Efficacy and Safety of Poly-l-Lactic Acid in Facial Aesthetics: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Research Question
2.2. Search Strategy and Selection Criteria
2.3. Selection of Studies
2.4. Analysis of Risk of Bias
2.5. Extraction of Data
3. Results
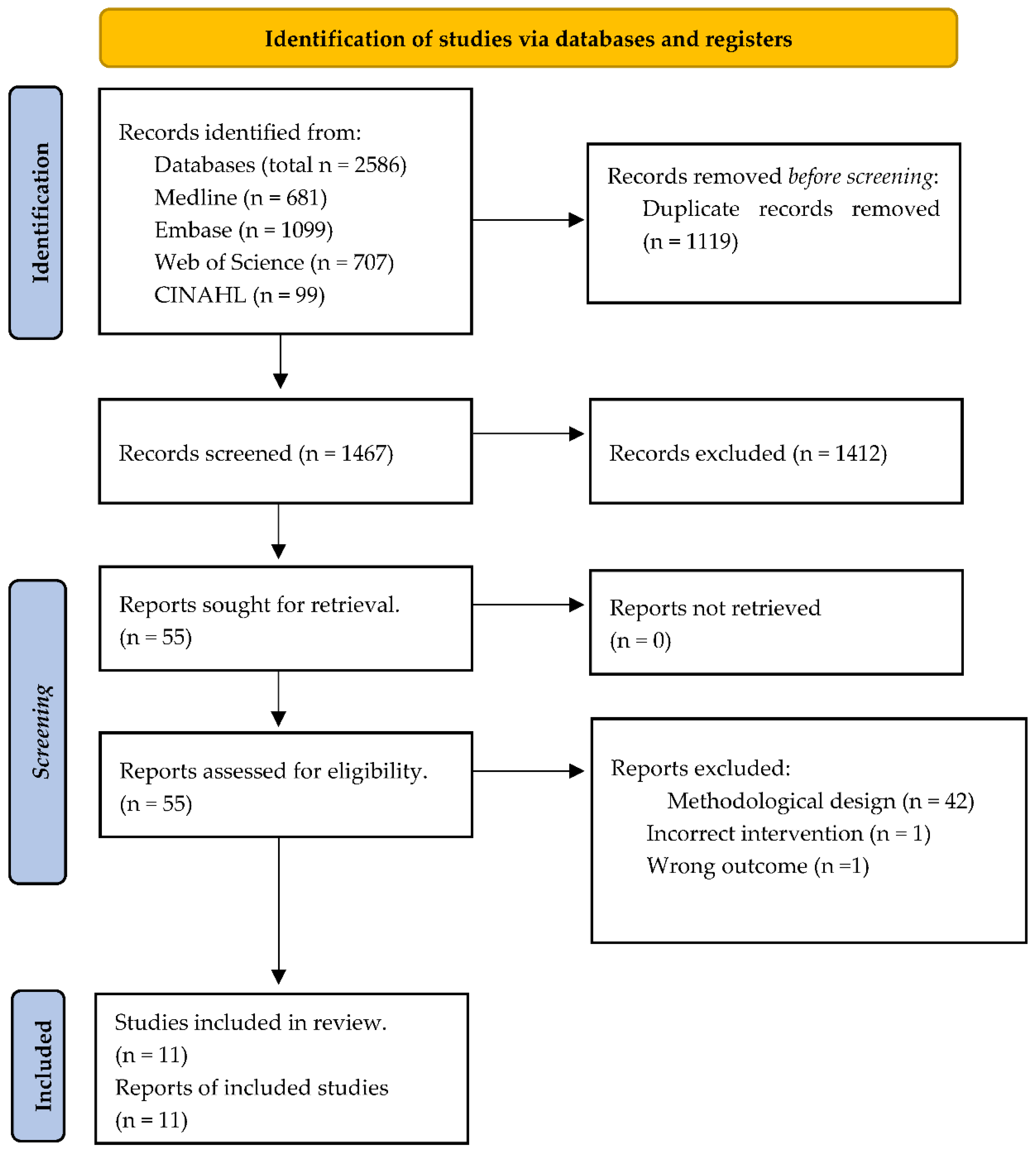
3.1. Literature Search Outflow
| Authors, Year | Population (P) | Intervention (I) | Comparison (C) | Outcomes (O) | Study Design (S) |
|---|---|---|---|---|---|
| Moyle, et al. 2004 [6] | N = 30 (15 in each group) Sex: 2 women; 28 men Age: mean 41.7 Subjects: HIV-related facial lipoatrophy | G1—immediate: three, PLLA (Newfill TM®) bilaterally injections session on day 1, 2 and 4 W later. Total, 4–5 mL/session D: not described F: 24 W A: facial photography, facial ultrasound, perceived changes in body shape (VAS) and HADS | G2—delayed: three, PLLA (Newfill TM®) bilaterally injections sessions on W 12, 14 and 16. Total, 4–5 mL. per session | A significant increase in dermal thickness in injected regions and in self-assessment scores was found in G1 compared to G2 at week 12. No differences in dermal thickness were observed between the groups at week 24. The severity score of lipoatrophy declined in both groups. AE: bruising and limited superficial local cellulitis. | Randomized, open label |
| Moyle, et al. 2006 [20] | N = 27 (G1: n = 13; G2: n = 14) Sex: 2 women; 25 men mean Age: mean 41 years Subjects: HIV-related facial lipoatrophy | G1—immediate: PLLA (Newfill TM®), three bilaterally injections session on 1, 2 and 4 W. Total, 4–5 mL/session D: 2 mL/sterile water and 1 mL/lidocaine F: 2 years A: perceived changes in body shape (VAS) and HADS | G2—delayed: three, PLLA (Newfill TM®) bilaterally injections sessions on 12, 14 and 16 W. Total, 4–5 mL/session | Patients’ self-perceived facial thinness was significantly more positive after 2 years than at baseline in both groups. Also, both groups were less depressed and anxious at the 2 years recall visit than at baseline. However, these improvements only reached statistical significance for depression in the delayed group. AE: From baseline to 2 years, 112 treatment AEs were reported and classified as mild, moderate and severe in intensity in 44, 47 and 7% of cases, respectively. A single case of injection-site induration and nine cases of injection-site nodules were noted at the 2 years recall. | Randomized, open label |
