New Antibacterial and Antioxidant Chitin Derivatives: Ultrasonic Preparation and Biological Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Methods
2.3. Synthesis of Alkene Chitin Derivatives
2.4. Synthesis of Cationic Chitin Derivatives
2.5. Preparation of Nanoparticles
2.6. Biological Experiments
2.7. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Cationic Chitin Derivatives by Ultrasonic Phenol-Ene Reaction
3.2. Preparation of Nanoparticles of Cationic Chitin-Based Polymer
3.3. Antibacterial Activity and Toxicity
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Stump, B. Click Bioconjugation: Modifying Proteins Using Click-Like Chemistry. ChemBioChem 2022, 23, e202200016. [Google Scholar] [CrossRef] [PubMed]
- Halay, E.; Acikbas, Y. Click chemistry: A fascinating, Nobel-winning method for the improvement of biological activity. Appl. Chem. Eng. 2023, 6, 1847. [Google Scholar] [CrossRef]
- Fantoni, N.Z.; El-Sagheer, A.H.; Brown, T. A Hitchhiker’s Guide to Click-Chemistry with Nucleic Acids. Chem. Rev. 2021, 121, 7122–7154. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Finn, M.G. Introduction: Click Chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef]
- Spruell, J.M. Efficient Templated Synthesis of Donor–Acceptor Rotaxanes Using Click Chemistry. In The Power of Click Chemistry for Molecular Machines and Surface Patterning; Spruell, J.M., Ed.; Springer: New York, NY, USA, 2011; pp. 19–33. [Google Scholar]
- Chandrasekaran, S. Click Reactions in Organic Synthesis; Wiley: Hoboken, NJ, USA, 2016; p. 341. [Google Scholar]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjug. Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Maity, M.; Hasnain, M.S.; Nayak, A.K. Chitosan: Source, chemistry, and properties. In Chitosan in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–22. [Google Scholar]
