Recent Advancements in Gel Polymer Electrolytes for Flexible Energy Storage Applications
Abstract
1. Introduction
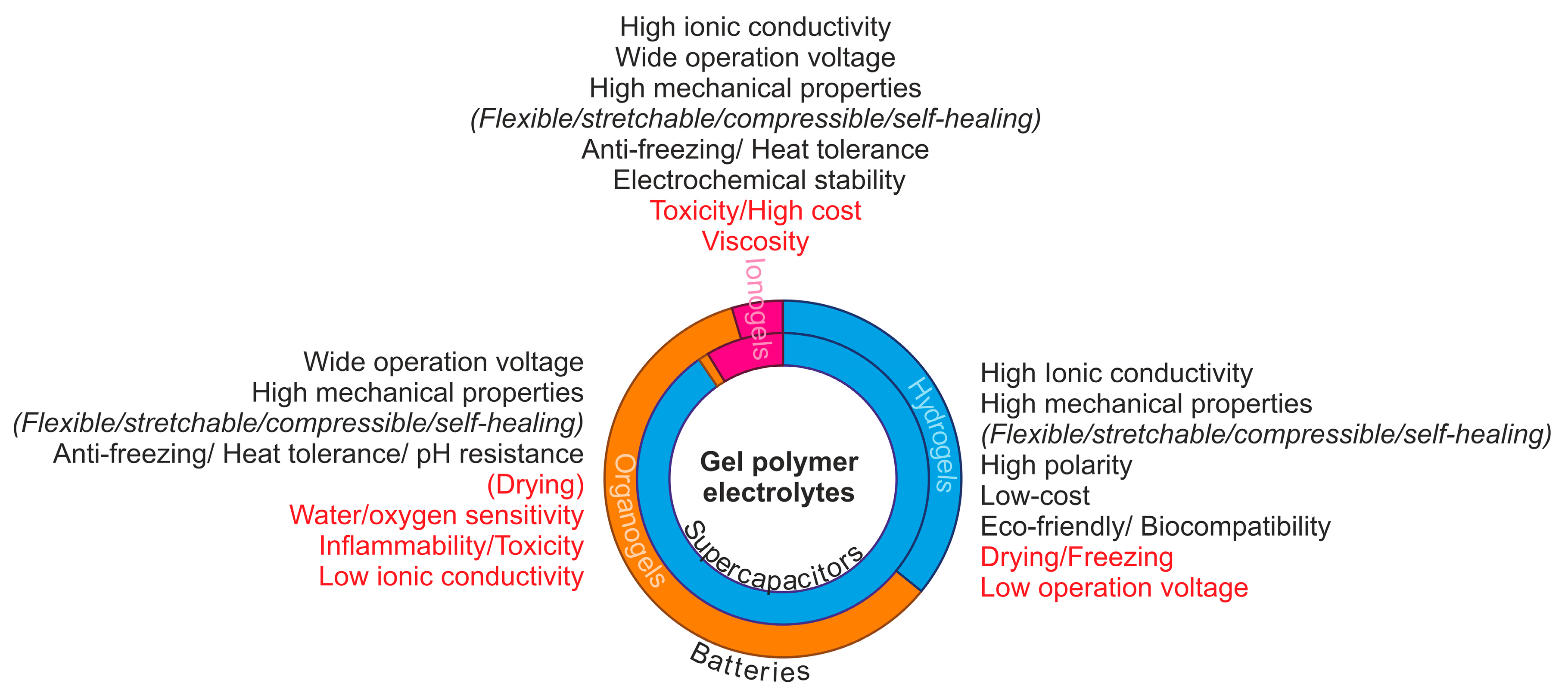
2. Design and Applications of GPE in Batteries and Supercapacitors
2.1. Ionic Liquid-Based Polymer Gel Electrolytes (IL-GPEs)
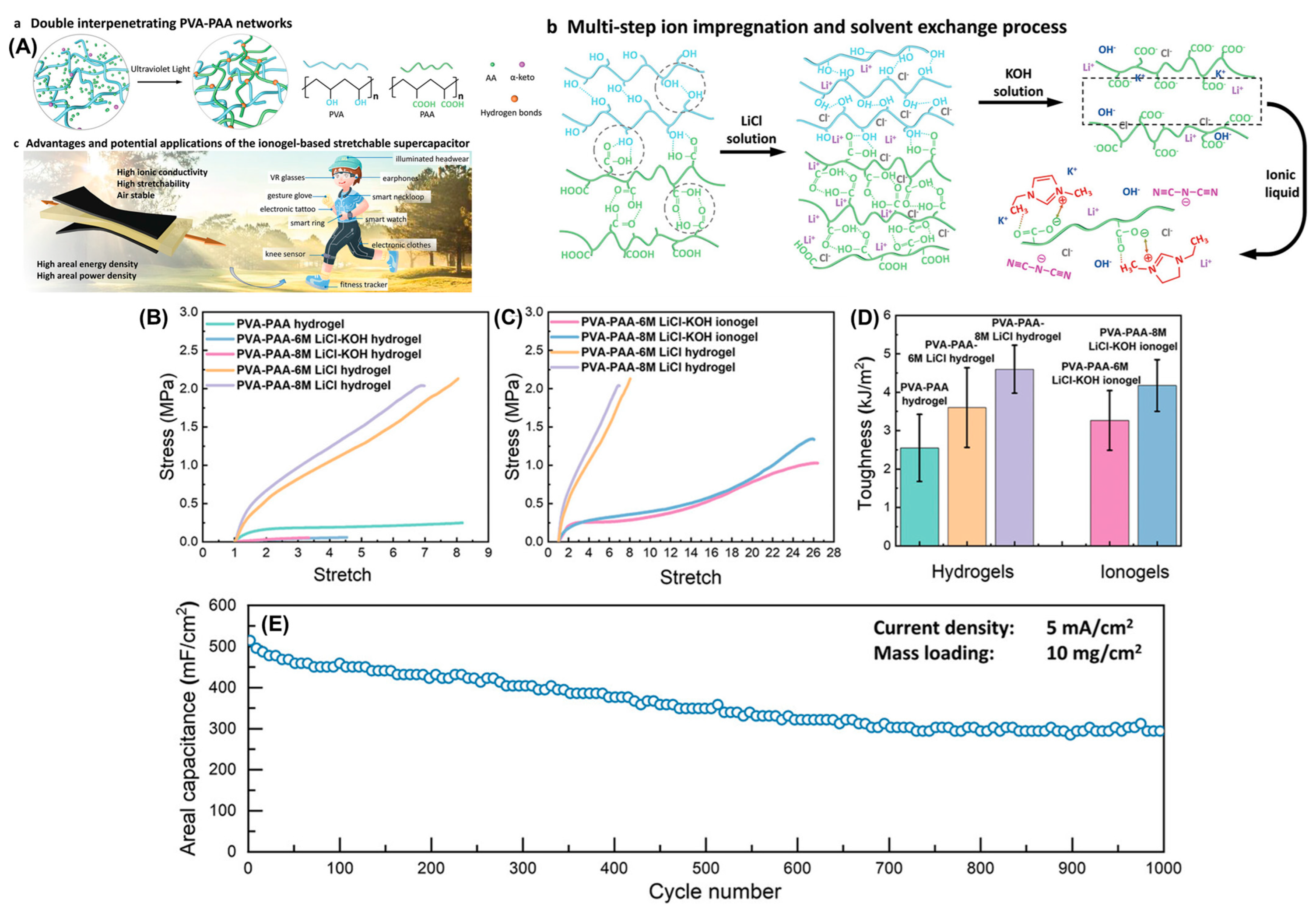
2.1.1. Ionogels for Battery Applications
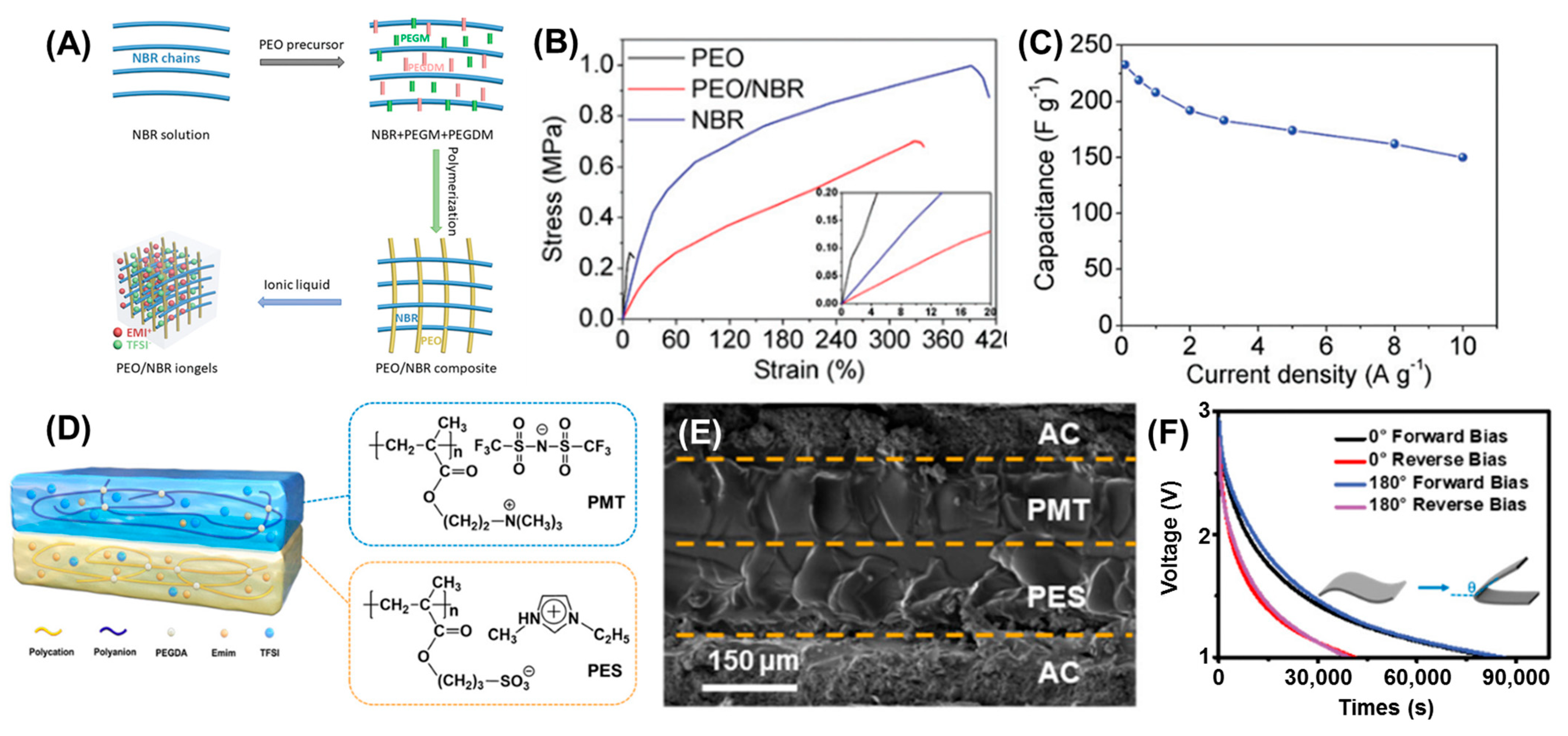
2.1.2. Ionogels for Supercapacitor Applications
2.2. Hydrogel
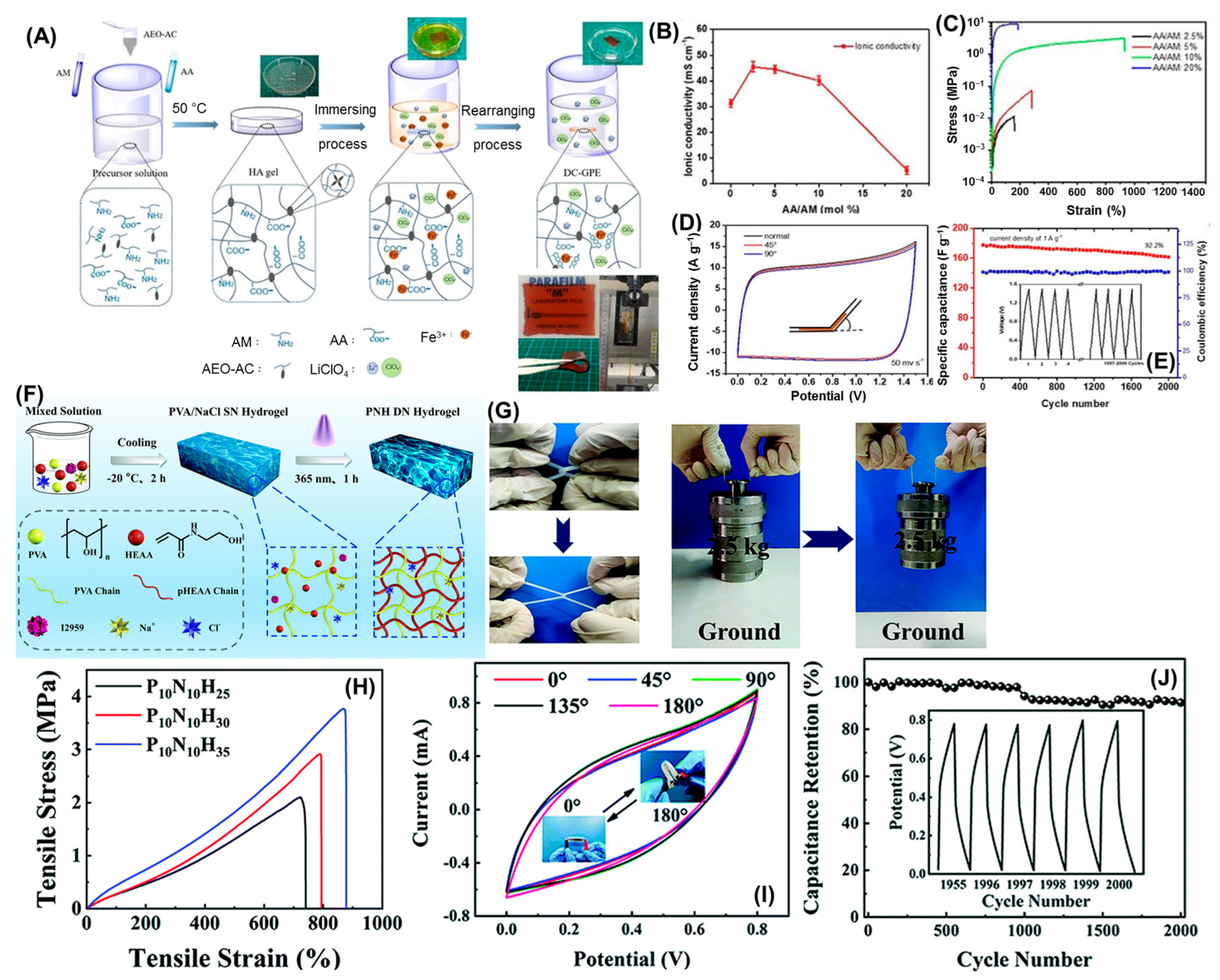
2.2.1. Hydrogels for Supercapacitor Applications
2.2.2. Hydrogels for Battery Applications
2.3. Organogels