| Carey, et al. 2007 [5] | N = 100 (G1 n = 50; G2 n = 50) Sex: 8 women; 92 men Age: mean 49.8 Subjects: HIV-related facial lipoatrophy and immunocompetent | G1—immediate: PLLA 4 bilaterally injection sessions at 2 W interval. 5 mL/side/session D: 5 mL/sterile water F: −1, 1, 3, 5, 7, 12 and 24 W A: CT and MBSRQ-AS | G2—delayed: PLLA bilaterally injections after 24 W | PLLA did not increase FSTV. At 24 W FSTV scores do not differ significantly between groups. Facial lipoatrophy severity was improved in (90%) in G1 compared with 18% G2 at 12 and 24 W. MBSRQ-AS scores were improved in G1 compared with G2 at 12 and 24 W. AE: 96% of patients experienced at least 1 procedure/product-related AE, like pain/discomfort, localized edema, and erythema. Most events were grade mild or moderate and of short duration. At 24 W, noninflammatory nodules and papule were reported in 6 participants. | Randomized, open label, multicenter |
| Brandt, et al. 2010 [2] | N = 233 Sex: 220 women; 13 men Age: mean 51.4 Subjects: Immunocompetent | G1: PLLA (Sculptra®), 1 to 4 bilaterally injection sessions at 3 W interval. Maximum of 2.5 mL/side or 5 mL total/session D: 5 mL/sterile water, 2 h before injection F: 3 W; 3, 6, 9, 13 M A: IGE | G2: Human collagen (CosmoPlast®), 1 to 4 bilaterally injection sessions of 1 mL/side, at 3 W intervals | G1 reported a significantly higher improvement in IGE through all the follow-ups, compared to G2. AE: G1 demonstrated a favorable safety and tolerability profile comparable to G2. The majority of AE were of mild to moderate in intensity (papules and nodules). | Randomized, single-blinded, multicenter |
| Narins, et al. 2010 [10] | N = 233 (G1: n = 116; G2: n = 117) Sex: 220 women; 13 men Age: mean 51.4 years Subjects: Immunocompetent | G1: PLLA (Sculptra®), 1 to 4 bilaterally injection sessions at 3 W interval. Maximum of 2.5 mL/side or 5 mL total/session D: 5 mL/sterile water, 2 h before injection F: 3 W; 3, 6, 9, 13,19 and 25 M A: Photography and WAS scale | G2: Human collagen (CosmoPlast®), 1 to 4 bilaterally injection sessions of 1 mL/side at 3 W intervals | A significant improvement in the mean change from baseline in WAS score was reported for G1 compared to G2 at 13-month follow-up. Improvements (up to 25 months) were significantly greater just in G1. AE: The majority were mild to moderate in intensity and self-limiting. Higher incidence of AEs was reported on the collagen group (63.2%) compared with the injectable PLLA group (53.4%). | Randomized, single-blinded, multicenter |
| Brown, et al. 2011 [21] | N = 233 (G1 n = 116; G2 n = 117) Sex: 220 Women; 13 men Age: mean 51.4 Subjects: nasolabial fold wrinkles | G1: PLLA (Sculptra®), 1 to 4 bilaterally injection sessions at 3 W interval each. Maximum of 2.5 mL/side or 5 mL total/session D: 5 mL/sterile water, 2 h before injection F: 3 W; 3, 6, 9, 13,19 and 25 M A: SGE, SS | G2: Human collagen (CosmoPlast®), 1 to 4 bilaterally injection sessions of 1 mL/side at 3 W intervals each | Aesthetic improvement in G1 was maintained overall above 90% at the 13 M follow-up and above 81% at 19 and 25 M follow-ups. In contrast, G2 declined to 15% at 13 M. In G1, the proportion of subjects with good to excellent