- Cheaburu-Yilmaz, C.N.; Karavana, S.Y.; Yilmaz, O. Functionalization of Chitosan by Click Chemistry; AIP Conference Proceedings; Lepadatescu, B., Ed.; American Institute of Physics Inc.: College Park, MD, USA, 2017. [Google Scholar]
- Kritchenkov, A.S.; Skorik, Y.A. Click reactions in chitosan chemistry. Russ. Chem. Bull. 2017, 66, 769–781. [Google Scholar] [CrossRef]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.H.; Jiang, Z.; Fu, X. Chitin and chitin-based biomaterials: A review of advances in processing and food applications. Carbohydr. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Xu, C.; Shi, Z.; Liu, L.; Fan, Y.; Yu, J. Efficient preparation of fluorescent nanomaterials derived from chitin via a modification-first strategy assisted by click chemistry. Green Chem. 2024, 26, 7176–7187. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Abramovich, R.A.; Kurliuk, A.V.; Shakola, T.V.; Kultyshkina, E.K.; Ballesteros Meza, M.J.; Pavlova, A.V.; Suchkova, E.P.; Le Nhat Thuy, G.; et al. Water-soluble triazole chitin derivative and its based nanoparticles: Synthesis, characterization, catalytic and antibacterial properties. Carbohydr. Polym. 2021, 257, 117593. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Kletskov, A.V.; Egorov, A.R.; Kurasova, M.N.; Tskhovrebov, A.G.; Khrustalev, V.N. Ultrasound and click chemistry lead to a new chitin chelator. Its Pd(II) complex is a recyclable catalyst for the Sonogashira reaction in water. Carbohydr. Polym. 2021, 252, 117167. [Google Scholar] [CrossRef]
- Hu, Y.; Dziekonski, E.T.; Wang, D.M.; Gonzalez, L.E.; Cooks, R.G. Rapid Identification of Phenolics in Mixtures by Two-Dimensional Tandem Mass Spectrometry with Microdroplet Accelerated Derivatization Reactions. Anal. Sens. 2024, 4, e202300081. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, D.; Brinker, V.A.; Hoye, T.R. The Phenol-Ene Reaction: Biaryl Synthesis via Trapping Reactions between HDDA-Generated Benzynes and Phenolics. Org. Lett. 2016, 18, 5596–5599. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, V.P.; Il’ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/Chitosan and Its Derivatives: Fundamental Problems and Practical Approaches. Biochemistry 2020, 85, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Sahm, D.H. Antibacterial susceptibility tests: Dilution methods. In Manual of Clinical Microbiology; Murray, P.R., Ed.; ASM Press: Washington, DC, USA, 1991; pp. 1105–1116. [Google Scholar]
- Kritchenkov, A.S.; Zhaliazniak, N.V.; Egorov, A.R.; Lobanov, N.N.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V.; Rubanik, V.V.; et al. Chitosan derivatives and their based nanoparticles: Ultrasonic approach to the synthesis, antimicrobial and transfection properties. Carbohydr. Polym. 2020, 242, 116478. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gürer, F.; Kargl, R.; Bračič, M.; Makuc, D.; Thonhofer, M.; Plavec, J.; Mohan, T.; Kleinschek, K.S. Water-based carbodiimide mediated synthesis of polysaccharide-amino acid conjugates: Deprotection, charge and structural analysis. Carbohydr. Polym. 2021, 267, 118226. [Google Scholar] [CrossRef]
- Sashiwa, H.; Yamamori, N.; Ichinose, Y.; Sunamoto, J.; Aiba, S.I. Michael reaction of chitosan with various acryl reagents in water. Biomacromolecules 2003, 4, 1250–1254. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Artemjev, A.A.; Kurliuk, A.V.; Anh Le, T.; Hieu Truong, H.; Le-Nhat-Thuy, G.; Van Tran Thi, T.; Van Tuyen, N.; et al. Novel biopolymer-based nanocomposite food coatings that exhibit active and smart properties due to a single type of nanoparticles. Food Chem. 2021, 343, 128676. [Google Scholar] [CrossRef]
- Wan Yusof, W.R.; Awang, N.Y.F.; Azhar Laile, M.A.; Azizi, J.; Awang Husaini, A.A.S.; Seeni, A.; Wilson, L.D.; Sabar, S. Chemically modified water-soluble chitosan derivatives: Modification strategies, biological activities, and applications. Polym.-Plast. Technol. Mater. 2023, 62, 2182–2220. [Google Scholar] [CrossRef]
