Mechanical Properties and Thermal Decomposition Mechanism of Glycidyl Azide Polyol Energetic Thermoplastic Elastomer Binder with RDX Composite
Abstract
1. Introduction
2. Experiments
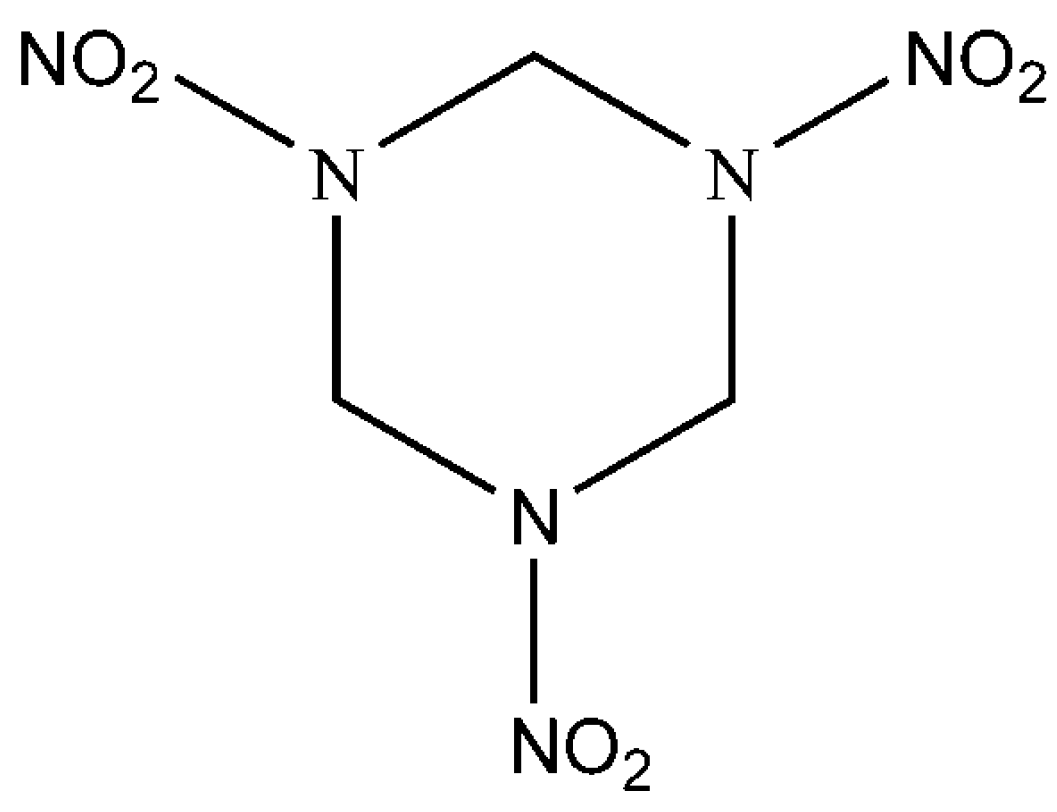
2.1. Materials
2.2. Measurements
3. Results and Discussion
