Polymers as Efficient Non-Viral Gene Delivery Vectors: The Role of the Chemical and Physical Architecture of Macromolecules
Abstract
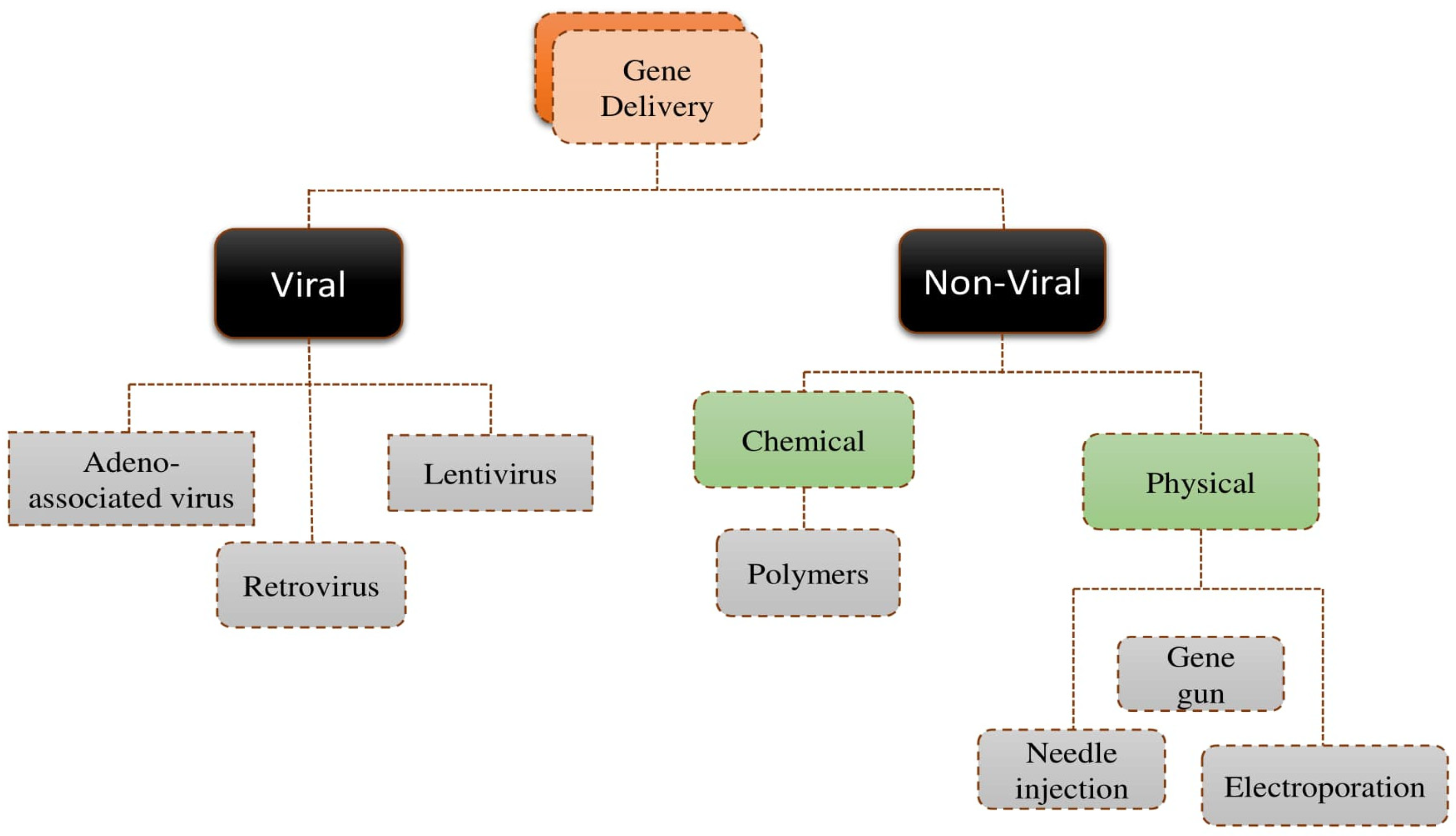
1. Introduction and Background to Gene Delivery
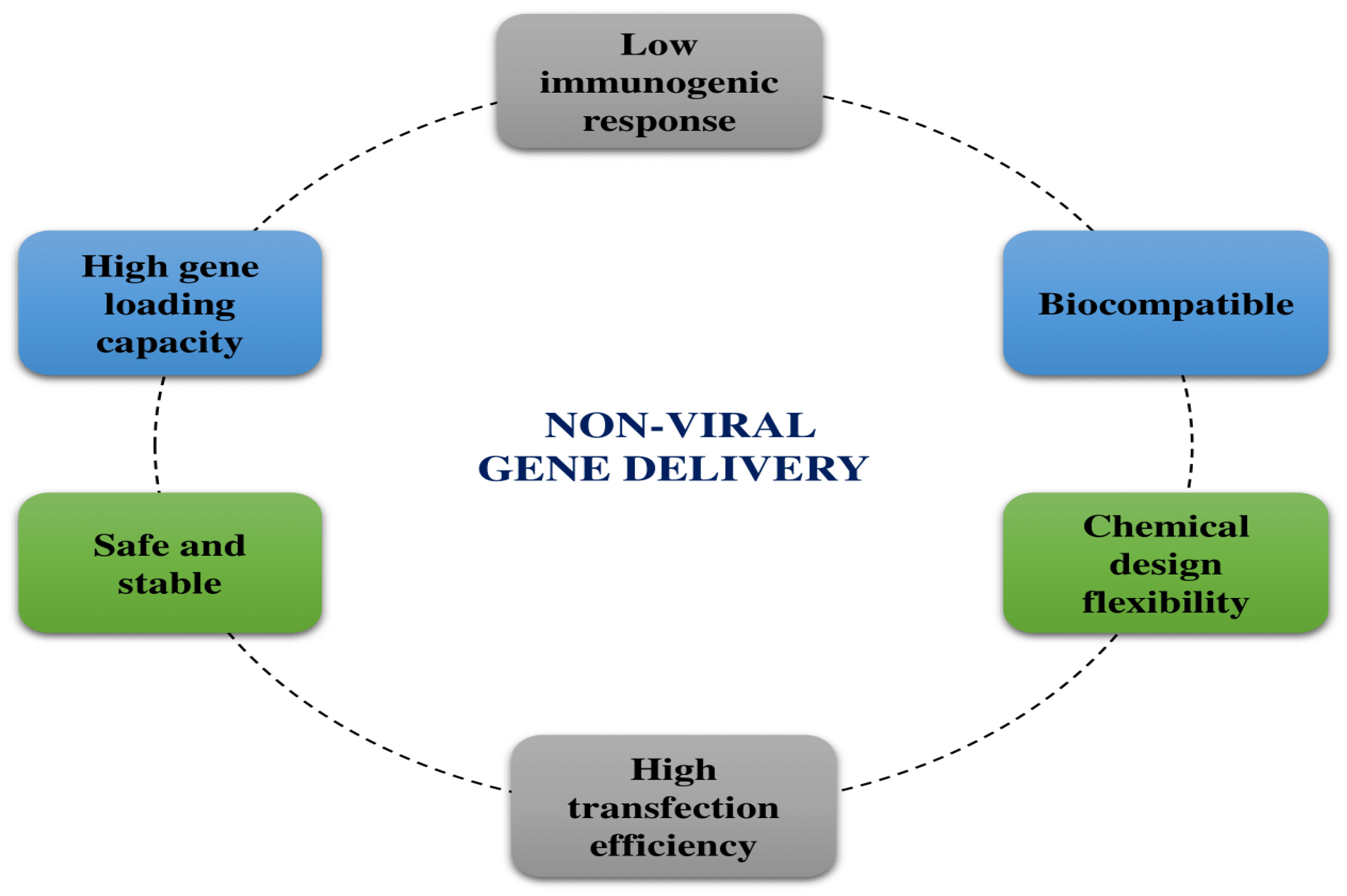
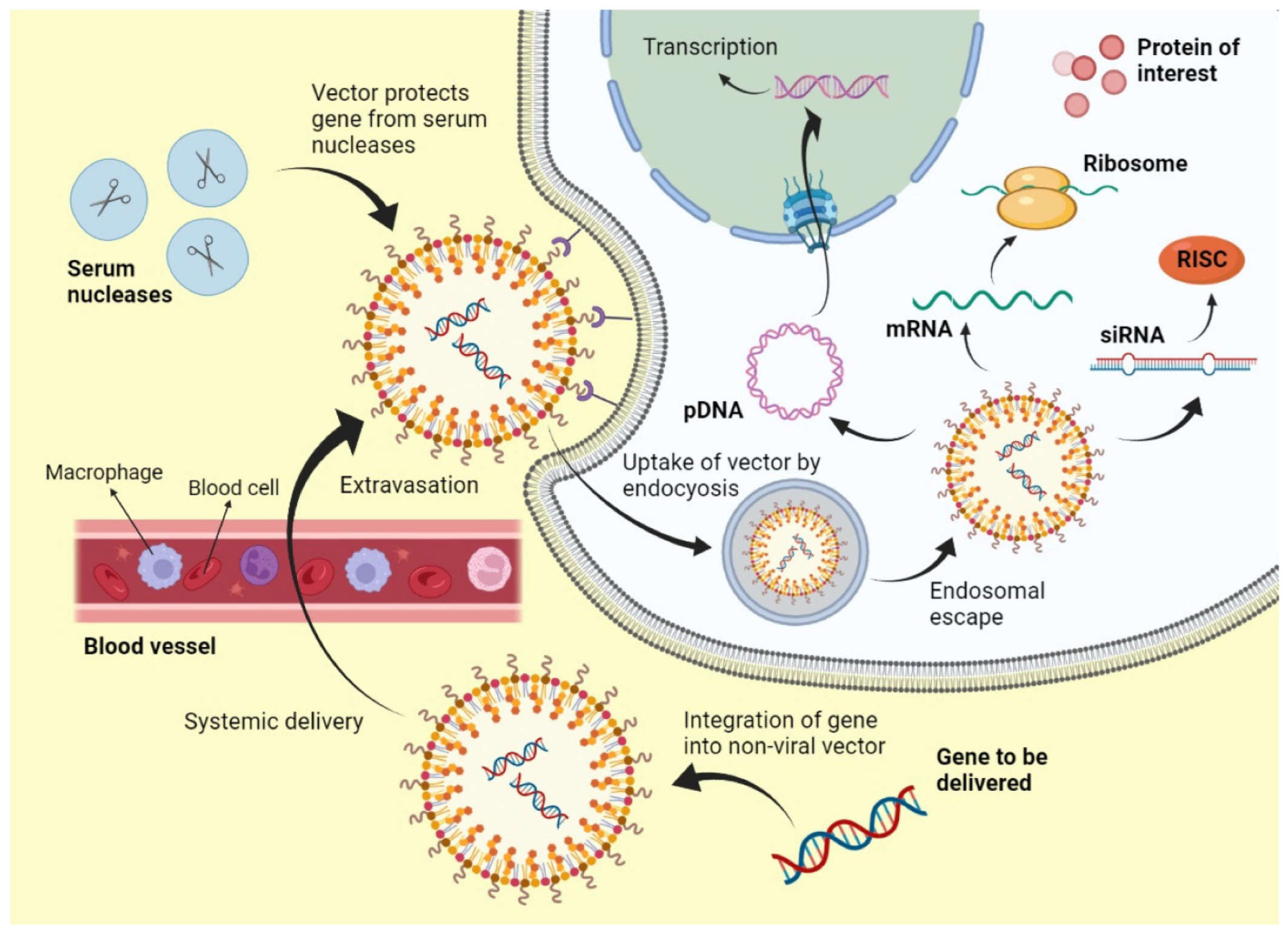
2. Introduction and Background to Non-Viral Systems
3. Macromolecules Used in Gene Delivery
3.1. Role of the Chemical and Physical Architecture of Macromolecules for Non-Viral Gene Therapy
3.1.1. Linear Polymers
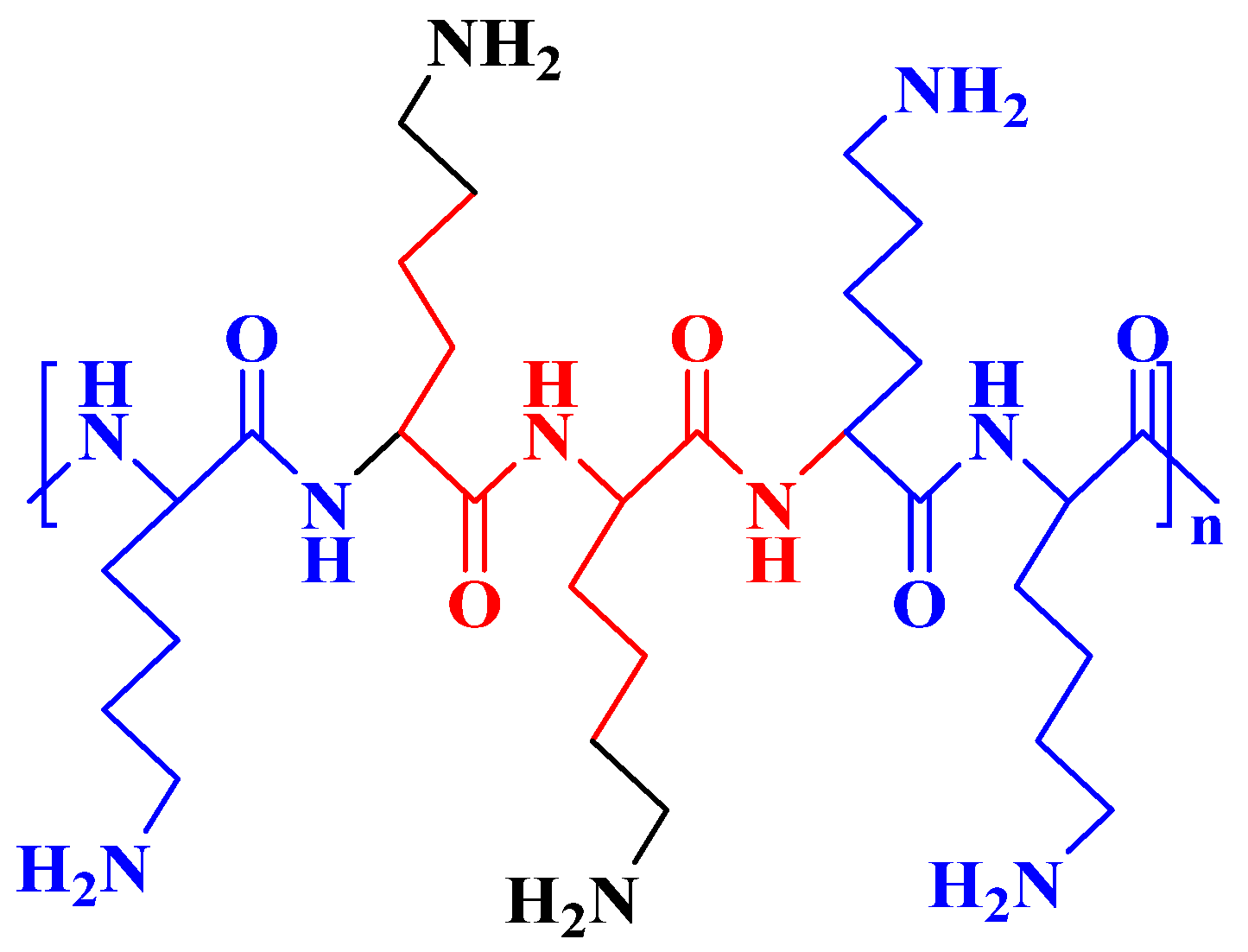
Poly-L-lysine (PLL)
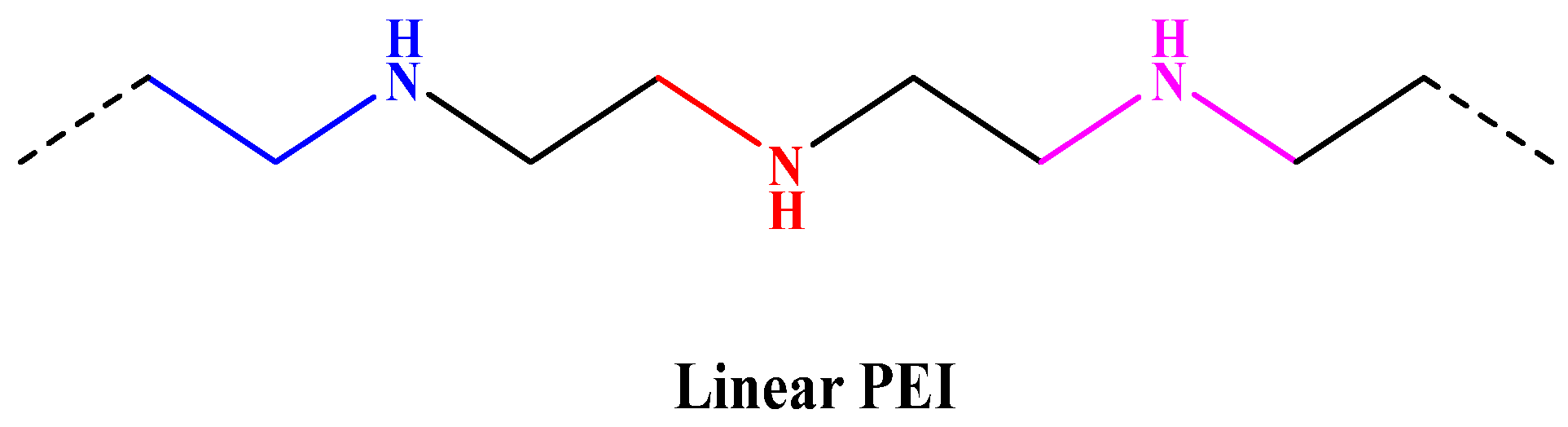
Linear Poly(ethylene imine) (PEI)
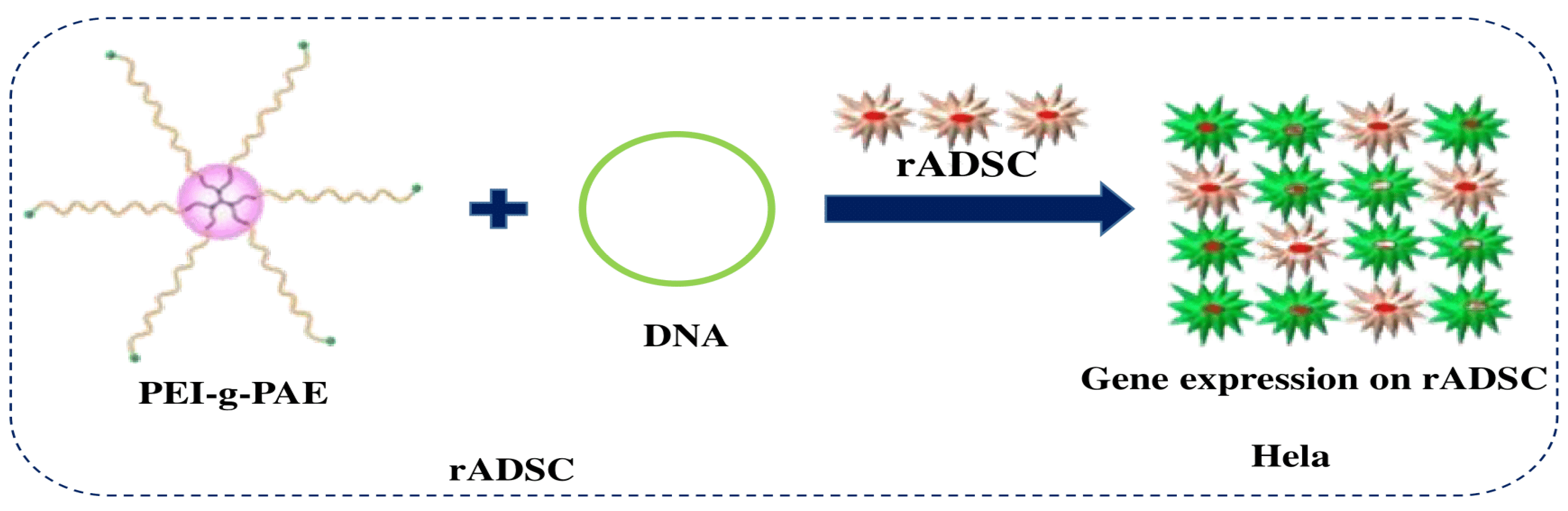
PEI as a Co-Delivery System for Drugs
3.1.2. Hyperbranched Polymers
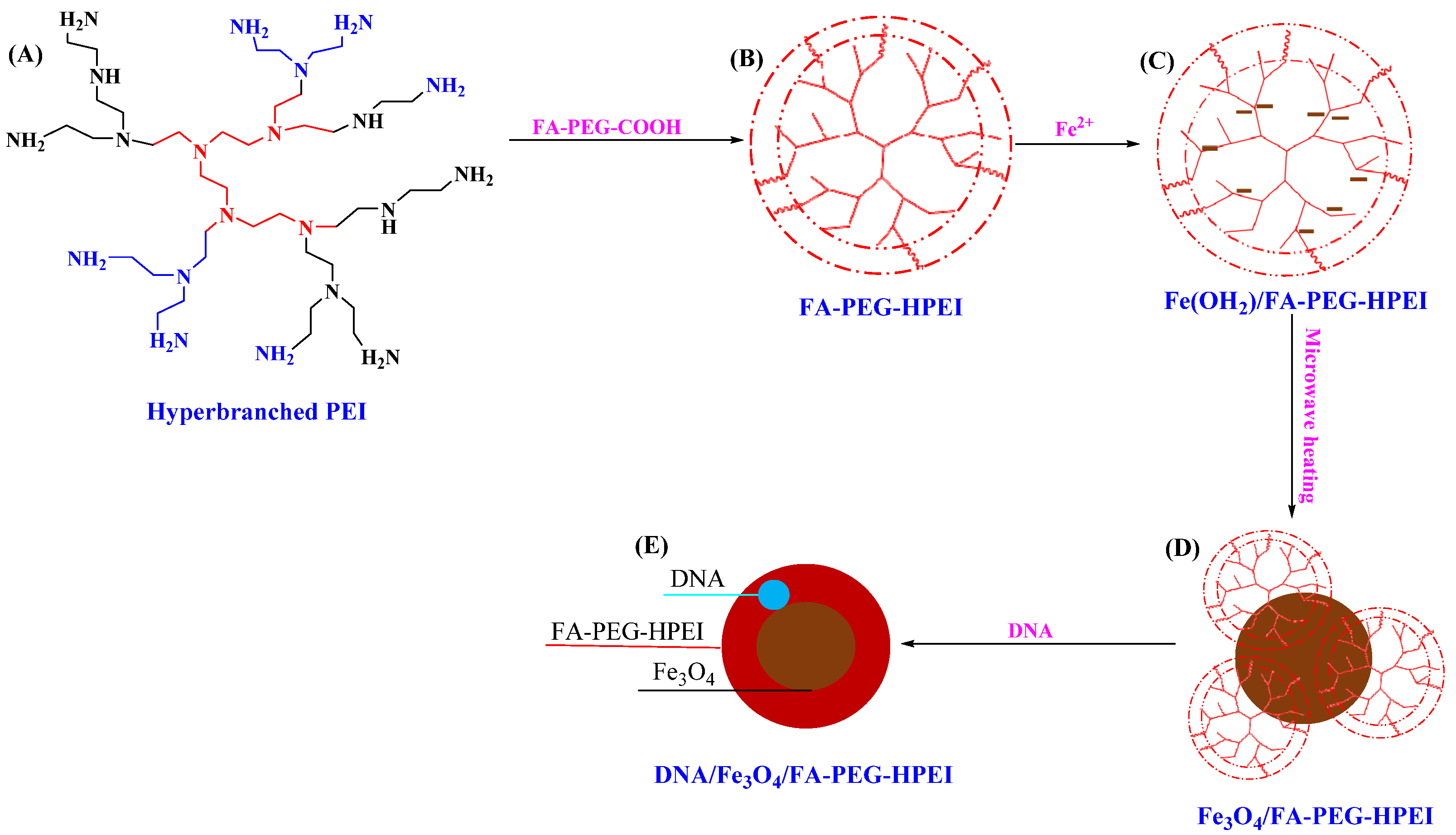
Hyperbranched PEI
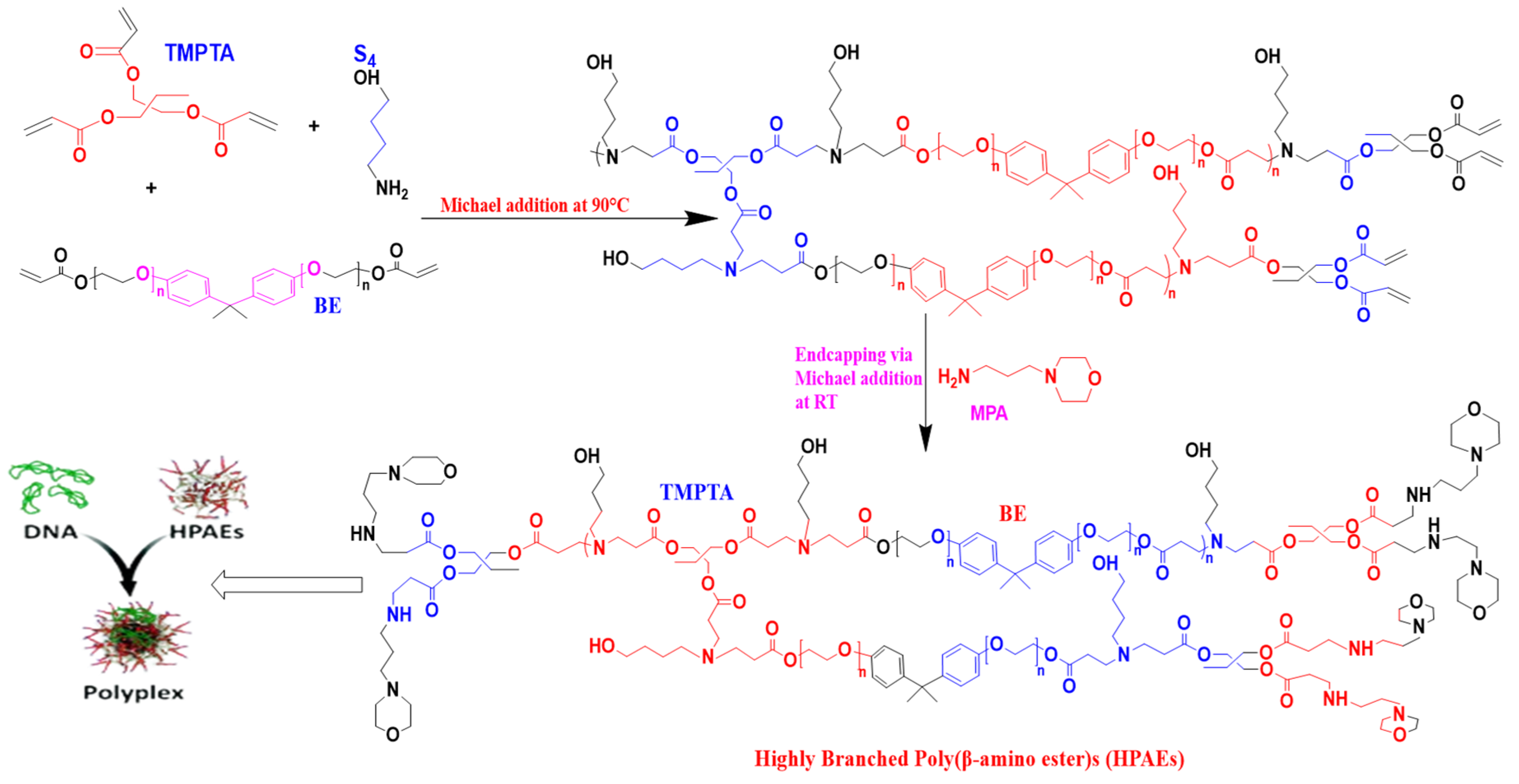
Poly(β-amino ester) (PβAE)
3.1.3. Dendritic Polymers
Dendrimers
PAMAM–Polyamidoamine
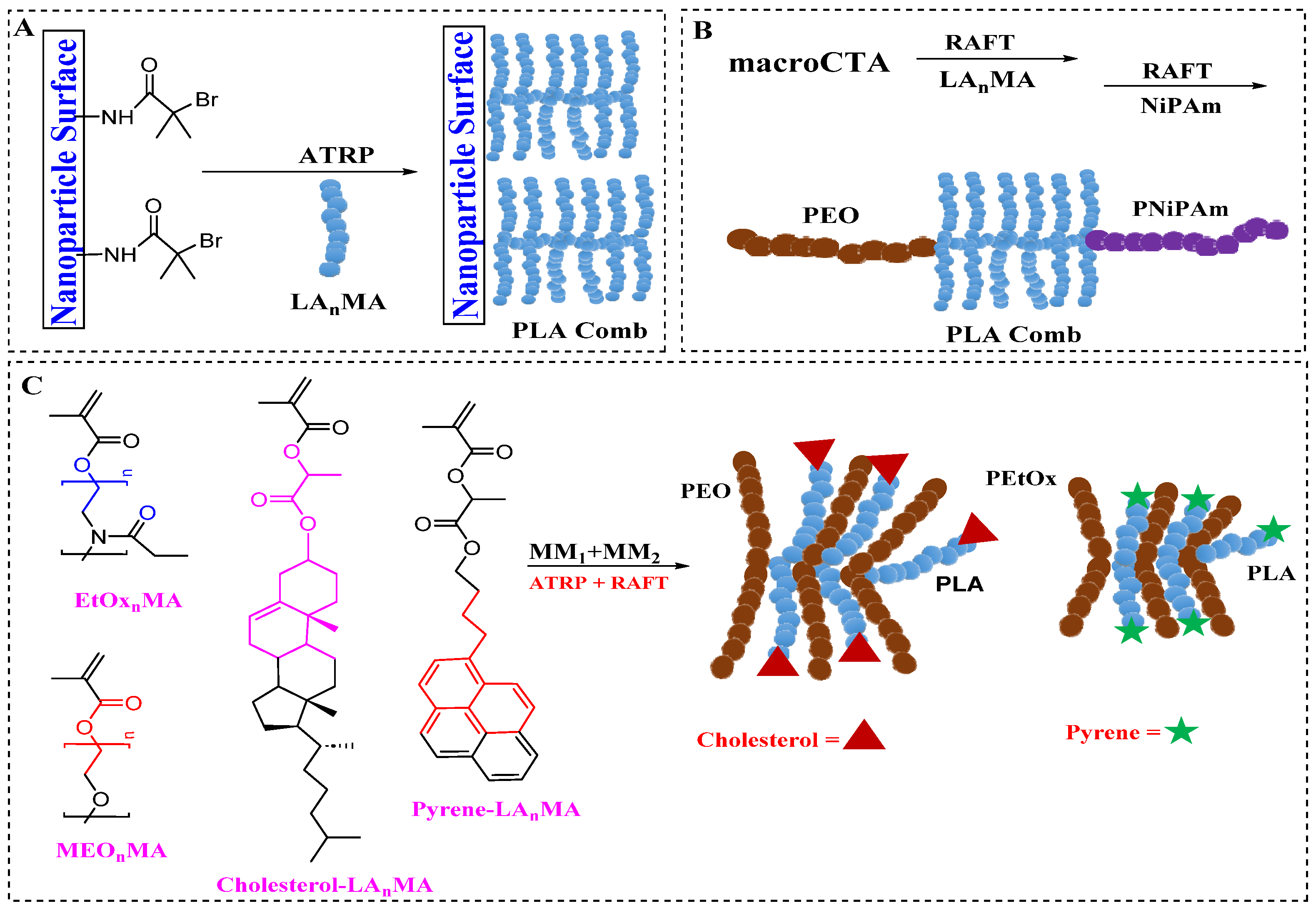
3.1.4. Comb Polymers
Poly(L-lysine)-grafted-poly(ethylene glycol) (PLL-g-PEG)
3.1.5. Brush Polymers
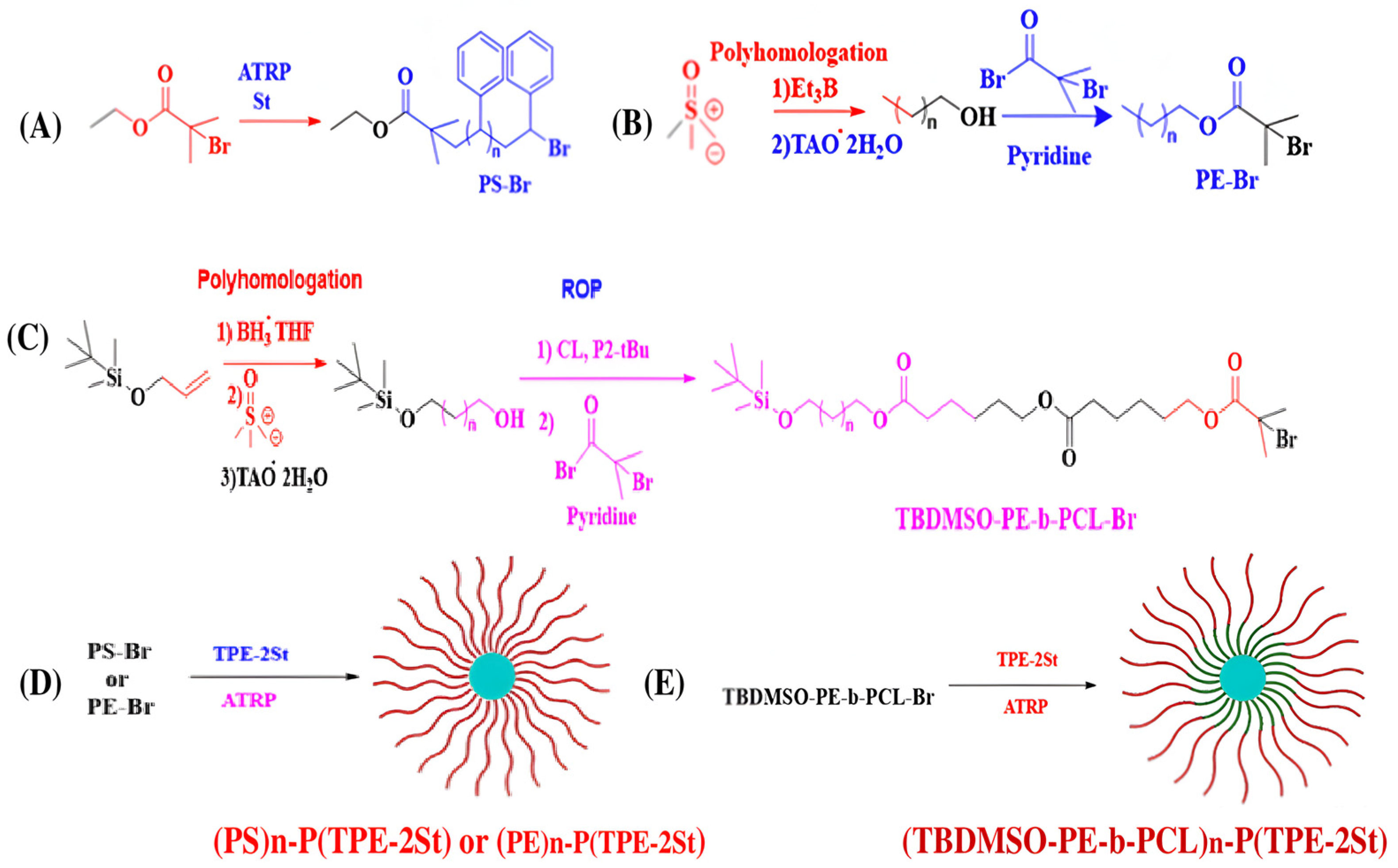
3.1.6. Star Polymers
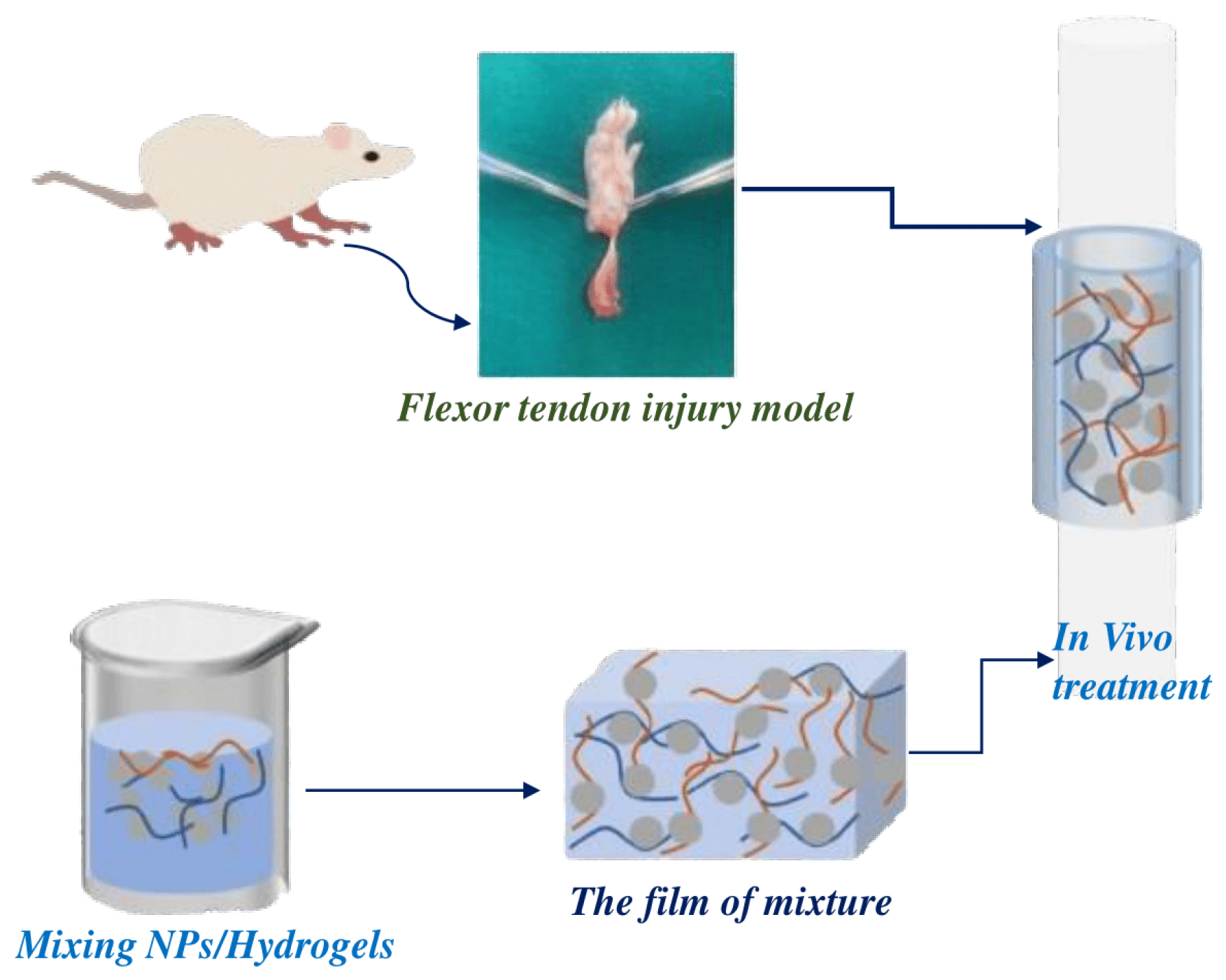
3.1.7. Hydrogels
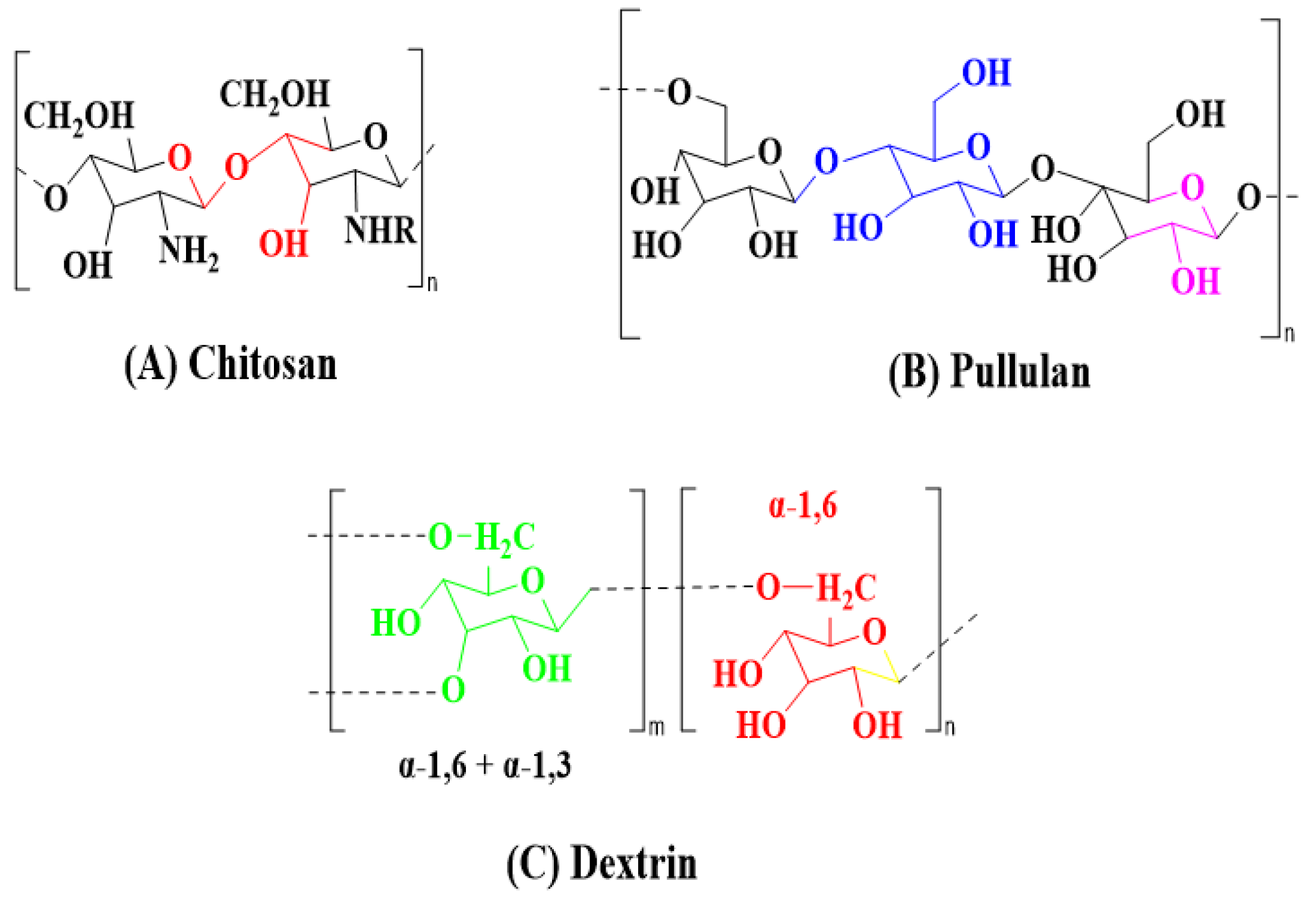
3.1.8. Natural Polymers/Modification of Natural Polymers
- Chitosan derivatives:
- Pullulan:
- Pullulan as a carrier for gene delivery:
- Dextran:
- Hyaluronic acid:
4. Challenges Facing Polymeric-Based Materials in Non-Viral Gene Delivery
4.1. Stabilizing Genes during Delivery
4.2. Increasing Capacity for Gene Cargo
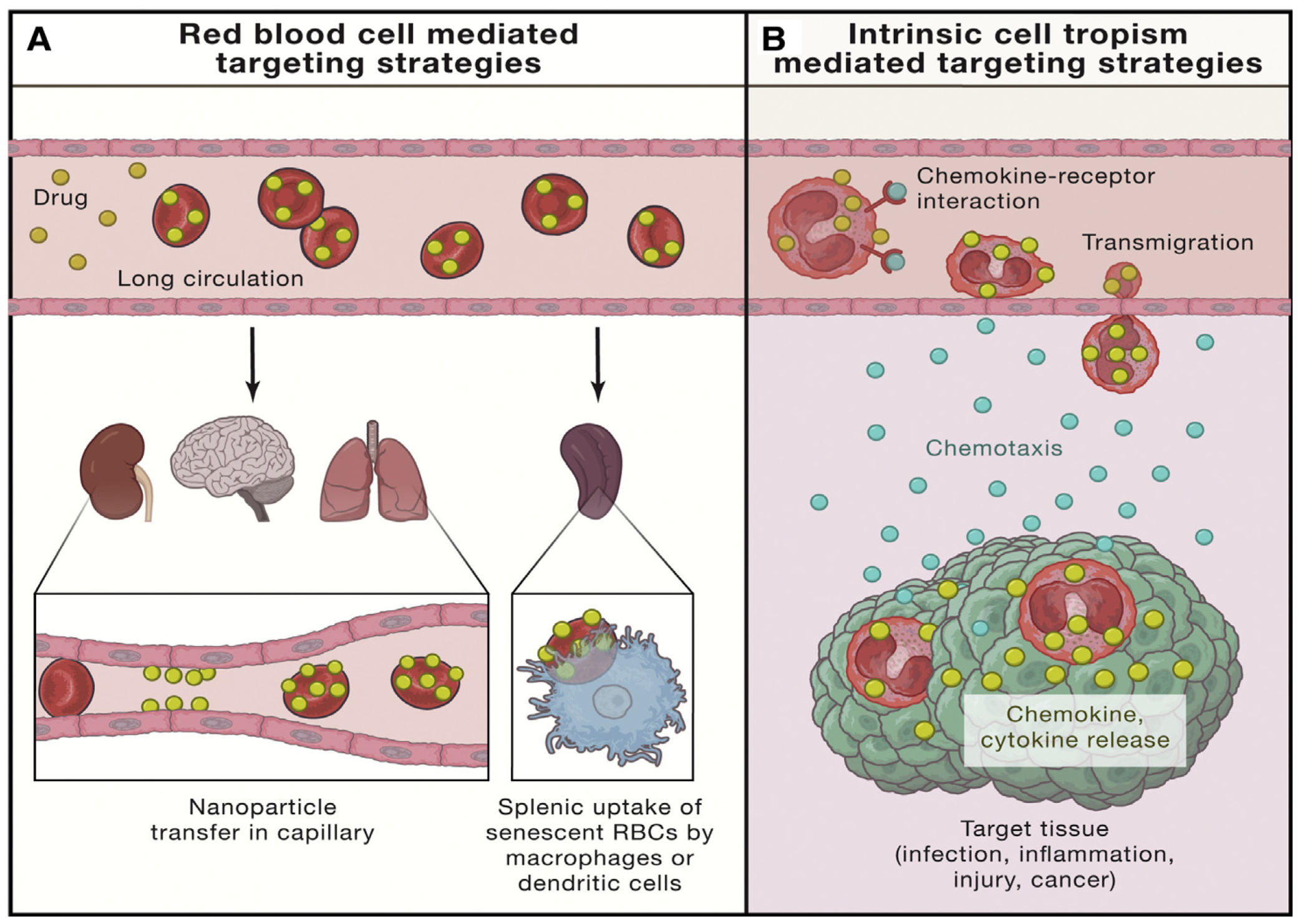
4.3. Targeting Specific Sells
4.4. Combining Gene and Drug Delivery
4.5. Cellular Uptake and Intracellular Trafficking and Localization
- Prospects:
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Picanço-Castro, V.; Pereira, C.G.; Covas, D.T.; Porto, G.S.; Athanassiadou, A.; Figueiredo, M.L. Emerging Patent Landscape for Non-Viral Vectors Used for Gene Therapy. Nat. Biotechnol. 2020, 38, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Chen, C.-K.; Ravikrishnan, A.; Rane, S.; Pfeifer, B.A. Overcoming Nonviral Gene Delivery Barriers: Perspective and Future. Mol. Pharm. 2013, 10, 4082–4098. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, M.J.; Kwon, I.C.; Roberts, T.M. Delivery Strategies and Potential Targets for SiRNA in Major Cancer Types. Adv. Drug Deliv. Rev. 2016, 104, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Huang, P.-K.; Law, W.-C.; Chu, C.-H.; Chen, N.-T.; Lo, L.-W. Biodegradable Polymers for Gene-Delivery Applications. Int. J. Nanomed. 2020, 2020, 2131–2150. [Google Scholar] [CrossRef] [PubMed]
- Mali, S. Delivery Systems for Gene Therapy. Indian J. Hum. Genet. 2013, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, J.; Sailor, M.J. Rekindling RNAi Therapy: Materials Design Requirements for In Vivo SiRNA Delivery. Adv. Mater. 2019, 31, 1903637. [Google Scholar] [CrossRef]
- Maier, P.; Von Kalle, C.; Laufs, S. Retroviral Vectors for Gene Therapy. Future Microbiol. 2010, 5, 1507–1523. [Google Scholar] [CrossRef]
- Li, Z.; Dullmann, J.; Schiedlmeier, B.; Schmidt, M.; Von Kalle, C.; Meyer, J.; Forster, M.; Stocking, C.; Wahlers, A.; Frank, O. Murine Leukemia Induced by Retroviral Gene Marking. Science 2002, 296, 497. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-Viral Vectors for Gene-Based Therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent Advances in the Development of Gene Delivery Systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef]
- Patil, S.; Gao, Y.-G.; Lin, X.; Li, Y.; Dang, K.; Tian, Y.; Zhang, W.-J.; Jiang, S.-F.; Qadir, A.; Qian, A.-R. The Development of Functional Non-Viral Vectors for Gene Delivery. Int. J. Mol. Sci. 2019, 20, 5491. [Google Scholar] [CrossRef] [PubMed]
- Procházková, N.; Nguyenová, M.-T.; Řehořová, M.; Kudláček, J.; Chvojka, J.; Ziak, J.; Balaštík, M.; Otáhal, J.; Jiruška, P.; Novák, O. NeuroPorator: An Open-Source, Current-Limited Electroporator for Safe in Utero Gene Transfer. J. Neurosci. Methods 2024, 406, 110126. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Kim, W.; Kang, H.-C.; Kim, S.H.; Park, A.H.; Park, E.K.; Cho, Y.-W.; Kim, S.; Kim, H.M.; Kim, J.A. Brain Somatic Mutations in MTOR Cause Focal Cortical Dysplasia Type II Leading to Intractable Epilepsy. Nat. Med. 2015, 21, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, F.; Donehower, L.A.; Scheurer, M.E.; Creighton, C.J. A Pediatric Brain Tumor Atlas of Genes Deregulated by Somatic Genomic Rearrangement. Nat. Commun. 2021, 12, 937. [Google Scholar] [CrossRef]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A Review on Electroporation-Based Intracellular Delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef] [PubMed]
- Vats, S.; Ballesteros, C.; Hung, S.; Sparapani, S.; Wong, K.; Haruna, J.; Li, C.; Authier, S. An Overview of Gene Editing Modalities and Related Non-Clinical Testing Considerations. Int. J. Toxicol. 2023, 42, 207–218. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Takahashi, G.; Gurumurthy, C.B.; Wada, K.; Miura, H.; Sato, M.; Ohtsuka, M. GONAD: G Enome-Editing via O Viductal N Ucleic A Cids D Elivery System: A Novel Microinjection Independent Genome Engineering Method in Mice. Sci. Rep. 2015, 5, 11406. [Google Scholar] [CrossRef]
- Sato, M.; Takabayashi, S.; Akasaka, E.; Nakamura, S. Recent Advances and Future Perspectives of In Vivo Targeted Delivery of Genome-Editing Reagents to Germ Cells, Embryos, and Fetuses in Mice. Cells 2020, 9, 799. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Kondos, N.; Sneed, K.; Pathak, Y. Recent Trends in Nano Drug Delivery Systems to Treat Cancers: With Special Focus on Liposomal Drug Delivery Systems. SciBase Oncol 2024, 2, 1–7. [Google Scholar]
- Francia, V.; Schiffelers, R.M.; Cullis, P.R.; Witzigmann, D. The Biomolecular Corona of Lipid Nanoparticles for Gene Therapy. Bioconjug. Chem. 2020, 31, 2046–2059. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T. An Introduction to Biotechnology: The Science, Technology and Medical Applications; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 1908818484. [Google Scholar]
- Lim, M.; Badruddoza, A.Z.M.; Firdous, J.; Azad, M.; Mannan, A.; Al-Hilal, T.A.; Cho, C.-S.; Islam, M.A. Engineered Nanodelivery Systems to Improve DNA Vaccine Technologies. Pharmaceutics 2020, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-Viral in Vitro Gene Delivery: It Is Now Time to Set the Bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-G.; Shi, Y.-D.; Zhang, Y.; Hu, J.; Lu, Z.-L.; He, L. A Naphthalimide-Based [12]AneN3 Compound as an Effective and Real-Time Fluorescence Tracking Non-Viral Gene Vector. Chem. Commun. 2015, 51, 16695–16698. [Google Scholar]
- Jeong, G.-W.; Nah, J.-W. Evaluation of Disulfide Bond-Conjugated LMWSC-g-BPEI as Non-Viral Vector for Low Cytotoxicity and Efficient Gene Delivery. Carbohydr. Polym. 2017, 178, 322–330. [Google Scholar] [CrossRef]
- Hidai, C.; Kitano, H. Nonviral Gene Therapy for Cancer: A Review. Diseases 2018, 6, 57. [Google Scholar] [CrossRef]
- Jones, C.H.; Hill, A.; Chen, M.; Pfeifer, B.A. Contemporary Approaches for Nonviral Gene Therapy. Discov. Med. 2015, 19, 447. [Google Scholar]
- Mirón-Barroso, S.; Domènech, E.B.; Trigueros, S. Nanotechnology-Based Strategies to Overcome Current Barriers in Gene Delivery. Int. J. Mol. Sci. 2021, 22, 8537. [Google Scholar] [CrossRef]
- Pezzoli, D.; Candiani, G. Non-Viral Gene Delivery Strategies for Gene Therapy: A “Ménage à Trois” among Nucleic Acids, Materials, and the Biological Environment: Stimuli-Responsive Gene Delivery Vectors. J. Nanopart. Res. 2013, 15, 1523. [Google Scholar] [CrossRef]