- Kahya, N. Water Soluble Chitosan Derivatives and their Biological Activities: A Review. Polym. Sci. 2019, 5, 3. [Google Scholar] [CrossRef]
- Kurita, K.; Inoue, S.; Nishimura, S.-I. Preparation of soluble chitin derivatives as reactive precursors for controlled modifications: Tosyl- and iodo-chitins. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 937–939. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Seidi, F.; Salimi, H. Synthesis of Novel Water-Soluble Aminodeoxychitin Derivatives. Starch-Stärke 2007, 59, 557–562. [Google Scholar] [CrossRef]
- Peng, N.; Ai, Z.; Fang, Z.; Wang, Y.; Xia, Z.; Zhong, Z.; Fan, X.; Ye, Q. Homogeneous synthesis of quaternized chitin in NaOH/urea aqueous solution as a potential gene vector. Carbohydr. Polym. 2016, 150, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.Y.; Shi, X.W.; Li, X.X.; Cai, J.; Duan, B.; Du, Y.M. Homogeneous synthesis and characterization of quaternized chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2012, 87, 422–426. [Google Scholar] [CrossRef]
- Park, I.K.; Park, Y.H. Preparation and structural characterization of water-soluble O-hydroxypropyl chitin derivatives. J. Appl. Polym. Sci. 2001, 80, 2624–2632. [Google Scholar] [CrossRef]
- Nishi, N.; Nishimura, S.-I.; Ebina, A.; Tsutsumi, A.; Tokura, S. Preparation and characterization of water-soluble chitin phosphate. Int. J. Biol. Macromol. 1984, 6, 53–54. [Google Scholar] [CrossRef]
- Kurita, K.; Koyama, Y.; Inoue, S.; Nishimura, S. ((Diethylamino)ethyl)chitins: Preparation and properties of novel aminated chitin derivatives. Macromolecules 1990, 23, 2865–2869. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Q.; Zhang, L.; Zhuo, R.; Jiang, X. Synthesis of carboxymethyl chitin in aqueous solution and its thermo- and pH-sensitive behaviors. Carbohydr. Polym. 2016, 137, 600–607. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- El-Azab, H.; Remiz, Y.; Radwan, M.; Sadek, M. Synthesis and Characterization of Chitosan based Catalyst for Catalysis Applications. Int. J. Adv. Trends Comput. Sci. Eng. 2020, 9, 521–527. [Google Scholar] [CrossRef]
