Polyaniline Composites Containing Eco-Friendly Biomass Carbon from Agricultural-Waste Coconut Husk for Enhancing Gas Sensor Performance in Hydrogen Sulfide Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Instruments
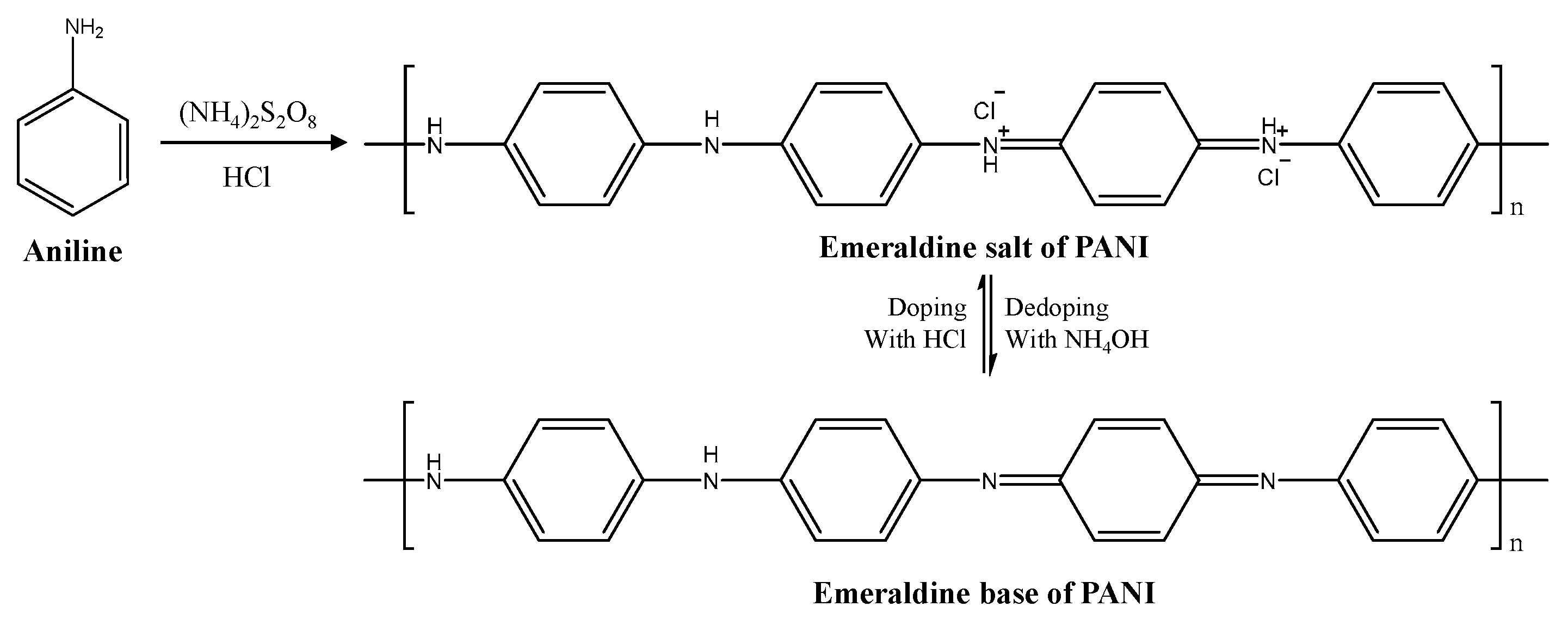
2.3. Synthesis of PANI
2.4. Preparation of CC and AC Using Agricultural-Waste Coconut Husk
2.5. Preparation of PANI-AC Composites
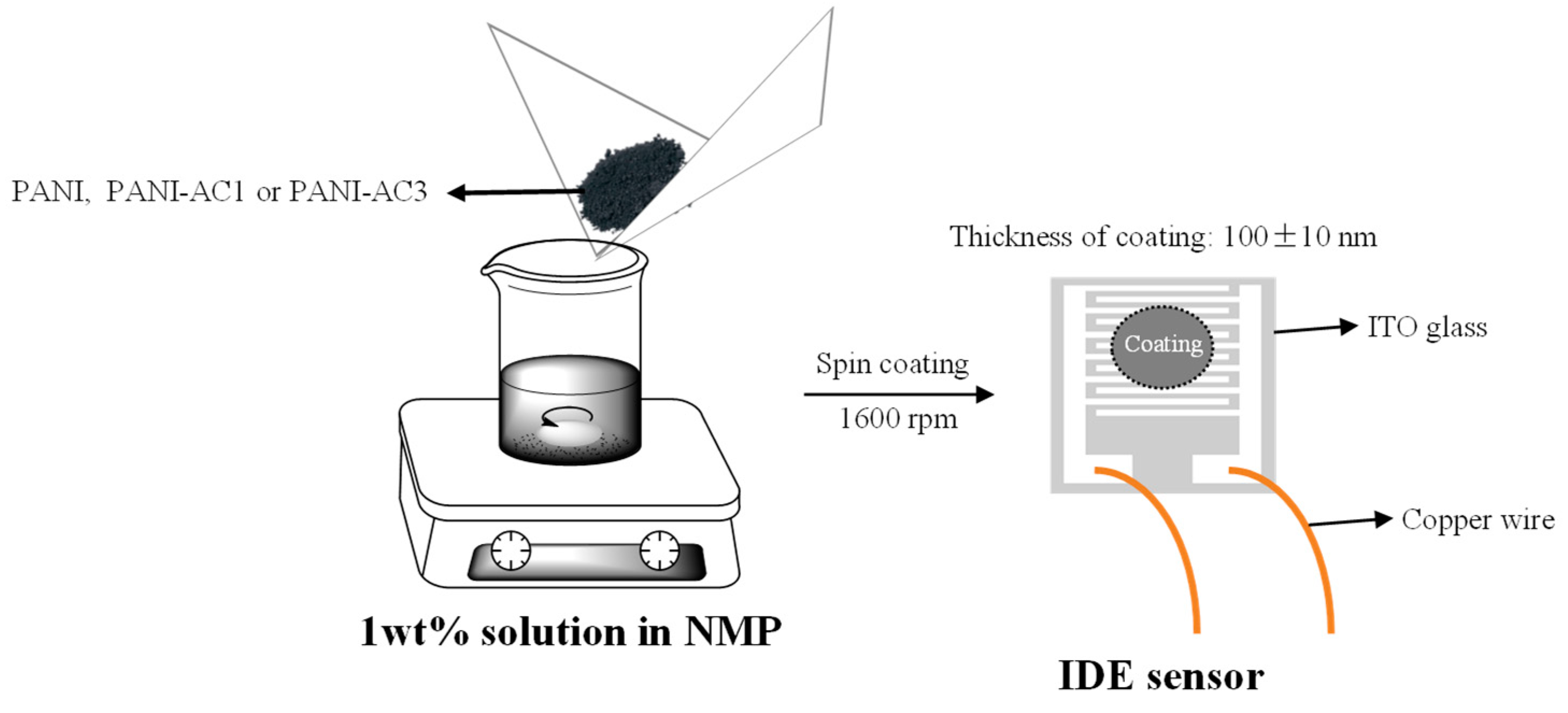
2.6. Preparation of PANI-AC Composites-Coated IDE Sensors
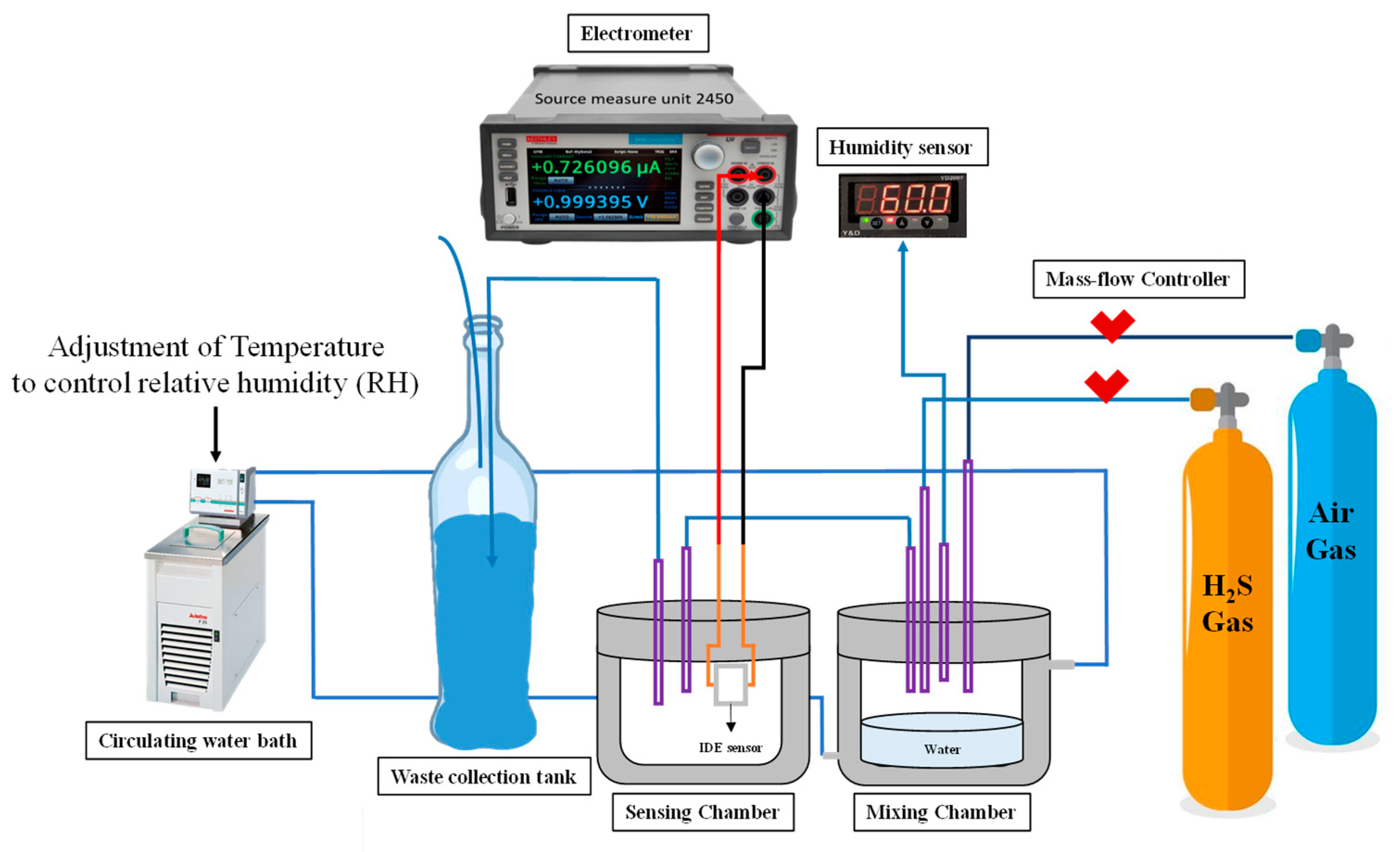
2.7. Gas-Sensing Experiment
3. Results and Discussion
3.1. Material Characterization
3.1.1. Raman Analyses of CC and AC
3.1.2. FTIR Analyses of CC, AC, PANI, and PANI-AC Composites
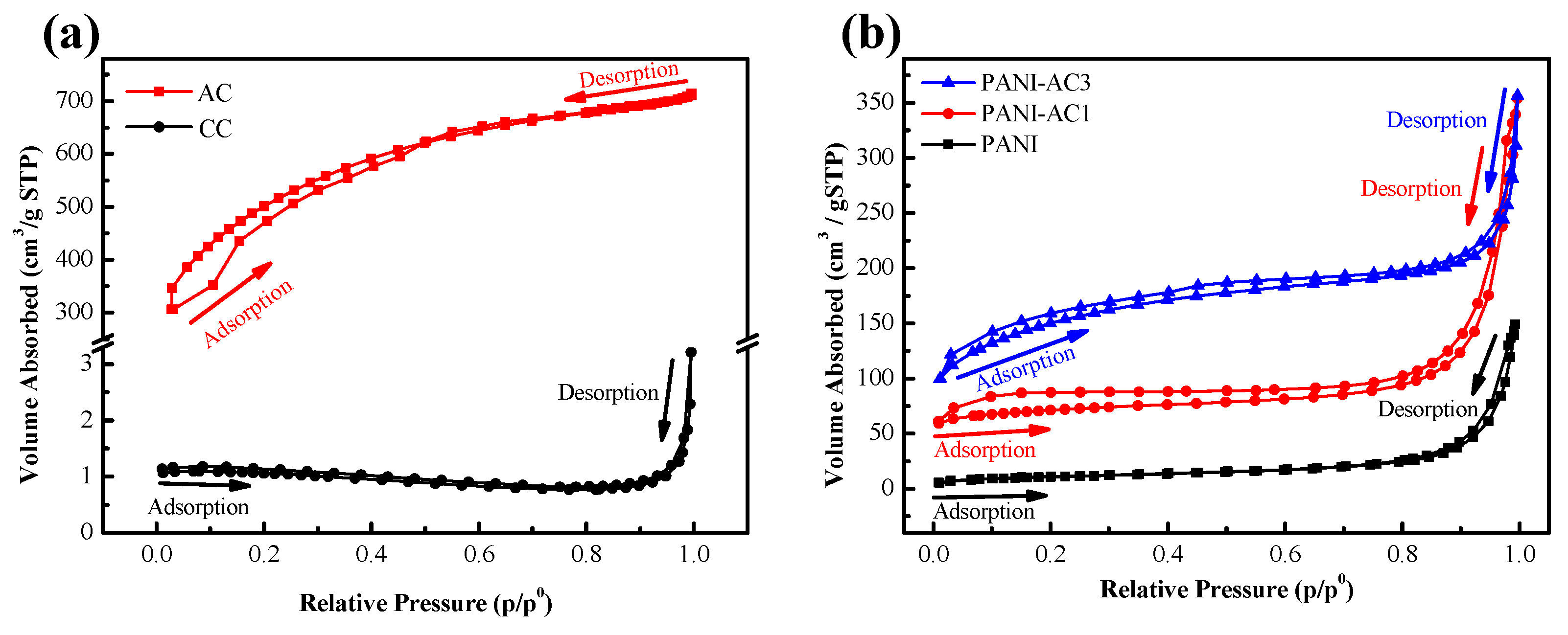
3.2. Pore Structure Characterization via the BET Analyses of CC, AC, PANI, and PANI-AC Composites
3.3. Morphologies of CC, AC, PANI, and PANI-AC Composites
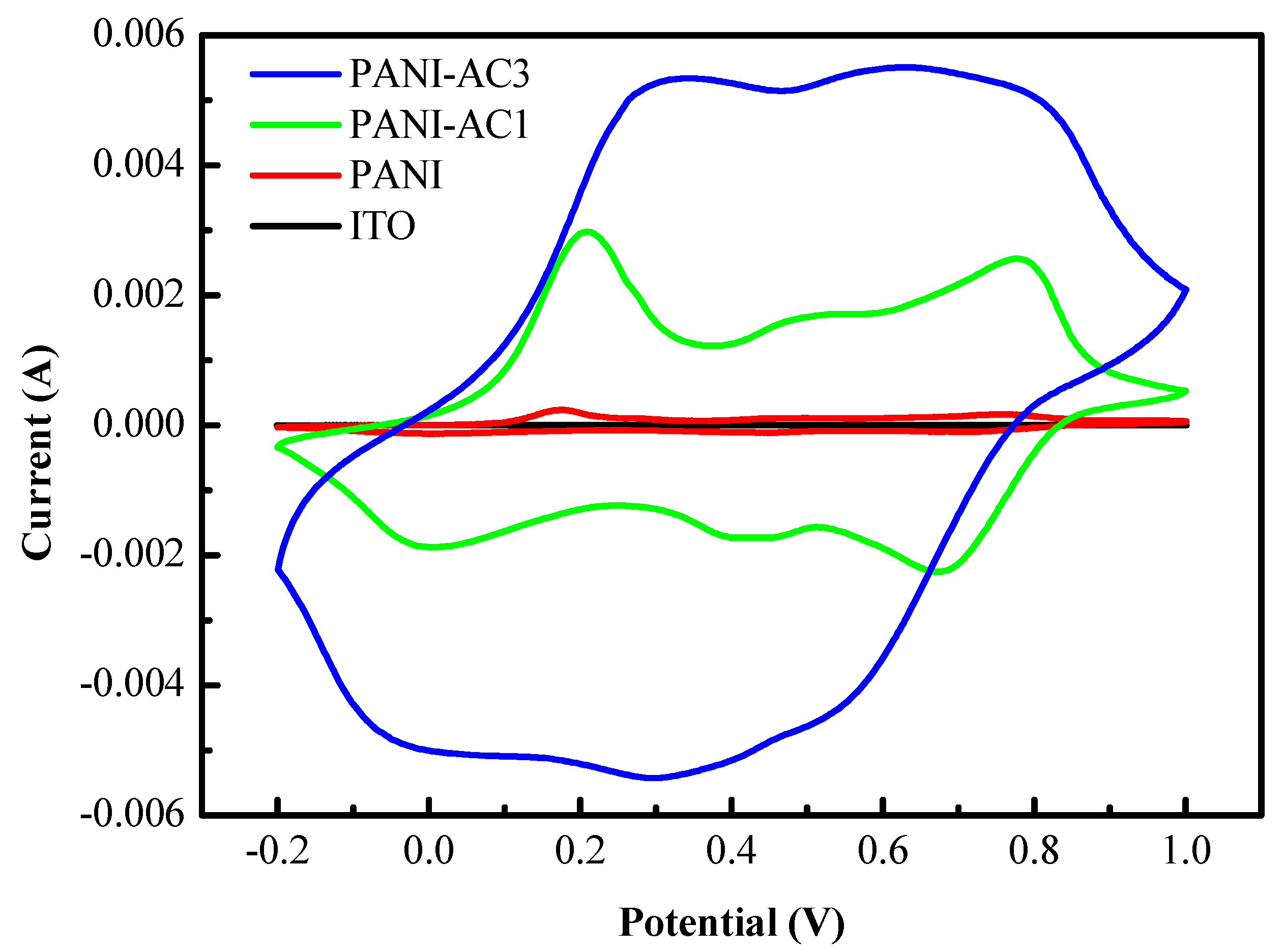
3.4. Electrochemical Redox Properties of PANI-AC Composites
3.5. Electrochemical Doping Properties of PANI-AC Composites
3.6. Gas-Sensing Performance
3.6.1. Sensitivity and Effect of Humidity
3.6.2. Repeatability, Selectivity, and Stability
3.7. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Yang, W.; Liu, L.; Chen, H.; Liu, Y. Enhanced H2S Gas-Sensing Performance of Ni-Doped ZnO Nanowire Arrays. ACS Omega 2023, 8, 7595–7601. [Google Scholar] [CrossRef] [PubMed]
- Zuo, P.; Wang, R.; Li, F.; Wu, F.; Xu, G.; Niu, W. A trace ppb-level electrochemical H2S sensor based on ultrathin Pt nanotubes. Talanta 2021, 233, 122539. [Google Scholar] [CrossRef] [PubMed]
- Laurent, D.; Barré, G.; Durlet, C.; Cartigny, P.; Carpentier, C.; Paris, G.; Collon, P.; Pironon, J.; Gaucher, E.C. Unravelling biotic versus abiotic processes in the development of large sulfuric-acid karsts. Geology 2023, 51, 262–267. [Google Scholar] [CrossRef]
- Sun, X.; Huang, W.; Ji, L.; Xu, H.; Qu, Z.; Yan, N. Establishing a Self-supporting System of H2S Production from SO2 with Induced Catalytic Reduction Process for Mercury Capture with Super-large Enrichment. Chem. Eng. J. 2023, 459, 141493. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Liu, Y.; Tong, X.; Zhou, Y. Hydrogen sulfide poisoning in forensic pathology and toxicology: Mechanism and metabolites quantification analysis. Crit. Rev. Toxicol. 2023, 52, 742–756. [Google Scholar] [CrossRef]