- Hogan, M.J.; Pardi, N. MRNA Vaccines in the COVID-19 Pandemic and Beyond. Annu. Rev. Med. 2022, 73, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Al Fayez, N.; Nassar, M.S.; Alshehri, A.A.; Alnefaie, M.K.; Almughem, F.A.; Alshehri, B.Y.; Alawad, A.O.; Tawfik, E.A. Recent Advancement in MRNA Vaccine Development and Applications. Pharmaceutics 2023, 15, 1972. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.; Shieh, J.C. Guide to Research Techniques in Neuroscience; Academic Press: Cambridge, MA, USA, 2015; ISBN 0128005971. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Zha, Z.; Li, H.; Toh, K.; Dirisala, A.; Matsumoto, Y.; Osada, K.; Kataoka, K.; Ge, Z. Ternary Polyplex Micelles with PEG Shells and Intermediate Barrier to Complexed DNA Cores for Efficient Systemic Gene Delivery. J. Control. Release 2015, 209, 77–87. [Google Scholar] [CrossRef]
- Pagels, R.F.; Prud’Homme, R.K. Polymeric Nanoparticles and Microparticles for the Delivery of Peptides, Biologics, and Soluble Therapeutics. J. Control. Release 2015, 219, 519–535. [Google Scholar] [CrossRef]
- Guo, Q.; Jiang, C. Delivery Strategies for Macromolecular Drugs in Cancer Therapy. Acta Pharm. Sin. B 2020, 10, 979–986. [Google Scholar] [CrossRef]
- Tyagi, P.; Santos, J.L. Macromolecule Nanotherapeutics: Approaches and Challenges. Drug Discov. Today 2018, 23, 1053–1061. [Google Scholar] [CrossRef]
- Uddin, S.N.; Islam, K.K. Cationic Polymers and Its Uses in Non-Viral Gene Delivery Systems: A Conceptual Research. Trends Med. Res. 2006, 1, 86–99. [Google Scholar]
- Jinturkar, K.A.; Rathi, M.N.; Misra, A. Gene Delivery Using Physical Methods. In Challenges in Delivery of Therapeutic Genomics and Proteomics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 83–126. [Google Scholar]
- Mehier-Humbert, S.; Guy, R.H. Physical Methods for Gene Transfer: Improving the Kinetics of Gene Delivery into Cells. Adv. Drug Deliv. Rev. 2005, 57, 733–753. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. The Practical Application of Gene Vectors in Cancer Therapy. Integr. Cancer Sci Ther. 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Future Directions for Atom Transfer Radical Polymerizations. Chem. Mater. 2024, 36, 1775–1778. [Google Scholar] [CrossRef]
- Skandalis, A.; Sentoukas, T.; Selianitis, D.; Balafouti, A.; Pispas, S. Using RAFT Polymerization Methodologies to Create Branched and Nanogel-Type Copolymers. Materials 2024, 17, 1947. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, G.-F. Peptide-Based Vectors for Gene Delivery. Chemistry 2023, 5, 1696–1718. [Google Scholar] [CrossRef]
- Shi, B.; Zheng, M.; Tao, W.; Chung, R.; Jin, D.; Ghaffari, D.; Farokhzad, O.C. Challenges in DNA Delivery and Recent Advances in Multifunctional Polymeric DNA Delivery Systems. Biomacromolecules 2017, 18, 2231–2246. [Google Scholar] [CrossRef] [PubMed]
- Nayvelt, I.; Thomas, T.; Thomas, T.J. Mechanistic Differences in DNA Nanoparticle Formation in the Presence of Oligolysines and Poly-L-Lysine. Biomacromolecules 2007, 8, 477–484. [Google Scholar] [CrossRef]
- Korolev, N.; Berezhnoy, N.V.; Eom, K.D.; Tam, J.P.; Nordenskiöld, L. A Universal Description for the Experimental Behavior of Salt-(in) Dependent Oligocation-Induced DNA Condensation. Nucleic Acids Res. 2009, 37, 7137–7150. [Google Scholar] [CrossRef]
- Tian, H.; Lin, L.; Jiao, Z.; Guo, Z.; Chen, J.; Gao, S.; Zhu, X.; Chen, X. Polylysine-Modified Polyethylenimine Inducing Tumor Apoptosis as an Efficient Gene Carrier. J. Control. Release 2013, 172, 410–418. [Google Scholar] [CrossRef]
- Malik, Y.S.; Sheikh, M.A.; Xing, Z.; Guo, Z.; Zhu, X.; Tian, H.; Chen, X. Polylysine-Modified Polyethylenimine Polymer Can Generate Genetically Engineered Mesenchymal Stem Cells for Combinational Suicidal Gene Therapy in Glioblastoma. Acta Biomater. 2018, 80, 144–153. [Google Scholar] [CrossRef]
- Kodama, Y.; Nakamura, T.; Kurosaki, T.; Egashira, K.; Mine, T.; Nakagawa, H.; Muro, T.; Kitahara, T.; Higuchi, N.; Sasaki, H. Biodegradable Nanoparticles Composed of Dendrigraft Poly-L-Lysine for Gene Delivery. Eur. J. Pharm. Biopharm. 2014, 87, 472–479. [Google Scholar] [CrossRef]
- Chen, B.; Yu, L.; Li, Z.; Wu, C. Design of Free Triblock Polylysine-b-Polyleucine-b-Polylysine Chains for Gene Delivery. Biomacromolecules 2018, 19, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Deng, C.; Tian, H.; Lu, T.; Chen, X.; Jing, X. Chemo-Physical and Biological Evaluation of Poly (L-lysine)-Grafted Chitosan Copolymers Used for Highly Efficient Gene Delivery. Macromol. Biosci. 2011, 11, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, H. Chaotic Multi-Swarm Whale Optimizer Boosted Support Vector Machine for Medical Diagnosis. Appl. Soft Comput. 2020, 88, 105946. [Google Scholar] [CrossRef]
- Chen, H.; Heidari, A.A.; Chen, H.; Wang, M.; Pan, Z.; Gandomi, A.H. Multi-Population Differential Evolution-Assisted Harris Hawks Optimization: Framework and Case Studies. Futur. Gener. Comput. Syst. 2020, 111, 175–198. [Google Scholar] [CrossRef]
- Kianfar, E.; Cao, V. Polymeric Membranes on Base of PolyMethyl Methacrylate for Air Separation: A Review. J. Mater. Res. Technol. 2021, 10, 1437–1461. [Google Scholar] [CrossRef]
- Yuan, W.; Li, H. Polymer-Based Nanocarriers for Therapeutic Nucleic Acids Delivery. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 445–460. [Google Scholar]
- Fahira, A.I.; Amalia, R.; Barliana, M.I.; Gatera, V.A.; Abdulah, R. Polyethyleneimine (PEI) as a Polymer-Based Co-Delivery System for Breast Cancer Therapy. Breast Cancer Targets Ther. 2023, 8, 71–83. [Google Scholar] [CrossRef]
- Yamagata, M.; Kawano, T.; Shiba, K.; Mori, T.; Katayama, Y.; Niidome, T. Structural Advantage of Dendritic Poly (L-Lysine) for Gene Delivery into Cells. Bioorg. Med. Chem. 2007, 15, 526–532. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, F.; Zhang, F.; Chen, G.; Wang, K.; Sun, M.; Li, J.; Oupický, D. Cyclam-Modified PEI for Combined VEGF SiRNA Silencing and CXCR4 Inhibition to Treat Metastatic Breast Cancer. Biomacromolecules 2018, 19, 392–401. [Google Scholar] [CrossRef]
- Gupta, R.B.; Kompella, U.B. Nanoparticle Technology for Drug Delivery; Taylor & Francis: New York, NY, USA, 2006; Volume 53. [Google Scholar]
- He, F.; Wang, C.-F.; Jiang, T.; Han, B.; Zhuo, R.-X. Poly [(5-Methyl-5-Allyloxycarbonyl-Trimethylene Carbonate)-Co-(5, 5-Dimethyl-Trimethylene Carbonate)] with Grafted Polyethylenimine as Biodegradable Polycations for Efficient Gene Delivery. Biomacromolecules 2010, 11, 3028–3035. [Google Scholar]
- Wang, C.-F.; Lin, Y.-X.; Jiang, T.; He, F.; Zhuo, R.-X. Polyethylenimine-Grafted Polycarbonates as Biodegradable Polycations for Gene Delivery. Biomaterials 2009, 30, 4824–4832. [Google Scholar] [CrossRef]
- Li, T.; Tong, Z.; Gao, B.; Li, Y.C.; Smyth, A.; Bayabil, H.K. Polyethyleneimine-Modified Biochar for Enhanced Phosphate Adsorption. Environ. Sci. Pollut. Res. 2020, 27, 7420–7429. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, P.; Singh, R.N. Polyethyleneimine-Functionalized Magnetic Fe3O4 and Nanodiamond Particles as a Platform for Amoxicillin Delivery. J. Nanosci. Nanotechnol. 2020, 20, 3957–3970. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, W.; Li, H.; Zheng, Y.; Chen, Z.; Lin, H.; Shen, Y. N-Acetyl-l-Leucine-Polyethyleneimine-Mediated Delivery of CpG Oligodeoxynucleotides 2006 Inhibits RAW264. 7 Cell Osteoclastogenesis. Drug Des. Devel. Ther. 2020, 10, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, T.; Shimada, N.; Iijima, K.; Hashizume, M.; Yoshimoto, K. Polyethyleneimine-Induced Astaxanthin Accumulation in the Green Alga Haematococcus Pluvialis by Increased Oxidative Stress. J. Biosci. Bioeng. 2019, 128, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Assi, T.; Rassy, E.; Farhat, F.; Kattan, C.; Kattan, J. Docetaxel Rechallenge in Patients with Metastatic Prostate Cancer: A Comprehensive Review. Oncol. Res. Treat. 2020, 43, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-Delivery of Resveratrol and Docetaxel via Polymeric Micelles to Improve the Treatment of Drug-Resistant Tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, X.; Yang, J. TAT Modified and Lipid–PEI Hybrid Nanoparticles for Co-Delivery of Docetaxel and PDNA. Biomed. Pharmacother. 2016, 84, 954–961. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, B. Polyethyleneimine-Based Drug Delivery Systems for Cancer Theranostics. J. Funct. Biomater. 2022, 14, 12. [Google Scholar] [CrossRef]
