Oil Sorption Properties of Centrifugally Spun Polyisobutylene-Based Thermoplastic Elastomer Microfibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Preparation
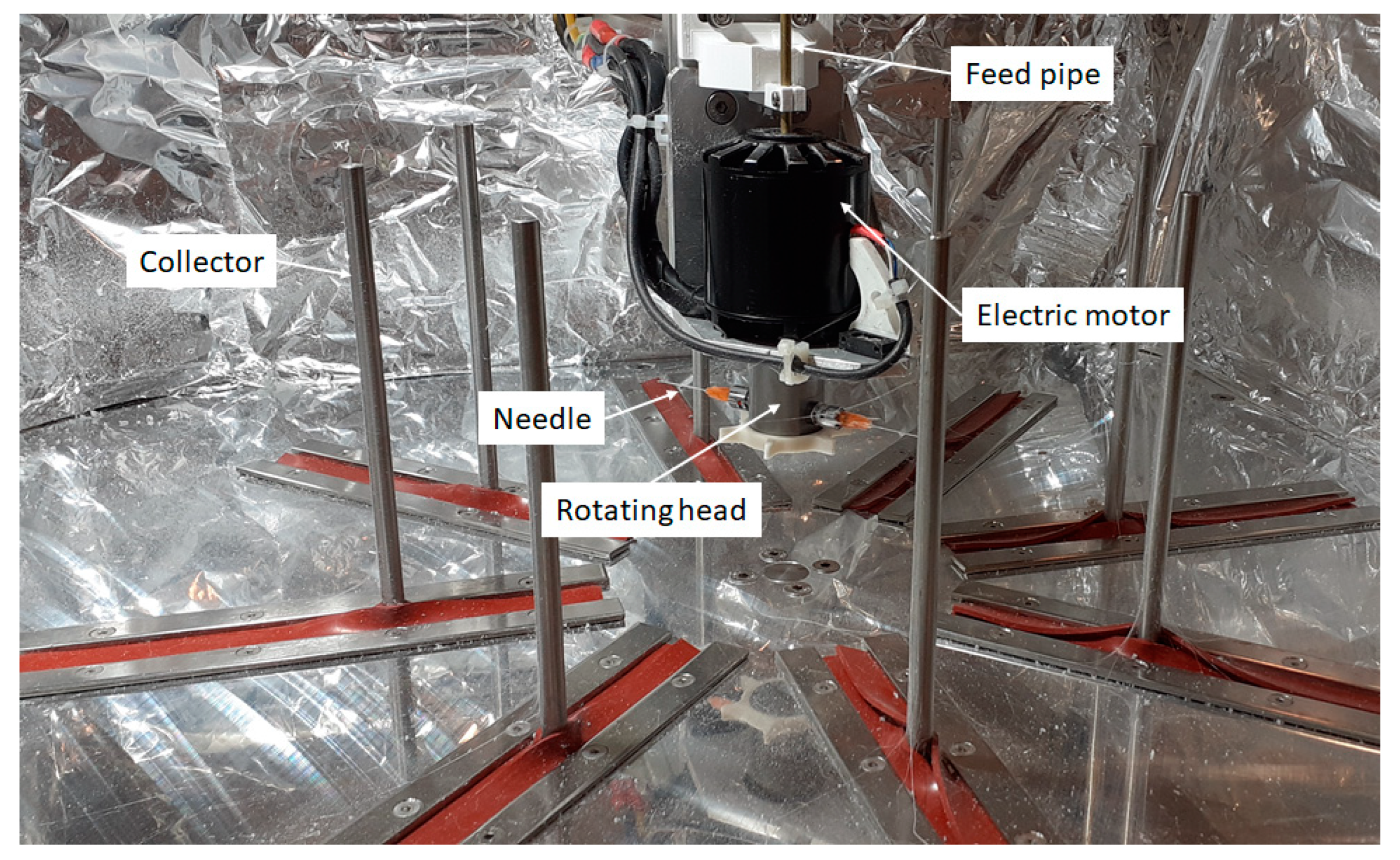
2.3. Centrifugal Spinning
2.4. Microscopy
2.5. Viscosity Measurement
2.6. Contact Angle Measurement (CA)
2.7. Oil Sorption Studies
- Specimens of 300–400 mg (SIBS and control) and 200 mg (PS) were cut from the fiber mats. The reason for the difference in mass between the SIBS and PS samples was the difference in volume, as the average density of the PS fiber mat was lower, and the testing equipment would not have been able to properly contain a 300 mg PS specimen. In the contact phase, the SIBS and PS specimens were placed in a steel mesh basket and the basket was placed in a stainless steel vessel containing 200 mL of oil. The PP specimens were placed on the oil surface by themselves. The devices used to handle the specimens can be seen in Figure 2. The different meshes were necessary as neither the oil-soaked SIBS nor the PS specimens could be held by a hook. Since the PP samples maintained their structural integrity throughout the whole experiment, a simple hook was enough for handling. This, however, was not true for the SIBS and PS samples, and a supporting mesh was required. The SIBS specimens were contained in a smaller mesh basket, while the larger size of the PS specimens warranted the use of a larger basket, as shown in Figure 2.
- 2.
- Water (45 mL) and motor oil (1 g) were added to a beaker. The beaker was placed on a shaker (Grant-Bio PMS-1000i; Grant Instruments Ltd., Cambridge, UK) set to 260 cycles/min. Specimens of 150–200 mg (SIBS and PP control) and 100 mg (PS) were placed on top of the liquid surface. After 15 min, the shaker was stopped, and the system was allowed to sit for 2 min. Following that, the specimens were removed, drained for 1 min, and weighed. A second weight measurement was performed 24 h after the first one. The specimens were also tested with pure water, to obtain a baseline water uptake value. The steps were the same as with the oil–water mixture with an additional step to remove all of the water using paper towels and drying in open air for 24 h. Each measurement was performed with 3 specimens.