- Deng, G.; Muqadas, M.; Adlat, S.; Zheng, H.; Li, G.; Zhu, P.; Nasser, M.I. Protective Effect of Hydrogen Sulfide on Cerebral Ischemia–Reperfusion Injury. Cell. Mol. Neurobiol. 2023, 43, 15–25. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Xiao, D.; Wang, S.; Zhang, T.; Yang, X.; Heng, S.; Sun, M. CuO/WO3 hollow microsphere P-N heterojunction sensor for continuous cycle detection of H2S gas. Sens. Actuators B Chem. 2023, 374, 132823. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Song, Z.; Kan, H.; Yu, H.; Liu, Q.; Zhang, G.; Liu, H. Sensitive H2S gas sensors employing colloidal zinc oxide quantum dots. Sens. Actuators B Chem. 2017, 249, 558–563. [Google Scholar] [CrossRef]
- Bang, J.H.; Mirzaei, A.; Choi, M.S.; Han, S.; Lee, H.Y.; Kim, S.S.; Kim, H.W. Decoration of multi-walled carbon nanotubes with CuO/Cu2O nanoparticles for selective sensing of H2S gas. Sens. Actuators B Chem. 2021, 344, 130176. [Google Scholar] [CrossRef]
- Keshtkar, S.; Rashidi, A.; Kooti, M.; Askarieh, M.; Pourhashem, S.; Ghasemy, E.; Izadi, N. A novel highly sensitive and selective H2S gas sensor at low temperatures based on SnO2 quantum dots-C60 nanohybrid: Experimental and theory study. Talanta 2018, 188, 531–539. [Google Scholar] [CrossRef]
- Kapse, V.D.; Ghosh, S.A.; Chaudhari, G.N.; Raghuwanshi, F.C. Nanocrystalline In2O3-based H2S sensors operable at low temperatures. Talanta 2008, 76, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Bodade, A.B.; Bodade, A.B.; Wankhade, H.G.; Chaudhari, G.N.; Kothari, D.C. Conduction mechanism and gas sensing properties of CoFe2O4 nanocomposite thick films for H2S gas. Talanta 2012, 89, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zhang, S.; Huang, C.; Guo, X.; Zhu, Y.; Thomas, T.; Guo, H.; Attfield, J.P.; Yang, M. Surface Functionalized Sensors for Humidity-Independent Gas Detection. Angew. Chem. Int. Ed. Engl. 2021, 60, 6561–6566. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.K.; Panda, S. Effect of moisture and molecular weight of polyaniline on H2S sensing characteristics. Sens. Actuators B Chem. 2021, 344, 130323. [Google Scholar] [CrossRef]
- Yavarinasab, A.; Janfaza, S.; Tahmooressi, H.; Ghazi, M.; Tasnim, N.; Hoorfar, M. A selective polypyrrole-based sub-ppm impedimetric sensor for the detection of dissolved hydrogen sulfide and ammonia in a mixture. J. Hazard. Mater. 2021, 416, 125892. [Google Scholar] [CrossRef] [PubMed]
- Agbor, N.; Petty, M.; Monkman, A. Polyaniline thin films for gas sensing. Sens. Actuators B Chem. 1995, 28, 173–179. [Google Scholar] [CrossRef]
