Preparations of Polyurethane Foam Composite (PUFC) Pads Containing Micro-/Nano-Crystalline Cellulose (MCC/NCC) toward the Chemical Mechanical Polishing Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Symbol Details
2.2. Materials
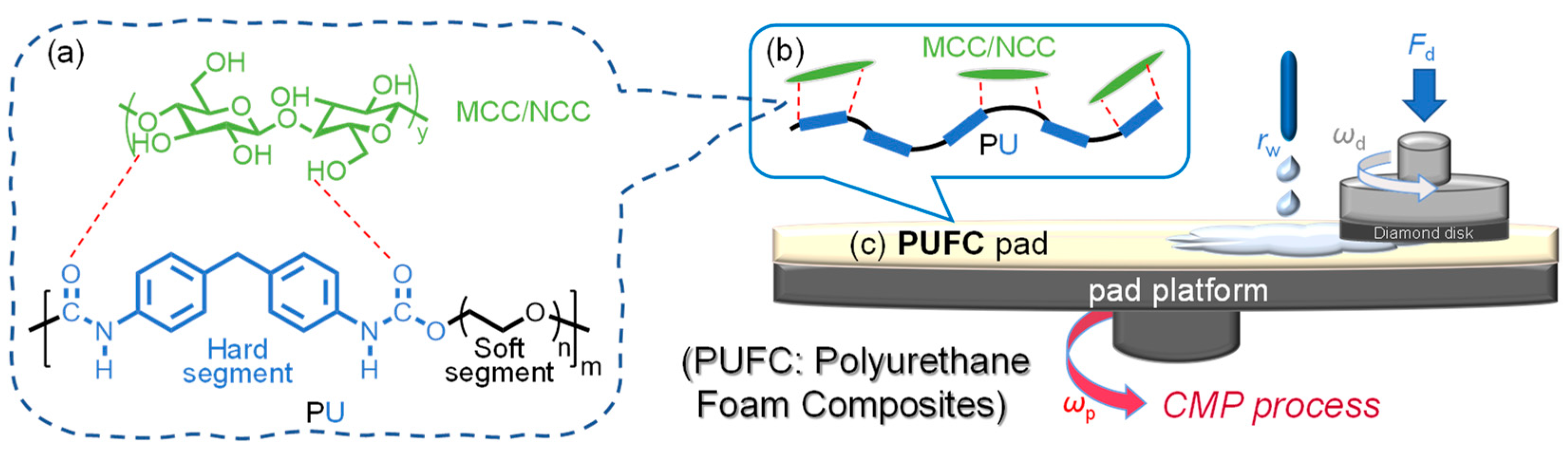
2.3. Preparing PUF and PUF Incorporated with MCC/NCC
2.4. Characterization
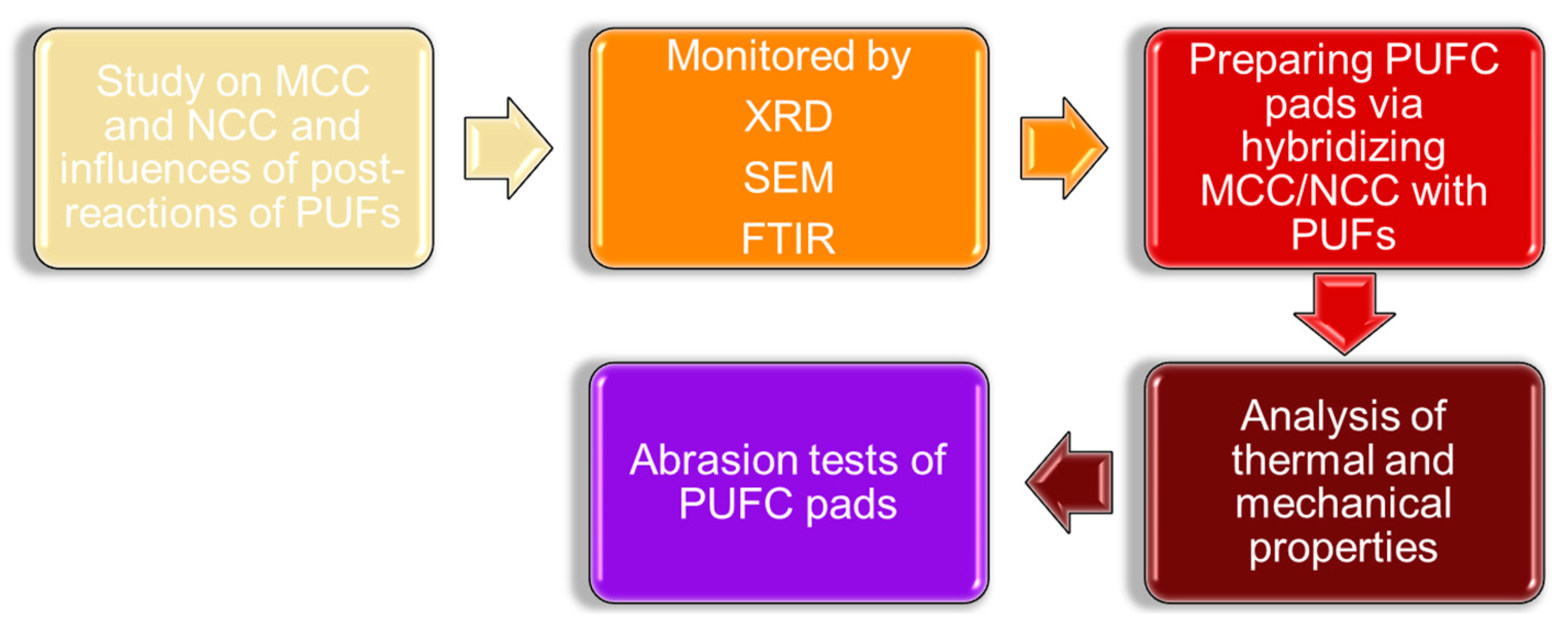
2.5. Research Workflow
3. Results and Discussion
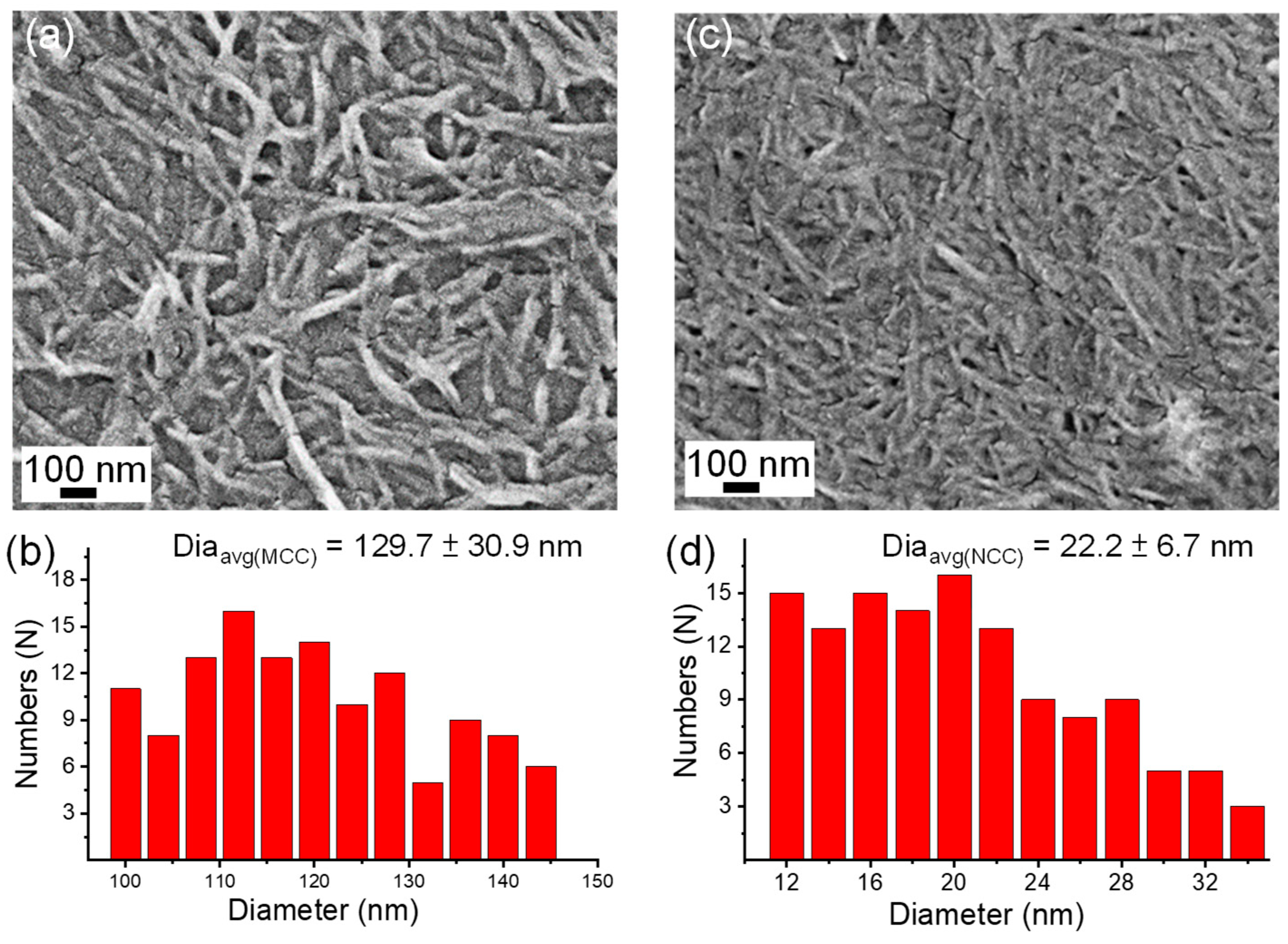
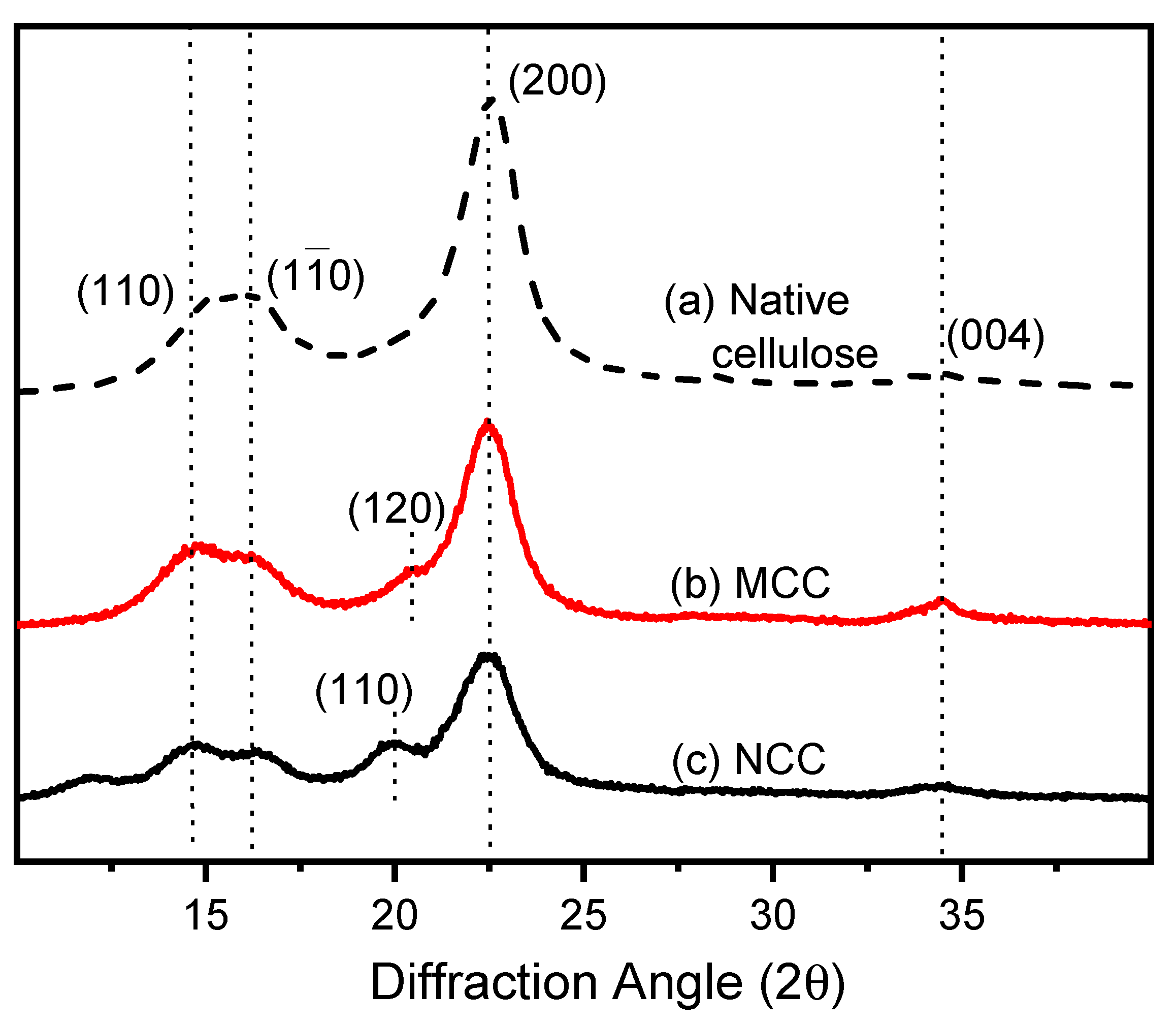
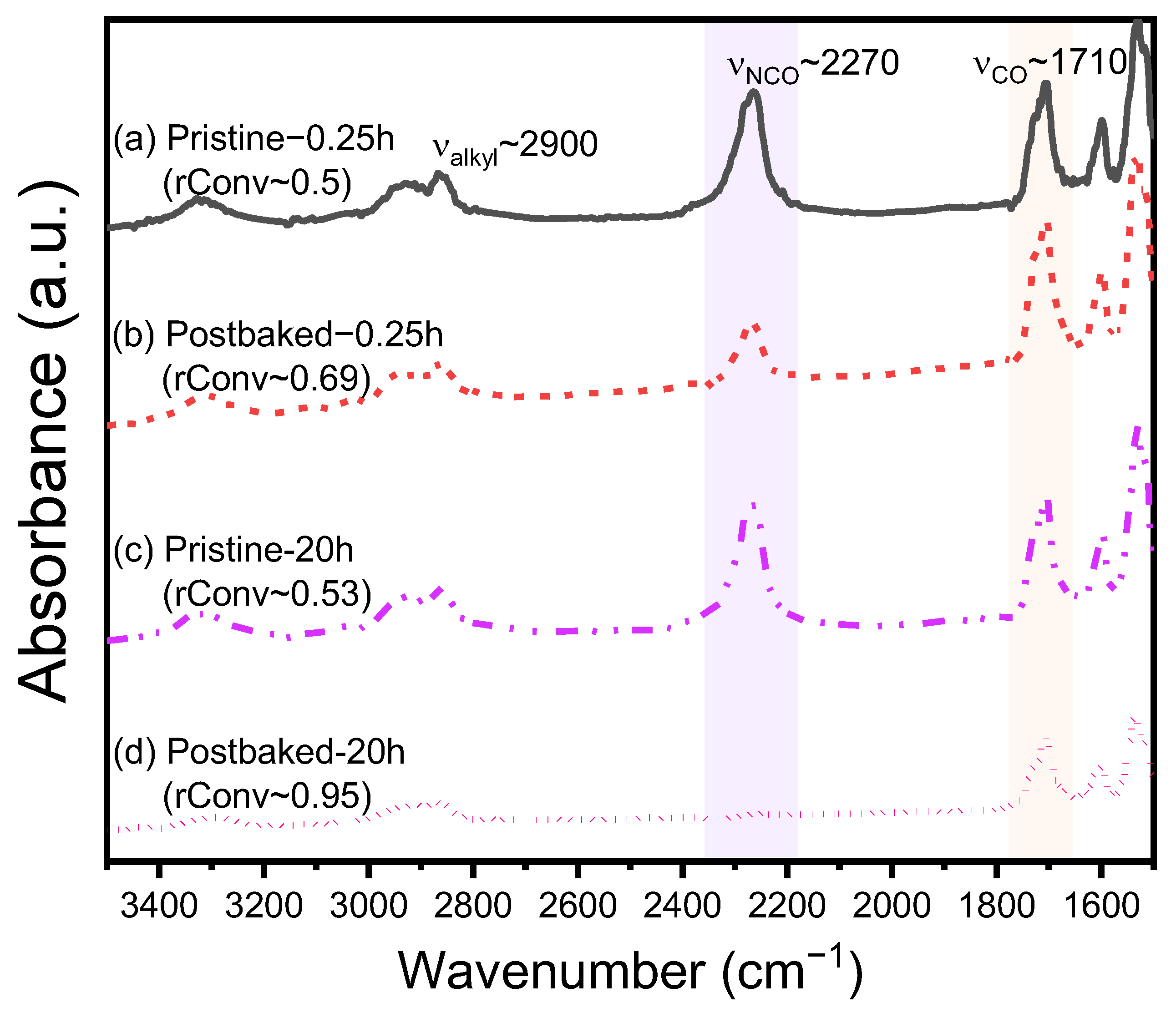
3.1. Characterization of MCC and NCC and Influence of Postbaked on PU Pads
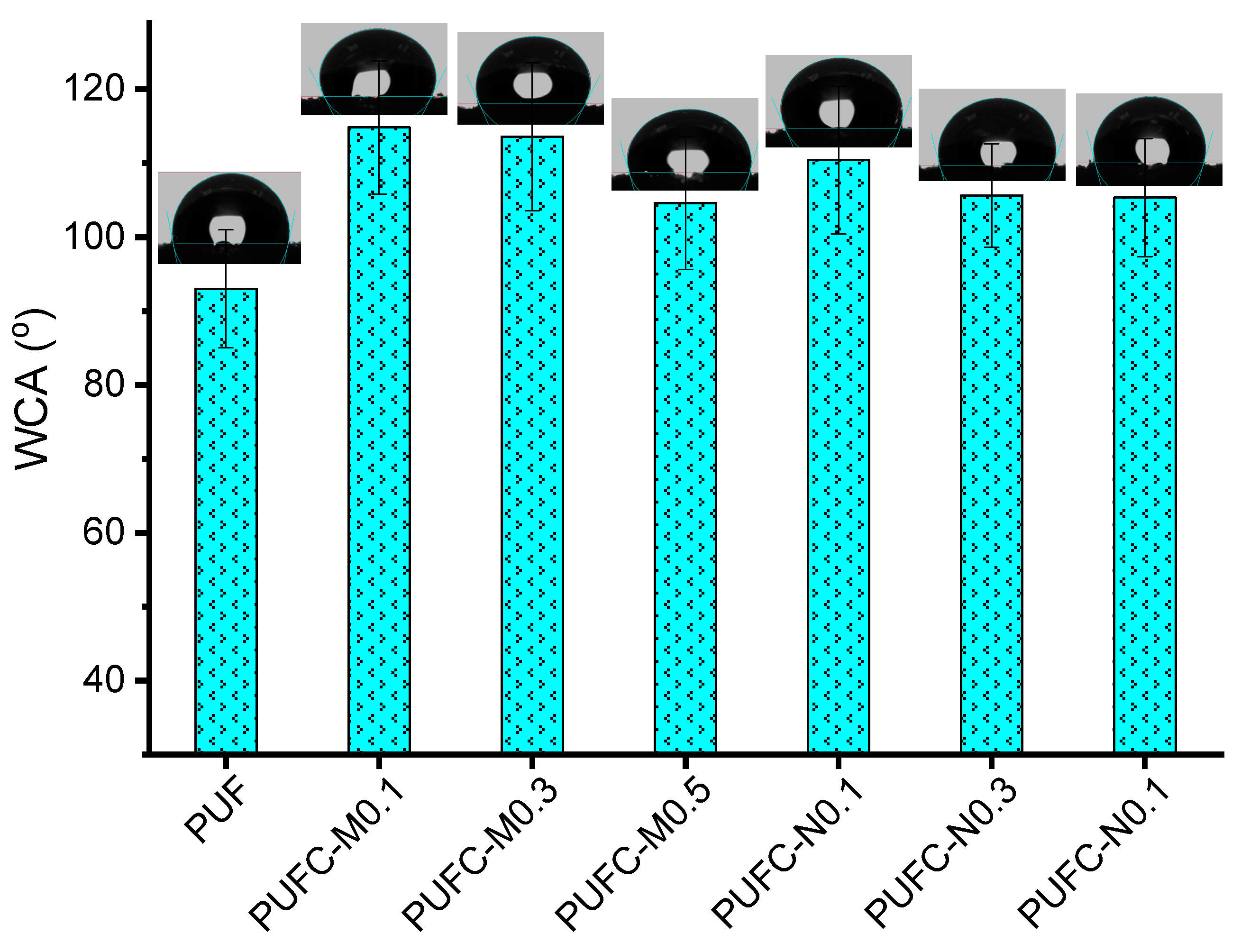
3.2. Thermal and Surface Properties of the PUF and MCC/NCC Composites
3.3. Mechanical and Abrasion Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, S.-Y.; Ahmad, N.; Jeffrey Kuo, C.F. Study of synthesis of dual-curing thermoplastic polyurethane hot-melt adhesive and optimization by using gray relational analysis to apply in fabric industry to solve seamless bonding issues. Polymers 2024, 16, 467. [Google Scholar] [CrossRef]
- de Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to polyurethane chemistry. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; Gupta, R.K., Kahol, P.K., Eds.; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, pp. 1–24. [Google Scholar] [CrossRef]
- Wongsamut, C.; Suwanpreedee, R.; Manuspiya, H. Thermoplastic polyurethane-based polycarbonate diol hot melt adhesives: The effect of hard-soft segment ratio on adhesion properties. Int. J. Adhes. Adhes. 2020, 102, 102677. [Google Scholar] [CrossRef]