2.8. Porosity Calculations
3. Results and Discussion
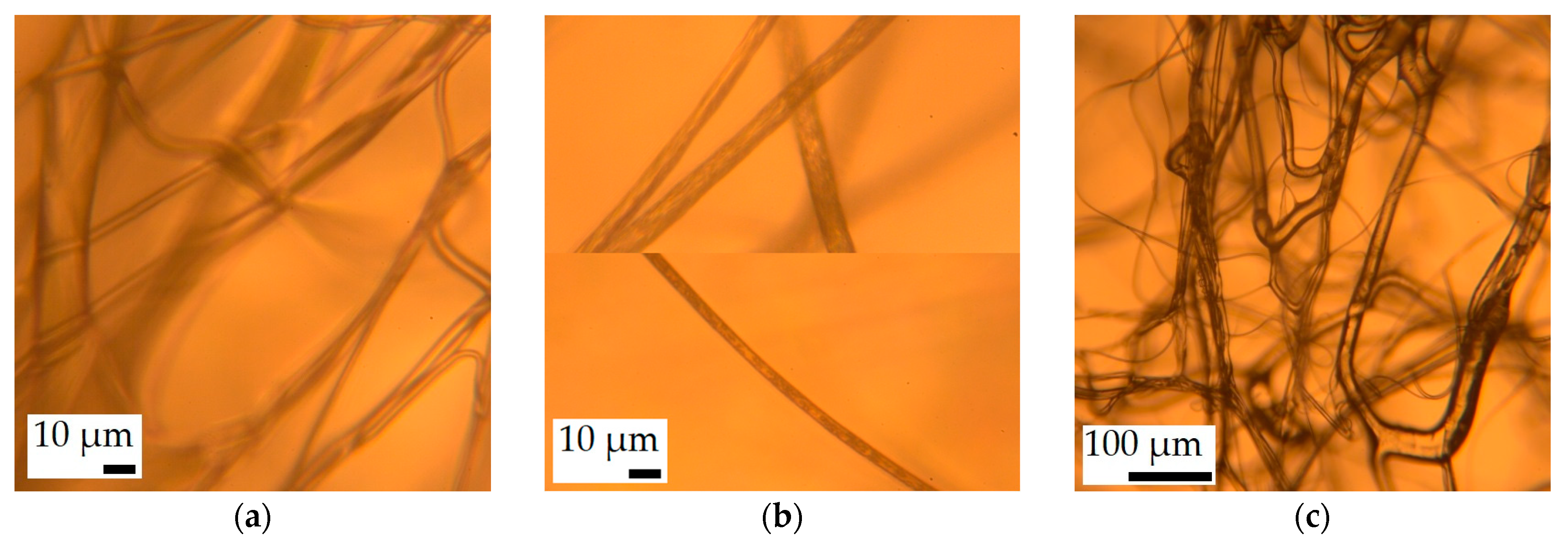
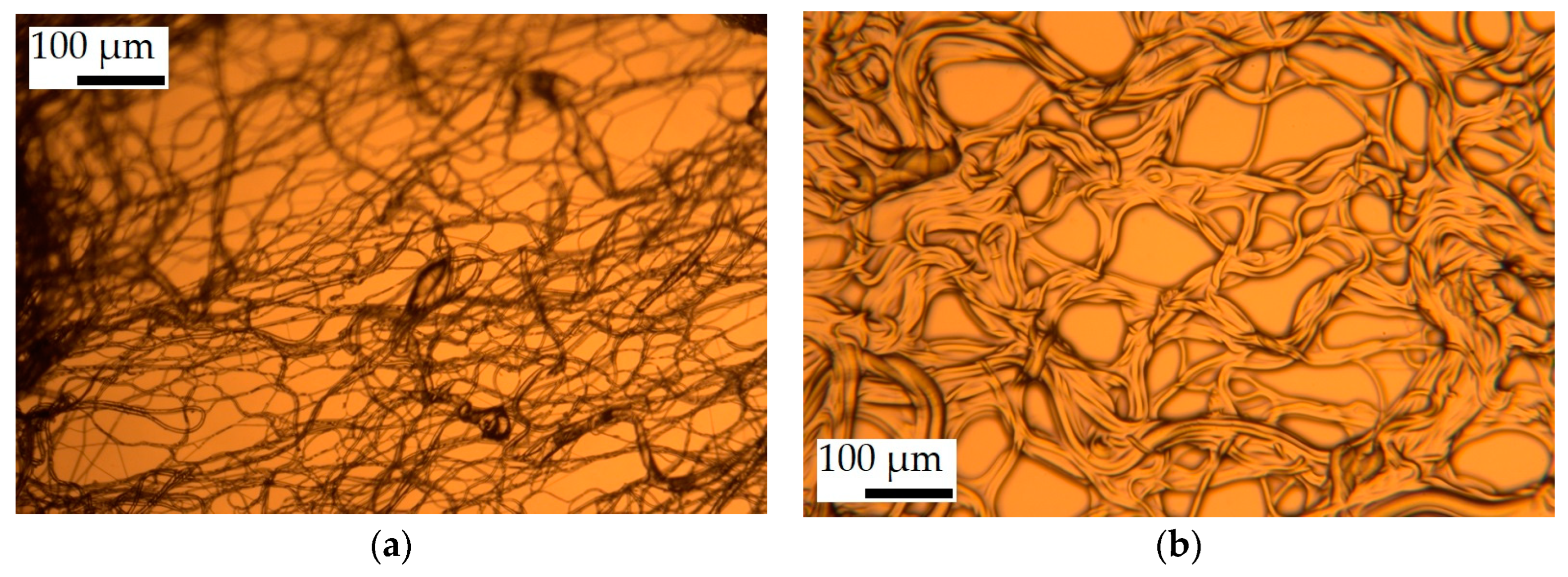
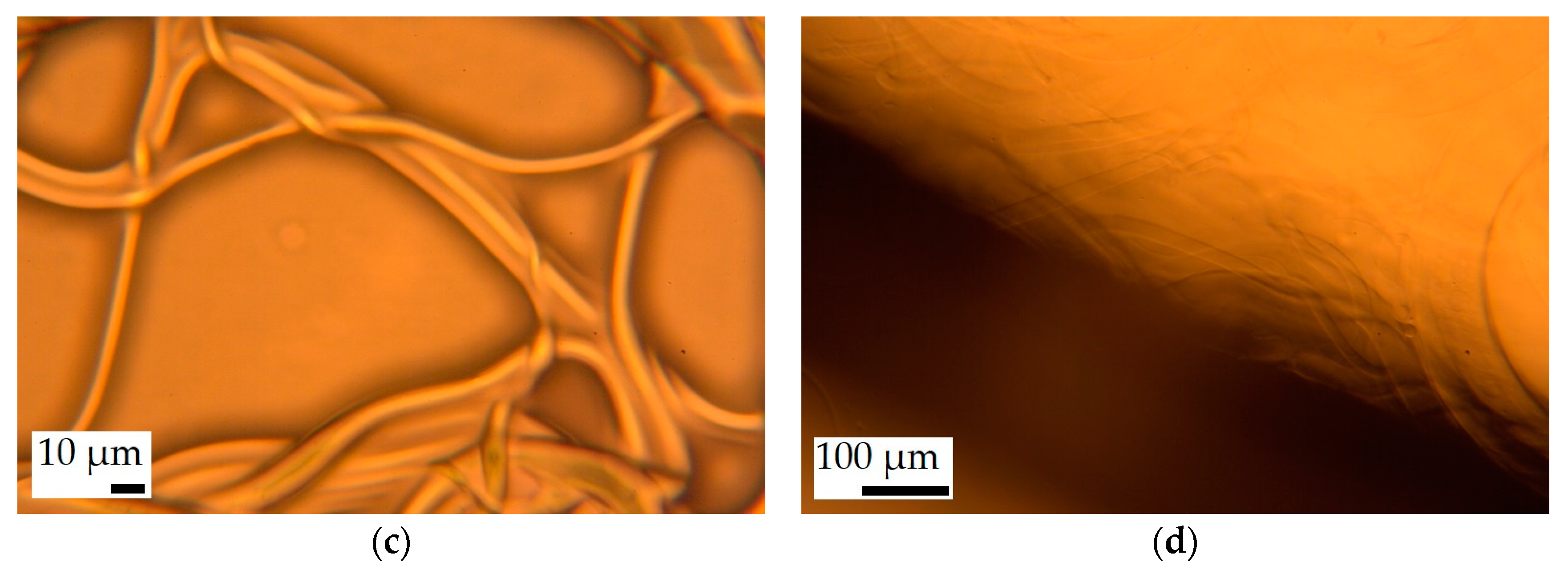
3.1. Fiber Morphology
3.2. Oil Viscosity
3.3. Contact Angles and Sorption Kinetics
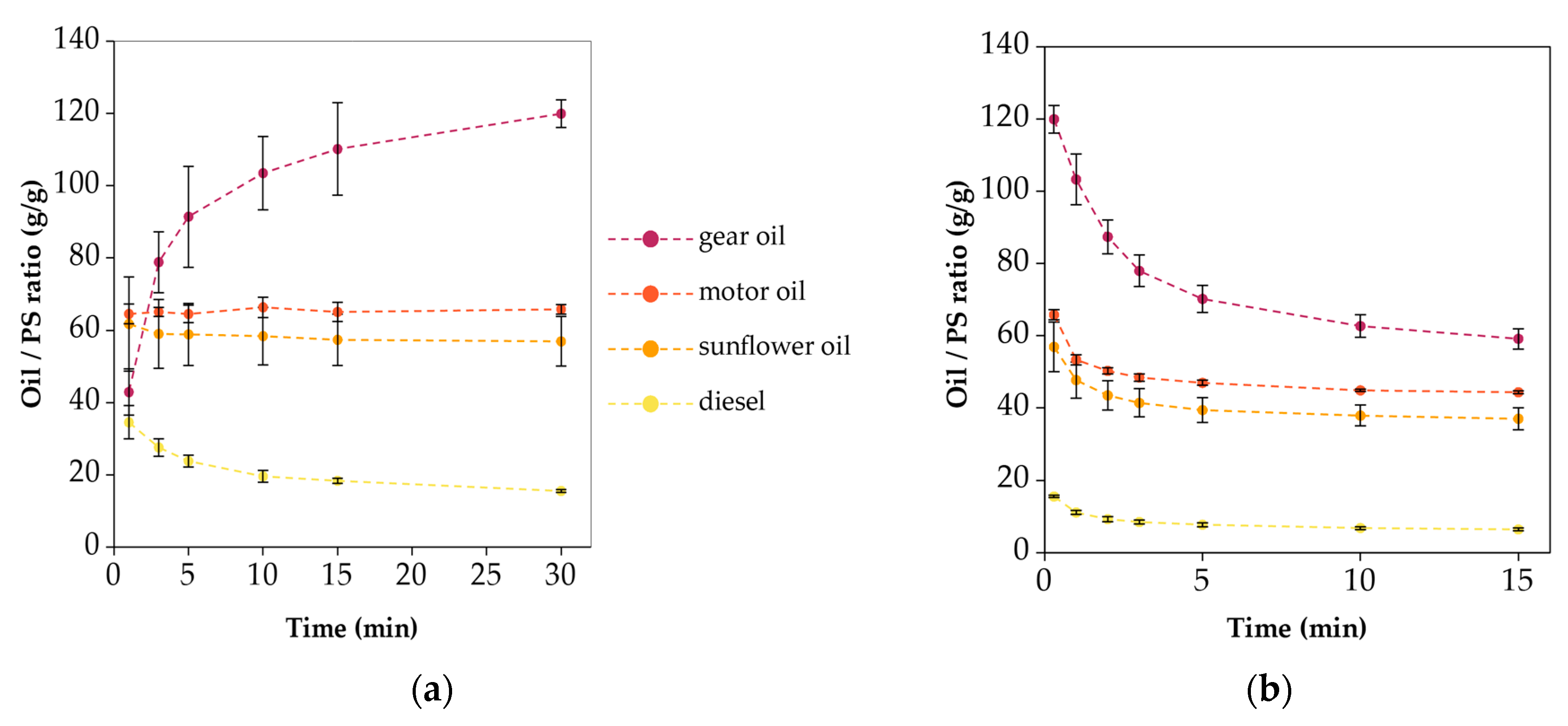
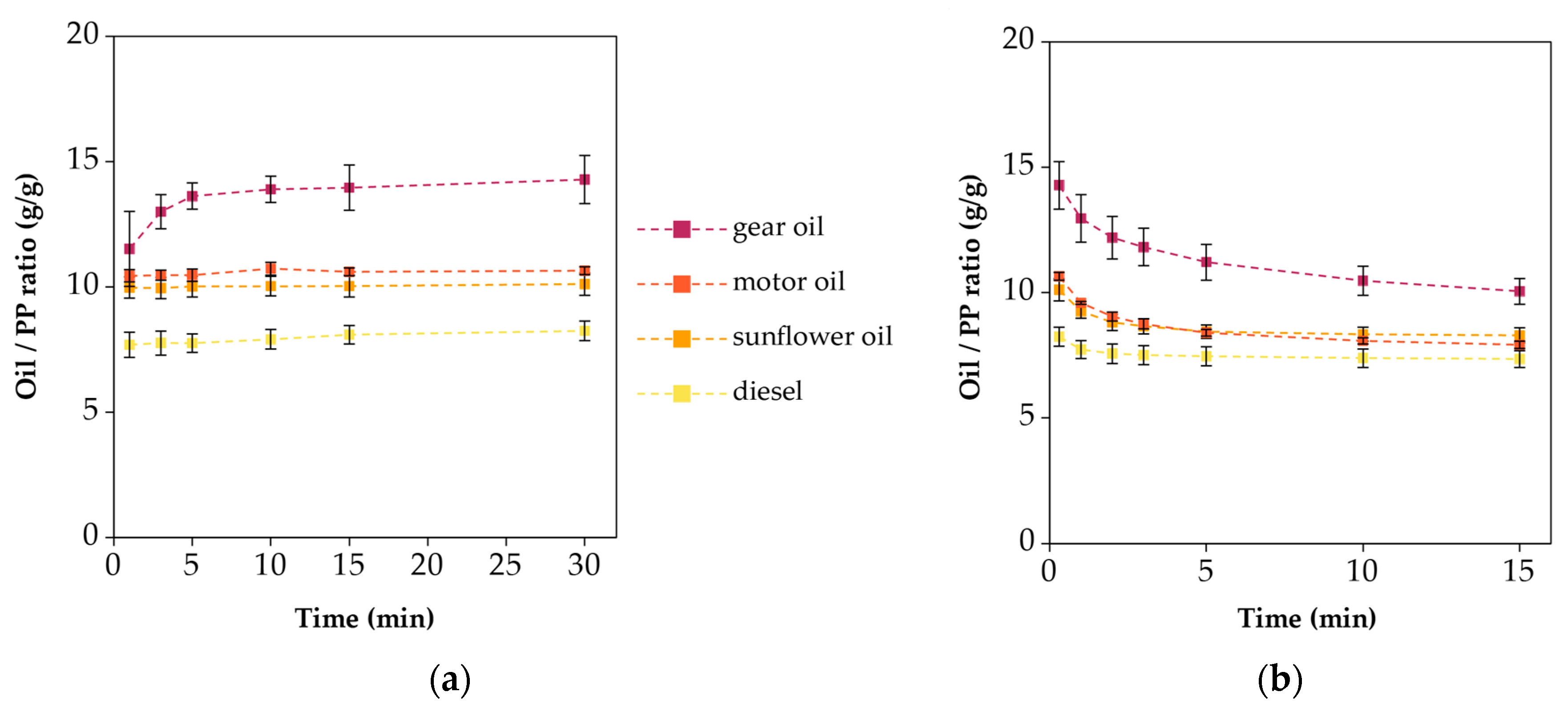
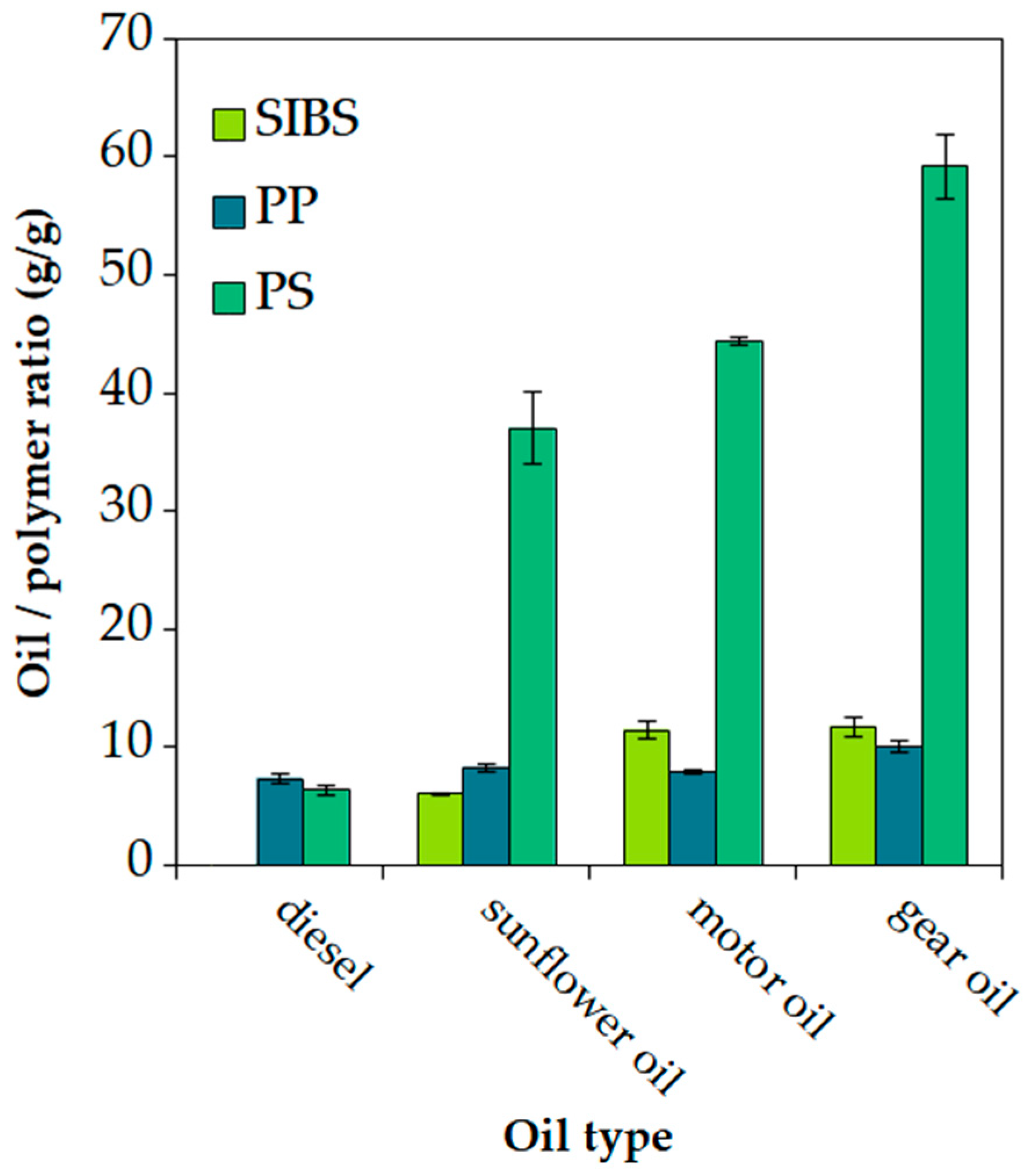
3.4. Oil Sorption
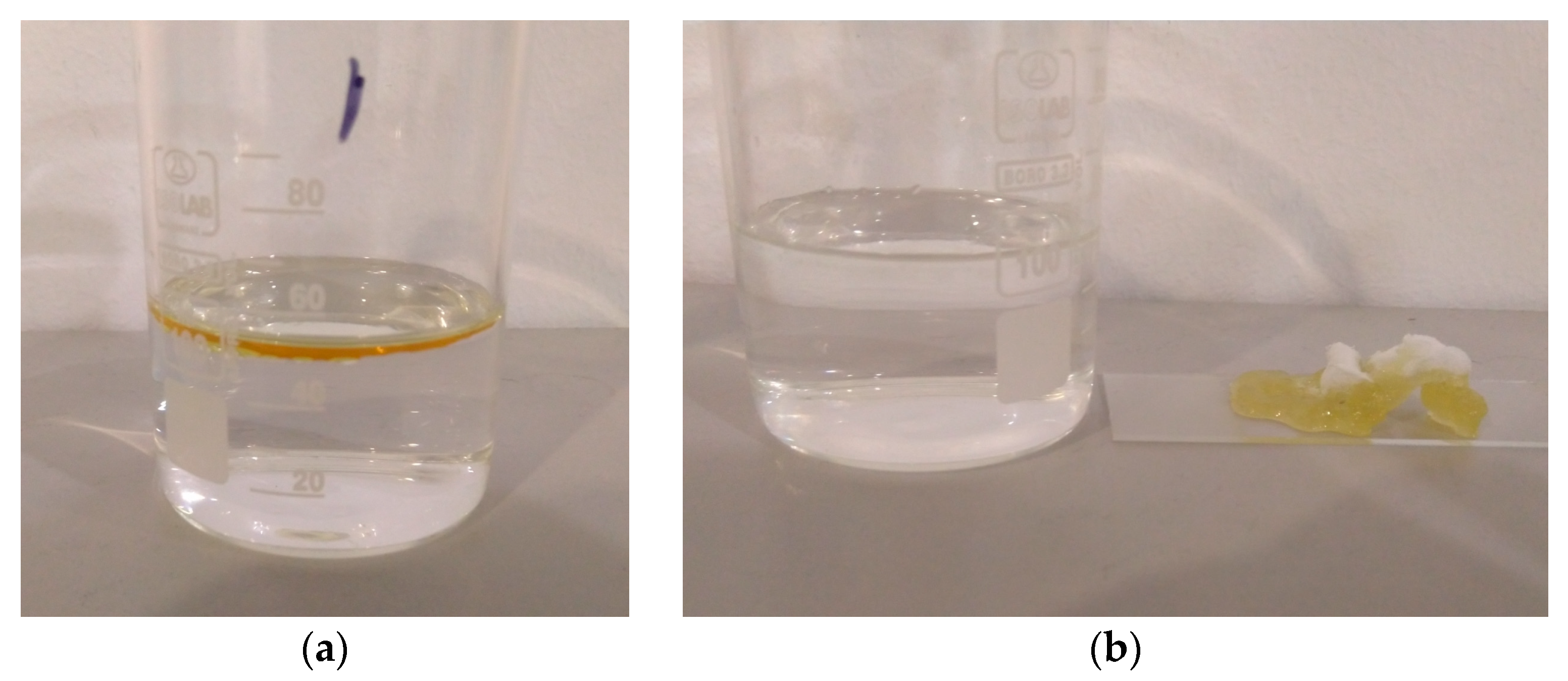
3.5. Oil–Water Separation
3.6. The Influence of Fiber Mat Porosity on the Oil Sorption Capacity
3.7. Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Beyer, J.; Trannum, H.C.; Bakke, T.; Hodson, P.V.; Collier, T.K. Environmental Effects of the Deepwater Horizon Oil Spill: A Review. Mar. Pollut. Bull. 2016, 110, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Asif, Z.; Chen, Z.; An, C.; Dong, J. Environmental Impacts and Challenges Associated with Oil Spills on Shorelines. J. Mar. Sci. Eng. 2022, 10, 762. [Google Scholar] [CrossRef]
- Ventikos, N.P.; Vergetis, E.; Psaraftis, H.N.; Triantafyllou, G. A High-Level Synthesis of Oil Spill Response Equipment and Countermeasures. J. Hazard. Mater. 2004, 107, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V.; Elkin, A.A.; Makarov, S.O.; Cunningham, C.J.; Peshkur, T.A.; Atlas, R.M.; Philp, J.C. Oil Spill Problems and Sustainable Response Strategies through New Technologies. Environ. Sci. Process. Impacts 2015, 17, 1201–1219. [Google Scholar] [CrossRef]
- Ge, J.; Zhao, H.-Y.; Zhu, H.-W.; Huang, J.; Shi, L.-A.; Yu, S.-H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef]
- F20 Committee ASTM International. Test Method for Sorbent Performance of Adsorbents; F20 Committee ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- Gergely, A.; Kántor, J.; Bitay, E.; Biró, D. Electrospinning of Polymer Fibres Using Recycled PET. Acta Mater. Transylvanica 2019, 2, 19–26. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre Diameter Fibres of Polymer, Produced by Electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Gilmore, K.J.; Higgins, M.J.; Liu, Y.; Wallace, G.G. Guidance of Neurite Outgrowth on Aligned Electrospun Polypyrrole/Poly(Styrene-β-Isobutylene-β-Styrene) Fiber Platforms. J. Biomed. Mater. Res. Part A 2010, 94, 1004–1011. [Google Scholar] [CrossRef]
- Jindal, A.; Puskas, J.E.; McClain, A.; Nedic, K.; Luebbers, M.T.; Baker, J.R.; dos Santos, B.P.; Camassola, M.; Jennings, W.; Einsporn, R.L.; et al. Encapsulation and Release of Zafirlukast from Electrospun Polyisobutylene-Based Thermoplastic Elastomeric Fiber Mat. Eur. Polym. J. 2018, 98, 254–261. [Google Scholar] [CrossRef]
- Yan, G.; Niu, H.; Lin, T. Needle-Less Electrospinning. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–247. ISBN 978-0-323-51270-1. [Google Scholar]
- Vo, P.; Doan, H.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Huynh, D. Centrifugally Spun Recycled PET: Processing and Characterization. Polymers 2018, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Merchiers, J.; Meurs, W.; Deferme, W.; Peeters, R.; Buntinx, M.; Reddy, N.K. Influence of Polymer Concentration and Nozzle Material on Centrifugal Fiber Spinning. Polymers 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Skrivanek, J.; Holec, P.; Batka, O.; Bilek, M.; Pokorny, P. Optimization of the Spinneret Rotation Speed and Airflow Parameters for the Nozzleless Forcespinning of a Polymer Solution. Polymers 2022, 14, 1042. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.J.; Phatak, A.; Giles, D.W.; Macosko, C.W.; Bates, F.S. Melt Blown Nanofibers: Fiber Diameter Distributions and Onset of Fiber Breakup. Polymer 2007, 48, 3306–3316. [Google Scholar] [CrossRef]
- Guo, M.; Liang, H.; Luo, Z.; Chen, Q.; Wei, W. Study on Melt-Blown Processing, Web Structure of Polypropylene Nonwovens and Its BTX Adsorption. Fibers Polym. 2016, 17, 257–265. [Google Scholar] [CrossRef]