- Kim, T.-H.; Choi, H.; Yu, G.S.; Lee, J.; Choi, J.S. Novel Hyperbranched Polyethyleneimine Conjugate as an Efficient Non-Viral Gene Delivery Vector. Macromol. Res. 2013, 21, 1097–1104. [Google Scholar] [CrossRef]
- Kumar, R.; Santa Chalarca, C.F.; Bockman, M.R.; Van Bruggen, C.; Grimme, C.J.; Dalal, R.J.; Hanson, M.G.; Hexum, J.K.; Reineke, T.M. Polymeric Delivery of Therapeutic Nucleic Acids. Chem. Rev. 2021, 121, 11527–11652. [Google Scholar] [CrossRef]
- Chen, B.; Liu, M.; Zhang, L.; Huang, J.; Yao, J.; Zhang, Z. Polyethylenimine-Functionalized Graphene Oxide as an Efficient Gene Delivery Vector. J. Mater. Chem. 2011, 21, 7736–7741. [Google Scholar] [CrossRef]
- Cook, A.B.; Peltier, R.; Zhang, J.; Gurnani, P.; Tanaka, J.; Burns, J.A.; Dallmann, R.; Hartlieb, M.; Perrier, S. Hyperbranched Poly (Ethylenimine-Co-Oxazoline) by Thiol–Yne Chemistry for Non-Viral Gene Delivery: Investigating the Role of Polymer Architecture. Polym. Chem. 2019, 10, 1202–1212. [Google Scholar] [CrossRef]
- Shi, Y.; Lei, G.; Li, Y.; Zhang, X.; Peng, R.; Hu, J.; Yuan, Z.; Liu, Y.; Shen, X.; Sun, N. In Situ Preparation of Non-Viral Gene Vectors with Folate/Magnetism Dual Targeting by Hyperbranched Polymers. Eur. Polym. J. 2020, 127, 109584. [Google Scholar] [CrossRef]
- Lynn, D.M.; Anderson, D.G.; Putnam, D.; Langer, R. Accelerated Discovery of Synthetic Transfection Vectors: Parallel Synthesis and Screening of a Degradable Polymer Library. J. Am. Chem. Soc. 2001, 123, 8155–8156. [Google Scholar] [CrossRef] [PubMed]
- Cutlar, L.; Zhou, D.; Gao, Y.; Zhao, T.; Greiser, U.; Wang, W.; Wang, W. Highly Branched Poly (β-Amino Esters): Synthesis and Application in Gene Delivery. Biomacromolecules 2015, 16, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.M.; Langer, R. Degradable Poly (β-Amino Esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Liu, S.; Gao, Y.; Zhou, D.; Zeng, M.; Alshehri, F.; Newland, B.; Lyu, J.; O’Keeffe-Ahern, J.; Greiser, U.; Guo, T. Highly Branched Poly (β-Amino Ester) Delivery of Minicircle DNA for Transfection of Neurodegenerative Disease Related Cells. Nat. Commun. 2019, 10, 3307. [Google Scholar] [CrossRef]
- Cai, X.; Dou, R.; Guo, C.; Tang, J.; Li, X.; Chen, J.; Zhang, J. Cationic Polymers as Transfection Reagents for Nucleic Acid Delivery. Pharmaceutics 2023, 15, 1502. [Google Scholar] [CrossRef]
- Cordeiro, R.A.; Serra, A.; Coelho, J.F.J.; Faneca, H. Poly (β-Amino Ester)-Based Gene Delivery Systems: From Discovery to Therapeutic Applications. J. Control. Release 2019, 310, 155–187. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM Dendrimers as Efficient Drug and Gene Delivery Nanosystems for Cancer Therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Toba, R.; Quintela, J.M.; Peinador, C.; Román, E.; Kaifer, A.E. A New Series of Dendrimers with 4, 4′-Bipyridinium Cores Capable of Fast Electron Transfer Reactions. Chem. Commun. 2001, 9, 857–858. [Google Scholar] [CrossRef]
- Kozaki, M.; Okada, K. Snowflake-like Dendrimers via Site-Selective Synthesis of Dendrons. Org. Lett. 2004, 6, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Patrick, T.B.; Juehne, T.; Reeb, E.; Hennessy, D. Zinc (II) Promoted Conversion of Aryltriazenes to Aryl Iodides and Aryl Nitriles. Tetrahedron Lett. 2001, 42, 3553–3554. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.-S.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. Biomed Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef]
- de Araújo, R.V.; Santos, S.d.S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging Non-Viral Vectors for Gene Delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef]
- Ita, K. Polyplexes for Gene and Nucleic Acid Delivery: Progress and Bottlenecks. Eur. J. Pharm. Sci. 2020, 150, 105358. [Google Scholar] [CrossRef]
- Pishavar, E.; Attaranzadeh, A.; Alibolandi, M.; Ramezani, M.; Hashemi, M. Modified PAMAM Vehicles for Effective TRAIL Gene Delivery to Colon Adenocarcinoma: In Vitro and In Vivo Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 503–513. [Google Scholar] [CrossRef]
- Lee, S.; Son, S.J.; Song, S.J.; Ha, T.H.; Choi, J.S. Polyamidoamine (PAMAM) Dendrimers Modified with Cathepsin-B Cleavable Oligopeptides for Enhanced Gene Delivery. Polymers 2017, 9, 224. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Du, L.; Liu, Y. A Reactive Oxygen Species-Responsive Dendrimer with Low Cytotoxicity for Efficient and Targeted Gene Delivery. Chin. Chem. Lett. 2020, 31, 275–280. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Lu, Y.; Song, B.; Zhao, M.; Hu, H.; Chen, D. A Novel Disulfide Bond-Mediated Cleavable RGD-Modified PAMAM Nanocomplex Containing Nuclear Localization Signal HMGB1 for Enhancing Gene Transfection Efficiency. Int. J. Nanomed. 2018, 13, 7135–7153. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Alves, C.S.; Wang, J.; Li, A.; Liu, J.; Shen, M.; Rodrigues, J.; Tomás, H.; Shi, X. Zwitterion-Functionalized Dendrimer-Entrapped Gold Nanoparticles for Serum-Enhanced Gene Delivery to Inhibit Cancer Cell Metastasis. Acta Biomater. 2019, 99, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hu, H.; Wang, D. Preparation of Gene Drug Delivery Systems of Cationic Peptide Lipid with 0G-PAMAM as Hydrophilic End and Its Biological Properties Evaluation. Chem. Phys. Lipids 2019, 224, 104685. [Google Scholar] [CrossRef] [PubMed]
- Jativa, S.D.; Thapar, N.; Broyles, D.; Dikici, E.; Daftarian, P.; Jiménez, J.J.; Daunert, S.; Deo, S.K. Enhanced Delivery of Plasmid DNA to Skeletal Muscle Cells Using a DLC8-Binding Peptide and ASSLNIA-Modified PAMAM Dendrimer. Mol. Pharm. 2019, 16, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Liaw, K.; Sharma, A.; Jimenez, A.; Chang, M.; Salazar, S.; Amlani, I.; Kannan, S.; Kannan, R.M. Glycosylation of PAMAM Dendrimers Significantly Improves Tumor Macrophage Targeting and Specificity in Glioblastoma. J. Control. Release 2021, 337, 179–192. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-Based Drug and Imaging Conjugates: Design Considerations for Nanomedical Applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as Nanocarrier for Drug Delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Gautam, S.P.; Gupta, A.K.; Sharma, A.; Gautam, T. Synthesis and Analytical Characterization of Ester and Amine Terminated PAMAM Dendrimers. Glob. J Med. Res. Pharma, Drug Dis. Toxicol. Med. 2013, 13, 7–15. [Google Scholar]
- Parsian, M.; Mutlu, P.; Yalcin, S.; Tezcaner, A.; Gunduz, U. Half Generations Magnetic PAMAM Dendrimers as an Effective System for Targeted Gemcitabine Delivery. Int. J. Pharm. 2016, 515, 104–113. [Google Scholar] [CrossRef]
- da Silva Santos, S.; Igne Ferreira, E.; Giarolla, J. Dendrimer Prodrugs. Molecules 2016, 21, 686. [Google Scholar] [CrossRef]
- Mastorakos, P.; Kambhampati, S.P.; Mishra, M.K.; Wu, T.; Song, E.; Hanes, J.; Kannan, R.M. Hydroxyl PAMAM Dendrimer-Based Gene Vectors for Transgene Delivery to Human Retinal Pigment Epithelial Cells. Nanoscale 2015, 7, 3845–3856. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-T.; Chen, G.; Nie, X.; Wang, L.-H.; Ding, S.-G.; You, Y.-Z. Low Generation PAMAM-Based Nanomicelles as ROS-Responsive Gene Vectors with Enhanced Transfection Efficacy and Reduced Cytotoxicity in Vitro. New J. Chem. 2017, 41, 3273–3279. [Google Scholar] [CrossRef]