SS scores remained above 84% throughout the follow-up periods, while in G2, subjects with good to excellent SS scores decreased progressively until the 13 M follow-up. AE: The most common AEs were injection-site erythema, pain and pruritus in both groups. Product-related application-site papules and nodules were found in both groups after 13 M, decreasing in G1 at 25 M. All events were of mild to moderate intensity. | Randomized, parallel, multicenter |
| Lafaurie, et al. 2012 [22] | N = 148 (G1: n = 73; G2: n = 75) Sex: 10 women; 138 men Age: mean 47 years Subjects: HIV-related facial lipoatrophy | G1: PLLA (Newfill TM®), 3 to 7 injection sessions of every 4 W until 24 W D: 4 mL/sterile water and 1 mL/lidocaine, 2 h before injections F: 96 W A: VAS, photographic images, MOS-HIV | G2: PH (Eutrophill®) gel | A significant improvement from baseline in lipoatrophy severity in both groups at week 48 and 96 was found. No significant differences were found in patients’ satisfaction scores and lipoatrophy severity between treatments at 48 and 96 weeks. AE: Bleeding, hematoma at injection site, vagal hypertonia during injections, and edema post-injections were reported in both groups and considered mild or moderate. Subcutaneous nodules were reported in 41% and 37% of cases in the PLLA and PH. | Prospective, randomized, single-blinded non inferiority |
| Bohnert, et al. 2019 [3] | N = 40 (G1 n = 20; G2 n = 20) Sex: women Age: 30–60 Subjects: shallow to deep nasolabial fold, other facial wrinkles | G1: PLLA (Sculptra Aesthetic®) 3 bilaterally injection session at intervals of 4 W. 5 to 6 mL/side/session. D: 5 mL/sterile water 24 h before injections, after this, 2 mL/sterile water. Immediately before injections, 2 mL/1% lidocaine was added. Total of 9 mL. F: 6, 9 and 12 M A: Skin Quality Rating (VAS), Subject Satisfaction, Standardized Photography, Skin Physiology (Corneometer, Tewameter and Cutometer). | G2: Saline Solution | All skin quality assessments were significantly improved at the 12 M visit in G1 compared with G2. Patient satisfaction was higher in G1 compared with G2 through all the study. At the 12 M follow-up, the G1 exhibited a greater reduction in trans epidermal water loss compared to G2. Elasticity significantly increased in G1 compared with G2 in all follow-ups. AE: No adverse events related to the treatment were reported. Temporary mild swelling was reported in three patients. | Randomized, controlled, double-blinded, multicenter |
