L. Interpretation of Infrared Spectra of Soil Minerals. Soil Sci. 1971, 112, 22–31. [Google Scholar] [CrossRef]
- Huang, C.W.; Sun, Y.M.; Huang, W.F. Curing Kinetics of the Synthesis of Poly(2-Hydroxyethyl Methacrylate) (PHEMA) with Ethylene Glycol Dimethacrylate (EGDMA) as a Crosslinking Agent. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 1873–1889. [Google Scholar] [CrossRef]
- Singh, N.K.; Lesser, A.J. A Physical and Mechanical Study of Prestressed Competitive Double Network Thermoplastic Elastomers. Macromolecules 2011, 44, 1480–1490. [Google Scholar] [CrossRef]
- Morita, S.; Ye, S.; Li, G.; Osawa, M. Effect of Glass Transition Temperature (Tg) on the Absorption of Bisphenol A in Poly(Acrylate)s Thin Films. Vib. Spectrosc. 2004, 35, 15–19. [Google Scholar] [CrossRef]
- Choi, S.S.; Hong, J.P.; Seo, Y.S.; Chung, S.M.; Nah, C. Fabrication and Characterization of Electrospun Polybutadiene Fibers Crosslinked by UV Irradiation. J. Appl. Polym. Sci. 2006, 101, 2333–2337. [Google Scholar] [CrossRef]
- Zalucha, D.J.; Abbey, K.J. The Chemistry of Structural Adhesives: Epoxy, Urethane, and Acrylic Adhesives. In Handbook of Industrial Chemistry and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 591–622. [Google Scholar] [CrossRef]
- De Freitas, P.S.; Wirz, D.; Stolz, M.; Göpfert, B.; Friederich, N.F.; Daniels, A.U. Pulsatile Dynamic Stiffness of Cartilage-like Materials and Use of Agarose Gels to Validate Mechanical Methods and Models. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2006, 78, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Q.; Jiang, J.; Miller, D.E.; Guo, X.E.; Mow, V.C.; Lu, H.H. Matrix Deposition Modulates the Viscoelastic Shear Properties of Hydrogel-Based Cartilage Grafts. Tissue Eng.-Part A 2011, 17, 1111–1122. [Google Scholar] [CrossRef]
- Buckley, C.T.; Thorpe, S.D.; O’Brien, F.J.; Robinson, A.J.; Kelly, D.J. The Effect of Concentration, Thermal History and Cell Seeding Density on the Initial Mechanical Properties of Agarose Hydrogels. J. Mech. Behav. Biomed. Mater. 2009, 2, 512–521. [Google Scholar] [CrossRef]
- Yu, B.; Kang, S.Y.; Akthakul, A.; Ramadurai, N.; Pilkenton, M.; Patel, A.; Nashat, A.; Anderson, D.G.; Sakamoto, F.H.; Gilchrest, B.A.; et al. An Elastic Second Skin. Nat. Mater. 2016, 15, 911–918. [Google Scholar] [CrossRef]




| Polymer Sample | Feed Monomer Ratio | HEMA (mol/g) | AAm (mol/g) | MBA (mol.-%) | DOPA | Yield (%) |
|---|---|---|---|---|---|---|
| P(HEMA-co-AAm) 1:1 | 1:1 | 0.015/1.950 | 0.015/1.065 | Without | without | 95 |