- Martinez, C.S.; Igartúa, D.E.; Calienni, M.N.; Feas, D.A.; Siri, M.; Montanari, J.; Chiaramoni, N.S.; Alonso, S.d.V.; Prieto, M.J. Relation between Biophysical Properties of Nanostructures and Their Toxicity on Zebrafish. Biophys. Rev. 2017, 9, 775–791. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer Toxicity: Let’s Meet the Challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, W.; Zhu, Y.; Xu, H.; Yang, X. Protective Effect of PEGylation against Poly (Amidoamine) Dendrimer-induced Hemolysis of Human Red Blood Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. An Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 93, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Najlah, M.; Freeman, S.; Khoder, M.; Attwood, D.; D’Emanuele, A. In Vitro Evaluation of Third Generation PAMAM Dendrimer Conjugates. Molecules 2017, 22, 1661. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Studzian, M.; Petersen, J.F.; Ficker, M.; Paolucci, V.; Christensen, J.B.; Tomalia, D.A.; Klajnert-Maculewicz, B. Modified PAMAM Dendrimer with 4-Carbomethoxypyrrolidone Surface Groups-Its Uptake, Efflux, and Location in a Cell. Colloids Surf. B Biointerfaces 2017, 159, 211–216. [Google Scholar] [CrossRef]
- Janaszewska, A.; Gorzkiewicz, M.; Ficker, M.; Petersen, J.F.; Paolucci, V.; Christensen, J.B.; Klajnert-Maculewicz, B. Pyrrolidone Modification Prevents PAMAM Dendrimers from Activation of Pro-Inflammatory Signaling Pathways in Human Monocytes. Mol. Pharm. 2018, 15, 12–20. [Google Scholar] [CrossRef]
- Salameh, J.W.; Zhou, L.; Ward, S.M.; Santa Chalarca, C.F.; Emrick, T.; Figueiredo, M.L. Polymer-mediated Gene Therapy: Recent Advances and Merging of Delivery Techniques. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1598. [Google Scholar] [CrossRef]
- Olden, B.R.; Cheng, Y.; Jonathan, L.Y.; Pun, S.H. Cationic Polymers for Non-Viral Gene Delivery to Human T Cells. J. Control. Release 2018, 282, 140–147. [Google Scholar] [CrossRef]
- Yildirim, I.; Weber, C.; Schubert, U.S. Old Meets New: Combination of PLA and RDRP to Obtain Sophisticated Macromolecular Architectures. Prog. Polym. Sci. 2018, 76, 111–150. [Google Scholar] [CrossRef]
- Wu, C.; Ying, A.; Ren, S. Synthesis of Stimuli Responsive Graft Triblock Polymers via Combination of Reversible Addition-Fragmentation Chain Transfer Polymerization and Ring Opening Polymerization. Asian J. Chem. 2013, 25, 3344. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The Big Picture on Nanomedicine: The State of Investigational and Approved Nanomedicine Products. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal Nanocarriers: A Review on Formulation Technology, Types and Applications toward Targeted Drug Delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M. Peptide and Protein PEGylation: A Review of Problems and Solutions. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Modi, M.W.; Pedder, S. Use of Peginterferon Alfa-2a (40 KD)(Pegasys®) for the Treatment of Hepatitis C. Adv. Drug Deliv. Rev. 2002, 54, 571–586. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernandez, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef]
- Rausch, K.; Reuter, A.; Fischer, K.; Schmidt, M. Evaluation of Nanoparticle Aggregation in Human Blood Serum. Biomacromolecules 2010, 11, 2836–2839. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. ‘Stealth’Corona-Core Nanoparticles Surface Modified by Polyethylene Glycol (PEG): Influences of the Corona (PEG Chain Length and Surface Density) and of the Core Composition on Phagocytic Uptake and Plasma Protein Adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and Development of Polymers for Gene Delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Dong, C.; Dong, H.; Wang, G.; Pauletti, G.M.; Pan, X.; Wen, H.; Mehl, I.; Li, Y.; Shi, D. Effective Gene Delivery Using Stimulus-Responsive Catiomer Designed with Redox-Sensitive Disulfide and Acid-Labile Imine Linkers. Biomacromolecules 2012, 13, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, Y.; Yue, D.; Yi, Q.; Li, S.; Shi, D.; Gu, Z. Reversible PEGylation and Schiff-Base Linked Imidazole Modification of Polylysine for High-Performance Gene Delivery. J. Mater. Chem. B 2015, 3, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tian, H.; Xing, Z.; Zhang, D.; Guo, Y.; Guo, Z.; Zhu, X.; Chen, X. A Non-Viral Suicide Gene Delivery System Traversing the Blood Brain Barrier for Non-Invasive Glioma Targeting Treatment. J. Control. Release 2016, 243, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Nian, S.; Huang, B.; Freychet, G.; Zhernenkov, M.; Cai, L.-H. Unexpected Folding of Bottlebrush Polymers in Melts. Macromolecules 2023, 56, 2551–2559. [Google Scholar] [CrossRef]
- Zorn, A.; Junkers, T.; Barner-Kowollik, C. Synthesis of a Macromonomer Library from High-Temperature Acrylate Polymerization. Macromol. Rapid Commun. 2009, 30, 2028–2035. [Google Scholar] [CrossRef]
- Yin, L.; Liu, L.; Zhang, N. Brush-like Polymers: Design, Synthesis and Applications. Chem. Commun. 2021, 57, 10484–10499. [Google Scholar] [CrossRef]
- Feng, C.; Huang, X. Polymer Brushes: Efficient Synthesis and Applications. Acc. Chem. Res. 2018, 51, 2314–2323. [Google Scholar] [CrossRef]
- Li, S.; Omi, M.; Cartieri, F.; Konkolewicz, D.; Mao, G.; Gao, H.; Averick, S.E.; Mishina, Y.; Matyjaszewski, K. Cationic Hyperbranched Polymers with Biocompatible Shells for SiRNA Delivery. Biomacromolecules 2018, 19, 3754–3765. [Google Scholar] [CrossRef]
- Burdyńska, J.; Daniel, W.; Li, Y.; Robertson, B.; Sheiko, S.S.; Matyjaszewski, K. Molecular Bottlebrushes with Bimodal Length Distribution of Side Chains. Macromolecules 2015, 48, 4813–4822. [Google Scholar] [CrossRef]
- Nese, A.; Lebedeva, N.V.; Sherwood, G.; Averick, S.; Li, Y.; Gao, H.; Peteanu, L.; Sheiko, S.S.; Matyjaszewski, K. PH-Responsive Fluorescent Molecular Bottlebrushes Prepared by Atom Transfer Radical Polymerization. Macromolecules 2011, 44, 5905–5910. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Li, H.; Dai, Y.; Hu, Z.; Huang, H.; Shi, Y.; Li, Y.; Chen, Y. Molecular Bottlebrushes Featuring Brush-on-Brush Architecture. ACS Macro Lett. 2019, 8, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, W.; Chi, L.; Fuchs, H. Polymer Brush and Inorganic Oxide Hybrid Nanodielectrics for High Performance Organic Transistors. J. Phys. Chem. B 2010, 114, 5315–5319. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, M.; Shen, W.; Du, B.; Yang, J.; Zhang, Q. A Polycationic Brush Mediated Co-Delivery of Doxorubicin and Gene for Combination Therapy. Polymers 2019, 11, 60. [Google Scholar] [CrossRef]
- Wang, D.; Lin, J.; Jia, F.; Tan, X.; Wang, Y.; Sun, X.; Cao, X.; Che, F.; Lu, H.; Gao, X. Bottlebrush-Architectured Poly (Ethylene Glycol) as an Efficient Vector for RNA Interference In Vivo. Sci. Adv. 2019, 5, eaav9322. [Google Scholar] [CrossRef]
- Blum, A.P.; Nelles, D.A.; Hidalgo, F.J.; Touve, M.A.; Sim, D.S.; Madrigal, A.A.; Yeo, G.W.; Gianneschi, N.C. Peptide Brush Polymers for Efficient Delivery of a Gene Editing Protein to Stem Cells. Angew. Chem. 2019, 131, 15793–15796. [Google Scholar] [CrossRef]
- O’Keeffe Ahern, J.; Zhou, D.; Gao, Y.; Lyu, J.; Meng, Z.; Cutlar, L.; Pierucci, L.; Wang, W. Brushlike Cationic Polymers with Low Charge Density for Gene Delivery. Biomacromolecules 2017, 19, 1410–1415. [Google Scholar] [CrossRef]
- Nie, J.-J.; Zhao, W.; Hu, H.; Yu, B.; Xu, F.-J. Controllable Heparin-Based Comb Copolymers and Their Self-Assembled Nanoparticles for Gene Delivery. ACS Appl. Mater. Interfaces 2016, 8, 8376–8385. [Google Scholar] [CrossRef]