- Lv, D.; Shen, W.; Chen, W.; Wang, Y.; Tan, R.; Zhao, M.; Song, W. One-step preparation of flexible citric acid-doped polyaniline gas sensor for ppb-level ammonia detection at room temperature. Sens. Actuators A Phys. 2023, 350, 114120. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, X.; Zhang, H.; Wu, C.; Su, Y.; Li, P.; Jiang, J.; Liu, A.; Li, H.; Yang, Q. Investigation on the electromagnetic interference shielding performance of hydrochloric acid doped polyaniline: An insight into the protonation. J. Mater. Sci. Mater. Electron. 2023, 34, 769. [Google Scholar] [CrossRef]
- Liu, S.; Qin, Y.; Bai, Y. Highly response and humidity-resistant gas sensor based on polyaniline-functionalized Bi2MoO6 with UV activation. Electrochim. Acta 2022, 427, 140863. [Google Scholar] [CrossRef]
- Kumar, A.; Kostikov, Y.; Zanatta, M.; Sorarù, G.D.; Orberger, B.; Nessim, G.D.; Mariotto, G. Carbon nanotubes synthesis using siliceous breccia as a catalyst source. Diam. Relat. Mater. 2019, 97, 107433. [Google Scholar] [CrossRef]
- Barraza-García, F.d.J.; Caballero-Briones, F.; Morelos-Gómez, A.; Martínez-Villegas, N.; Hernández-Martínez, J.L.; Endo, M.; López-Urías, F.; Muñoz-Sandoval, E. The synthesis of sponge-type nitrogen-doped multiwall carbon nanotubes using ball-milled natural red-leptosol as catalyst precursor: A cycle voltammetry study. Carbon 2022, 196, 510–524. [Google Scholar] [CrossRef]
- Rathour, R.K.; Devi, M.; Dahiya, P.; Sharma, N.; Kaushik, N.; Kumari, D.; Kumar, P.; Baadhe, R.R.; Walia, A.; Bhatt, A.K. Recent trends, opportunities and challenges in sustainable management of rice straw waste biomass for green biorefinery. Energies 2023, 16, 1429. [Google Scholar] [CrossRef]
- Adetona, A.B.; Layzell, D.B. Diverting residual biomass to energy use: Quantifying the global warming potential of biogenic CO2 (GWPbCO2). GCB Bioenergy 2023, 15, 697–709. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, S.; Kaur, A.; Tokas, D.; Kathi, S.; Singh, A.N. Harnessing Energy from Animal Waste: A Win–Win Approach for India. In Manure Technology and Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2023; pp. 283–304. [Google Scholar]
- Jayanthi, V.; Avudaiappan, S.; Amran, M.; Arunachalam, K.P.; Qader, D.N.; Delgado, M.C.; Flores, E.I.S.; Rashid, R.S. Innovative use of micronized biomass silica-GGBS as agro-industrial by-products for the production of a sustainable high-strength geopolymer concrete. Case Stud. Constr. Mater. 2023, 18, e01782. [Google Scholar] [CrossRef]