| Palm, et al. 2021 [23] | N = 80 Sex: 76 women; 4 men Aged: mean 51.5 Subjects: deficiencies in nasolabial contour | G1: PLLA (Sculptra Aesthetic®), up to 4 injections sessions with 4 W intervals. Maximum volume 9 mL/session (4.5 mL per nasolabial fold). D: 8 mL/sterile water and 1 mL of 2% lidocaine hydrochloride. Total volume of 9 mL. No standing time was required. F: 16, 24, 32, 40 and 48 W A: WAS, GAIS, FACE-Q and a subject questionnaire. | G2: PLLA (Sculptra Aesthetic®), up to 4 injections sessions with 4 W intervals. Maximum volume 5 mL/session (2.5 mL per nasolabial fold). D: 5 mL/sterile water. Two to 72 h standing time. | WAS scores were slightly higher for G2 than for G1 at earlier time points; however, values were similar between the groups at 48 W. GAIS scores showed that all subjects improved at all visits. Individuals in both groups were satisfied with the appearance of their nasolabial folds after 48 W. AE: 11.9% in G1 and 33.3% in G2 reported AEs related to the study product or injection procedure. The most common AEs were headache, rhinorrhea, and perioral hypoesthesia, all of mild intensity in the treatment group. | Randomized, single-blinded, multicenter |
| Han, et al. 2023 [24] | N = 55 (G1 n = 55; G2 n = 55) Sex: 48 women; 7 men Age: mean 53.8 Subjects: nasolabial folds wrinkles | G1: PLLA (Sculptra) a maximum of 1 mL. Optionally, touch-up injection at 6 W D: 8 mL/sterile water 12 h before injection. F: 0, 2 W, 3 and 6 M A: WSRS score, and GAIS | G2: PLLA (GANA V), a maximum of 1 mL. Optionally, touch-up injection at 6 W D:15 mL/sterile | WSRS scores showed a significant improvement for G2 and no improvements in G1 after 6 months. No significant differences were observed between the two groups in GAIS scores in all follow-ups. A higher satisfaction score in the immediate outcome assessment was reported in G2 compared with G1. G2 has an acceptable 6 M effectiveness compared with G1, which is in line with the established non-inferiority margin AE: Both groups experienced erythema, tenderness, firmness, swelling, bumps, bruising, and pigmentation. There were no significant differences in the rate of injection site reactions between the two groups. | Randomized, double-blind, non-inferiority, split face controlled |
| Fabi, et al. 2024 [8] | N = 149 (G1 n = 97; G2 n = 52) Sex: (144 women, 5 men) Age: mean 60.7 Subjects: moderate to severe cheek wrinkles. | G1: PLLA-SCA (Sculptra®) 1 bilaterally injection session, with 3 more injection sessions permitted at monthly intervals. D: 8 mL/ sterile water and 1 mL/lidocaine (2%) F: 7, 9, and 12 M A: GCWS, GAIS, and FACE-Q questionnaire | G2: No treatment | GCWS scores at rest and dynamic were significantly higher in G1 compared to G2 at 7, 9, 12 M GAIS scores showed responders to treatment just in G1 through all the study. FACE-Q scores showed a significant increase in G1 in all follow-up points when compared with G2. AE: The most common post-treatment symptoms were tenderness, bruising, swelling, and pain, being mild to moderate in intensity. The most common treatment-related AEs included injection site bruising, dizziness, and headache. No serious treatment-related AEs were reported. | Randomized, single-blinded, no-controlled |