- Qi, B.; Hu, X.; Cui, S.; Liu, H.; Li, Y.; Li, Y.; Lu, J.; Bao, M. Rapid Fabrication of Superhydrophobic Magnetic Melt-Blown Fiber Felt for Oil Spill Recovery and Efficient Oil–Water Separation. Sep. Purif. Technol. 2023, 306, 122486. [Google Scholar] [CrossRef]
- Doan, H.N.; Nguyen, D.K.; Vo, P.P.; Hayashi, K.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Huynh, D.P. Facile and Scalable Fabrication of Porous Polystyrene Fibers for Oil Removal by Centrifugal Spinning. ACS Omega 2019, 4, 15992–16000. [Google Scholar] [CrossRef]
- Zhang, L.; Narita, C.; Himeda, Y.; Honma, H.; Yamada, K. Development of Highly Oil-Absorbent Polylactic-Acid Microfibers with a Nanoporous Structure via Simple One-Step Centrifugal Spinning. Sep. Purif. Technol. 2022, 282, 120156. [Google Scholar] [CrossRef]
- Li, H.; Wu, W.; Bubakir, M.M.; Chen, H.; Zhong, X.; Liu, Z.; Ding, Y.; Yang, W. Polypropylene Fibers Fabricated via a Needleless Melt-Electrospinning Device for Marine Oil-Spill Cleanup. J. Appl. Polym. Sci. 2014, 131, 40080. [Google Scholar] [CrossRef]
- Ceylan, D.; Dogu, S.; Karacik, B.; Yakan, S.D.; Okay, O.S.; Okay, O. Evaluation of Butyl Rubber as Sorbent Material for the Removal of Oil and Polycyclic Aromatic Hydrocarbons from Seawater. Environ. Sci. Technol. 2009, 43, 3846–3852. [Google Scholar] [CrossRef]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.-M.; Kennedy, J.P. Medical Applications of Poly(Styrene-Block-Isobutylene-Block-Styrene) (“SIBS”). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Strickler, F.; Richard, R.; McFadden, S.; Lindquist, J.; Schwarz, M.C.; Faust, R.; Wilson, G.J.; Boden, M. In Vivo and In Vitro Characterization of Poly(Styrene-b-isobutylene-b-styrene) Copolymer Stent Coatings for Biostability, Vascular Compatibility and Mechanical Integrity. J. Biomed. Mater. Res 2010, 92A, 773–782. [Google Scholar] [CrossRef]