- Polvara, E.; Gallego, E.; Invernizzi, M.; Perales, J.F.; Sironi, S. Chemical characterization of odorous emissions: A comparative performance study of different sampling methods. Talanta 2023, 253, 124110. [Google Scholar] [CrossRef]
- Safian, M.T.-U.; Sekeri, S.H.; Yaqoob, A.A.; Serrà, A.; Jamudin, M.D.; Mohamad Ibrahim, M.N. Utilization of lignocellulosic biomass: A practical journey towards the development of emulsifying agent. Talanta 2022, 239, 123109. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Maji, S.; Shrestha, R.G.; Shrestha, R.L.; Shrestha, T.; Ariga, K.; Shrestha, L.K. Jackfruit Seed-Derived Nanoporous Carbons as the Electrode Material for Supercapacitors. C 2020, 6, 73. [Google Scholar] [CrossRef]
- Wang, N.; Ye, J.-X.; Sun, J.-B.; Zhang, X.-F.; Deng, Z.-P.; Xu, Y.-M.; Huo, L.-H.; Gao, S. Rapid and accurate detection of highly toxic NO2 gas based on catkins biomass-derived porous In2O3 microtubes at low temperature. Sens. Actuators B Chem. 2022, 361, 131692. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, H.; Wang, H.; Zhao, X.; Sun, S.; Shi, J.; Huang, M.; Liu, W.; Zheng, Y.; Li, P. Dual-doped hierarchical porous carbon derived from biomass for advanced supercapacitors and lithium ion batteries. RSC Adv. 2019, 9, 32382–32394. [Google Scholar] [CrossRef]
- Kaipannan, S.; Marappan, S. Fabrication of 9.6 V High-performance Asymmetric Supercapacitors Stack Based on Nickel Hexacyanoferrate-derived Ni(OH)2 Nanosheets and Bio-derived Activated Carbon. Sci. Rep. 2019, 9, 1104. [Google Scholar] [CrossRef]
- Liu, S.; Pan, M.; Feng, Z.; Qin, Y.; Wang, Y.; Tan, L.; Sun, T. Ultra-high adsorption of tetracycline antibiotics on garlic skin-derived porous biomass carbon with high surface area. New J. Chem. 2020, 44, 1097–1106. [Google Scholar] [CrossRef]
- Ghosh, S.; Santhosh, R.; Jeniffer, S.; Raghavan, V.; Jacob, G.; Nanaji, K.; Kollu, P.; Jeong, S.K.; Grace, A.N. Natural biomass derived hard carbon and activated carbons as electrochemical supercapacitor electrodes. Sci. Rep. 2019, 9, 16315. [Google Scholar] [CrossRef] [PubMed]
- Yorgun, S.; Vural, N.; Demiral, H. Preparation of high-surface area activated carbons from Paulownia wood by ZnCl2 activation. Microporous Mesoporous Mater. 2009, 122, 189–194. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Cheng, C.K.; Luque, R.; Thomas, S.; Banh, T.L.; Nguyen, X.P. Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review. Chemosphere 2022, 287, 131959. [Google Scholar] [CrossRef]