- Li, J.; Qian, J.; Xu, Y.; Yan, S.; Shen, J.; Yin, M. A Facile-Synthesized Star Polycation Constructed as a Highly Efficient Gene Vector in Pest Management. ACS Sustain. Chem. Eng. 2019, 7, 6316–6322. [Google Scholar] [CrossRef]
- Fus-Kujawa, A.; Sieroń, Ł.; Dobrzyńska, E.; Chajec, Ł.; Mendrek, B.; Jarosz, N.; Głowacki, Ł.; Dubaj, K.; Dubaj, W.; Kowalczuk, A. Star Polymers as Non-Viral Carriers for Apoptosis Induction. Biomolecules 2022, 12, 608. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, Z.; Yang, Y.; Wu, Q.; Cheng, Z.; Liang, L.; Dai, H.; Huang, K.; Lu, W.; Zhang, Z. Efficacy and Safety of Low Dose Recombinant Tissue-Type Plasminogen Activator for the Treatment of Acute Pulmonary Thromboembolism: A Randomized, Multicenter, Controlled Trial. Chest 2010, 137, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Mendrek, B.; Sieroń, Ł.; Żymełka-Miara, I.; Binkiewicz, P.; Libera, M.; Smet, M.; Trzebicka, B.; Sieroń, A.L.; Kowalczuk, A.; Dworak, A. Nonviral Plasmid DNA Carriers Based on N, N′-Dimethylaminoethyl Methacrylate and Di (Ethylene Glycol) Methyl Ether Methacrylate Star Copolymers. Biomacromolecules 2015, 16, 3275–3285. [Google Scholar] [CrossRef] [PubMed]
- Fus-Kujawa, A.; Teper, P.; Botor, M.; Klarzyńska, K.; Sieroń, Ł.; Verbelen, B.; Smet, M.; Sieroń, A.L.; Mendrek, B.; Kowalczuk, A. Functional Star Polymers as Reagents for Efficient Nucleic Acids Delivery into HT-1080 Cells. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 356–370. [Google Scholar] [CrossRef]
- Mendrek, B.; Sieroń, Ł.; Libera, M.; Smet, M.; Trzebicka, B.; Sieroń, A.L.; Dworak, A.; Kowalczuk, A. Polycationic Star Polymers with Hyperbranched Cores for Gene Delivery. Polymer 2014, 55, 4551–4562. [Google Scholar] [CrossRef]
- Ding, H.; Park, S.; Zhong, M.; Pan, X.; Pietrasik, J.; Bettinger, C.J.; Matyjaszewski, K. Facile Arm-First Synthesis of Star Block Copolymers via ARGET ATRP with Ppm Amounts of Catalyst. Macromolecules 2016, 49, 6752–6760. [Google Scholar] [CrossRef]
- Zhang, Z.; Bilalis, P.; Zhang, H.; Gnanou, Y.; Hadjichristidis, N. Core Cross-Linked Multiarm Star Polymers with Aggregation-Induced Emission and Temperature Responsive Fluorescence Characteristics. Macromolecules 2017, 50, 4217–4226. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Kanazawa, A.; Kanaoka, S.; Aoshima, S. Quantitative and Ultrafast Synthesis of Well-Defined Star-Shaped Poly (p-Methoxystyrene) via One-Pot Living Cationic Polymerization. Macromolecules 2016, 49, 71–79. [Google Scholar] [CrossRef]
- Cho, H.Y.; Averick, S.E.; Paredes, E.; Wegner, K.; Averick, A.; Jurga, S.; Das, S.R.; Matyjaszewski, K. Star Polymers with a Cationic Core Prepared by ATRP for Cellular Nucleic Acids Delivery. Biomacromolecules 2013, 14, 1262–1267. [Google Scholar] [CrossRef]
- Huang, J.; Liang, H.; Cheng, D.; Lu, J. Polypeptide–Poly (Ethylene Glycol) Miktoarm Star Copolymers with a Fluorescently Labeled Core: Synthesis, Delivery and Imaging of SiRNA. Polym. Chem. 2016, 7, 1792–1802. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, D.; Zeng, M.; Alshehri, F.; Li, X.; O’Keeffe-Ahern, J.; Gao, Y.; Pierucci, L.; Greiser, U.; Yin, G. Star Poly (β-Amino Esters) Obtained from the Combination of Linear Poly (β-Amino Esters) and Polyethylenimine. ACS Macro Lett. 2017, 6, 575–579. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhao, P.; Chen, Z.; Lin, Q. Hyperbranched-Star PEI-g-PEG as a Nonviral Vector with Efficient Uptake and Hypotoxicity for Retinoblastoma Gene Therapy Application. Colloid Interface Sci. Commun. 2022, 50, 100647. [Google Scholar] [CrossRef]
- No, Y.J.; Castilho, M.; Ramaswamy, Y.; Zreiqat, H. Role of Biomaterials and Controlled Architecture on Tendon/Ligament Repair and Regeneration. Adv. Mater. 2020, 32, 1904511. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Mallapragada, S.K.; Agarwal, A. Synthetic Sustained Gene Delivery Systems. Curr. Top. Med. Chem. 2008, 8, 311–330. [Google Scholar] [CrossRef]
- Bosteels, J.; Weyers, S.; D’Hooghe, T.M.; Torrance, H.; Broekmans, F.J.; Chua, S.J.; Mol, B.W.J.; Cochrane Gynaecology and Fertility Group. Anti-Adhesion Therapy Following Operative Hysteroscopy for Treatment of Female Subfertility. Cochrane Database Syst. Rev. 2017, 11, CD011110. [Google Scholar] [CrossRef]
- Jin, J.; Yang, Q.Q.; Zhou, Y.L. Non-Viral Delivery of Gene Therapy to the Tendon. Polymers 2022, 14, 3338. [Google Scholar] [CrossRef]
- Schulze, J.; Hendrikx, S.; Schulz-Siegmund, M.; Aigner, A. Microparticulate Poly (Vinyl Alcohol) Hydrogel Formulations for Embedding and Controlled Release of Polyethylenimine (PEI)-Based Nanoparticles. Acta Biomater. 2016, 45, 210–222. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Benoit, D.S.W. Degradable Poly (Ethylene Glycol)(PEG)-Based Hydrogels for Spatiotemporal Control of SiRNA/Nanoparticle Delivery. J. Control. Release 2018, 287, 58–66. [Google Scholar] [CrossRef]
- Nie, H.; Soh, B.W.; Fu, Y.; Wang, C. Three-dimensional Fibrous PLGA/HAp Composite Scaffold for BMP-2 Delivery. Biotechnol. Bioeng. 2008, 99, 223–234. [Google Scholar] [CrossRef]
- Kong, H.J.; Kim, E.S.; Huang, Y.-C.; Mooney, D.J. Design of Biodegradable Hydrogel for the Local and Sustained Delivery of Angiogenic Plasmid DNA. Pharm. Res. 2008, 25, 1230–1238. [Google Scholar] [CrossRef]
- Trentin, D.; Hall, H.; Wechsler, S.; Hubbell, J.A. Tissue Engineering Special Feature: Peptide-Matrix-Mediated Gene Transfer of an Oxygen-Insensitive Hypoxia-Inducible Factor-1 Variant for Local Induction of Angiogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 2506–2511. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H.; Tanaka, E.; Fukuyama, N.; Sato, E.; Sakamoto, H.; Tabata, Y.; Ando, K.; Iseki, H.; Shinozaki, Y.; Kimura, K. Biodegradable Gelatin Hydrogel Potentiates the Angiogenic Effect of Fibroblast Growth Factor 4 Plasmid in Rabbit Hindlimb Ischemia. J. Am. Coll. Cardiol. 2003, 41, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomas, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abd-Elwakil, M.M.; Abd-Elsalam, K.; Elsayed, M.T.; Hashem, Y.; Mohamed, O. Natural Polymeric Nanoparticles for Brain-Targeting: Implications on Drug and Gene Delivery. Curr. Pharm. Des. 2016, 22, 3305–3323. [Google Scholar] [CrossRef]
- Sarvari, R.; Nouri, M.; Agbolaghi, S.; Roshangar, L.; Sadrhaghighi, A.; Seifalian, A.M.; Keyhanvar, P. A Summary on Non-Viral Systems for Gene Delivery Based on Natural and Synthetic Polymers. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 246–265. [Google Scholar] [CrossRef]
- Dang, J.M.; Leong, K.W. Natural Polymers for Gene Delivery and Tissue Engineering. Adv. Drug Deliv. Rev. 2006, 58, 487–499. [Google Scholar] [CrossRef]
- Madkhali, O.; Mekhail, G.; Wettig, S.D. Modified Gelatin Nanoparticles for Gene Delivery. Int. J. Pharm. 2019, 554, 224–234. [Google Scholar] [CrossRef]
- Thomas, T.J.; Tajmir-Riahi, H.-A.; Pillai, C.K.S. Biodegradable Polymers for Gene Delivery. Molecules 2019, 24, 3744. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-Based Formulations for Delivery of DNA and SiRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Zhang, H.; Bahamondez-Canas, T.F.; Zhang, Y.; Leal, J.; Smyth, H.D.C. PEGylated Chitosan for Nonviral Aerosol and Mucosal Delivery of the CRISPR/Cas9 System in Vitro. Mol. Pharm. 2018, 15, 4814–4826. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.-C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in Vitro and in Vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jia, X.; Yang, Q.; Yang, Y.; Zhao, Y.; Fan, Y.; Yuan, X. Targeted Delivery of MicroRNA-126 to Vascular Endothelial Cells via REDV Peptide Modified PEG-Trimethyl Chitosan. Biomater. Sci. 2016, 4, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Xing, L.; Luo, C.-Q.; Zhou, T.-J.; Li, H.-S.; Cho, C.-S. Chemical Modification of Chitosan as a Gene Transporter. Curr. Org. Chem. 2018, 22, 668–689. [Google Scholar] [CrossRef]