- Lim, G.T.; Puskas, J.E.; Reneker, D.H.; Jákli, A.; Horton, W.E. Highly Hydrophobic Electrospun Fiber Mats from Polyisobutylene-Based Thermoplastic Elastomers. Biomacromolecules 2011, 12, 1795–1799. [Google Scholar] [CrossRef]
- Kovačević, A.; Radoičić, M.; Marković, D.; Ponjavić, M.; Nikodinovic-Runic, J.; Radetić, M. Non-Woven Sorbent Based on Recycled Jute Fibers for Efficient Oil Spill Clean-up: From Production to Biodegradation. Environ. Technol. Innov. 2023, 31, 103170. [Google Scholar] [CrossRef]
- Condurache, B.-C.; Cojocaru, C.; Samoila, P.; Ignat, M.; Harabagiu, V. Data-Driven Modeling and Optimization of Oil Spill Sorption by Wool Fibers: Retention Kinetics and Recovery by Centrifugation. Int. J. Environ. Sci. Technol. 2022, 19, 367–378. [Google Scholar] [CrossRef]
- Blaquera, A.L.M.; Herrera, M.U.; Manalo, R.D.; Maguyon-Detras, M.C.; Futalan, C.C.M.; Balela, M.D.L. Oil Adsorption Kinetics of Calcium Stearate-Coated Kapok Fibers. Polymers 2023, 15, 452. [Google Scholar] [CrossRef]
- Singh, C.J.; Mukhopadhyay, S.; Rengasamy, R.S. A Sustainable Approach to Oil Spill Cleanup by Kapok and Waste Cotton Needle Punched Nonwoven Blends. Ind. Crops Prod. 2023, 191, 115939. [Google Scholar] [CrossRef]
- Kántor, J.; Farmos, R.L.; Gergely, A.L. Optimization of Oil Sorbent Thermoplastic Elastomer Microfiber Production by Centrifugal Spinning. Polymers 2023, 15, 3368. [Google Scholar] [CrossRef]
- Fábián, H.; Gergely, A. Design of a High Performance Fiber-Producing Machine. Acta Mater. Transylvanica 2022, 5, 62–65. [Google Scholar] [CrossRef]
- Bazargan, A.; Tan, J.; McKay, G. Standardization of Oil Sorbent Performance Testing. J. Test. Eval. 2015, 43, 1271–1278. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Tung, S.-H. One-Step Electrospinning To Produce Nonsolvent-Induced Macroporous Fibers with Ultrahigh Oil Adsorption Capability. Macromolecules 2017, 50, 2528–2534. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Electrospun Porous Structure Fibrous Film with High Oil Adsorption Capacity. ACS Appl. Mater. Interfaces 2012, 4, 3207–3212. [Google Scholar] [CrossRef]