| P(HEMA-co-AAm) 1:1/0.1% MBA | 1:1 | 0.015/1.950 | 0.015/1.065 | 0.1 | without | 90 |
| P(HEMA-co-AAm) 1:1/0.3% MBA | 1:1 | 0.015/1.950 | 0.015/1.065 | 0.3 | without | 92 |
| P(HEMA-co-AAm) 1:1/0.5% MBA | 1:1 | 0.015/1.950 | 0.015/1.065 | 0.5 | without | 92 |
| P(HEMA-co-AAm) 1:1/0.1% MBA/0.1% DOPA | 1:1 | 0.015/1.950 | 0.015/1.065 | 0.1 | 0.1 | 90 |
| P(HEMA-co-AAm) 1:1/0.3% MBA/0.3% DOPA | 1:1 | 0.015/1.950 | 0.015/1.065 | 0.3 | 0.3 | 90 |
| P(HEMA-co-AAm) 1:1/0.5% MBA/0.5% DOPA | 1:1 | 0.015/1.950 | 0.015/1.065 | 0.5 | 0.5 | 90 |
| System | Vibration Bands [ν (cm−1)] | |||||
|---|---|---|---|---|---|---|
| Stretching | ||||||
| O-H: -OH | C-H: >CH, -CH2-, -CH3 | C-O: -CO2R | C-O: H2NCO- | N-H: H2NCO- | C-H: >CH-, -CH2-, -CH3 | |
| P(HEMA-co-AAm) 1:1 | 3343 | 3000-2867 | 1717 | 1660 | 1610 | 1446 |
| P(HEMA-co-AAm) 1:1/0.1 mol.-% MBA | 3340 | 3000-2865 | 1716 | 1660 | 1607 | 1460 |
| P(HEMA-co-AAm) 1:1/0.3 mol.-% MBA | 3347 | 3000-2860 | 1713 | 1665 | 1600 | 1453 |
| P(HEMA-co-AAm) 1:1/0.5 mol.-% MBA | 3440 | 3000-2857 | 1714 | 1660 | 1608 | 1437 |
| P(HEMA-co-AAm) 1:1/0.5 mol.- % DOPA | 3348 | 2982-2835 | 1715 | 1662 | 1607 | 1447 |
| P(HEMA-co-AAm) 1:1/0.5 mol.-% MBA/DOPA | 3445 | 3000-2854 | 1714 | 1662 | 1611 | 1450 |
| Hydrogel 1:1 0.0 mol.-% MBA 127.4 | Glass Transition Temperature (Tg, °C) | ||
| 0.1 mol.-% MBA | 0.3 mol.-% MBA | 0.5 mol.-% MBA | |
| 100.5 | 112.6 | 115.2 | |
| P(HEMA-co-AAm) 1:1 | 0.1 mol.-% MBA/DOPA | 0.3 mol.-% MBA/DOPA | 0.5 mol.-% MBA/DOPA |
| 108.3 | 115.6 | 118.5 | |
| Hydrogel Samples | Young’s Modulus (E) (kPa) |
|---|---|
| P(HEMA-co-AAm) 1:1 (MBA 0.1 mol.-% MBA) | 30.42 ± 3.43 |
| P(HEMA-co-AAm) 1:1 (MBA 0.3 mol.-% MBA) | 50.80 ± 1.57 |
| P(HEMA-co-AAm) 1:1 (MBA 0.5 mol.-% MBA) | 56.05 ± 4.38 |
| P(HEMA-co-AAm) 1:1 (0.1 mol.-% DOPA) | 33.77 ± 2.74 |
| P(HEMA-co-AAm) 1:1 (0.1 mol.-% MBA and DOPA) | 48.27 ± 1.71 |
| P(HEMA-co-AAm) 1:1 (0.3 mol.-% MBA and DOPA) | 54.63 ± 3.14 |
| P(HEMA-co-AAm) 1:1 (0.5 mol.-% MBA and DOPA) | 58.86 ± 2.04 |
| Sample | Failure Shear Stress (kPa) | Shear Modulus of Elasticity (kPa) |
|---|---|---|
| P(HEMA-co-AAm) 1:1 /0.1 mol.-% MBA | 1.37 ± 0.07 | 0.58 ± 0.10 |
| P(HEMA-co-AAm) 1:1 /0.3 mol.-% MBA | 1.19 ± 0.16 | 1.58 ± 0.36 |
| P(HEMA-co-AAm) 1:1 0.5 mol.-% MBA | 1.88 ± 0.17 | 1.14 ± 0.27 |
| P(HEMA-co-AAm) 1:1 (0.1 mol.-% MBA/0.1 mol.-% DOPA) | 3.58 ± 0.94 | 0.97 ± 0.32 |
| P(HEMA-co-AAm) 1:1 (0.3 mol.-% MBA/0.3 mol.-% DOPA) | 9.67 ± 1.55 | 7.11 ± 1.83 |