- Nicholas, J.; Mohamed, M.; Dhaliwal, G.S.; Anandan, S.; Chandrashekhara, K. Effects of accelerated environmental aging on glass fiber reinforced thermoset polyurethane composites. Compos. Part B-Eng. 2016, 94, 370–378. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Ahmad Bhatti, I. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Engels, H.-W.; Pirkl, H.-G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethane: Vielseitige materialien und nachhaltige problemlöser für aktuelle anforderungen. Angew. Chem. Int. Ed. 2013, 125, 9596–9616. [Google Scholar] [CrossRef]
- Martin, D.J.; Osman, A.F.; Andriani, Y.; Edwards, G.A. Thermoplastic polyurethane (TPU)-based polymer nanocomposites. In Advances in Polymer Nanocomposites; Woodhead Publishing: Sawston, UK, 2012; pp. 321–350. [Google Scholar] [CrossRef]
- Kwon, H.; Shin, S.; Yu, Y.; Lee, W.; Park, H.; Lee, S.Y.; Woo, E.; Ahn, D.; Baek, M.-J.; Lee, D.W. Polyol-dependent adhesion mechanism of XDI- and H6XDI-based polyurethanes. Polym. Test. 2023, 129, 108260. [Google Scholar] [CrossRef]
- Heath, R. Chapter 28—Isocyanate-based polymers: Polyurethanes, polyureas, polyisocyanurates, and their copolymers. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 799–835. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Liang, Y.; Meng, F.; Cui, Z.; Chen, T.; Yang, Y.; Fan, C.; Yu, T.; Zhao, J. Modelling of polyurethane polishing pad surface topography and fixed-point polished surface profile. Tribol. Int. 2024, 195, 109646. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Z.; Zhu, M.; Wang, B.; Chen, J.; Wang, W. Effect of secondary foaming on the structural properties of polyurethane polishing pad. Materials 2024, 17, 2759. [Google Scholar] [CrossRef] [PubMed]