- Isık, T.; Demir, M.M. Tailored Electrospun Fibers from Waste Polystyrene for High Oil Adsorption. Sustain. Mater. Technol. 2018, 18, e00084. [Google Scholar] [CrossRef]
- Budlayan, M.L.M.; Patricio, J.N.; Lagare-Oracion, J.P.; Arco, S.D.; Alguno, A.C.; Basilio, A.; Latayada, F.S.; Capangpangan, R.Y. Improvised Centrifugal Spinning for the Production of Polystyrene Microfibers from Waste Expanded Polystyrene Foam and Its Potential Application for Oil Adsorption. J. Eng. Appl. Sci. 2021, 68, 25. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, G.; Zhao, Y.; Kang, H.; Chen, Z.; Zhao, M.; Sun, Z.; Gao, C.; Xue, L. Porous Structures of C-Shaped Polypropylene Fibers and Oil-Absorbing Performance of Their Spun-Bond Non-Woven Fabrics. Adv. Fiber Mater. 2024, 6, 1092–1107. [Google Scholar] [CrossRef]
- Le, Q.P.; Olekhnovich, R.O.; Uspenskaya, M.V.; Vu, T.H.N. Study on Polyvinyl Chloride Nanofibers Ability for Oil Spill Elimination. Iran. Polym. J. 2021, 30, 473–483. [Google Scholar] [CrossRef]
- Zhu, H.; Qiu, S.; Jiang, W.; Wu, D.; Zhang, C. Evaluation of Electrospun Polyvinyl Chloride/Polystyrene Fibers as Sorbent Materials for Oil Spill Cleanup. Environ. Sci. Technol. 2011, 45, 4527–4531. [Google Scholar] [CrossRef]
- Crawford, E.A.; Gupta, S.; Trinh, B.M.; Mekonnen, T.H. Styrene-Ethylene-Butylene-Styrene (SEBS) Melt-Blown Fibers for Oil Spill Remediation and Oil Barrier Geotextiles. Polymer 2024, 298, 126942. [Google Scholar] [CrossRef]





| Shear Rate (1/s) | 2.89 | 3.62 | 7.25 | 14.5 |
|---|---|---|---|---|
| Oil Type | Dynamic Viscosity (mPa·s) | |||
| Gear oil | 725 | - | - | - |
| Motor oil | - | 126.6 | 128.7 | - |
| Sunflower oil | - | - | 57.6 | 66 |