- Chuan, D.; Jin, T.; Fan, R.; Zhou, L.; Guo, G. Chitosan for Gene Delivery: Methods for Improvement and Applications. Adv. Colloid Interface Sci. 2019, 268, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, A.S.; Andranovitš, S.; Skorik, Y.A. Chitosan and Its Derivatives: Vectors in Gene Therapy. Russ. Chem. Rev. 2017, 86, 231. [Google Scholar] [CrossRef]
- Inamdar, N.; Mourya, V.K. Chitosan and Anionic Polymers—Complex Formation and Applications. In Polysaccharide: Development, Properties and Applications; Nova Science: Hauppauge, NY, USA, 2011; pp. 333–377. [Google Scholar]
- Yan, J.; Du, Y.-Z.; Chen, F.-Y.; You, J.; Yuan, H.; Hu, F.-Q. Effect of Proteins with Different Isoelectric Points on the Gene Transfection Efficiency Mediated by Stearic Acid Grafted Chitosan Oligosaccharide Micelles. Mol. Pharm. 2013, 10, 2568–2577. [Google Scholar] [CrossRef]
- Meng, T.; Wu, J.; Yi, H.; Liu, J.; Lu, B.; Yuan, M.; Huang, X.; Yuan, H.; Hu, F. A Spermine Conjugated Stearic Acid-g-Chitosan Oligosaccharide Polymer with Different Types of Amino Groups for Efficient P53 Gene Therapy. Colloids Surf. B Biointerfaces 2016, 145, 695–705. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K. Pullulan-Hyperproducing Color Variant Strain of Aureobasidium Pullulans FB-1 Newly Isolated from Phylloplane of Ficus Sp. Bioresour. Technol. 2008, 99, 3896–3899. [Google Scholar] [CrossRef]
- West, T.P. Production of the Polysaccharide Pullulan by Aureobasidium Pullulans Cell Immobilization. Polysaccharides 2022, 3, 544–555. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An Exopolysaccharide and Its Various Applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Nagata, Y.; Ishikawa, T.; Abe, T.; Murakami, M.; Kojima, T.; Taniguchi, K.; Shimada, H.; Hirano, S.; Ueda, S. Randomized Phase II Clinical Trial of NY-ESO-1 Protein Vaccine Combined with Cholesteryl Pullulan (CHP-NY-ESO-1) in Resected Esophageal Cancer Patients. Ann. Oncol. 2019, 30, v496. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and Pullulan Derivatives as Promising Biomolecules for Drug and Gene Targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Scomparin, A.; Salmaso, S.; Bersani, S.; Satchi-Fainaro, R.; Caliceti, P. Novel Folated and Non-Folated Pullulan Bioconjugates for Anticancer Drug Delivery. Eur. J. Pharm. Sci. 2011, 42, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Kaneo, Y.; Tanaka, T.; Nakano, T.; Yamaguchi, Y. Evidence for Receptor-Mediated Hepatic Uptake of Pullulan in Rats. J. Control. Release 2001, 70, 365–373. [Google Scholar] [CrossRef]
- Wang, J.; Dou, B.; Bao, Y. Efficient Targeted PDNA/SiRNA Delivery with Folate–Low-Molecular-Weight Polyethyleneimine–Modified Pullulan as Non-Viral Carrier. Mater. Sci. Eng. C 2014, 34, 98–109. [Google Scholar] [CrossRef]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F. Autophagy Modulation for Cancer Therapy. Cancer Biol. Ther. 2011, 11, 169–176. [Google Scholar] [CrossRef]
- Chen, L.; Ji, F.; Bao, Y.; Xia, J.; Guo, L.; Wang, J.; Li, Y. Biocompatible Cationic Pullulan-g-Desoxycholic Acid-g-PEI Micelles Used to Co-Deliver Drug and Gene for Cancer Therapy. Mater. Sci. Eng. C 2017, 70, 418–429. [Google Scholar] [CrossRef]
- Chen, L.; Qian, M.; Zhang, L.; Xia, J.; Bao, Y.; Wang, J.; Guo, L.; Li, Y. Co-Delivery of Doxorubicin and ShRNA of Beclin1 by Folate Receptor Targeted Pullulan-Based Multifunctional Nanomicelles for Combinational Cancer Therapy. RSC Adv. 2018, 8, 17710–17722. [Google Scholar] [CrossRef]
- Moraes, F.C.; Antunes, J.C.; Ramirez, L.M.F.; Aprile, P.; Franck, G.; Chauvierre, C.; Chaubet, F.; Letourneur, D. Synthesis of Cationic Quaternized Pullulan Derivatives for MiRNA Delivery. Int. J. Pharm. 2020, 577, 119041. [Google Scholar] [CrossRef]
- Hosseinkhani, H.; Aoyama, T.; Ogawa, O.; Tabata, Y. Liver Targeting of Plasmid DNA by Pullulan Conjugation Based on Metal Coordination. J. Control. Release 2002, 83, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D. Polysaccharides Ii; Springer Science & Business Media: Berlin, Germany, 2006; Volume 205, ISBN 3540371028. [Google Scholar]
- Huang, G.; Huang, H. Application of Dextran as Nanoscale Drug Carriers. Nanomedicine 2018, 13, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Bisazza, A.; Lembo, D. Micro-and Nanobubbles: A Versatile Non-Viral Platform for Gene Delivery. Int. J. Pharm. 2013, 456, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Sherly, M.C.D.; Rekha, M.R.; Harikrishnan, V.S. Cationised Dextran and Pullulan Modified with Diethyl Aminoethyl Methacrylate for Gene Delivery in Cancer Cells. Carbohydr. Polym. 2020, 242, 116426. [Google Scholar] [CrossRef] [PubMed]
- González, J.F.; Encinar, J.M.; González-García, C.M.; Sabio, E.; Ramiro, A.; Canito, J.L.; Gañán, J. Preparation of Activated Carbons from Used Tyres by Gasification with Steam and Carbon Dioxide. Appl. Surf. Sci. 2006, 252, 5999–6004. [Google Scholar] [CrossRef]
- Li, Y.; Jia, F.; Gao, Y.; Wang, X.; Cui, X.; Pan, Z.; Wang, W.; Li, M.; Lu, J.; Wu, Y. Self-Assembled Nanocomposites of Carboxymethyl β-Dextran/Protamine Sulfate for Enhanced Chemotherapeutic Drug Sensitivity of Triple-Negative Breast Cancer by Autophagy Inhibition via a Ternary Collaborative Strategy. Int. J. Biol. Macromol. 2023, 233, 123663. [Google Scholar] [CrossRef]
- Chen, Z.; Krishnamachary, B.; Mironchik, Y.; Banerjee, S.R.; Pomper, M.G.; Bhujwalla, Z.M. PSMA-Specific Degradable Dextran for Multiplexed Immunotargeted SiRNA Therapeutics against Prostate Cancer. Nanoscale 2022, 14, 14014–14022. [Google Scholar] [CrossRef]
- Kopka, K.; Benešová, M.; Bařinka, C.; Haberkorn, U.; Babich, J. Glu-Ureido–Based Inhibitors of Prostate-Specific Membrane Antigen: Lessons Learned during the Development of a Novel Class of Low-Molecular-Weight Theranostic Radiotracers. J. Nucl. Med. 2017, 58, 17S–26S. [Google Scholar] [CrossRef]
- Tekie, F.S.M.; Soleimani, M.; Zakerian, A.; Dinarvand, M.; Amini, M.; Dinarvand, R.; Arefian, E.; Atyabi, F. Glutathione Responsive Chitosan-Thiolated Dextran Conjugated MiR-145 Nanoparticles Targeted with AS1411 Aptamer for Cancer Treatment. Carbohydr. Polym. 2018, 201, 131–140. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Bambury, R.M.; Van Allen, E.M.; Drabkin, H.A.; Lara, P.N.; Harzstark, A.L.; Wagle, N.; Figlin, R.A.; Smith, G.W.; Garraway, L.A. A Phase II Trial of AS1411 (a Novel Nucleolin-Targeted DNA Aptamer) in Metastatic Renal Cell Carcinoma. Investig. New Drugs 2014, 32, 178–187. [Google Scholar] [CrossRef]
- Tong, X.; Ga, L.; Ai, J.; Wang, Y. Progress in Cancer Drug Delivery Based on AS1411 Oriented Nanomaterials. J. Nanobiotechnol. 2022, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Jariyal, H.; Srivastava, A. Hyaluronic Acid: More than a Carrier, Having an Overpowering Extracellular and Intracellular Impact on Cancer. Carbohydr. Polym. 2023, 317, 121081. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- de Paula, M.C.; Carvalho, S.G.; Silvestre, A.L.P.; Dos Santos, A.M.; Meneguin, A.B.; Chorilli, M. The Role of Hyaluronic Acid in the Design and Functionalization of Nanoparticles for the Treatment of Colorectal Cancer. Carbohydr. Polym. 2023, 320, 121257. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical Modifications of Hyaluronic Acid for the Synthesis of Derivatives for a Broad Range of Biomedical Applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic Acid (Hyaluronan): A Review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Robert, L. Hyaluronan, a Truly “Youthful” Polysaccharide. Its Medical Applications. Pathol. Biol. 2015, 63, 32–34. [Google Scholar] [CrossRef]