- Safari, J.; Azizi, F.; Sadeghi, M. Chitosan nanoparticles as a green and renewable catalyst in the synthesis of 1,4-dihydropyridine under solvent-free conditions. New J. Chem. 2015, 39, 1905–1909. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Hoang, N.H.; Le Thanh, T.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan Nanoparticles-Based Ionic Gelation Method: A Promising Candidate for Plant Disease Management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial cationic polymers: From structural design to functional control. Polym. J. 2018, 50, 33–44. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Lipkan, N.A.; Kurliuk, A.V.; Shakola, T.V.; Egorov, A.R.; Volkova, O.V.; Meledina, T.V.; Suchkova, E.P.; Zabodalova, L.A.; Dysin, A.P. Synthesis and Antibacterial Activity of Chitin Tetrazole Derivatives. Pharm. Chem. J. 2020, 54, 138–141. [Google Scholar] [CrossRef]
- Dupont, H.; Montravers, P. Chapter 21—Rat Polymicrobial Peritonitis Infection Model. In Handbook of Animal Models of Infection; Zak, O., Sande, M.A., Eds.; Academic Press: London, UK, 1999; pp. 189–194. [Google Scholar]
- Shakola, T.V.; Rubanik, V.V.; Kurliuk, A.V.; Kirichuk, A.A.; Tskhovrebov, A.G.; Egorov, A.R.; Kritchenkov, A.S. Benzothiazole Derivatives of Chitosan and Their Derived Nanoparticles: Synthesis and In Vitro and In Vivo Antibacterial Effects. Polymers 2023, 15, 3469. [Google Scholar] [CrossRef]