- Huy, L.N.Q.; Lin, C.Y.; Chen, C.C.A. Development of modeling to investigate polyurethane pad hardness in chemical mechanical planarization/polishing (CMP) process. Jpn. J. Appl. Phys. 2022, 61, SJ1002. [Google Scholar] [CrossRef]
- Hooper, B.J.; Byrne, G.; Galligan, S. Pad conditioning in chemical mechanical polishing. J. Mater. Process. Technol. 2002, 123, 107–113. [Google Scholar] [CrossRef]
- Bajaj, R.; Desai, M.; Jairath, R.; Stell, M.; Tolles, R. Effect of Polishing Pad Material Properties on Chemical Mechanical Polishing (CMP) Processes; MRS Online Proceedings Library: Warrendale, PA, USA, 1994; Volume 337, pp. 637–644. [Google Scholar]
- McGrath, J.; Davis, C. Polishing pad surface characterisation in chemical mechanical planarisation. J. Mater. Process. Technol. 2004, 153–154, 666–673. [Google Scholar] [CrossRef]
- Lu, J.; Coppeta, J.; Rogers, C.; Manno, V.; Racz, L.; Philipossian, A.; Moinpour, M.; Kaufmanc, F. The Effect of Wafer Shape on Slurry Film Thickness and Friction Coefficients in Chemical Mechanical Planarization; MRS Online Proceedings Library: Warrendale, PA, USA, 2000; Volume 613, p. 121. [Google Scholar] [CrossRef]
- Chandrashekhar, A.; Gopi, J.A.; Prabhu, T.N. Development of flexible bio-based porous polyurethane nanocellulose composites for wastewater treatment. In Proceedings of the International Conference on Advances in Materials Research, Bangalore, India, 3 October 2020. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Shih, Y.F.; Lin, S.H.; Xu, J.P.; Su, C.J.; Huang, C.F.; Hsu, S.H. Stretchable and biodegradable chitosan-polyurethane-cellulose nanofiber composites as anisotropic materials. Int. J. Biol. Macromol. 2023, 230, 12311. [Google Scholar] [CrossRef]