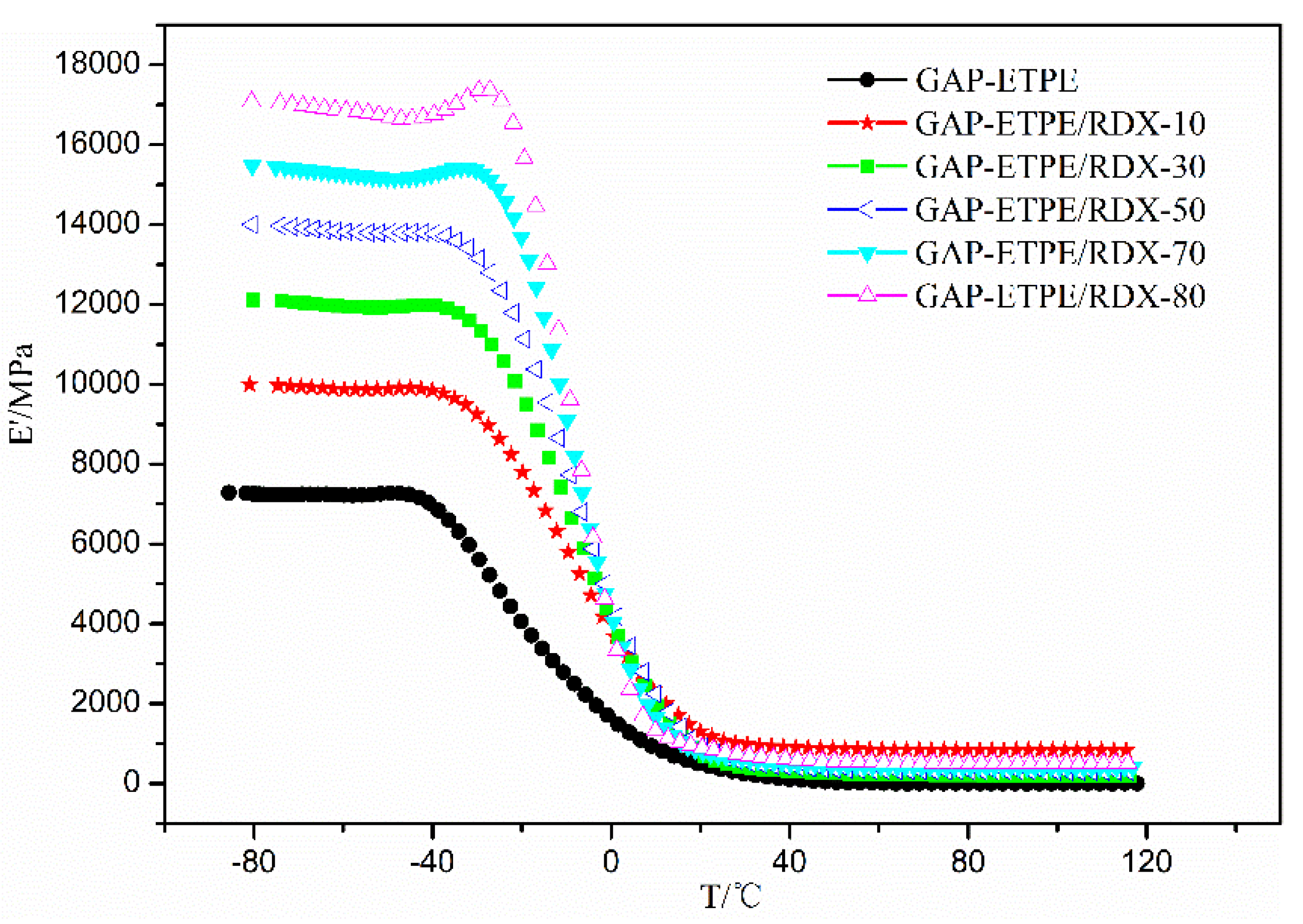
3.1. Dynamic Mechanical Analysis
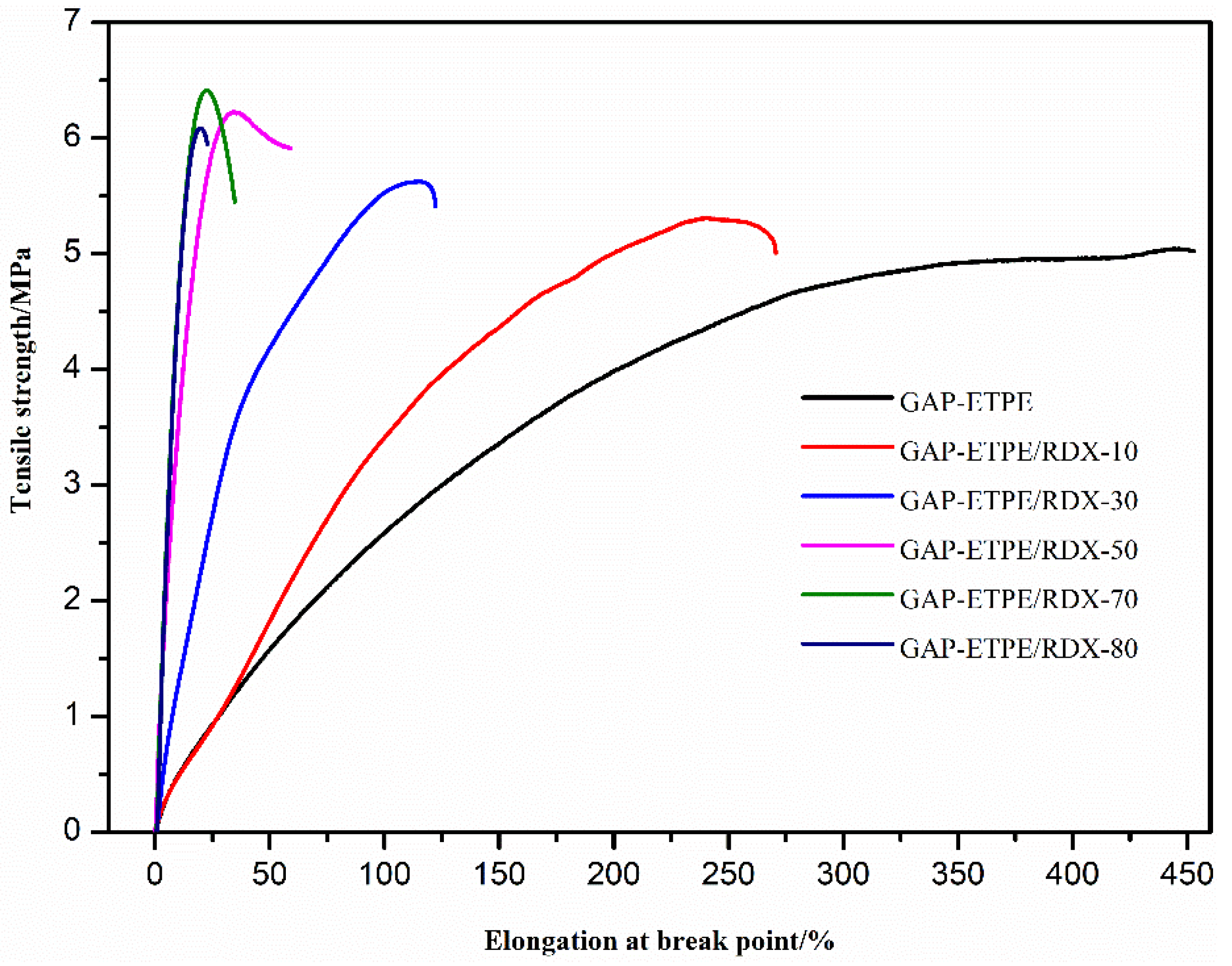
3.2. Tensile Mechanical Properties Analysis
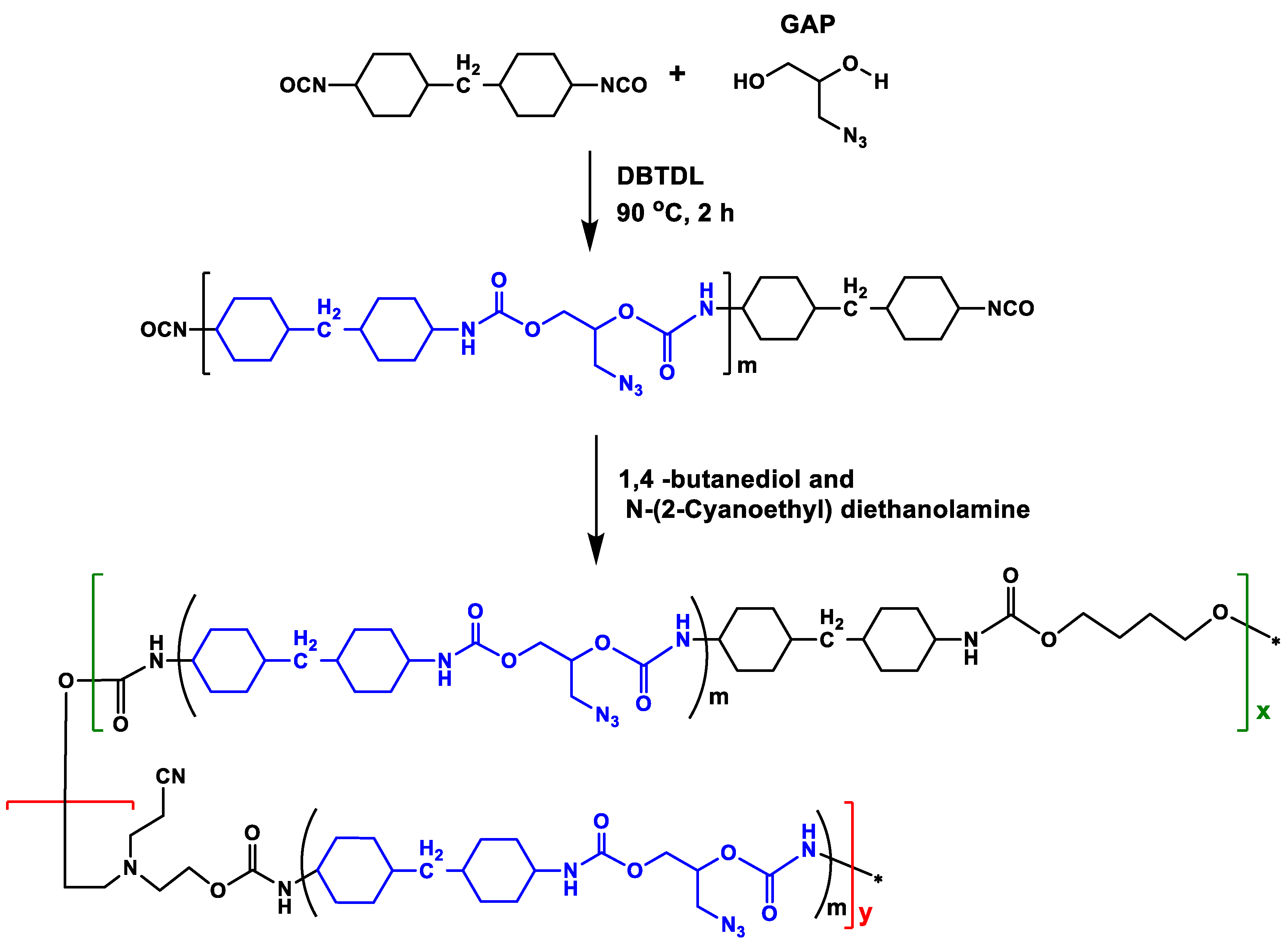
3.3. Interaction-Enhanced Mechanism Analysis
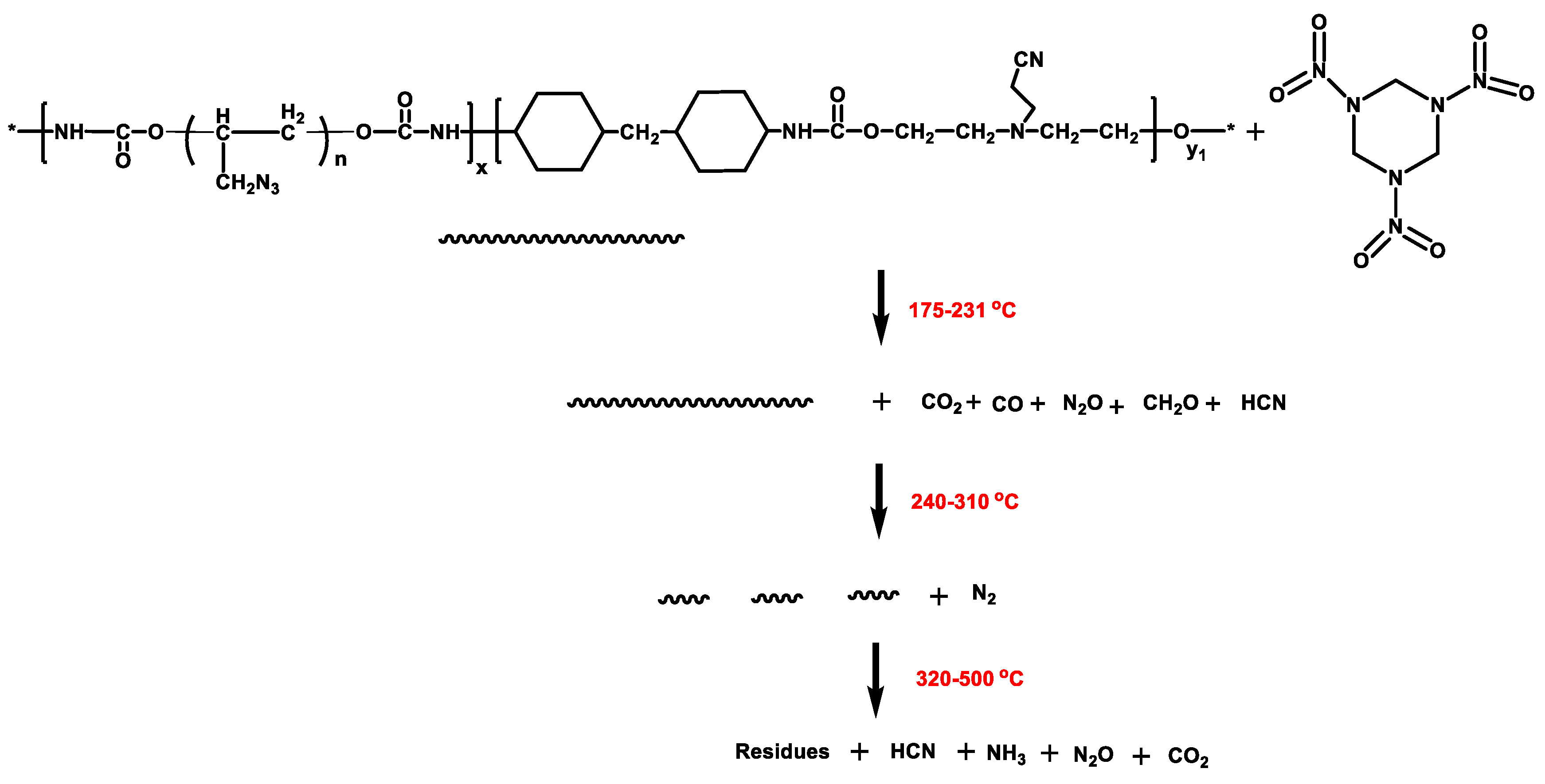