- Egorov, A.R.; Kirichuk, A.A.; Rubanik, V.V.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan and Its Derivatives: Preparation and Antibacterial Properties. Materials 2023, 16, 6076. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Kongkaoroptham, P.; Piroonpan, T.; Pasanphan, W. Chitosan nanoparticles based on their derivatives as antioxidant and antibacterial additives for active bioplastic packaging. Carbohydr. Polym. 2021, 257, 117610. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Natera, J.; Massad, W.; García, N.A. The role of vitamin B6 as an antioxidant in the presence of vitamin B2-photogenerated reactive oxygen species. A kinetic and mechanistic study. Photochem. Photobiol. Sci. 2012, 11, 938–945. [Google Scholar] [CrossRef]
- Matxain, J.M.; Ristilä, M.; Strid, Å.; Eriksson, L.A. Theoretical study of the antioxidant properties of pyridoxine. J. Phys. Chem. A 2006, 110, 13068–13072. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]

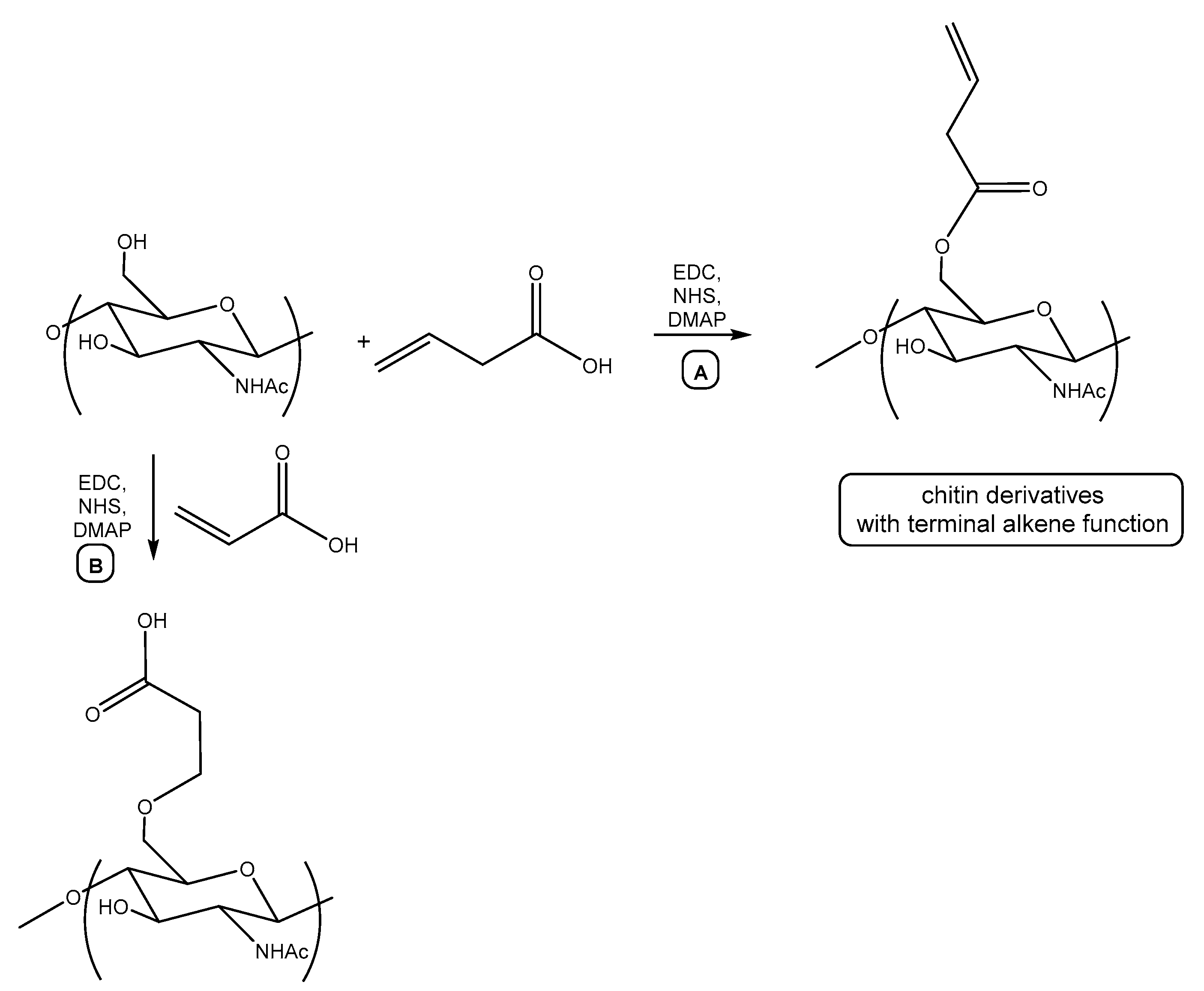
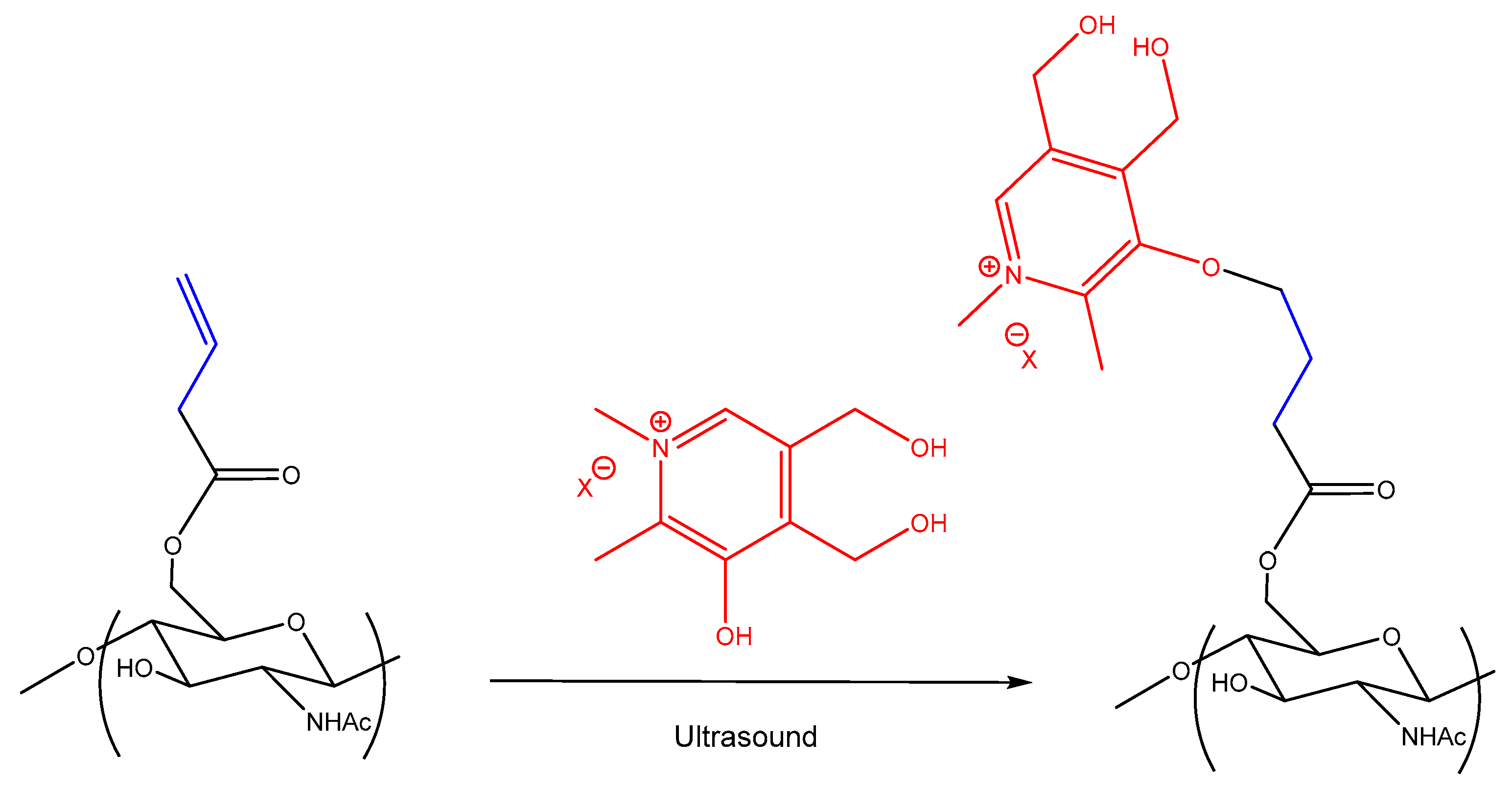
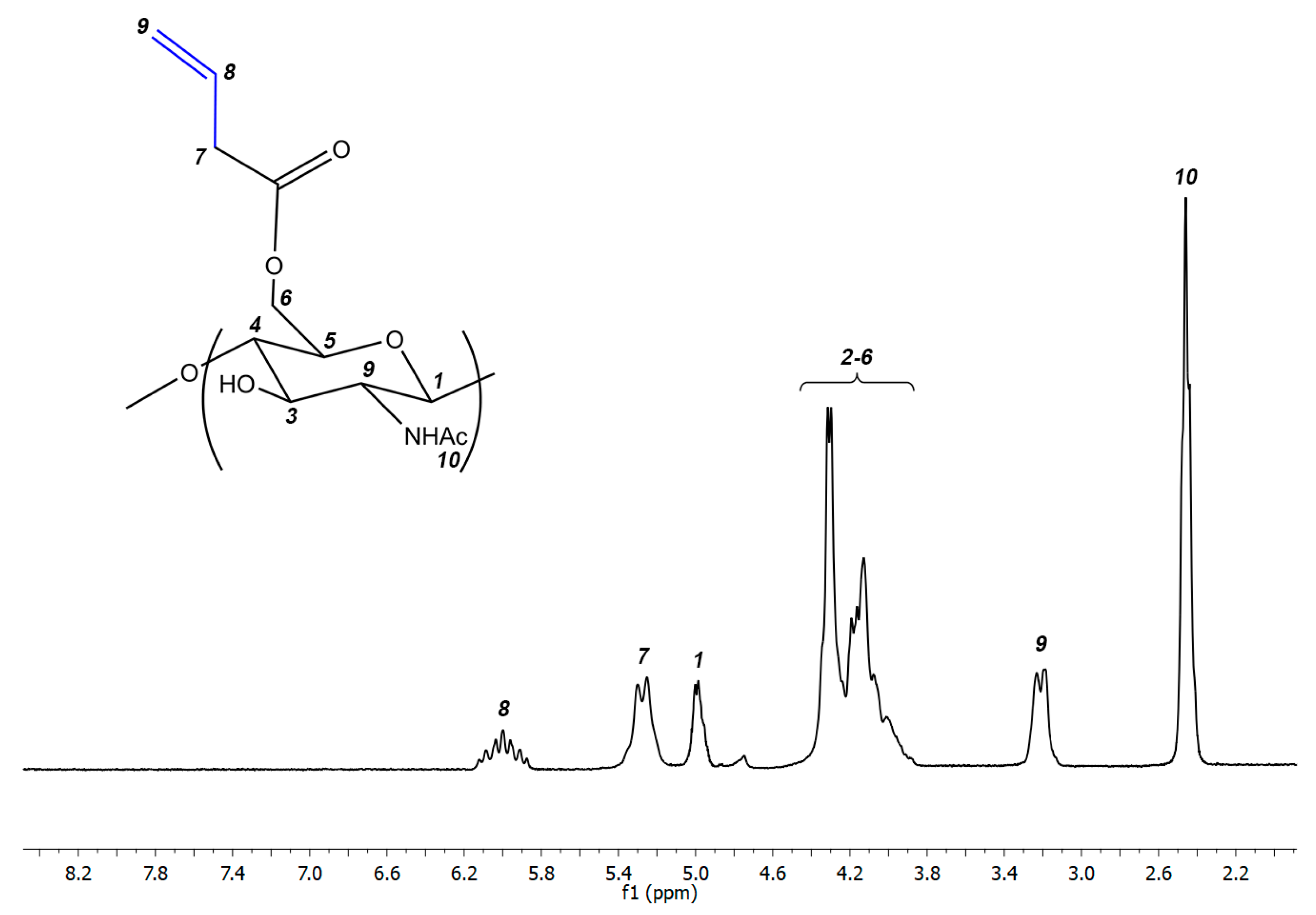

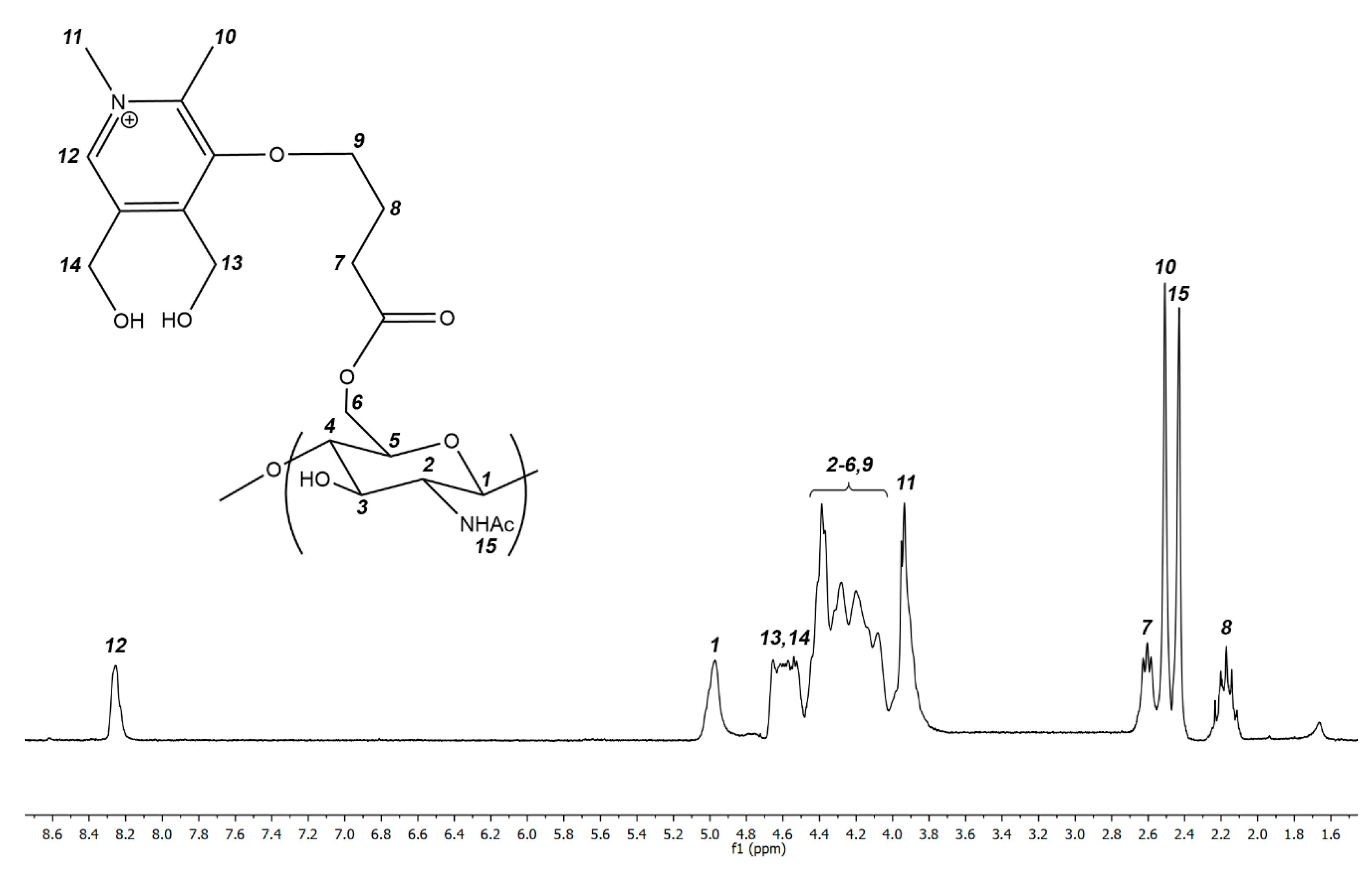


| Name of Alkene Chitin Derivative | Name of Cationic Chitin Derivative | Molecular Weight of the Original Chitin | Degree of Substitution of Chitin Derivative |
|---|---|---|---|
| A-Ch-I-A | C-Ch-I-A | 3.4 × 104 | 0.15 |
| A-Ch-I-B | C-Ch-I-B | 7.2 × 104 | 0.15 |
| A-Ch-I-C | C-Ch-I-C | 18.8 × 104 | 0.15 |
| A-Ch-II-A | C-Ch-II-A | 3.4 × 104 | 0.46 |
| A-Ch-II-B | C-Ch-II-B | 7.2 × 104 | 0.44 |
| A-Ch-II-C | C-Ch-II-C | 18.8 × 104 | 0.45 |
| A-Ch-III-A | C-Ch-III-A | 3.4 × 104 | 0.67 |
| A-Ch-III-B | C-Ch-III-B | 7.2 × 104 | 0.66 |
| A-Ch-III-C | C-Ch-III-C | 18.8 × 104 | 0.65 |
| Type of Nanoparticles | Mean Hydrodynamic Diameter, nm * | TPP:Polymer Mass Ratio | V(TPP), mL * | Polydispersity Index * | ζ-Potential, mV * |
|---|---|---|---|---|---|
| Prepared from C-Ch-I-B | |||||
| NP-1-I | 400 ± 5 | 1:228 | 0.35 | 0.11 ± 0.02 | 53.1 ± 0.1 |
| NP-2-I | 202 ± 7 | 1:94 | 0.85 | 0.11 ± 0.05 | 48.3 ± 0.2 |
| NP-3-I | 108 ± 4 | 1:67 | 1.20 | 0.10 ± 0.03 | 42.7 ± 0.3 |