3.2. Studies’ Results
3.2.1. Efficacy and Durability of PLLA: Immediate × Delayed Protocol
3.2.2. PLLA × Human Collagen
3.2.3. Other Comparisons
3.2.4. Adverse Events
3.2.5. Dilution Protocol
3.2.6. Quality Assessment
4. Discussion
4.1. Study Strengths and Limitations
4.2. Clinical Implications and Generalizability
4.3. Future Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christen, M.-O. Collagen stimulators in body applications: A review focused on poly-l-lactic acid (PLLA). Clin. Cosmet. Investig. Dermatol. 2022, 15, 997–1019. [Google Scholar] [CrossRef] [PubMed]
- Narins, R.S.; Baumann, L.; Brandt, F.S.; Fagien, S.; Glazer, S.; Lowe, N.J.; Monheit, G.D.; Rendon, M.I.; Rohrich, R.J.; Werschler, W.P. A randomized study of the efficacy and safety of injectable poly-l-lactic acid versus human-based collagen implant in the treatment of nasolabial fold wrinkles. J. Am. Acad. Dermatol. 2010, 62, 448–462. [Google Scholar] [CrossRef]
- Bohnert, K.; Dorizas, A.; Lorenc, P.; Sadick, N.S. Randomized, controlled, multicentered, double-blind investigation of injectable poly-l-lactic acid for improving skin quality. Dermatol. Surg. 2019, 45, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.; Vleggaar, D. Facial volume restoration of the aging face with poly-l-lactic acid. Dermatol. Ther. 2011, 24, 2–27. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.L.; Baker, D.; Rogers, G.D.; Petoumenos, K.; Chuah, J.; Easey, N.; Machon, K.; Cooper, D.A.; Emery, S.; Carr, A. A randomized, multicenter, open-label study of poly-l-lactic acid for HIV-1 facial lipoatrophy. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 46, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Moyle, G.; Lysakova, L.; Brown, S.; Sibtain, N.; Healy, J.; Priest, C.; Mandalia, S.; Barton, S. A randomized open-label study of immediate versus delayed polylactic acid injections for the cosmetic management of facial lipoatrophy in persons with HIV infection. HIV Med. 2004, 5, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Azizzadeh, B.; Graivier, M. Injectable poly-l-lactic acid (Sculptra): Technical considerations in soft-tissue contouring. Plast. Reconstr. Surg. 2006, 118, 55S–63S. [Google Scholar] [CrossRef] [PubMed]
- Fabi, S.; Hamilton, T.; LaTowsky, B.; Kazin, R.; Marcus, K.; Mayoral, F.; Joseph, J.; Hooper, D.; Shridharani, S.; Hicks, J. Effectiveness and Safety of Sculptra Poly-l-Lactic Acid Injectable Implant in the Correction of Cheek Wrinkles. J. Drugs Dermatol. JDD 2024, 23, 1297–1305. [Google Scholar] [CrossRef]
- Food and Drug Administration FDA. Summary of Safety and Effectiveness Data (SSES). 2023. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160035B.pdf (accessed on 3 May 2024).