- Idris, M.O.; Guerrero–Barajas, C.; Kim, H.-C.; Yaqoob, A.A.; Ibrahim, M.N.M. Scalability of biomass-derived graphene derivative materials as viable anode electrode for a commercialized microbial fuel cell: A systematic review. Chin. J. Chem. Eng. 2022, 55, 277–292. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, X.-F.; Xu, T.-T.; Deng, Z.-P.; Xu, Y.-M.; Huo, L.-H.; Gao, S. A spendable gas sensor with higher sensitivity and lowest detection limit towards H2S: Porous α-Fe2O3 hierarchical tubule derived from poplar branch. Chem. Eng. J. 2020, 392, 123679. [Google Scholar] [CrossRef]
- Li, C.; Song, B.-Y.; Teng, Y.; Zhang, X.-F.; Deng, Z.-P.; Xu, Y.-M.; Huo, L.-H.; Gao, S. Biomass-derived hierarchical porous ZnO microtubules for highly selective detection of ppb-level nitric oxide at low temperature. Sens. Actuators B Chem. 2021, 333, 129627. [Google Scholar] [CrossRef]
- Zhao, D.-L.; Zeng, X.-W.; Shen, Z.-M. Synthesis of carbon nanotube/polyaniline composite nanotube and its microwave permittivity. Acta Phys. Sin. 2005, 54, 3878–3883. [Google Scholar] [CrossRef]
- Zheng, B.-n.; Gao, C. Preparation of graphene nanoscroll/polyaniline composites and their use in high performance supercapacitors. New Carbon Mater. 2016, 31, 315–320. [Google Scholar] [CrossRef]
- Tran, T.P.; Do, Q.H. High-Performance Supercapacitor Electrode Based on Buckypaper/Polyaniline Composite. J. Electron. Mater. 2017, 46, 6056–6062. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Megha, R.; Ravikiran, Y.T.; Chethan, B.; Raj Prakash, H.G.; Vijaya Kumari, S.C.; Thomas, S. Effect of mechanical mixing method of preparation of polyaniline-transition metal oxide composites on DC conductivity and humidity sensing response. J. Mater. Sci. Mater. Electron. 2018, 29, 7253–7261. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Shrestha, T.; Tamrakar, B.M.; Shrestha, R.G.; Maji, S.; Ariga, K.; Shrestha, L.K. Nanoporous carbon materials derived from washnut seed with enhanced supercapacitance. Materials 2020, 13, 2371. [Google Scholar] [CrossRef] [PubMed]
- Hirst, E.A.; Taylor, A.; Mokaya, R. A simple flash carbonization route for conversion of biomass to porous carbons with high CO2 storage capacity. J. Mater. Chem. A 2018, 6, 12393–12403. [Google Scholar] [CrossRef]
- Valencia, A.; Muñiz-Valencia, R.; Ceballos-Magaña, S.G.; Rojas-Mayorga, C.K.; Bonilla-Petriciolet, A.; González, J.; Aguayo-Villarreal, I.A. Cyclohexane and benzene separation by fixed-bed adsorption on activated carbons prepared from coconut shell. Environ. Technol. Innov. 2022, 25, 102076. [Google Scholar] [CrossRef]