3.4. Thermal Decomposition Mechanism of GAP–ETPE/RDX
- (1)
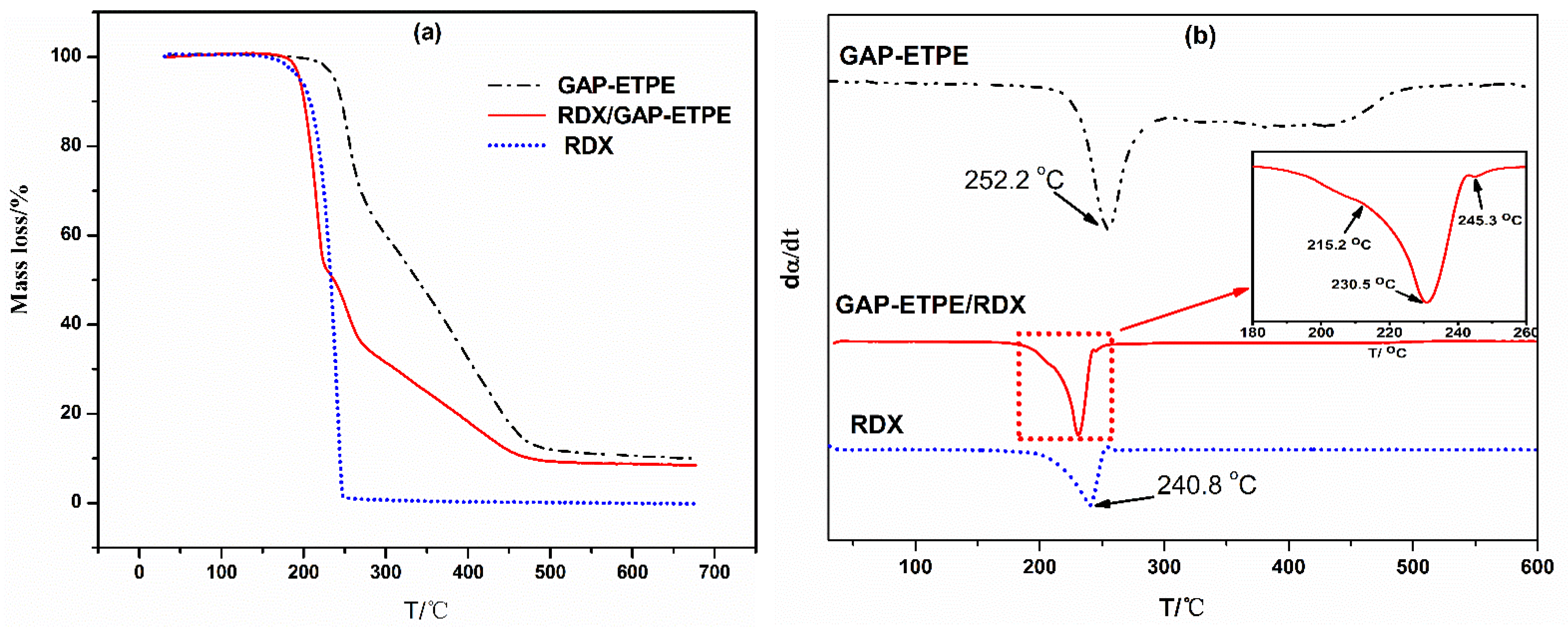
- TG/DTG analysis of GAP–ETPE/RDX
- (2)
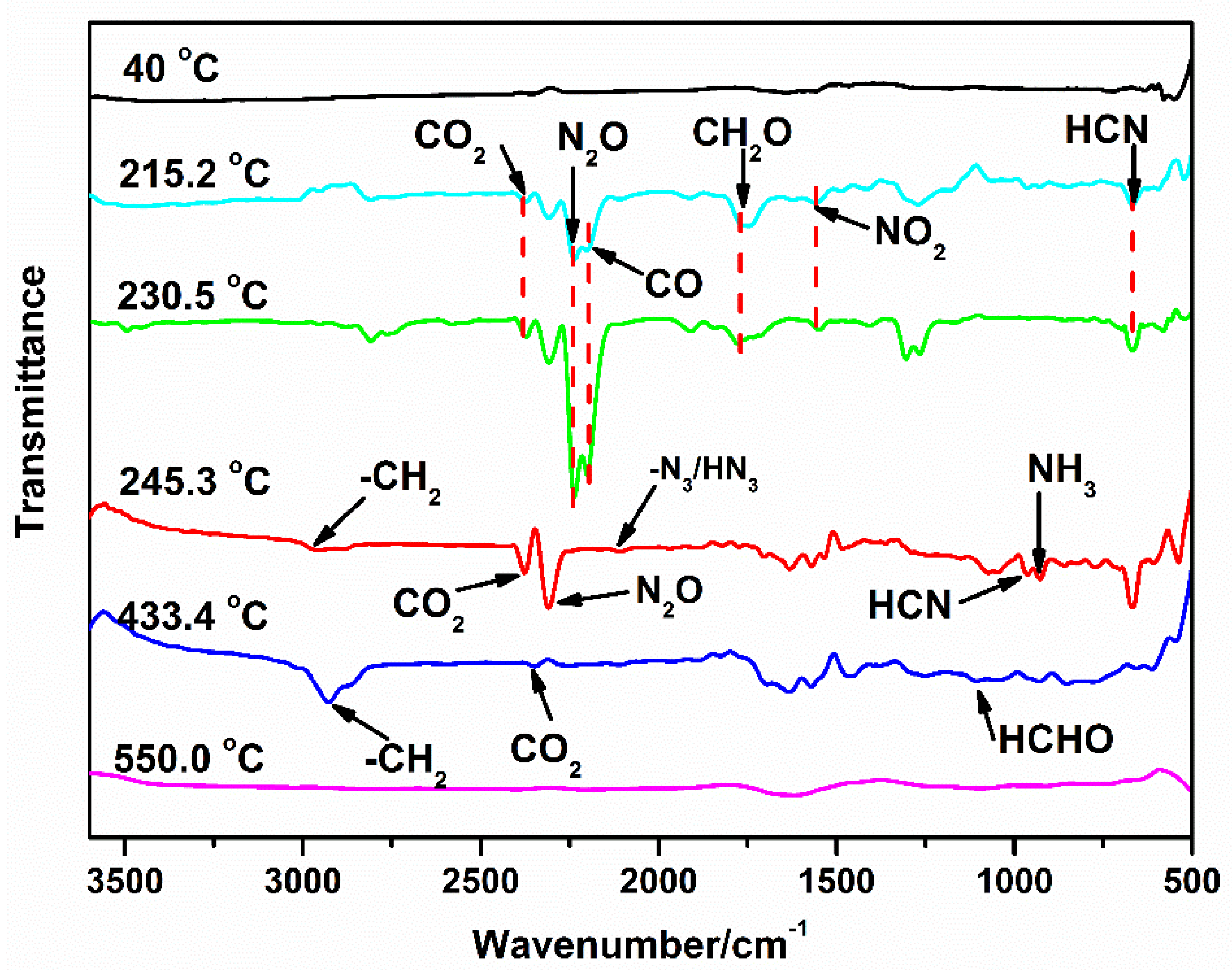
- TG/FTIR analysis of GAP–ETPE/RDX
4. Conclusions
- DMA data showed the E’ value of GAP–ETPE/RDX composite increased from 7280 MPa to 17,000 MPa, and the Tg value was shifted to higher temperatures, ranging from −30.1 °C to −26.1 °C with the RDX percentages.
- The static mechanical test showed that GAP–ETPE/RDX composite did not display a de-wetting phenomenon because of the “induce effect” between –CN and –NO2 group, which led to a stronger interfacial adhesion force for the GAP–ETPE/RDX model propellant.
- The TG/FTIR results show that there are four stages during the GAP–ETPE/RDX thermal decomposition: the first two stages are attributed to RDX decomposition process (175–231 °C), which is divided into two stages, one is slow rate decomposition, and the other is self-catalyzed accelerated decomposition stage. The third stage is the azide group (240–310 °C), and the last stage corresponds to the polyether and polyurethane decomposition (320–500 °C).