- Chen, J.K.; Huang, H.Y.; Tu, C.W.; Lee, L.T.; Jamnongkan, T.; Huang, C.F. SI ATRP for the surface modifications of optically transparent paper films made by TEMPO-oxidized cellulose nanofibers. Polymers 2022, 14, 946. [Google Scholar] [CrossRef]
- Chen, R.D.; Huang, C.F.; Hsu, S.H. Composites of waterborne polyurethane and cellulose nanofibers for 3D printing and bioapplications. Carbohydr. Polym. 2019, 212, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Hong, J.Y.; Huang, C.F.; Wu, J.Y.; Wang, T.L.; Wu, T.M.; Lee, R.H. Enhanced photovoltaic properties of perovskite solar cells by the addition of cellulose derivatives to mapbi3-based photoactive layer. Cellulose 2019, 26, 9229–9239. [Google Scholar] [CrossRef]
- Huang, C.F.; Chen, J.K.; Tsai, T.Y.; Hsieh, Y.A.; Lin, K.Y.A. Dual-functionalized cellulose nanofibrils prepared through TEMPO-mediated oxidation and surface-initiated ATRP. Polymer 2015, 72, 395–405. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Heish, Y.T.; Tsai, T.Y.; Huang, C.F. TEMPO-oxidized pulp as an efficient and recyclable sorbent to remove paraquat from water. Cellulose 2015, 22, 3261–3274. [Google Scholar] [CrossRef]
- Amin, K.N.M.; Amiralian, N.; Annamalai, P.K.; Edwards, G.; Chaleat, C.; Martin, D.J. Scalable processing of thermoplastic polyurethane nanocomposites toughened with nanocellulose. Chem. Eng. J. 2016, 302, 406–416. [Google Scholar] [CrossRef]