- Brandt, F.S.; Cazzaniga, A.; Baumann, L.; Fagien, S.; Glazer, S.; Kenkel, J.M.; Lowe, N.J.; Monheit, G.D.; Narins, R.S.; Rendon, M.I. Investigator global evaluations of efficacy of injectable poly-l-lactic acid versus human collagen in the correction of nasolabial fold wrinkles. Aesthetic Surg. J. 2011, 31, 521–528. [Google Scholar] [CrossRef]
- Haddad, A.; Kadunc, B.V.; Guarnieri, C.; Noviello, J.S.; da Cunha, M.G.; Parada, M.B. Conceitos atuais no uso do ácido poli-l-láctico para rejuvenescimento facial: Revisão e aspectos práticos. Surg. Cosmet. Dermatol. 2017, 9, 60–71. [Google Scholar]
- Akinbiyi, T.; Othman, S.; Familusi, O.; Calvert, C.; Card, E.B.; Percec, I. Better results in facial rejuvenation with fillers. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2763. [Google Scholar] [CrossRef] [PubMed]
- Butterwick, K.; Lowe, N.J. Injectable poly-l-lactic acid for cosmetic enhancement: Learning from the European experience. J. Am. Acad. Dermatol. 2009, 61, 281–293. [Google Scholar] [CrossRef]
- Riva, J.J.; Malik, K.M.; Burnie, S.J.; Endicott, A.R.; Busse, J.W. What is your research question? An introduction to the PICOT format for clinicians. J. Can. Chiropr. Assoc. 2012, 56, 167. [Google Scholar] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Clark, J.M.; Sanders, S.; Carter, M.; Honeyman, D.; Cleo, G.; Auld, Y.; Booth, D.; Condron, P.; Dalais, C.; Bateup, S. Improving the translation of search strategies using the Polyglot Search Translator: A randomized controlled trial. J. Med. Libr. Assoc. JMLA 2020, 108, 195. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. JMLA 2016, 104, 240. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Moyle, G.J.; Brown, S.; Lysakova, L.; Barton, S.E. Long-term safety and efficacy of poly-l-lactic acid in the treatment of HIV-related facial lipoatrophy. HIV Med. 2006, 7, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Rohrich, R.J.; Baumann, L.; Brandt, F.S.; Fagien, S.; Glazer, S.; Kenkel, J.M.; Lowe, N.J.; Monheit, G.D.; Narins, R.S.; et al. Subject global evaluation and subject satisfaction using injectable poly-l-lactic acid versus human collagen for the correction of nasolabial fold wrinkles. Plast. Reconstr. Surg. 2011, 127, 1684–1692. [Google Scholar] [CrossRef]
- Lafaurie, M.; Dolivo, M.; Girard, P.M.; May, T.; Bouchaud, O.; Carbonnel, E.; Madelaine, I.; Loze, B.; Porcher, R.; Molina, J.M. Polylactic acid vs. polyacrylamide hydrogel for treatment of facial lipoatrophy: A randomized controlled trial [Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS) 132 SMILE]. HIV Med. 2013, 14, 410–420. [Google Scholar] [CrossRef]
- Palm, M.; Weinkle, S.; Cho, Y.; LaTowsky, B.; Prather, H. A Randomized Study on PLLA Using Higher Dilution Volume and Immediate Use Following Reconstitution. J. Drugs Dermatol. 2021, 20, 760–766. [Google Scholar]
- Han, W.Y.; Kim, H.J.; Kwon, R.; Kang, S.M.; Yon, D.K. Safety and efficacy of Poly-l-Lactic acid filler (Gana V vs. Sculptra) injection for correction of the nasolabial fold: A double-blind, non-inferiority, randomized, split-face controlled trial. Aesthetic Plast. Surg. 2023, 47, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, J.H.; Kim, H.M.; Batsukh, S.; Sung, M.J.; Lim, T.H.; Lee, M.H.; Son, K.H.; Byun, K. Poly-l-lactic acid fillers improved dermal collagen synthesis by modulating m2 macrophage polarization in aged animal skin. Cells 2023, 12, 1320. [Google Scholar] [CrossRef] [PubMed]