| P(HEMA-co-AAm) 1:1 (0.5 mol.-% MBA/0.5 mol.-% DOPA) | 4.07 ± 0.44 | 5.18 ± 0.56 |
| Sample | Feed Monomer Ratio | Water Absorbency Capacity g/g at 5 h of Hydration | |||||
| 0.1 mol.-% MBA | 0.3 mol.-% MBA | 0.5 mol.-% MBA | |||||
| pH | (%) | pH | (%) | pH | (%) | ||
| P(HEMA-co-AAm) | 1:1 | pH 3 | 938 ± 21 | pH 3 | 364 ± 20 | pH 3 | 324 ± 20 |
| P(HEMA-co-AAm) | 1:1 | pH 7 | 1392 ± 30 | pH 7 | 411 ± 25 | pH 7 | 371 ± 26 |
| P(HEMA-co-AAm) | 1:1 | pH 10 | 1806 ± 36 | pH 10 | 608 ± 95 | pH 10 | 476 ± 12 |
| 0.1 mol.-% MBA/DOPA | 0.3 mol.-% MBA/DOPA | 0.5 mol.-% MBA/DOPA | |||||
| pH | (%) | pH | (%) | pH | (%) | ||
| P(HEMA-co-AAm) | 1:1 | pH 3 | 408 ± 12 | pH 3 | 280 ± 15 | pH 3 | 305 ± 34 |
| P(HEMA-co-AAm) | 1:1 | pH 7 | 709 ± 63 | pH 7 | 299 ± 14 | pH 7 | 359 ± 54 |
| P(HEMA-co-AAm) | 1:1 | pH 10 | 824 ± 21 | pH 10 | 346 ± 21 | pH 10 | 439 ± 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Gilbert, S.; Castro-García, M.; Díaz-Chamorro, H.; Marambio, O.G.; Sánchez, J.; Martin-Trasancos, R.; Inostroza, M.; García-Herrera, C.; Pizarro, G.d.C. Synthesis, Characterization and Catechol-Based Bioinspired Adhesive Properties in Wet Medium of Poly(2-Hydroxyethyl Methacrylate-co-Acrylamide) Hydrogels. Polymers 2024, 16, 187. https://doi.org/10.3390/polym16020187
Romero-Gilbert S, Castro-García M, Díaz-Chamorro H, Marambio OG, Sánchez J, Martin-Trasancos R, Inostroza M, García-Herrera C, Pizarro GdC. Synthesis, Characterization and Catechol-Based Bioinspired Adhesive Properties in Wet Medium of Poly(2-Hydroxyethyl Methacrylate-co-Acrylamide) Hydrogels. Polymers. 2024; 16(2):187. https://doi.org/10.3390/polym16020187
Chicago/Turabian StyleRomero-Gilbert, Sebastian, Matías Castro-García, Héctor Díaz-Chamorro, Oscar G. Marambio, Julio Sánchez, Rudy Martin-Trasancos, Matías Inostroza, Claudio García-Herrera, and Guadalupe del C. Pizarro. 2024. "Synthesis, Characterization and Catechol-Based Bioinspired Adhesive Properties in Wet Medium of Poly(2-Hydroxyethyl Methacrylate-co-Acrylamide) Hydrogels" Polymers 16, no. 2: 187. https://doi.org/10.3390/polym16020187
APA StyleRomero-Gilbert, S., Castro-García, M., Díaz-Chamorro, H., Marambio, O. G., Sánchez, J., Martin-Trasancos, R., Inostroza, M., García-Herrera, C., & Pizarro, G. d. C. (2024). Synthesis, Characterization and Catechol-Based Bioinspired Adhesive Properties in Wet Medium of Poly(2-Hydroxyethyl Methacrylate-co-Acrylamide) Hydrogels. Polymers, 16(2), 187. https://doi.org/10.3390/polym16020187