- Vijayan, J.G.; Chandrashekar, A.; Ag, J.; Prabhu, T.N.; Kalappa, P. Polyurethane and its composites derived from bio-sources: Synthesis, characterization and adsorption studies. Polym. Polym. Compos. 2022, 30, 09673911221110347. [Google Scholar] [CrossRef]
- Abd El-Fattah, M.; Hasan, A.M.A.; Keshawy, M.; El Saeed, A.M.; Aboelenien, O.M. Nanocrystalline cellulose as an eco-friendly reinforcing additive to polyurethane coating for augmented anticorrosive behavior. Carbohydr. Polym. 2018, 183, 311–318. [Google Scholar] [CrossRef]
- Aguayo, M.G.; Pérez, A.F.; Reyes, G.; Oviedo, C.; Gacitúa, W.; Gonzalez, R.; Uyarte, O. Isolation and characterization of cellulose nanocrystals from rejected fibers originated in the kraft pulping process. Polymers 2018, 10, 1145. [Google Scholar] [CrossRef]
- Nindiyasari, F.; Griesshaber, E.; Zimmermann, T.; Manian, A.P.; Randow, C.; Zehbe, R.; Fernandez-Diaz, L.; Ziegler, A.; Fleck, C.; Schmahl, W.W. Characterization and mechanical properties investigation of the cellulose/gypsum composite. J. Compos. Mater. 2016, 50, 657–672. [Google Scholar] [CrossRef]
- SriBala, G.; Chennuru, R.; Mahapatra, S.; Vinu, R. Effect of alkaline ultrasonic pretreatment on crystalline morphology and enzymatic hydrolysis of cellulose. Cellulose 2016, 23, 1725–1740. [Google Scholar] [CrossRef]
- Wada, M.; Sugiyama, J.; Okano, T. Native celluloses on the basis of 2 crystalline phase (Iα/Iβ) system. J. Appl. Polym. Sci. 1993, 49, 1491–1496. [Google Scholar] [CrossRef]