- Rawal, S.; Joshi, B.; Kumar, Y. Synthesis and characterization of activated carbon from the biomass of Saccharum bengalense for electrochemical supercapacitors. J. Energy Storage 2018, 20, 418–426. [Google Scholar] [CrossRef]
- Sujiono, E.H.; Zabrian, D.; Zurnansyah; Mulyati; Zharvan, V.; Samnur; Humairah, N.A. Fabrication and characterization of coconut shell activated carbon using variation chemical activation for wastewater treatment application. Results Chem. 2022, 4, 100291. [Google Scholar] [CrossRef]
- Kesavan, T.; Partheeban, T.; Vivekanantha, M.; Kundu, M.; Maduraiveeran, G.; Sasidharan, M. Hierarchical nanoporous activated carbon as potential electrode materials for high performance electrochemical supercapacitor. Microporous Mesoporous Mater. 2019, 274, 236–244. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Chonat, A.; Palatty, S. Enhanced electrochemical performance of a hybrid supercapacitive material based on ternary doped polyaniline/activated carbon composite. Energy Fuels 2020, 34, 10148–10159. [Google Scholar] [CrossRef]
- Divyashree, A.; Manaf, S.A.B.A.; Yallappa, S.; Chaitra, K.; Kathyayini, N.; Hegde, G. Low cost, high performance supercapacitor electrode using coconut wastes: Eco-friendly approach. J. Energy Chem. 2016, 25, 880–887. [Google Scholar]
- Li, S.; Zhang, N.; Zhou, H.; Li, J.; Gao, N.; Huang, Z.; Jiang, L.; Kuang, Y. An all-in-one material with excellent electrical double-layer capacitance and pseudocapacitance performances for supercapacitor. Appl. Surf. Sci. 2018, 453, 63–72. [Google Scholar] [CrossRef]
- Zaihua, D.; Qiuni, Z.; Si, W.; Qi, H.; Zhen, Y.; Yajie, Z.; Yadong, J.; Huiling, T. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B Chem. 2020, 317, 128204. [Google Scholar]
- Wang, J.; Zhang, D.; Gao, Y.; Chen, F.; Wang, T.; Xia, H.; Sui, X.; Wang, Z. Fast-response hydrogen sulfide gas sensor based on electrospinning Co3O4 nanofibers-modified CuO nanoflowers: Experimental and DFT calculation. Sens. Actuators B Chem. 2023, 396, 134579. [Google Scholar] [CrossRef]
- Ahmed, M.M.M.; Chi, Y.-T.; Hung, Y.-H.; Reyes, L.M.C.; Yeh, J.-M. UV-cured electroactive polyurethane acrylate coatings with superhydrophobic surface structure of biomimetic peacock feather for anticorrosion application. Prog. Org. Coat. 2022, 165, 106679. [Google Scholar] [CrossRef]
- Yoon, S.-B.; Yoon, E.-H.; Kim, K.-B. Electrochemical properties of leucoemeraldine, emeraldine, and pernigraniline forms of polyaniline/multi-wall carbon nanotube nanocomposites for supercapacitor applications. J. Power Sources 2011, 196, 10791–10797. [Google Scholar] [CrossRef]