| Diesel | - | - | 8.6 | 10.3 |
| Droplet Average Sorption Time (s) | ||||
|---|---|---|---|---|
| Specimen | Gear Oil | Motor Oil | Sunflower Oil | Diesel |
| SIBS | 3.96 ± 1.75 | 0.64 ± 0.07 | 0.58 ± 0.15 | 0.21 ± 0.09 |
| PS | 2.11 ± 0.48 | 0.48 ± 0.1 | 0.27 ± 0.12 | 0.11 ± 0.05 |
| PP | 4.24 ± 0.41 | 0.62 ± 0.09 | 0.42 ± 0.08 | 0.08 ± 0.03 |
| Material | Oil Type | Sorption Capacity (g/g) | Sample Size (mg) | Contact Time (s) | Drain Time (s) | Reference |
|---|---|---|---|---|---|---|
| PS | silicone oil pump oil vegetable oil | 32–47 34–48 28–45 | 10 | 3600 | 10 | [19] |
| PS | silicone oil pump oil sunflower oil diesel | 350–840 210–650 100–450 30–60 | 10 | 3600 | 60 | [35] |
| PS | silicone oil motor oil peanut oil diesel | 20–80 40–130 40–112 3–7 | 1000 | 2400 | 300 | [36] |
| PS | vegetable oil | 55–124 | 25 | 60 | 30 | [37] |
| PS (recycled EPS) | motor oil | 15 | 120 | 20 | n/a | [38] |
| PP | motor oil peanut oil | 38–65 25–52 | 100 | 3600 | 1800 | [21] |
| PP | diesel crude oil | 11.4 15.5 | 2000 | 300 | n/a | [22] |
| PP | silicone oil machine oil soybean oil | 35 45 32 | n/a | n/a | 30 | [39] |
| PVC | motor oil hydraulic oil crude oil | 35 24 16–19 | 250 | 3600 | 120 | [40] |
| PVC/PS | motor oil peanut oil diesel | 146 119 38 | 1000 | 3600 | 120 | [41] |
| SEBS | transformer oil diesel crude oil | 4.8 dissolved 4.3 | n/a | 30 | n/a | [42] |
| butyl rubber porous sheet (non-fibrous) | diesel crude oil | 20.3 23 | 2000 | n/a | 30 | [22] |
| PLA | silicone oil pump oil sunflower oil | 30 28 25 | 8 | n/a | 30 | [20] |
| Natural fibers | ||||||
| cellulose/lignin (jute) | motor oil 5w30 diesel crude oil | 7 7 6 | n/a | 900 | 1800 | [26] |
| sheep wool | motor oil 5w30 motor oil 15w40 | 9 11 | n/a | 900 | 1800 | [27] |
| modified kapok | motor oil diesel | 86.5 60.6 | 0.1 | 1800 | 30 | [28] |
| kapok/cotton | engine oil vegetable oil diesel | 45 44 40 | ASTM F716 | [29] | ||