- Sugiyama, J.; Persson, J.; Chanzy, H. Combined infrared and electron-diffraction study of the polymorphism of native celluloses. Macromolecules 1991, 24, 2461–2466. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Parcheta-Szwindowska, P.; KopczyE ska, K.; Kordyzon, M.J.; Datta, J. Fabrication and characterization of green polyurethane foams with enhanced vibration damping capability. ACS Sustain. Chem. Eng. 2023, 11, 14348–14357. [Google Scholar] [CrossRef]
- Guo, Q.; Ma, J.; Yin, T.; Jin, H.; Zheng, J.; Gao, H. Superhydrophobic non-metallic surfaces with multiscale nano/micro-structure: Fabrication and application. Molecules 2024, 29, 2098. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Wang, C.F.; Chen, J.K.; Hsieh, C.C.; Chen, P.A. Fast formation of hydrophilic and reactive polymer micropatterns by photocatalytic lithography method. Appl. Surf. Sci. 2013, 286, 280–286. [Google Scholar] [CrossRef]
- Wang, C.F.; Chiou, S.F.; Ko, F.H.; Chou, C.T.; Lin, H.C.; Huang, C.F.; Chang, F.C. Fabrication of biomimetic super-amphiphobic surfaces through plasma modification of benzoxazine films. Macromol. Rapid Commun. 2006, 27, 333–337. [Google Scholar] [CrossRef]
- Tu, C.W.; Tsai, C.H.; Wang, C.F.; Kuo, S.W.; Chang, F.C. Fabrication of superhydrophobic and superoleophilic polystyrene surfaces by a facile one-step method. Macromol. Rapid Commun. 2007, 28, 2262–2266. [Google Scholar] [CrossRef]
- Tu, C.W.; Tsai, F.C.; Chang, C.J.; Yang, C.H.; Kuo, S.W.; Zhang, J.; Chen, T.; Huang, C.F. Surface-initiated initiators for continuous activator regeneration (SI ICAR) ATRP of MMA from 2,2,6,6–tetramethylpiperidine–1–oxy (TEMPO) oxidized cellulose nanofibers for the preparations of PMMA nanocomposites. Polymers 2019, 11, 1631. [Google Scholar] [CrossRef]