- Dhand, C.; Arya, S.K.; Singh, S.P.; Singh, B.P.; Datta, M.; Malhotra, B.D. Preparation of polyaniline/multiwalled carbon nanotube composite by novel electrophoretic route. Carbon 2008, 46, 1727–1735. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.R.M.; Xian-Lun, L.; Sathishkumar, N.; Chen, C.-Y.; Santiago, K.S.; Chen, H.-T.; Lin, Y.-F.; Yeh, J.-M. Detection of hydrogen sulfide using polyaniline incorporated with graphene oxide aerogel. Synth. Met. 2021, 282, 116934. [Google Scholar] [CrossRef]
- Wang, L.; Huang, H.; Xiao, S.; Cai, D.; Liu, Y.; Liu, B.; Wang, D.; Wang, C.; Li, H.; Wang, Y.; et al. Enhanced Sensitivity and Stability of Room-Temperature NH3 Sensors Using Core–Shell CeO2 Nanoparticles@Cross-linked PANI with p–n Heterojunctions. ACS Appl. Mater. Interfaces 2014, 6, 14131–14140. [Google Scholar] [CrossRef]
- Akber, H.J.; Ibrahim, I.M.; Razeg, K.H. Hydrothermal Synthesis of Polyaniline Nano-fibers as H2S Gas Sensor. J. Phys. Conf. Ser. 2020, 1664, 012017. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, K.; Sun, J.; Zhang, D.; Luo, R.; Li, D.; Liu, C. Polythiophene-WO3 hybrid architectures for low-temperature H2S detection. Sens. Actuators B Chem. 2014, 197, 142–148. [Google Scholar] [CrossRef]
- Nerkar, D.M.; Jaware, S.E.; Padhye, G. Fabrication of a Novel Flexible Room Temperature Hydrogen Sulfide (H2S) Gas Sensor based on Polypyrrole Films. Int. J. Sci. Res. 2016, 5, 106–111. [Google Scholar] [CrossRef]
- Hosseini-Shokouh, S.H.; Zhou, J.; Berger, E.; Lv, Z.-P.; Hong, X.; Virtanen, V.; Kordas, K.; Komsa, H.-P. Highly Selective H2S Gas Sensor Based on Ti3C2Tx MXene–Organic Composites. ACS Appl. Mater. Interfaces 2023, 15, 7063–7073. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hani, A.F.S.; Awwad, F.; Greish, Y.E.; Ayesh, A.I.; Mahmoud, S.T. Design, fabrication, and characterization of low-power gas sensors based on organic-inorganic nano-composite. Org. Electron. 2017, 42, 284–292. [Google Scholar] [CrossRef]
- Hasan, M.; Ibrahim, I.M.; Bakr, N. The Sensitivity of PolyPyrrole NanoTube/(Ag NanoParticle, Ag-NiO nanocomposite) Against H2S Toxic Gas at Low Temperature. Sens. Transducers 2020, 243, 31–41. [Google Scholar]
- Garg, R.; Kumar, V.; Kumar, D.; Chakarvarti, S.K. Polypyrrole Microwires as Toxic Gas Sensors for Ammonia and Hydrogen Sulphide. J. Syst. Integr. 2015, 3, 1–13. [Google Scholar] [CrossRef]