| cotton | engine oil vegetable oil diesel | 21 20 16.5 | ASTM F716 | [29] | ||
| Specimen | Measured Water Uptake (g/g) | Possible Maximum Oil Uptake (g/g) | Measured Oil + Water Uptake at 1 min (g/g) | Measured Oil + Water Uptake at 24 h (g/g) |
|---|---|---|---|---|
| SIBS | 0.4 ± 0.07 | 4.90 | 6.00 ± 0.36 | 5.02 ± 0.37 |
| PS | 3.26 ± 0.5 | 10.67 | 15.89 ± 1.55 | 10.55 ± 0.58 |
| PP | 0.2 ± 0.1 | 4.96 | 7.67 ± 0.4 | 5.23 ± 0.1 |
| Specimen | Density (10−2 g/cm3) | Specific Volume (cm3/g) | Porosity | Average Fiber Diameter (μm) | Mass Specific Surface Area (m2/g) | Volume Specific Surface Area (m2/cm3) | Motor Oil Retention (g/cm3) | Gear Oil Retention (g/cm3) |
|---|---|---|---|---|---|---|---|---|
| SIBS | 6.57 ± 0.21 | 15.21 | 0.931 | 5.9 | 0.71 | 0.0467 | 0.754 | 0.773 |
| PS | 1.17 ± 0.34 | 85.02 | 0.988 | 8.5 | 0.47 | 0.0055 | 0.522 | 0.695 |
| PP | 7.73 ± 0.28 | 12.92 | 0.915 | 6.9 | 0.637 | 0.0493 | 0.612 | 0.777 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kántor, J.; Fekete, G.; Gergely, A.L. Oil Sorption Properties of Centrifugally Spun Polyisobutylene-Based Thermoplastic Elastomer Microfibers. Polymers 2024, 16, 2624. https://doi.org/10.3390/polym16182624
Kántor J, Fekete G, Gergely AL. Oil Sorption Properties of Centrifugally Spun Polyisobutylene-Based Thermoplastic Elastomer Microfibers. Polymers. 2024; 16(18):2624. https://doi.org/10.3390/polym16182624
Chicago/Turabian StyleKántor, József, Gusztáv Fekete, and Attila Levente Gergely. 2024. "Oil Sorption Properties of Centrifugally Spun Polyisobutylene-Based Thermoplastic Elastomer Microfibers" Polymers 16, no. 18: 2624. https://doi.org/10.3390/polym16182624
APA StyleKántor, J., Fekete, G., & Gergely, A. L. (2024). Oil Sorption Properties of Centrifugally Spun Polyisobutylene-Based Thermoplastic Elastomer Microfibers. Polymers, 16(18), 2624. https://doi.org/10.3390/polym16182624