- Lee, S.J.; Ghosh, S.C.; Han, H.D.; Stone, R.L.; Bottsford-Miller, J.; Shen, D.Y.; Auzenne, E.J.; Lopez-Araujo, A.; Lu, C.; Nishimura, M. Metronomic Activity of CD44-Targeted Hyaluronic Acid-Paclitaxel in Ovarian Carcinoma. Clin. Cancer Res. 2012, 18, 4114–4121. [Google Scholar] [CrossRef]
- Yang, Y.; Jing, L.; Li, X.; Lin, L.; Yue, X.; Dai, Z. Hyaluronic Acid Conjugated Magnetic Prussian Blue@ Quantum Dot Nanoparticles for Cancer Theranostics. Theranostics 2017, 7, 466. [Google Scholar] [CrossRef]
- Ding, L.; Agrawal, P.; Singh, S.K.; Chhonker, Y.S.; Sun, J.; Murry, D.J. Polymer-Based Drug Delivery Systems for Cancer Therapeutics. Polymers 2024, 16, 843. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Won, J.E.; Byeon, Y.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Park, Y.-Y.; Lee, J.-W.; Kang, T.H.; Jung, I.D. Selective Delivery of PLXDC1 Small Interfering RNA to Endothelial Cells for Anti-Angiogenesis Tumor Therapy Using CD44-Targeted Chitosan Nanoparticles for Epithelial Ovarian Cancer. Drug Deliv. 2018, 25, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef]
- He, W.; Turkeshi, A.; Li, X.; Zhang, H. Progress in Systemic Co-Delivery of MicroRNAs and Chemotherapeutics for Cancer Treatment by Using Lipid-Based Nanoparticles. Ther. Deliv. 2020, 11, 591–603. [Google Scholar] [CrossRef]
- de Wolf, H.K.; Johansson, N.; Thong, A.-T.; Snel, C.J.; Mastrobattista, E.; Hennink, W.E.; Storm, G. Plasmid CpG Depletion Improves Degree and Duration of Tumor Gene Expression after Intravenous Administration of Polyplexes. Pharm. Res. 2008, 25, 1654–1662. [Google Scholar] [CrossRef]
- Yin, H.; Song, C.-Q.; Suresh, S.; Wu, Q.; Walsh, S.; Rhym, L.H.; Mintzer, E.; Bolukbasi, M.F.; Zhu, L.J.; Kauffman, K. Structure-Guided Chemical Modification of Guide RNA Enables Potent Non-Viral in Vivo Genome Editing. Nat. Biotechnol. 2017, 35, 1179–1187. [Google Scholar] [CrossRef]
- Charlesworth, C.T.; Deshpande, P.S.; Dever, D.P.; Camarena, J.; Lemgart, V.T.; Cromer, M.K.; Vakulskas, C.A.; Collingwood, M.A.; Zhang, L.; Bode, N.M. Identification of Preexisting Adaptive Immunity to Cas9 Proteins in Humans. Nat. Med. 2019, 25, 249–254. [Google Scholar] [CrossRef]
- Shmakov, S.; Abudayyeh, O.O.; Makarova, K.S.; Wolf, Y.I.; Gootenberg, J.S.; Semenova, E.; Minakhin, L.; Joung, J.; Konermann, S.; Severinov, K. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 2015, 60, 385–397. [Google Scholar] [CrossRef]
- Choi, H.; Shin, J.; Woo, J. Effect of Electricity Generation Mix on Battery Electric Vehicle Adoption and Its Environmental Impact. Energy Policy 2018, 121, 13–24. [Google Scholar] [CrossRef]
- Nelwan, M. Hemophilia A and Induced Pluripotent Stem Cells. J. Adv. Biol. Biotechnol. 2017, 14, 1–11. [Google Scholar] [CrossRef]
- Yu, J.; Chau, K.F.; Vodyanik, M.A.; Jiang, J.; Jiang, Y. Efficient Feeder-Free Episomal Reprogramming with Small Molecules. PLoS ONE 2011, 6, e17557. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K. Pluripotent Stem Cells Induced from Mouse Somatic Cells by Small-Molecule Compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Stadtfeld, M.; Nagaya, M.; Utikal, J.; Weir, G.; Hochedlinger, K. Induced Pluripotent Stem Cells Generated without Viral Integration. Science 2008, 322, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Temperton, N.J.; Wright, E.; Scott, S.D. Retroviral Pseudotypes—From Scientific Tools to Clinical Utility. Encycl. Life Sci 2015, 10, a0021549. [Google Scholar]
- Pérez-Luz, S.; Díaz-Nido, J. Prospects for the Use of Artificial Chromosomes and Minichromosome-Like Episomes in Gene Therapy. Biomed Res. Int. 2010, 2010, 642804. [Google Scholar] [CrossRef][Green Version]
- Nelwan, M.L.; No, J.A.Y. The Tyrosinemia Type I. Methods 2013, 14, 1–14. [Google Scholar]
- Balciunas, D.; Wangensteen, K.J.; Wilber, A.; Bell, J.; Geurts, A.; Sivasubbu, S.; Wang, X.; Hackett, P.B.; Largaespada, D.A.; McIvor, R.S. Harnessing a High Cargo-Capacity Transposon for Genetic Applications in Vertebrates. PLoS Genet. 2006, 2, e169. [Google Scholar] [CrossRef]
- Kishino, Y.; Seki, T.; Fujita, J.; Yuasa, S.; Tohyama, S.; Kunitomi, A.; Tabei, R.; Nakajima, K.; Okada, M.; Hirano, A. Derivation of Transgene-Free Human Induced Pluripotent Stem Cells from Human Peripheral T Cells in Defined Culture Conditions. PLoS ONE 2014, 9, e97397. [Google Scholar] [CrossRef]
- Nakanishi, M.; Otsu, M. Development of Sendai Virus Vectors and Their Potential Applications in Gene Therapy and Regenerative Medicine. Curr. Gene Ther. 2012, 12, 410–416. [Google Scholar] [CrossRef]
- Bernal, J.A. RNA-Based Tools for Nuclear Reprogramming and Lineage-Conversion: Towards Clinical Applications. J. Cardiovasc. Transl. Res. 2013, 6, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski-Daspit, A.S.; Kauffman, A.C.; Bracaglia, L.G.; Saltzman, W.M. Polymeric Vehicles for Nucleic Acid Delivery. Adv. Drug Deliv. Rev. 2020, 156, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tao, Y.; Lamas, V.; Huang, M.; Yeh, W.-H.; Pan, B.; Hu, Y.-J.; Hu, J.H.; Thompson, D.B.; Shu, Y. Treatment of Autosomal Dominant Hearing Loss by in Vivo Delivery of Genome Editing Agents. Nature 2018, 553, 217–221. [Google Scholar] [CrossRef]
- Lee, K.; Conboy, M.; Park, H.M.; Jiang, F.; Kim, H.J.; Dewitt, M.A.; Mackley, V.A.; Chang, K.; Rao, A.; Skinner, C. Nanoparticle Delivery of Cas9 Ribonucleoprotein and Donor DNA in Vivo Induces Homology-Directed DNA Repair. Nat. Biomed. Eng. 2017, 1, 889–901. [Google Scholar] [CrossRef]
- Park, H.; Oh, J.; Shim, G.; Cho, B.; Chang, Y.; Kim, S.; Baek, S.; Kim, H.; Shin, J.; Choi, H. In Vivo Neuronal Gene Editing via CRISPR–Cas9 Amphiphilic Nanocomplexes Alleviates Deficits in Mouse Models of Alzheimer’s Disease. Nat. Neurosci. 2019, 22, 524–528. [Google Scholar] [CrossRef]
- Stephan, M.T.; Moon, J.J.; Um, S.H.; Bershteyn, A.; Irvine, D.J. Therapeutic Cell Engineering with Surface-Conjugated Synthetic Nanoparticles. Nat. Med. 2010, 16, 1035–1041. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Tang, W.; Wen, R.; Zhou, S.; Lee, C.; Wang, H.; Jiang, W.; Delahunty, I.M.; Zhen, Z. Nanoparticle-laden Macrophages for Tumor-tropic Drug Delivery. Adv. Mater. 2018, 30, 1805557. [Google Scholar] [CrossRef]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.; Chang, R.-K.; Hussain, M.A. Pharmaceutical Development and Regulatory Considerations for Nanoparticles and Nanoparticulate Drug Delivery Systems. J. Pharm. Sci. 2013, 102, 3867–3882. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; De Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.; Brown, D.G.; Lennard, S.; Anderton, M.J.; Barrett, J.C.; Eriksson, U.; Fidock, M.; Hamren, B.; Johnson, A.; March, R.E. Impact of a Five-Dimensional Framework on R&D Productivity at AstraZeneca. Nat. Rev. Drug Discov. 2018, 17, 167–181. [Google Scholar] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and Strategies in Anti-Cancer Nanomedicine Development: An Industry Perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, S.; Gao, W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano 2020, 14, 12281–12290. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of Pegylated Liposomal Doxorubicin: Review of Animal and Human Studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate Systems for Brain Delivery of Drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Park, I.H.; Sohn, J.H.; Kim, S.B.; Lee, K.S.; Chung, J.S.; Lee, S.H.; Kim, T.Y.; Jung, K.H.; Cho, E.K.; Kim, Y.S. An Open-Label, Randomized, Parallel, Phase III Trial Evaluating the Efficacy and Safety of Polymeric Micelle-Formulated Paclitaxel Compared to Conventional Cremophor EL-Based Paclitaxel for Recurrent or Metastatic HER2-Negative Breast Cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2017, 49, 569–577. [Google Scholar] [CrossRef]