| NP-4-I | 312 ± 4 | 1:55 | 1.45 | 0.13 ± 0.04 | 33.4 ± 0.1 |
| NP-5-I | 450 ± 6 | 1:47 | 1.70 | 0.15 ± 0.02 | 25.2 ± 0.2 |
| Prepared from C-Ch-II-B | |||||
| NP-1-II | 407 ± 4 | 1:133 | 0.60 | 0.13 ± 0.03 | 52.3 ± 0.3 |
| NP-2-II | 211 ± 8 | 1:76 | 1.05 | 0.14 ± 0.03 | 46.8 ± 0.1 |
| NP-3-II | 96 ± 3 | 1:50 | 1.60 | 0.11 ± 0.02 | 41.2 ± 0.4 |
| NP-4-II | 303 ± 9 | 1:43 | 1.85 | 0.12 ± 0.03 | 30.9 ± 0.2 |
| NP-5-II | 472 ± 4 | 1:39 | 2.05 | 0.11 ± 0.03 | 24.0 ± 0.1 |
| Prepared from C-Ch-III-B | |||||
| NP-1-III | 390 ± 9 | 1:80 | 1.00 | 0.10 ± 0.04 | 50.7 ± 0.2 |
| NP-2-III | 201 ± 4 | 1:57 | 1.40 | 0.12 ± 0.02 | 46.1 ± 0.5 |
| NP-3-III | 110 ± 6 | 1:50 | 1.60 | 0.10 ± 0.04 | 44.4 ± 0.3 |
| NP-4-III | 312 ± 3 | 1:35 | 2.30 | 0.11 ± 0.01 | 32.5 ± 0.1 |
| NP-5-III | 457 ± 3 | 1:33 | 2.45 | 0.11 ± 0.02 | 23.0 ± 0.3 |
| Sample | Bacillus subtilis | Klebsiella pneumoniae |
|---|---|---|
| Inhibition Zone, mm * | ||
| Ampicillin | 30.6 ± 0.2 | 23.3 ± 0.2 |
| Chitin | Effect Not Detected | Effect Not Detected |
| Polymers | ||
| C-Ch-I-A | 14.0 ± 0.3 | 11.5 ± 0.3 |
| C-Ch-I-B | 15.7 ± 0.4 | 12.7 ± 0.1 |
| C-Ch-I-C | 13.5 ± 0.1 | 11.2 ± 0.4 |
| C-Ch-II-A | 16.2 ± 0.3 | 15.1 ± 0.1 |
| C-Ch-II-B | 18.1 ± 0.1 | 16.6 ± 0.3 |
| C-Ch-II-C | 15.3 ± 0.1 | 14.2 ± 0.3 |
| C-Ch-III-A | 19.3 ± 0.2 | 18.2 ± 0.2 |
| C-Ch-III-B | 22.3 ± 0.3 | 19.7 ± 0.2 |
| C-Ch-III-C | 19.1 ± 0.2 | 17.5 ± 0.1 |
| Nanoparticles derived from C-Ch-I-B | ||
| NP-1-I | 15.9 ± 0.2 | 13.4 ± 0.2 |
| NP-2-I | 17.1 ± 0.4 | 14.1 ± 0.2 |
| NP-3-I | 19.3 ± 0.3 | 16.2 ± 0.4 |
| NP-4-I | 14.0 ± 0.1 | 13.2 ± 0.2 |
| NP-5-I | 12.5 ± 0.3 | 12.5 ± 0.3 |
| Nanoparticles derived from C-Ch-II-B | ||
| NP-1-II | 18.8 ± 0.1 | 17.8 ± 0.1 |
| NP-2-II | 20.1 ± 0.2 | 19.3 ± 0.3 |
| NP-3-II | 23.6 ± 0.1 | 21.7 ± 0.2 |
| NP-4-II | 21.3 ± 0.1 | 19.1 ± 0.2 |
| NP-5-II | 19.5 ± 0.3 | 18.4 ± 0.3 |
| Nanoparticles derived from C-Ch-III-B | ||
| NP-1-III | 24.4 ± 0.1 | 20.5 ± 0.3 |