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikder, A.K.; Reddy, S. Review on Energetic Thermoplastic elastomers (ETPEs) for military science. Propellants Explos. Pyrotech. 2013, 38, 14–28. [Google Scholar] [CrossRef]
- Wan, M.; Shi, C.; Qian, X.; Qin, Y.; Jing, J.; Che, H.; Ren, F.; Li, J.; Yu, B.; Zhou, K. Design of novel double-layer coated ammonium polyphosphate and its application in flame retardant thermoplastic polyurethanes. Chem. Eng. J. 2023, 459, 141448. [Google Scholar] [CrossRef]
- Miller, R.S. Research on new energetic materials. J. Mater. Res. Soc. Symp. Proc. 1996, 418, 3–14. [Google Scholar] [CrossRef]
- Murphy, E.A.; Ntozakhe, T.; Murphy, C.J.; Fay, J.J.; Sperling, L.H.; Manser, G.E. Characterization of poly(3,3-bisethoxymethyl oxetane) and poly(3,3-bisazidomethyl oxetane) and their block copolymers. J. Appl. Polym. Sci. 1989, 37, 267–281. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, N.; Deng, J.; Ge, Z.; Luo, Y. A kind of bonding functional energetic thermoplastic elastomers based on glycidyl azide polymer. J. Elastomers Plast. 2016, 48, 728–738. [Google Scholar] [CrossRef]
- Guo, M.; Ma, Z.; He, L.; He, W.; Wang, Y. Effect of varied proportion of GAP-ETPE/NC as binder on thermal decomposition behaviors, stability and mechanical properties of nitramine propellants. J. Therm. Anal. Calorim. 2017, 130, 909–918. [Google Scholar] [CrossRef]
- Min, B.S.; Ko, S.W. Characterization of segmented block copolyurethane network based on glycidyl azide polymer and polycaprolactone. Macromole Res. 2007, 15, 225–233. [Google Scholar] [CrossRef]
- Sun, Q.; Sang, C.; Wang, Z.; Luo, Y. The study of mechanical and creep properties of glycidyl azide polyol energetic thermoplastic elastomer binder with bonding group with RDX and its interface reinforcement mechanism. Mater. Res. Express 2018, 5, 025309. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Zhang, Z.; Ge, Z.; Luo, Y. Effect of hard-segment content on rheological properties of glycidyl azide polyol-based energetic thermoplastic polyurethane elastomers. Polym. Bull. 2016, 73, 3095–3104. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Zhao, B.; Luo, Y. Effect of nitrocellulose (NC) on morphology, rheological and mechanical properties of glycidyl azide polymer based energetic thermoplastic elastomer/NC blends. Polym. Int. 2017, 66, 705–711. [Google Scholar] [CrossRef]
- Louafi, E.; Boulkadid, M.K.; Belgacemi, R.; Touidjine, S.; Akbi, H.; Belkhiri, S.; Benaliouche, F. Decomposition reaction kinetics of double-base propellant catalyzed with graphene oxide–copper oxide nanocomposite. Int. J. Chem. Kinet. 2023, 55, 479–488. [Google Scholar] [CrossRef]
- Yang, W.; Liu, W.; Zheng, M.; Zhang, X.; Jin, P.; Li, T.; Luo, Y. Preparation of PDA@ AP composite particles by two-step method and its application in glycidyl azide polymer-energetic thermoplastic elastomer propellants. J. Appl. Polym. Sci. 2023, 140, e54654. [Google Scholar] [CrossRef]
- Nardai, M.M. Cohesion Properties in PBX and Composite Propellants Computational Results and Experimental Aspects; Fraunhofer ICT: Pfinztal, Germany; Karlsruhe, Germany, 2015. [Google Scholar]
- Ding, Y.; Hu, C.; Guo, X.; Che, Y.; Huang, J. Structure and mechanical properties of novel composites based on glycidyl azide polymer and propargyl-terminated polybutadiene as potential binder of solid propellant. J. Appl. Polym. Sci. 2014, 131, 40007. [Google Scholar] [CrossRef]
- Kimura, E.; Oyumi, Y. Effects of copolymerization ratio of BAMO/NMMO and catalyst on sensitivity and burning rate of HMX propellant. Propellants Explos. Pyrotech. 1995, 20, 215–221. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Wang, Z.; Zhang, Y.; Ge, Z.; Luo, Y. Synthesis and characterization of novel energetic thermoplastic elastomers based on glycidyl azide polymer (GAP) with bonding functions. Polym. Bull. 2015, 72, 1835–1847. [Google Scholar] [CrossRef]
- Sun, Q.; Sang, C.; Wang, Z.; Luo, Y. Improvement of the creep resistance of glycidyl azide polyol energetic thermoplastic elastomer-based propellant by nitrocellulose filler and its mechanism. J. Elastomers Plast. 2018, 50, 579–595. [Google Scholar] [CrossRef]
- GB/T 528-1998; Rubber, Vulcanized or Thermoplastic Determination of Tensile Stress-Strain Properties. Standards Press of China: Beijing, China, 1999.