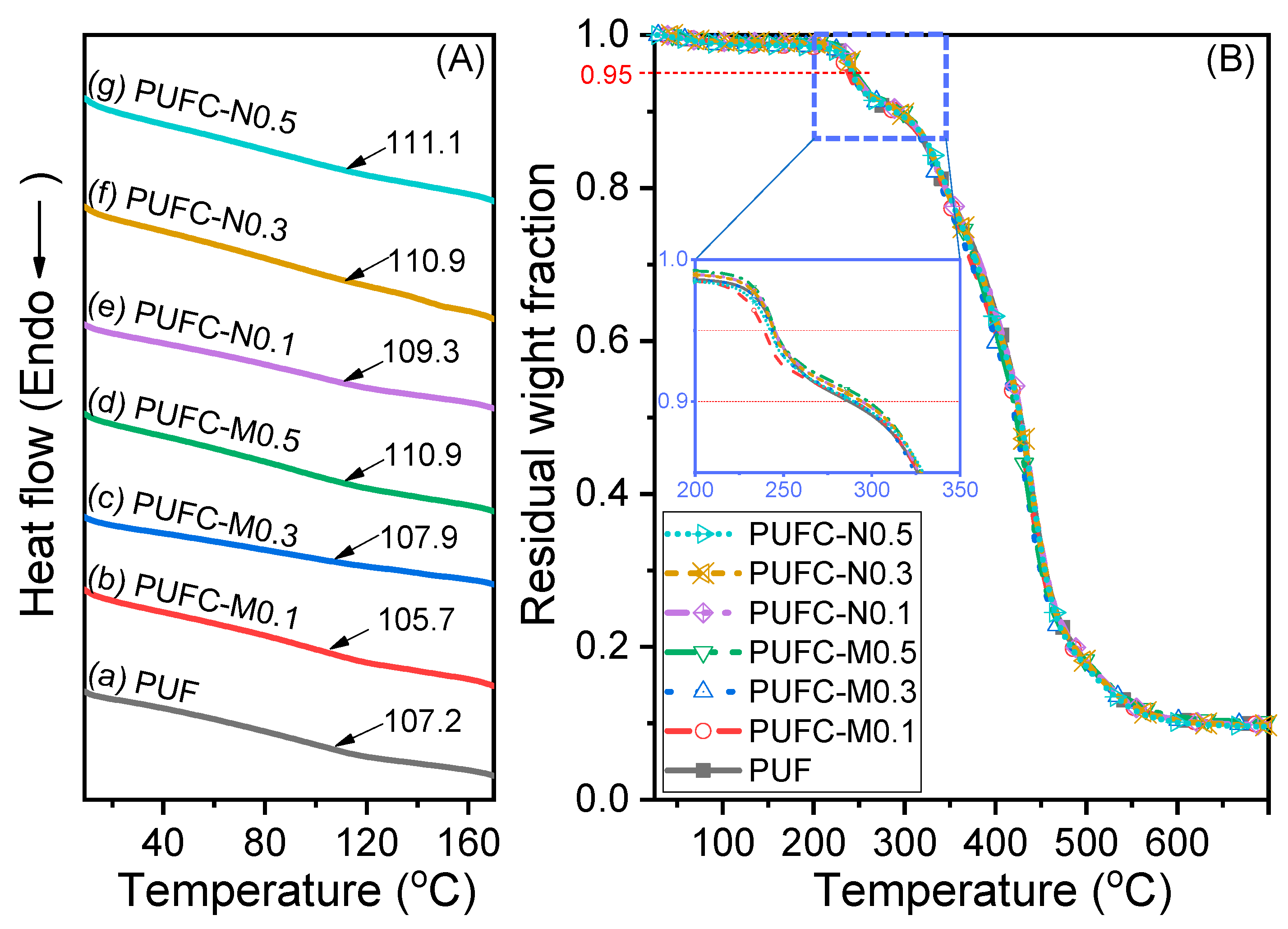
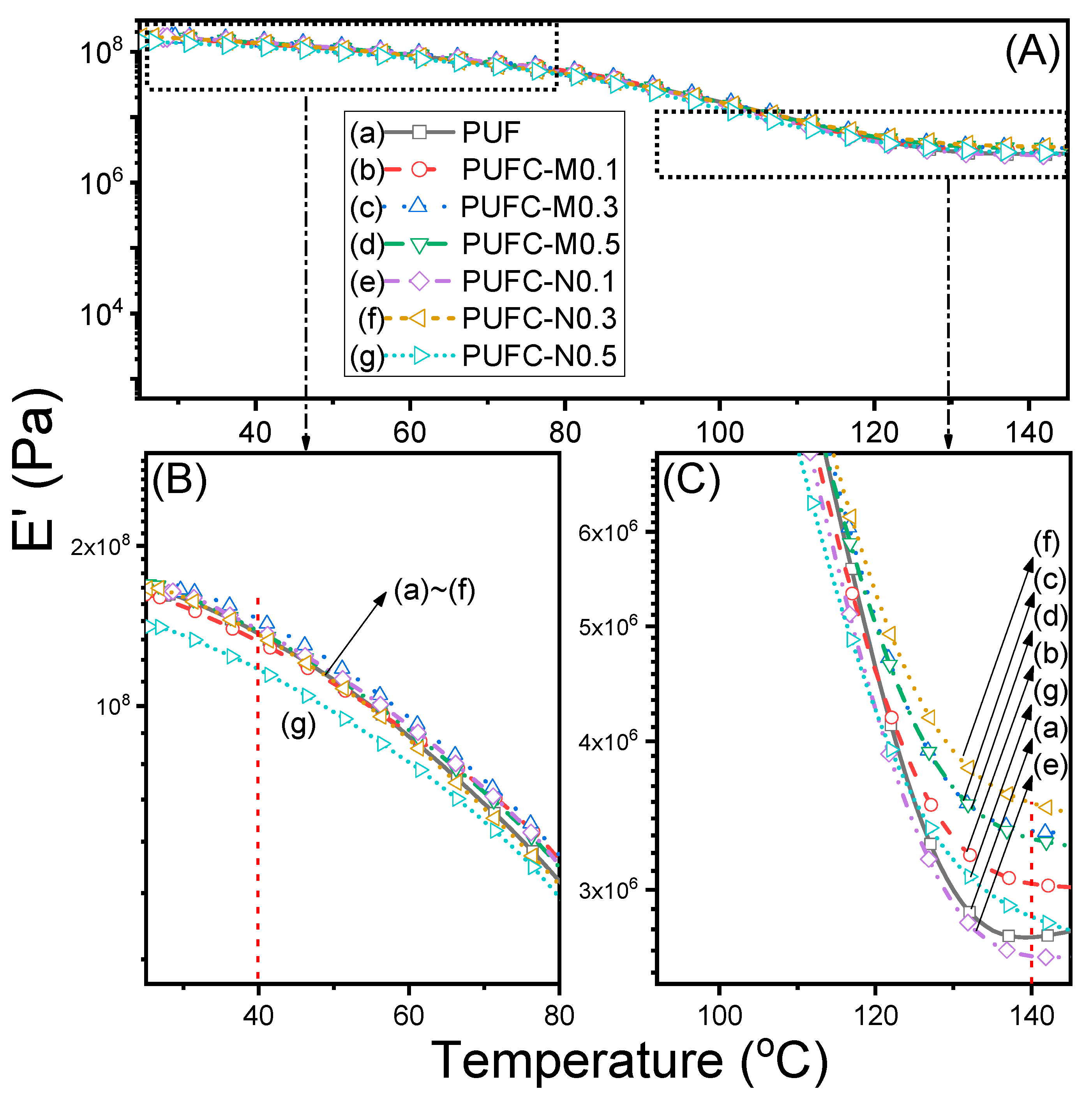
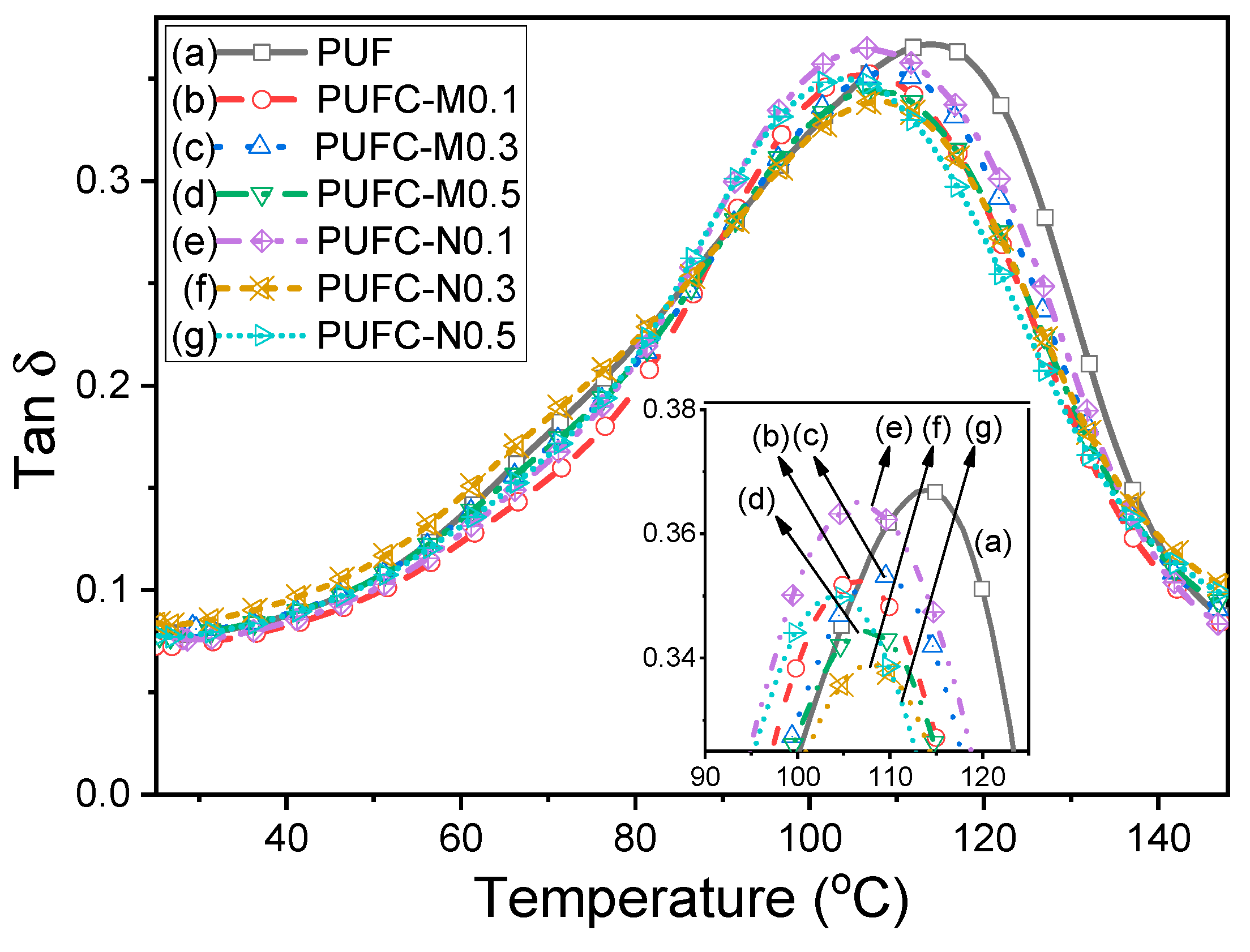
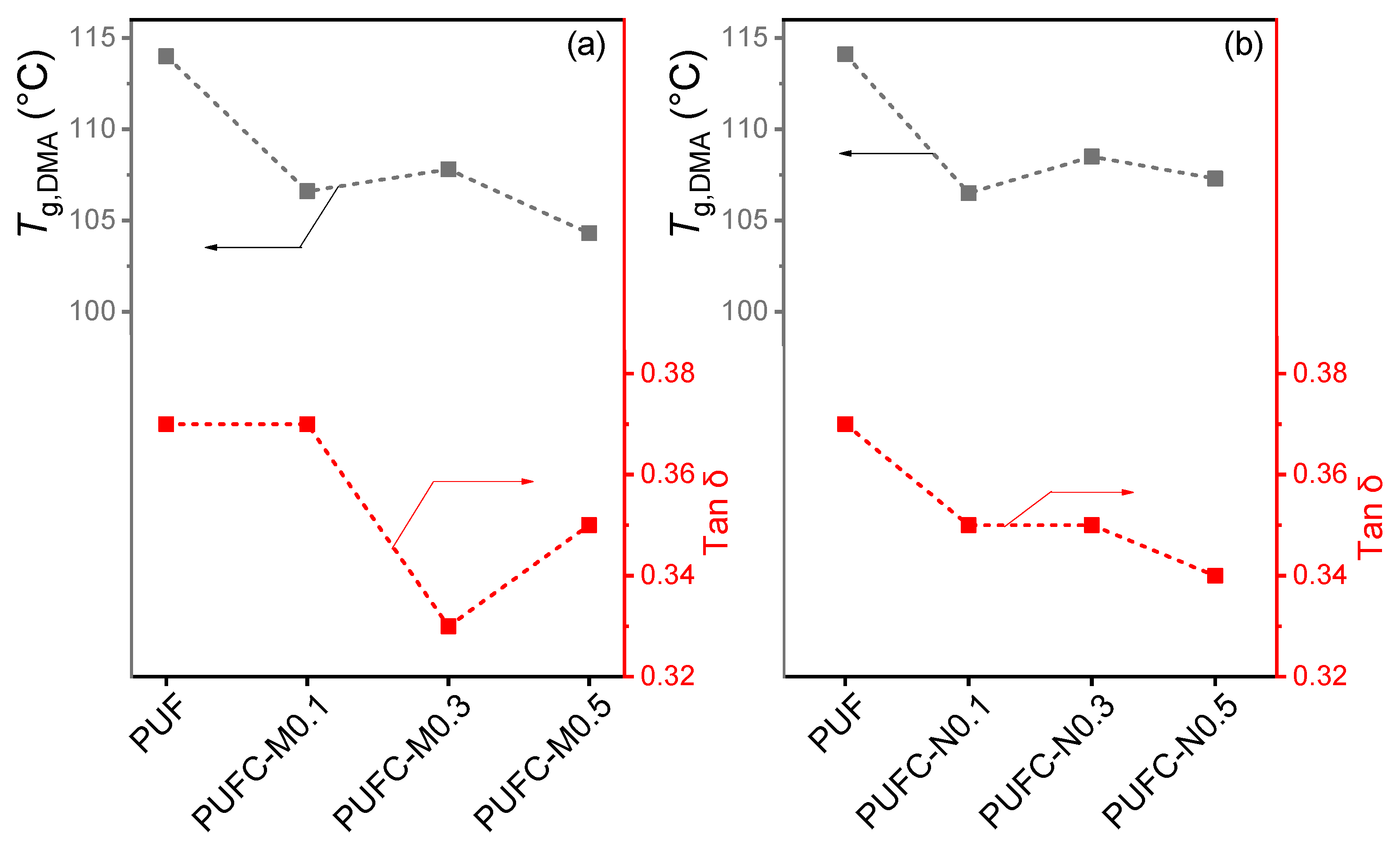
| Sample | Tg,DSC (°C) | Td5%1 (°C) | Td10%1 (°C) | Char Yield (%) | E’@40 °C (×10−8 Pa) | E’@140 °C (×10−6 Pa) | Tg,DMA2 (°C) | Tan δ |
|---|---|---|---|---|---|---|---|---|
| PUF | 105.8 | 244.3 | 277.5 | 9.9 | 1.35 | 2.72 | 114.1 | 0.37 |
| PUFC-M0.1 | 105.7 | 239.2 | 290.3 | 9.8 | 1.31 | 3.02 | 106.5 | 0.35 |
| PUFC-M0.3 | 107.9 | 244.4 | 290.1 | 9.8 | 1.45 | 3.38 | 108.5 | 0.35 |
| PUFC-M0.5 | 110.9 | 245.4 | 297.9 | 10.1 | 1.37 | 3.32 | 107.3 | 0.34 |
| PUFC-N0.1 | 109.3 | 245.3 | 294.2 | 9.7 | 1.39 | 2.64 | 106.6 | 0.37 |
| PUFC-N0.3 | 110.9 | 243.8 | 294.4 | 9.7 | 1.35 | 3.56 | 107.8 | 0.33 |
| PUFC-N0.5 | 111.1 | 243.4 | 290.4 | 9.5 | 1.16 | 2.83 | 104.3 | 0.35 |
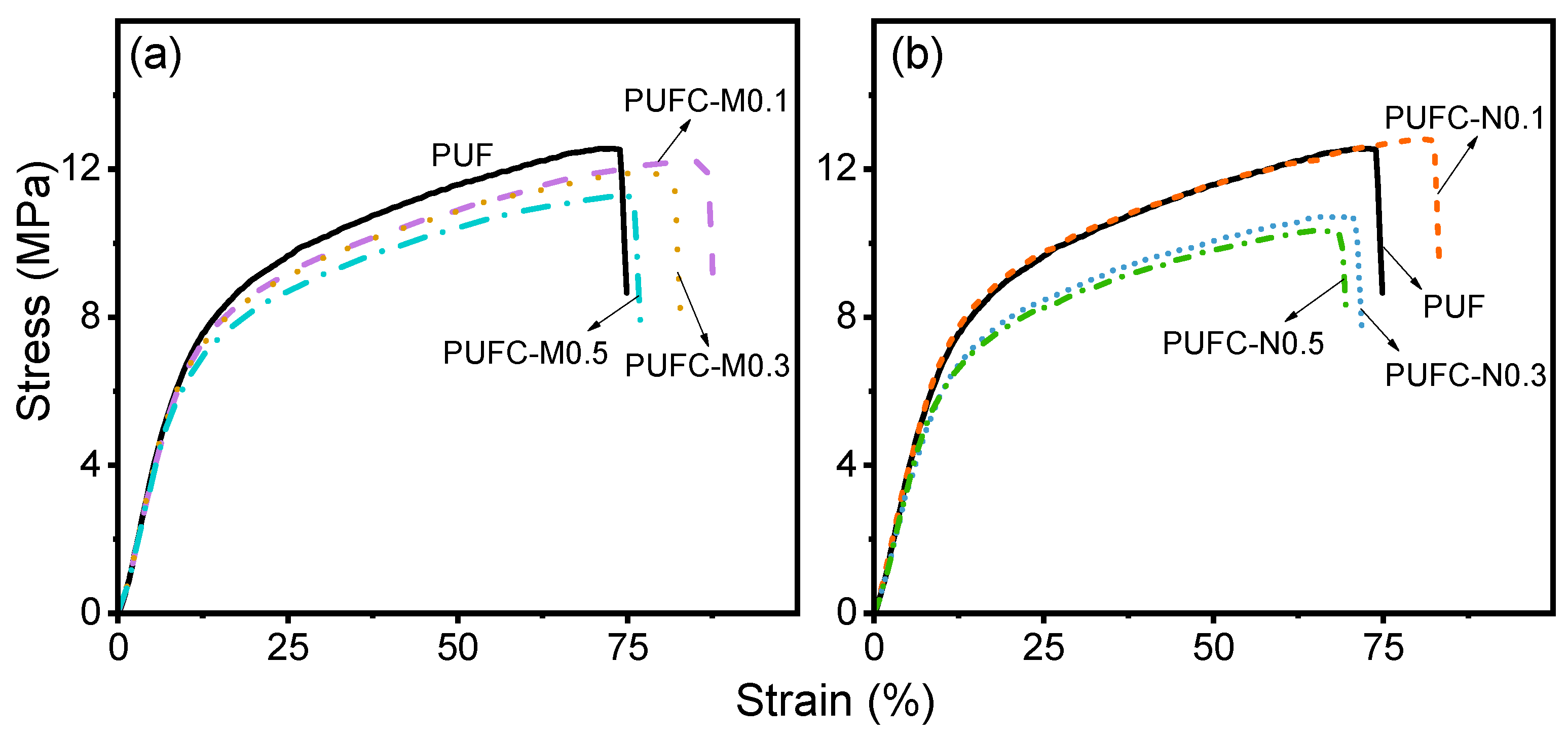
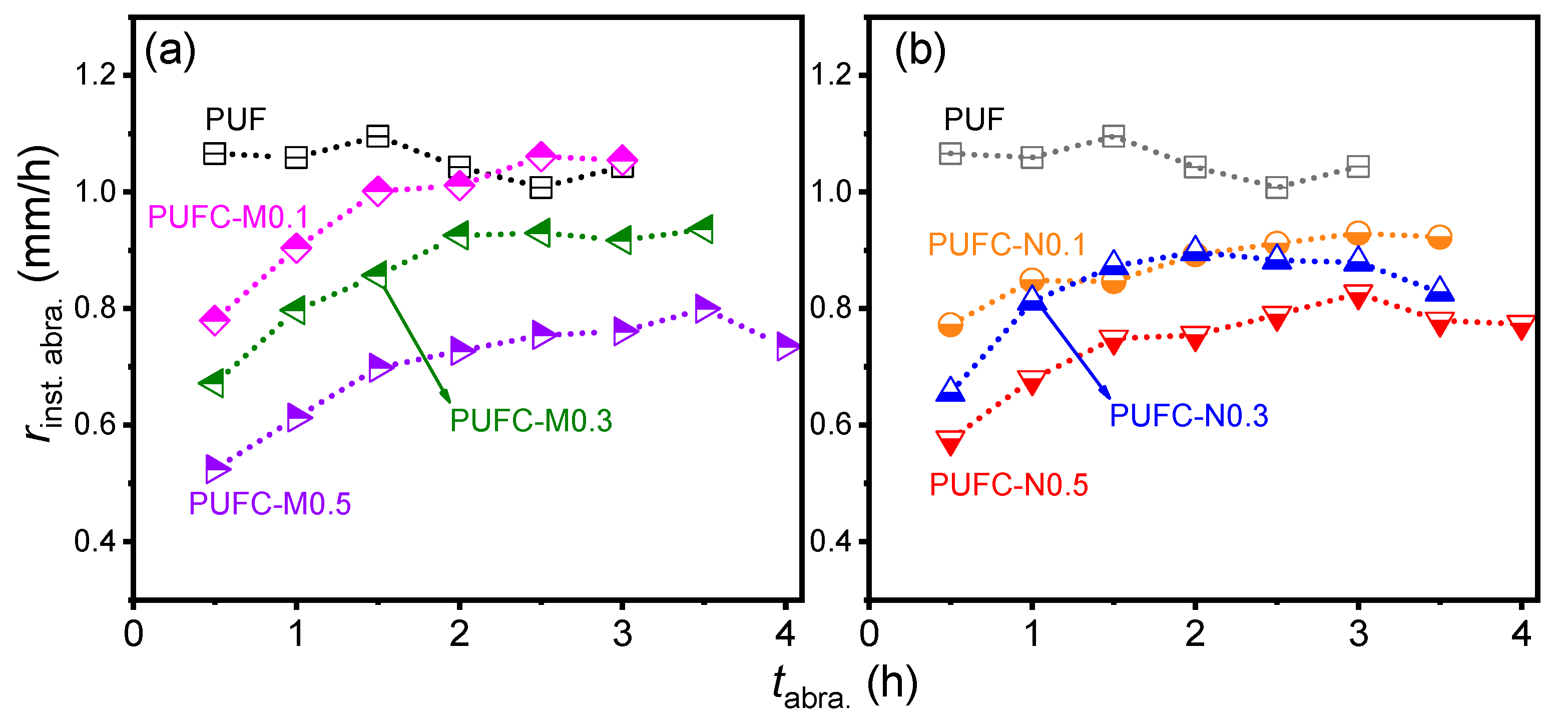
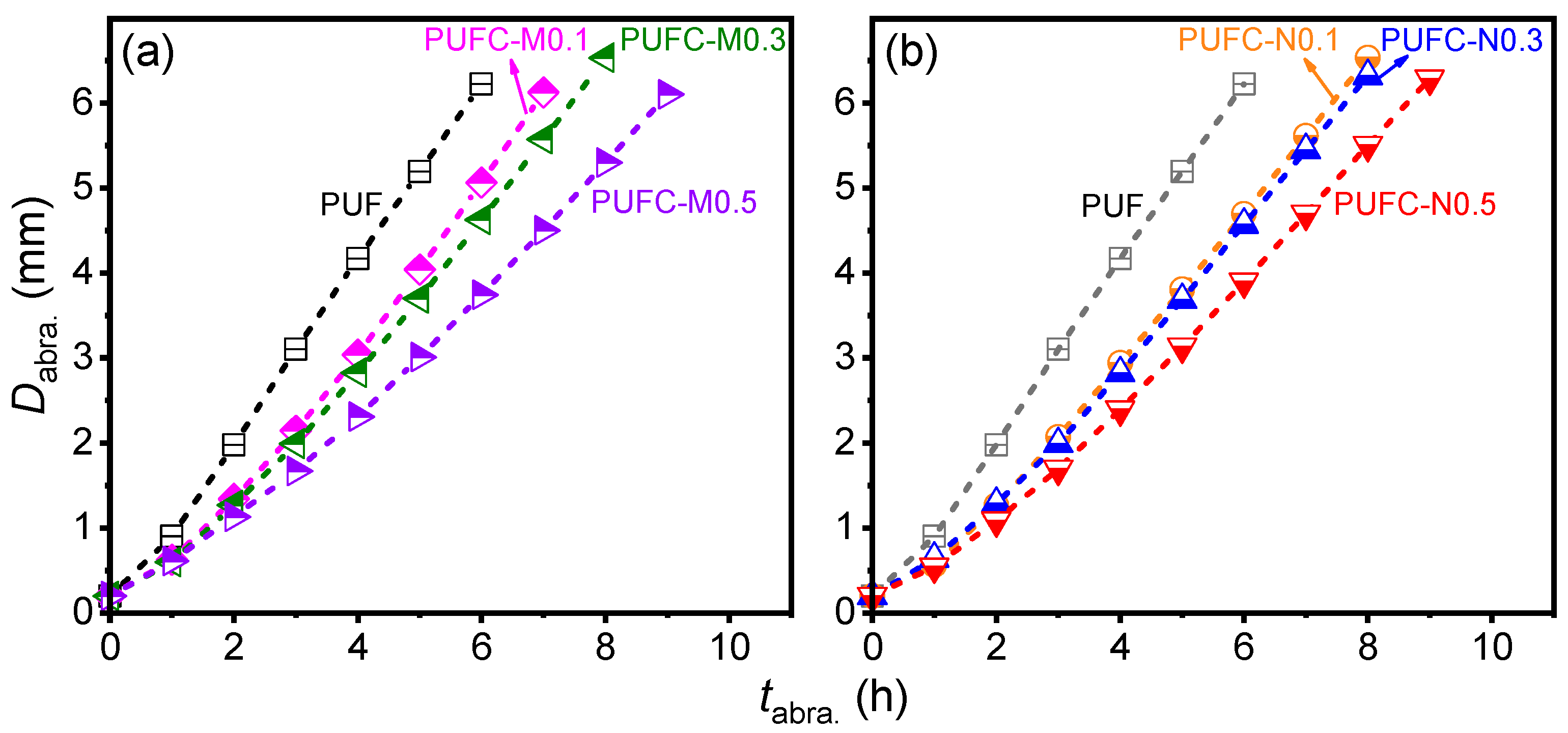
| Sample | Max. Stress (σm) 1 (MPa) | Max. Strain (εm) 1 (%) | Young’s Modulus 1 (MPa) | Toughness (U) 1 (kJ/m3) | Avg. rinst. abra. 2 (mm/h) | roverall abra.3 (mm/h) |
|---|---|---|---|---|---|---|
| PUF | 12.6 ± 0.1 | 71.2 ± 4.4 | 31 ± 0.6 | 19 ± 1.0 | 1.05 ± 0.03 | 1.04 |
| PUFC-M0.1 | 12.2 ± 0.4 | 84.2 ± 5.3 | 30 ± 1.1 | 21 ± 2.7 | 0.96 ± 0.11 | 0.98 |
| PUFC-M0.3 | 11.9 ± 0.3 | 76.9 ± 4.5 | 31 ± 0.9 | 20 ± 2.1 | 0.86 ± 0.10 | 0.91 |
| PUFC-M0.5 | 11.3 ± 0.1 | 73.5 ± 4.5 | 29 ± 0.8 | 17 ± 1.2 | 0.70 ± 0.09 | 0.76 |
| PUFC-N0.1 | 12.8 ± 0.5 | 80.2 ± 4.9 | 31 ± 0.9 | 21 ± 3.1 | 0.87 ± 0.05 | 0.89 |