- Geng, L. Gas sensitivity study of polypyrrole/WO3 hybrid materials to H2S. Synth. Met. 2010, 160, 1708–1711. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]






| Sample Code | SBET (m2/g) a | Vt (cm3/g) b | Dp (nm) c |
|---|---|---|---|
| CC | 5 | 0.01 | 42.7 |
| AC | 1718 | 1.10 | 2.6 |
| PANI | 38 | 0.21 | 22.8 |
| PANI-AC1 | 225 | 0.43 | 16.6 |
| PANI-AC3 | 416 | 0.93 | 4.2 |
| Sample Code | Conductivity (S/cm) | |||
|---|---|---|---|---|
| Undoped | Doped | Dedoped | Redoped | |
| PANI | 6.02 × 10−6 | 2.24 × 10−3 | 6.42 × 10−6 | 2.2 × 10−3 |
| PANI-AC1 | 6.35 × 10−6 | 4.96 × 10−3 | 7.07 × 10−6 | 4.46 × 10−3 |
| PANI-AC3 | 7.60 × 10−6 | 3.87 × 10−2 | 7.81 × 10−6 | 3.65 × 10−2 |
| Sample Code | Response (Ra/Rg) | Gas Concentration (ppm) | Operating Temperature (°C) | Ref. |
|---|---|---|---|---|
| PANI-NF | 7.32 | 25 | 150 | [62] |
| PTh-WO3 | 1.4 | 200 | 70 | [63] |
| PPy film | 5 | 10 | 25 | [64] |
| Ti3C2Tx/PDS-Cl | 2.2 | 5 | 30 | [65] |
| PVA-IL-WO3 | 15 | 10 | 80 | [66] |
| Ag NPs-PPy NTs | 1.3 | 20 | 25 | [67] |
| PPy microwire | 1.85 | 200 | 25 | [68] |
| PPy/WO3 | 1.61 | 1000 | 90 | [69] |
| PANI-AC3 | 8.6 | 10 | 25 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, K.-H.; Yan, M.; Hung, Y.-H.; Kuang, J.-Y.; Chang, H.-C.; Lai, Y.-J.; Yeh, J.-M. Polyaniline Composites Containing Eco-Friendly Biomass Carbon from Agricultural-Waste Coconut Husk for Enhancing Gas Sensor Performance in Hydrogen Sulfide Detection. Polymers 2023, 15, 4554. https://doi.org/10.3390/polym15234554
Luo K-H, Yan M, Hung Y-H, Kuang J-Y, Chang H-C, Lai Y-J, Yeh J-M. Polyaniline Composites Containing Eco-Friendly Biomass Carbon from Agricultural-Waste Coconut Husk for Enhancing Gas Sensor Performance in Hydrogen Sulfide Detection. Polymers. 2023; 15(23):4554. https://doi.org/10.3390/polym15234554
Chicago/Turabian StyleLuo, Kun-Hao, Minsi Yan, Yu-Han Hung, Jia-Yu Kuang, Hsing-Chih Chang, Ying-Jang Lai, and Jui-Ming Yeh. 2023. "Polyaniline Composites Containing Eco-Friendly Biomass Carbon from Agricultural-Waste Coconut Husk for Enhancing Gas Sensor Performance in Hydrogen Sulfide Detection" Polymers 15, no. 23: 4554. https://doi.org/10.3390/polym15234554
APA StyleLuo, K.-H., Yan, M., Hung, Y.-H., Kuang, J.-Y., Chang, H.-C., Lai, Y.-J., & Yeh, J.-M. (2023). Polyaniline Composites Containing Eco-Friendly Biomass Carbon from Agricultural-Waste Coconut Husk for Enhancing Gas Sensor Performance in Hydrogen Sulfide Detection. Polymers, 15(23), 4554. https://doi.org/10.3390/polym15234554