- Sedush, N.G.; Kalinin, K.T.; Azarkevich, P.N.; Gorskaya, A.A. Physicochemical characteristics and hydrolytic degradation of polylactic acid dermal fillers: A comparative study. Cosmetics 2023, 10, 110. [Google Scholar] [CrossRef]
- Baumann, K.; Alm, J.; Norberg, M.; Ejehorn, M. Immediate Use after Reconstitution of a Biostimulatory Poly-l-Lactic Acid Injectable Implant. J. Drugs Dermatol. JDD 2020, 19, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, V. New emerging trends in synthetic biodegradable polymers–Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Riddhesh, D. Global Poly-l-Lactic Acid (PLLA) Filler Market. 2023. Available online: https://dataintelo.com/report/global-poly-l-lactic-acid-plla-filler-market/ (accessed on 7 May 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Author, Year | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Results | Overall |
|---|---|---|---|---|---|---|
| Moyle et al., 2004 [6] | Some concerns | High risk | Low risk | Low risk | Some concerns | High risk |
| Moyle et al., 2006 [20] | Some concerns | High risk | Some concerns | Some concerns | Some concerns | High risk |
| Carey et al., 2007 [5] | Some concerns | High risk | Low risk | Low risk | Some concerns | High risk |
| Brandt et al., 2010 [2] | Some concerns | Low risk | Some concerns | Some concerns | Some concerns | Some concerns |
| Narins et al., 2011 [10] | Some concerns | Some concerns | Low risk | Low risk | Some concerns | Some concerns |
| Brown et al., 2011 [21] | Low risk | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Lafaurie et al., 2013 [22] | Low risk | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Bohnert et al., 2019 [3] | Low risk | Low risk | Some concerns | Low risk | Some concerns | Some concerns |
| Palm et al., 2021 [23] | Some concerns | High risk | Low risk | Low risk | Some concerns | High risk |
| Han et al., 2023 [24] | Low risk | Low risk | Some concerns | Low risk | Some concerns | Some concerns |
| Fabi et al., 2024 [8] | Some concerns | High risk | Low risk | Low risk | Some concerns | High risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signori, R.; Barbosa, A.d.P.; Cezar-dos-Santos, F.; Carbone, A.C.; Ventura, S.; Nobre, B.B.d.S.; Neves, M.L.B.B.; Câmara-Souza, M.B.; Poluha, R.L.; De la Torre Canales, G. Efficacy and Safety of Poly-l-Lactic Acid in Facial Aesthetics: A Systematic Review. Polymers 2024, 16, 2564. https://doi.org/10.3390/polym16182564
Signori R, Barbosa AdP, Cezar-dos-Santos F, Carbone AC, Ventura S, Nobre BBdS, Neves MLBB, Câmara-Souza MB, Poluha RL, De la Torre Canales G. Efficacy and Safety of Poly-l-Lactic Acid in Facial Aesthetics: A Systematic Review. Polymers. 2024; 16(18):2564. https://doi.org/10.3390/polym16182564
Chicago/Turabian StyleSignori, Roberta, Antony de Paula Barbosa, Fernando Cezar-dos-Santos, Ana Claudia Carbone, Silvio Ventura, Bryanne Brissian de Souza Nobre, Maria Luiza Boechat Borges Neves, Mariana Barbosa Câmara-Souza, Rodrigo Lorenzi Poluha, and Giancarlo De la Torre Canales. 2024. "Efficacy and Safety of Poly-l-Lactic Acid in Facial Aesthetics: A Systematic Review" Polymers 16, no. 18: 2564. https://doi.org/10.3390/polym16182564
APA StyleSignori, R., Barbosa, A. d. P., Cezar-dos-Santos, F., Carbone, A. C., Ventura, S., Nobre, B. B. d. S., Neves, M. L. B. B., Câmara-Souza, M. B., Poluha, R. L., & De la Torre Canales, G. (2024). Efficacy and Safety of Poly-l-Lactic Acid in Facial Aesthetics: A Systematic Review. Polymers, 16(18), 2564. https://doi.org/10.3390/polym16182564