| PUFC-N0.3 | 10.7 ± 0.3 | 68.5 ± 5.8 | 27 ± 0.5 | 15 ± 1.8 | 0.83 ± 0.08 | 0.85 |
| PUFC-N0.5 | 10.3 ± 0.2 | 64.9 ± 3.4 | 28 ± 0.5 | 14 ± 0.9 | 0.74 ± 0.07 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-S.; Huang, Y.-W.; Luo, Q.-W.; Lin, C.-H.; Srinophakun, P.; Alapol, S.; Lin, K.-Y.A.; Huang, C.-F. Preparations of Polyurethane Foam Composite (PUFC) Pads Containing Micro-/Nano-Crystalline Cellulose (MCC/NCC) toward the Chemical Mechanical Polishing Process. Polymers 2024, 16, 2738. https://doi.org/10.3390/polym16192738
Huang Y-S, Huang Y-W, Luo Q-W, Lin C-H, Srinophakun P, Alapol S, Lin K-YA, Huang C-F. Preparations of Polyurethane Foam Composite (PUFC) Pads Containing Micro-/Nano-Crystalline Cellulose (MCC/NCC) toward the Chemical Mechanical Polishing Process. Polymers. 2024; 16(19):2738. https://doi.org/10.3390/polym16192738
Chicago/Turabian StyleHuang, Yi-Shen, Yu-Wen Huang, Qiao-Wen Luo, Chao-Hsing Lin, Penjit Srinophakun, Supanicha Alapol, Kun-Yi Andrew Lin, and Chih-Feng Huang. 2024. "Preparations of Polyurethane Foam Composite (PUFC) Pads Containing Micro-/Nano-Crystalline Cellulose (MCC/NCC) toward the Chemical Mechanical Polishing Process" Polymers 16, no. 19: 2738. https://doi.org/10.3390/polym16192738
APA StyleHuang, Y.-S., Huang, Y.-W., Luo, Q.-W., Lin, C.-H., Srinophakun, P., Alapol, S., Lin, K.-Y. A., & Huang, C.-F. (2024). Preparations of Polyurethane Foam Composite (PUFC) Pads Containing Micro-/Nano-Crystalline Cellulose (MCC/NCC) toward the Chemical Mechanical Polishing Process. Polymers, 16(19), 2738. https://doi.org/10.3390/polym16192738




_Lin.png)