- Gerbase, A.E.; Petzhold, C.L.; Costa, A.O. Dynamic mechanical and thermal behavior of epoxy resins based on soybean oil. J. Am. Oil Chem. Soc. 2002, 8, 797–802. [Google Scholar] [CrossRef]
- Li, M.; Cho, U.R. Effectiveness of coupling agents in the poly (methyl methacrylate)-modified starch/styrene-butadiene rubber interfaces. Mater. Lett. 2013, 92, 132–135. [Google Scholar] [CrossRef]
- Pan, B.; Luo, Y.; Tan, H. Study on Adhesion Properties of CL-20 and Tree molecular Bonding Agent. Energ. Mater. 2004, 12, 199–202. [Google Scholar]
- Khoury Moussa, H.; Challita, G.; Badreddine, H.; Montay, G.; Guelorget, B.; Vallon, T.; Yared, W.; Abi Rizk, M.; Alhussein, A. Enhancement of mechanical properties of high modulus polypropylene grade for multilayer sewage pipes applications. J. Appl. Polym. Sci. 2023, 140, e53314. [Google Scholar] [CrossRef]
- Bauetdinov, Y.; Grekova, A.; Sangwan, R. Thermal stability and decomposition mechanisms of hexatetracarbon: Tight-binding molecular dynamics and density functional theory study. Mod. Phys. Lett. B 2023, 37, 2350023-1. [Google Scholar] [CrossRef]
- Felix, S.P.; Singh, G.; Sikder, A.; Aggrawal, J. Studies on energetic compounds: Part 33: Thermolysis of keto-RDX and its plastic bonded explosives containing thermally stable polymers. Thermochim. Acta 2005, 426, 53–60. [Google Scholar] [CrossRef]
- Patidar, L.; Thynell, S.T. Quantum mechanics investigation of initial reaction pathways and early ring-opening reactions in thermal decomposition of liquid-phase RDX. Combust. Flame 2017, 178, 7–20. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Y.; Li, X.; Li, G. Kinetics and mechanism of thermal decomposition reaction of BAMO/GAP tri-block copolymer. Polym. Mater. Sci. Eng. 2012, 11, 42–45. [Google Scholar]
- Zhang, Z.; Wang, G.; Luo, N.; Huang, M.; Jin, M.; Luo, Y. Thermal decomposition of energetic thermoplastic elastomers of poly (glycidyl nitrate). J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]




| Samples | RDX/% | Tg/°C |
|---|---|---|
| GAP–ETPE | 0 | −31.6 |
| GAP–ETPE/RDX-10 | 10 | −30.1 |
| GAP–ETPE/RDX-30 | 30 | −28.6 |
| GAP–ETPE/RDX-50 | 50 | −27.2 |
| GAP–ETPE/RDX-70 | 70 | −26.4 |
| GAP–ETPE/RDX-80 | 80 | −26.1 |
| Sample Name | Tensile Strength/MPa | E/MPa | εb/% |
|---|---|---|---|
| GAP–ETPE | 5.01 ± 0.52 | 1.1 ± 0.1 | 452.3 ± 11.2 |
| GAP–ETPE/RDX-10 | 5.32 ± 0.73 | 2.2 ± 0.2 | 269.2 ± 8.3 |
| GAP–ETPE/RDX-30 | 5.61 ± 0.51 | 4.6 ± 0.6 | 121.5 ± 14.1 |
| GAP–ETPE/RDX-50 | 6.12 ± 0.32 | 7.6 ± 0.4 | 85.9 ± 3.5 |
| GAP–ETPE/RDX-70 | 6.43 ± 0.65 | 20.1 ± 0.5 | 34.7 ± 4.6 |
| GAP–ETPE/RDX-80 | 6.07 ± 0.58 | 29.5 ± 2.5 | 22.5 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Yang, X.-M.; Yin, G.-Z. Mechanical Properties and Thermal Decomposition Mechanism of Glycidyl Azide Polyol Energetic Thermoplastic Elastomer Binder with RDX Composite. Polymers 2024, 16, 2626. https://doi.org/10.3390/polym16182626
Sun Q, Yang X-M, Yin G-Z. Mechanical Properties and Thermal Decomposition Mechanism of Glycidyl Azide Polyol Energetic Thermoplastic Elastomer Binder with RDX Composite. Polymers. 2024; 16(18):2626. https://doi.org/10.3390/polym16182626
Chicago/Turabian StyleSun, Qili, Xiao-Mei Yang, and Guang-Zhong Yin. 2024. "Mechanical Properties and Thermal Decomposition Mechanism of Glycidyl Azide Polyol Energetic Thermoplastic Elastomer Binder with RDX Composite" Polymers 16, no. 18: 2626. https://doi.org/10.3390/polym16182626
APA StyleSun, Q., Yang, X.-M., & Yin, G.-Z. (2024). Mechanical Properties and Thermal Decomposition Mechanism of Glycidyl Azide Polyol Energetic Thermoplastic Elastomer Binder with RDX Composite. Polymers, 16(18), 2626. https://doi.org/10.3390/polym16182626