- Brand, W.; Noorlander, C.W.; Giannakou, C.; De Jong, W.H.; Kooi, M.W.; Park, M.V.D.Z.; Vandebriel, R.J.; Bosselaers, I.E.M.; Scholl, J.H.G.; Geertsma, R.E. Nanomedicinal Products: A Survey on Specific Toxicity and Side Effects. Int. J. Nanomed. 2017, 12, 6107–6129. [Google Scholar] [CrossRef]
- Luan, X.; Yuan, H.; Song, Y.; Hu, H.; Wen, B.; He, M.; Zhang, H.; Li, Y.; Li, F.; Shu, P. Reappraisal of Anticancer Nanomedicine Design Criteria in Three Types of Preclinical Cancer Models for Better Clinical Translation. Biomaterials 2021, 275, 120910. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ong, Z.Y.; Wiradharma, N.; Attia, A.B.E.; Yang, Y. Advanced Materials for Co-delivery of Drugs and Genes in Cancer Therapy. Adv. Healthc. Mater. 2012, 1, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Qiu, R. Cargo-Mediated Activation of Cytoplasmic Dynein In Vivo. Front. Cell Dev. Biol. 2020, 8, 598952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, L. A Window onto SiRNA Delivery. Nat. Biotechnol. 2013, 31, 611–612. [Google Scholar] [CrossRef]
- Zhang, H.; Gerson, T.; Varney, M.L.; Singh, R.K.; Vinogradov, S. V Multifunctional Peptide-PEG Intercalating Conjugates: Programmatic of Gene Delivery to the Blood-Brain Barrier. Pharm. Res. 2010, 27, 2528–2543. [Google Scholar] [CrossRef]
- Li, W.; Nicol, F.; Szoka Jr, F.C. GALA: A Designed Synthetic PH-Responsive Amphipathic Peptide with Applications in Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2004, 56, 967–985. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Lv, Y.; Xin, X.; Qin, C.; Han, X.; Yang, L.; He, W.; Yin, L. Cytosolic Co-Delivery of MiRNA-34a and Docetaxel with Core-Shell Nanocarriers via Caveolae-Mediated Pathway for the Treatment of Metastatic Breast Cancer. Sci. Rep. 2017, 7, 46186. [Google Scholar] [CrossRef]
- Gascón, A.R.; del Pozo-Rodríguez, A.; Solinís, M.Á. Non-Viral Delivery Systems in Gene Therapy. In Gene Therapy-Tools and Potential Applications; IntechOpen: Rijeka, Croatia, 2013; ISBN 9535110144. [Google Scholar]
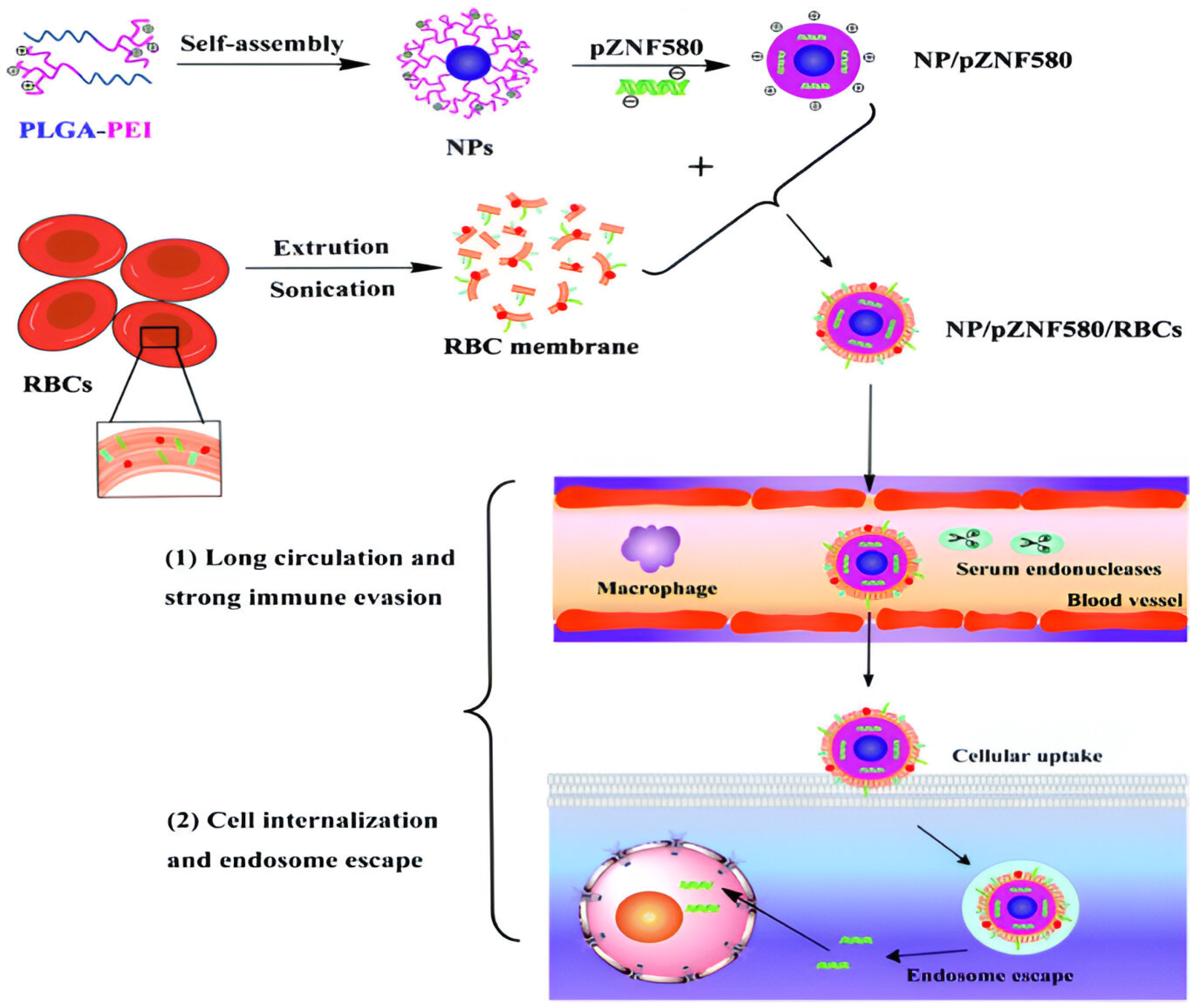
- Hao, X.; Li, Q.; Wang, H.; Muhammad, K.; Guo, J.; Ren, X.; Shi, C.; Xia, S.; Zhang, W.; Feng, Y. Red-Blood-Cell-Mimetic Gene Delivery Systems for Long Circulation and High Transfection Efficiency in ECs. J. Mater. Chem. B 2018, 6, 5975–5985. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and Pseudo-Stealth Nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Tabesh, F.; Haghverdi, G.; Devarakonda, K.P.; Massoud, T.F.; Paulmurugan, R. Synthesis, Characterization, and Application of a Biocompatible Gene Delivery Nanocarrier Constructed from Gold Nanostars and a Chitosan–Cyclodextrin–Poly (Ethylene Imine) Graft Polymer. Mater. Adv. 2024. [Google Scholar] [CrossRef]
- Alphandéry, E.; Grand-Dewyse, P.; Lefèvre, R.; Mandawala, C.; Durand-Dubief, M. Cancer Therapy Using Nanoformulated Substances: Scientific, Regulatory and Financial Aspects. Expert Rev. Anticancer Ther. 2015, 15, 1233–1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Y.; Xu, A.; Wang, C.; Li, N.; Huang, H.; Zhang, Y.; Yang, X. Non-Viral Nucleic Acid Delivery System for RNA Therapeutics. Adv. Ther. 2023, 6, 2300005. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-Viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.-Y.; Lu, A.; Wang, X.-Y.; Jiang, L.-X.; Wang, J.-C. Non-Viral Vectors for RNA Delivery. J. Control. Release 2022, 342, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Wang, K.; Stevens, W.F.; Kjems, J. Chitosan Composites for Biomedical Applications: Status, Challenges and Perspectives. Mater. Sci. Technol. 2008, 24, 1053–1061. [Google Scholar] [CrossRef]
- Maguire, C.A.; Ramirez, S.H.; Merkel, S.F.; Sena-Esteves, M.; Breakefield, X.O. Gene Therapy for the Nervous System: Challenges and New Strategies. Neurotherapeutics 2014, 11, 817–839. [Google Scholar] [CrossRef]



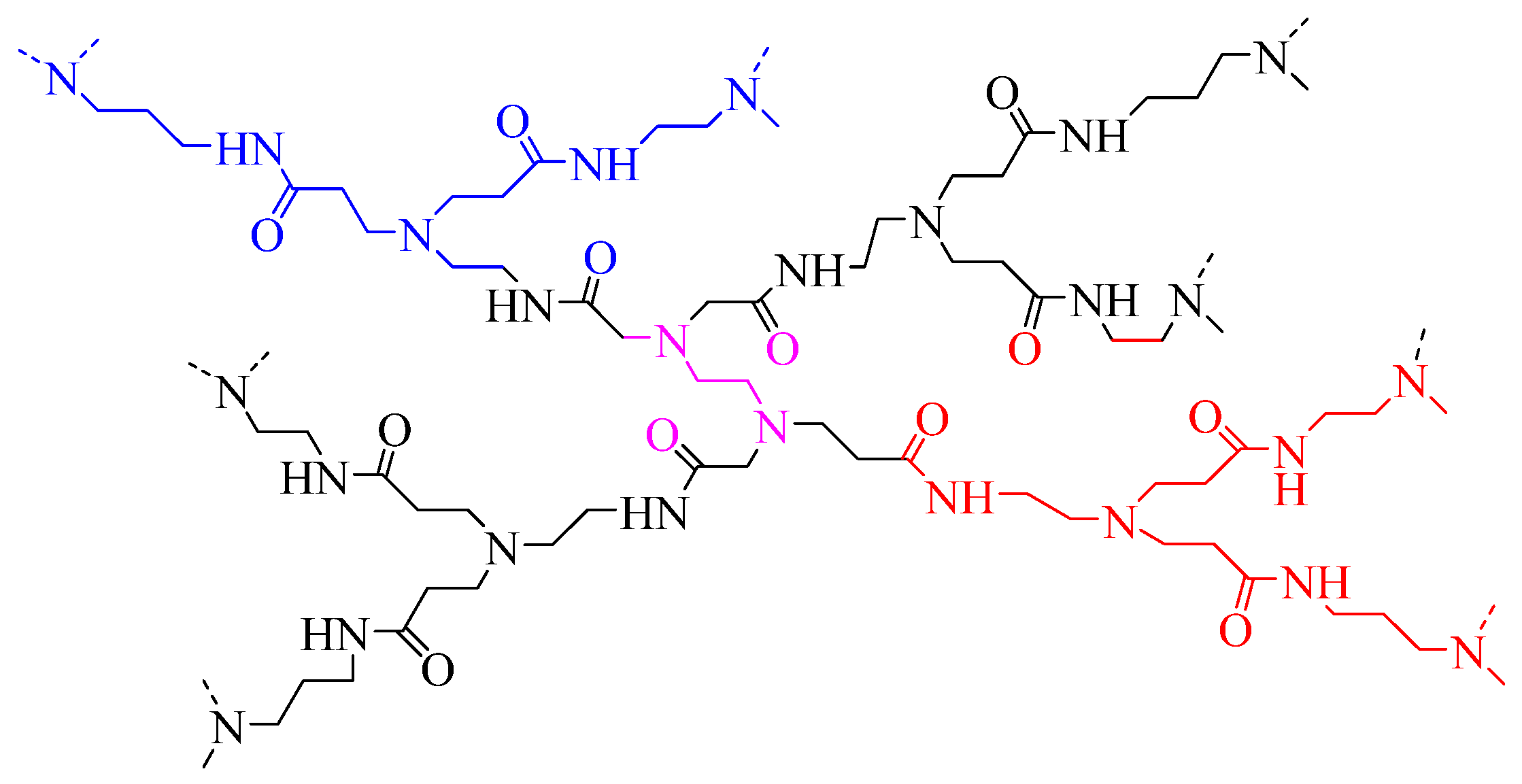

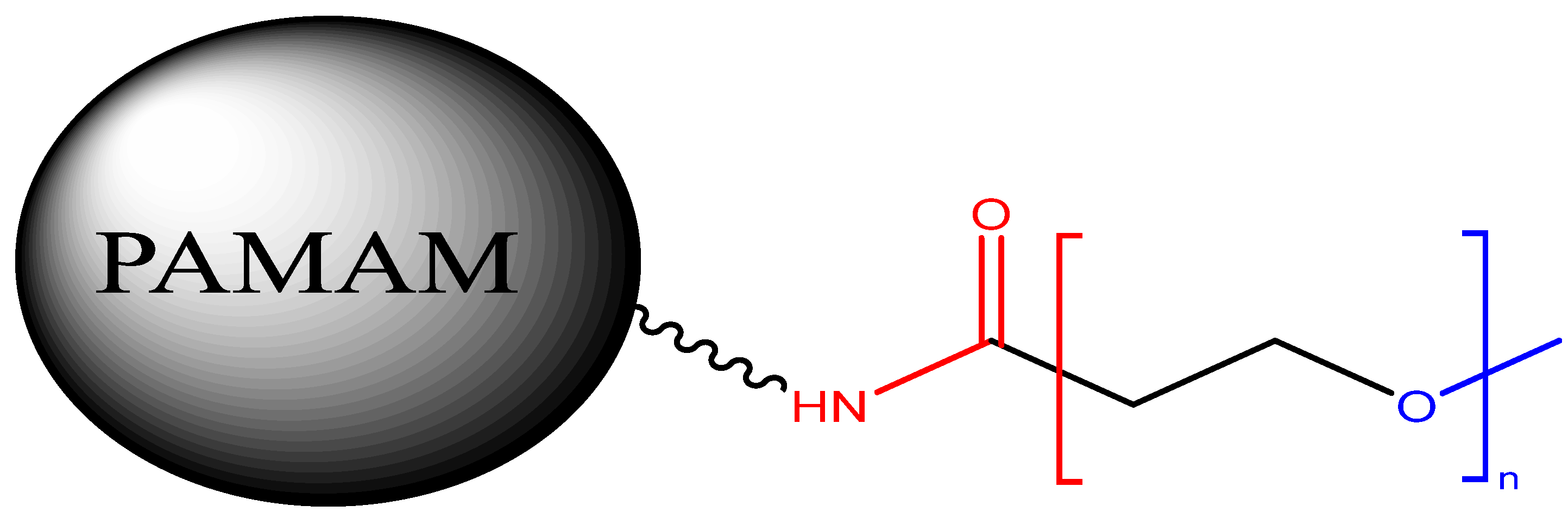
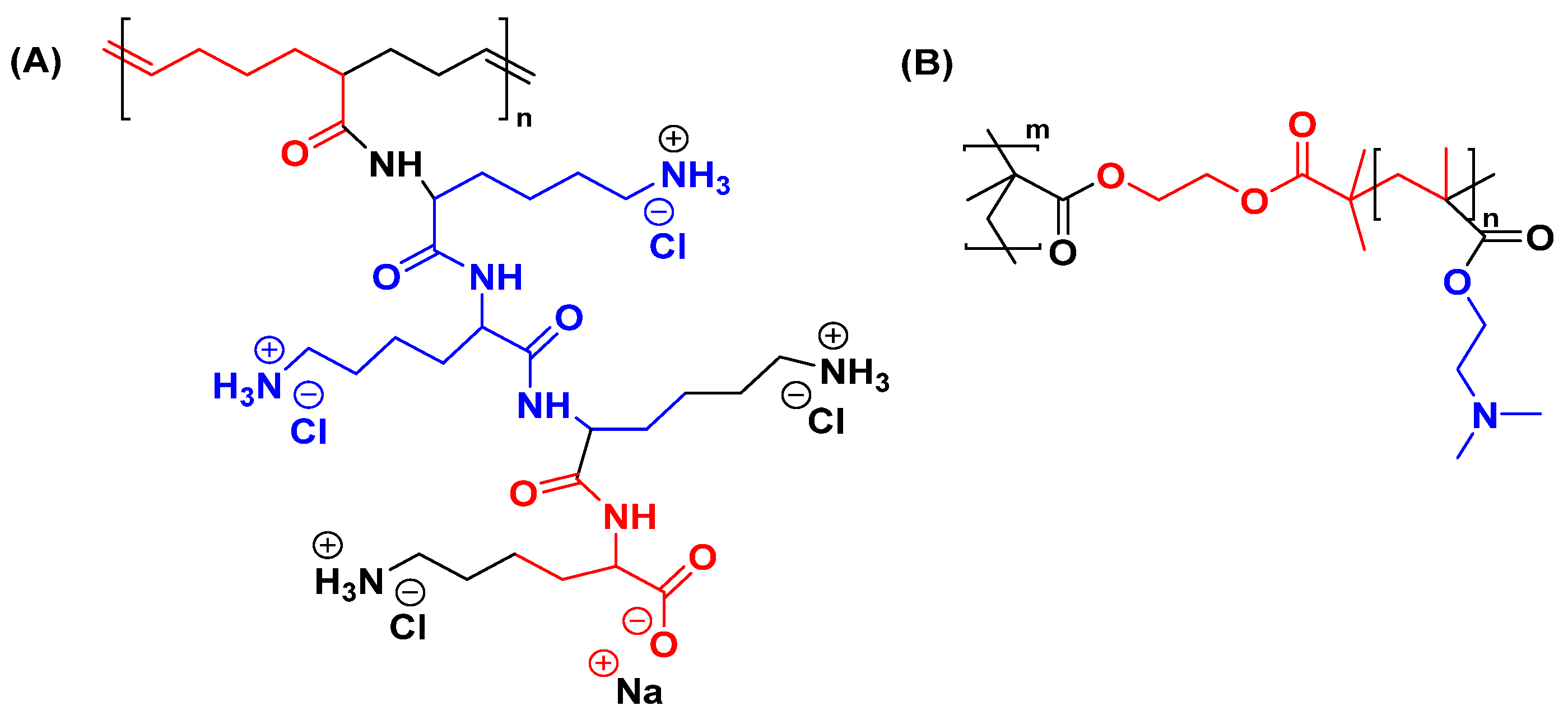



| Polymers | Structure | Characteristics | Limitations |
|---|---|---|---|
| Poly-L-lysine (PLL) | 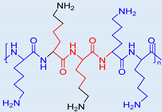 | Positively charged, good for DNA binding and delivery | High toxicity, degradable |
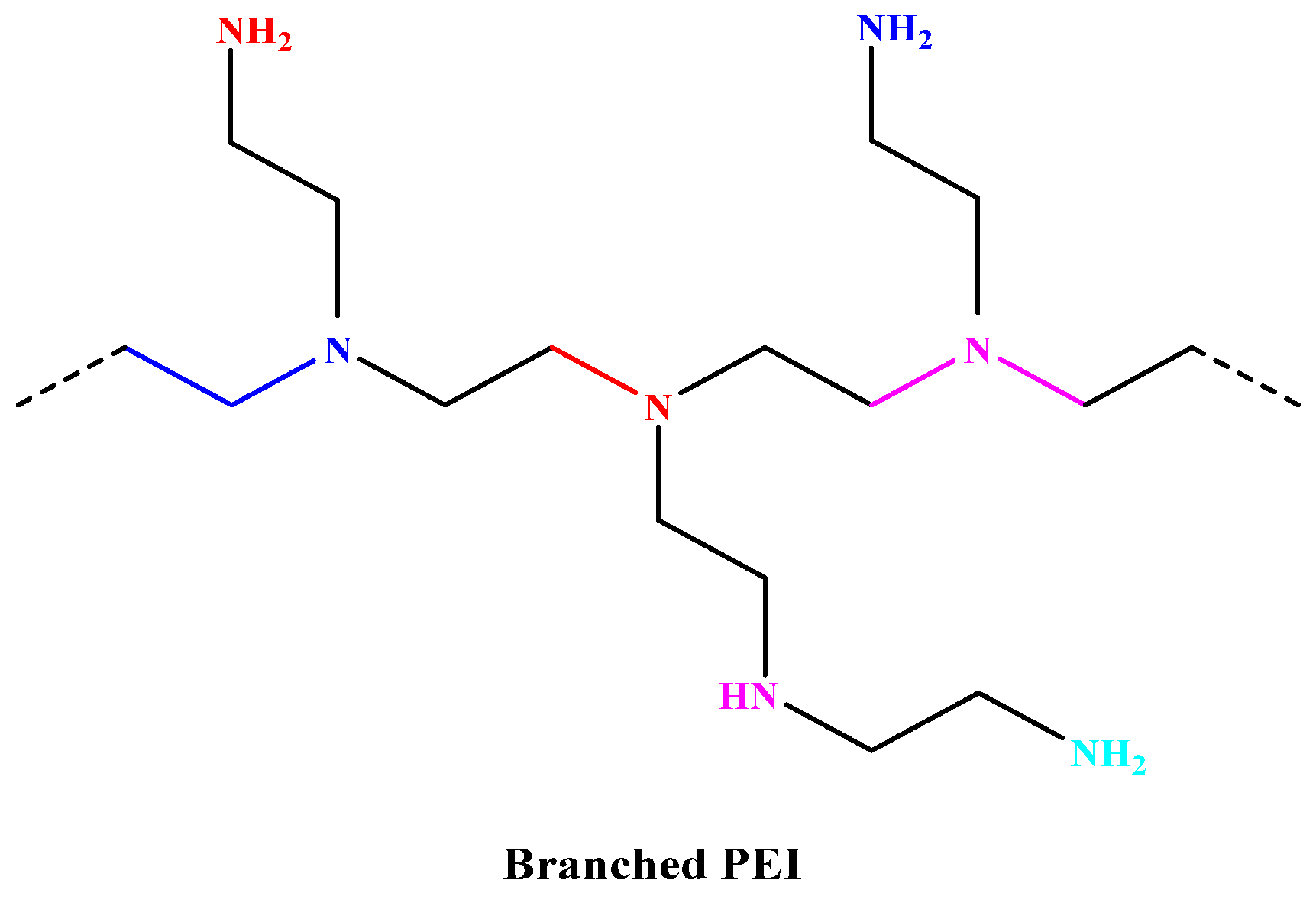
| Poly(ethylene imine) (PEI) |  | High transfection efficiency, strong DNA binding | High toxicity, non-degradable |
| Polyamidoamine (PAMAM) | 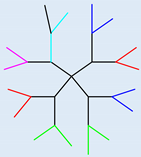 | Dendrimer structure, good biocompatibility, uniform size, high degree of branching, | Expensive, potential toxicity at higher generations |
| Poly-L-lysine-grafted-polyethylene glycol (PLL-g-PEG) | 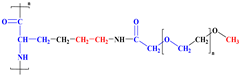 | Improved biocompatibility and stability | Complex synthesis, reduced DNA binding capacity |
| Hydrogels | Linear copolymer | Highly absorbent, good for controlled release | Potential for variable degradation rates, aggregation tendency with protein at high pHs |
| Natural polymers include chitosan, pullulan, dextrin, and hyaluronic acid. | 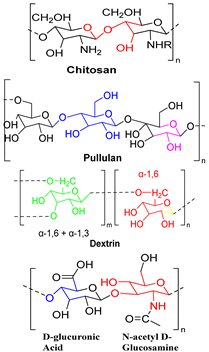 | Biodegradable, biocompatible, low toxicity | Limited mechanical properties, batch-to-batch variation, poor solubility in water, low transfection efficiency |
| Problem Being Solved | Modification | Ref. |
|---|---|---|
| Hydrophobicity-to-lipophilicity ratio | Alkyl carboxylate, PEG, and cholesteryl chloroformate | [93] |
| Improvement of biodegradability | GLFG oligopeptide, thioketal core | [94,95] |
| Prolonged blood circulation half-life and reduced clearance | PEG attached via a disulfide bond, carboxy betaine acrylamide | [96,97] |
| Enhanced cell binding and nucleic acid binding | Gold nanoparticle core with PAMAM-coated liposomes | [97,98] |
| Tissue targeting | ASSLNIA oligopeptide, monosaccharides (glucose and mannose) | [99,100] |
| Vectors | Cargo Capacity | Features | Ref. |
|---|---|---|---|
| Retrovirus | 7–10 kb | Integrating | [232] |
| Adenovirus | ~36 kb | Non-integrating | [233] |
| AAV | 4.7 kb | Safely integrating | [228] |
| HSV-1 Amplicon | 150 kb | Non-integrating | [234] |
| Tol2 Transposon | ~10 kb | Less integrating | [235] |
| Episomal | 172–660 kb | Non-integrating | [236] |
| Sendai virus | 15,384 kb | Non-integrating | [237,238] |
| Linear PEI | ~30 kb | Non-integrating, suitable for transient transfections, and good for plasmid DNA delivery. | [91] |
| Branched PEI | ~30 kb | Non-integrating, it has a higher transfection efficiency due to its branched structure, and it is often used for plasmid DNA and RNA delivery. | [239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M. Polymers as Efficient Non-Viral Gene Delivery Vectors: The Role of the Chemical and Physical Architecture of Macromolecules. Polymers 2024, 16, 2629. https://doi.org/10.3390/polym16182629
Khan M. Polymers as Efficient Non-Viral Gene Delivery Vectors: The Role of the Chemical and Physical Architecture of Macromolecules. Polymers. 2024; 16(18):2629. https://doi.org/10.3390/polym16182629
Chicago/Turabian StyleKhan, Majad. 2024. "Polymers as Efficient Non-Viral Gene Delivery Vectors: The Role of the Chemical and Physical Architecture of Macromolecules" Polymers 16, no. 18: 2629. https://doi.org/10.3390/polym16182629
APA StyleKhan, M. (2024). Polymers as Efficient Non-Viral Gene Delivery Vectors: The Role of the Chemical and Physical Architecture of Macromolecules. Polymers, 16(18), 2629. https://doi.org/10.3390/polym16182629