| NP-2-III | 26.9 ± 0.1 | 22.2 ± 0.2 |
| NP-3-III | 30.8 ± 0.4 | 24.8 ± 0.2 |
| NP-4-III | 26.5 ± 0.3 | 19.6 ± 0.1 |
| NP-5-III | 23.2 ± 0.2 | 17.3 ± 0.3 |
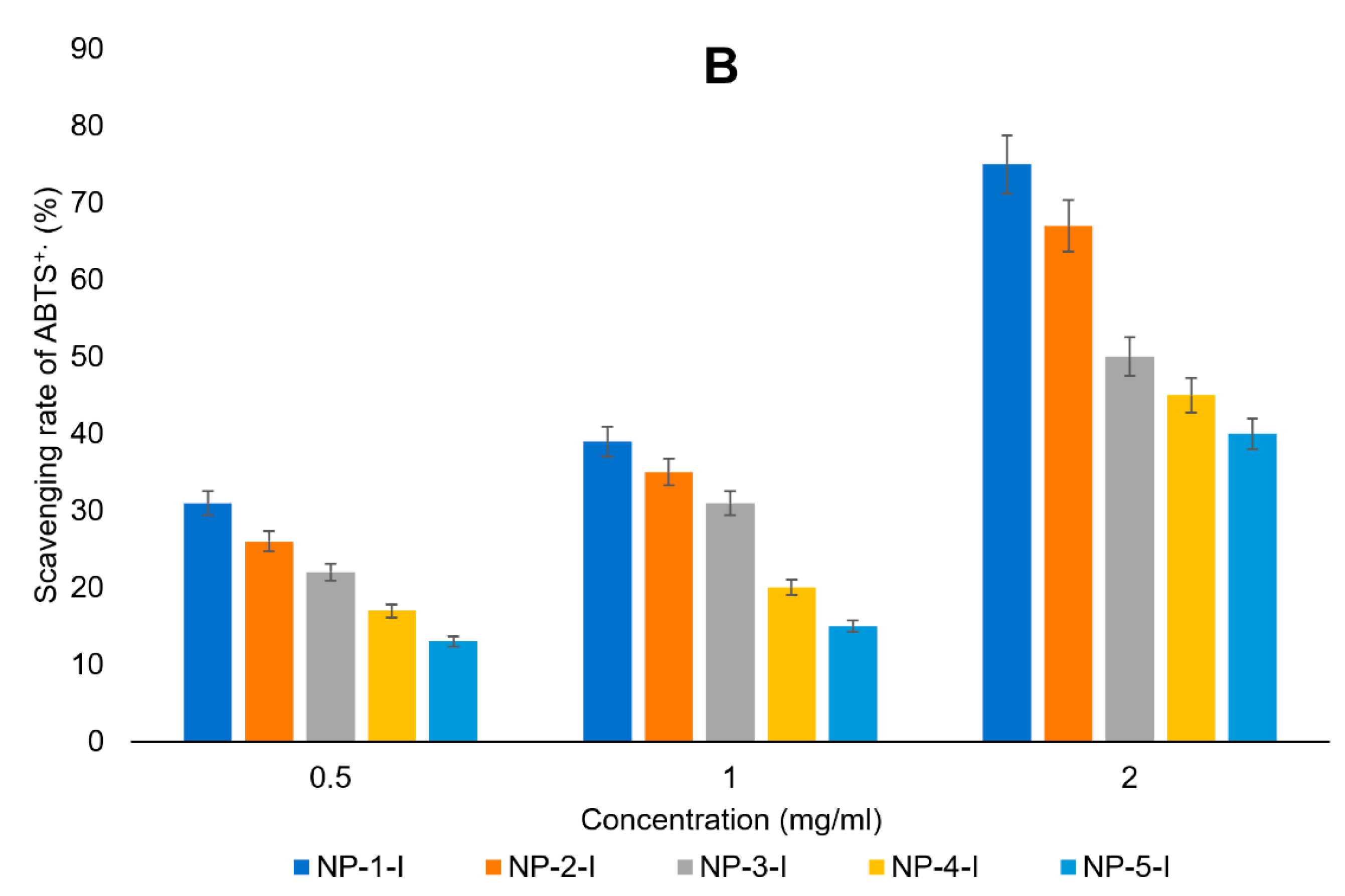
| Sample | IC50, mg/mL * | Sample | IC50, mg/mL * |
|---|---|---|---|
| C-Ch-I-B | 1.12 ± 0.02 | NP-3-I | 2.05 ± 0.03 |
| C-Ch-II-B | 0.90 ± 0.03 | NP-4-I | 1.48 ± 0.02 |
| C-Ch-III-B | 0.29 ± 0.01 | NP-5-I | 0.63 ± 0.03 |
| NP-1-I | 2.41 ± 0.04 | Ascorbic acid | 0.016 ± 0.003 |
| NP-2-I | 2.25 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorov, A.R.; Khubiev, O.M.; Golubev, R.A.; Semenkova, D.I.; Nikolaev, A.A.; Maharramov, A.M.; Mammadova, G.Z.; Liu, W.; Tskhovrebov, A.G.; Kritchenkov, A.S. New Antibacterial and Antioxidant Chitin Derivatives: Ultrasonic Preparation and Biological Effects. Polymers 2024, 16, 2509. https://doi.org/10.3390/polym16172509
Egorov AR, Khubiev OM, Golubev RA, Semenkova DI, Nikolaev AA, Maharramov AM, Mammadova GZ, Liu W, Tskhovrebov AG, Kritchenkov AS. New Antibacterial and Antioxidant Chitin Derivatives: Ultrasonic Preparation and Biological Effects. Polymers. 2024; 16(17):2509. https://doi.org/10.3390/polym16172509
Chicago/Turabian StyleEgorov, Anton R., Omar M. Khubiev, Roman A. Golubev, Daria I. Semenkova, Andrey A. Nikolaev, Abel M. Maharramov, Gunay Z. Mammadova, Wanjun Liu, Alexander G. Tskhovrebov, and Andreii S. Kritchenkov. 2024. "New Antibacterial and Antioxidant Chitin Derivatives: Ultrasonic Preparation and Biological Effects" Polymers 16, no. 17: 2509. https://doi.org/10.3390/polym16172509
APA StyleEgorov, A. R., Khubiev, O. M., Golubev, R. A., Semenkova, D. I., Nikolaev, A. A., Maharramov, A. M., Mammadova, G. Z., Liu, W., Tskhovrebov, A. G., & Kritchenkov, A. S. (2024). New Antibacterial and Antioxidant Chitin Derivatives: Ultrasonic Preparation and Biological Effects. Polymers, 16(17), 2509. https://doi.org/10.3390/polym16172509