2.3.1. Organogels for Battery Applications
2.3.2. Organogels for Supercapacitor Applications
2.4. Single-Ion Gel Electrolytes
2.5. Hybrid Gel Electrolytes
2.5.1. Hybrid GPEs for Battery Applications
2.5.2. Hybrid GPEs for Supercapacitor Applications
3. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Deng, D. Li-Ion Batteries: Basics, Progress, and Challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Leong, K.W.; Pan, W.; Yi, X.; Luo, S.; Zhao, X.; Zhang, Y.; Wang, Y.; Mao, J.; Chen, Y.; Xuan, J.; et al. Next-Generation Magnesium-Ion Batteries: The Quasi-Solid-State Approach to Multivalent Metal Ion Storage. Sci. Adv. 2023, 9, eadh1181. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yu, M. Flexible Solid-State Lithium-Sulfur Batteries Based on Structural Designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Charge Storage Mechanism in Nanoporous Carbons and Its Consequence for Electrical Double Layer Capacitors. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3457–3467. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islam, M.A.; Azad, A.K. Advanced Materials and Technologies for Hybrid Supercapacitors for Energy Storage—A Review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Rudra, S.; Seo, H.W.; Sarker, S.; Kim, D.M. Supercapatteries as Hybrid Electrochemical Energy Storage Devices: Current Status and Future Prospects. Molecules 2024, 29, 243. [Google Scholar] [CrossRef]
- Xia, L.; Yu, L.; Hu, D.; Chen, G.Z. Electrolytes for Electrochemical Energy Storage. Mater. Chem. Front. 2017, 1, 584–618. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic Conductivity and Ion Transport Mechanisms of Solid-State Lithium-Ion Battery Electrolytes: A Review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer Electrolytes for Lithium-Ion Batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Armand, M. Polymers with Ionic Conductivity. Adv. Mater. 1990, 2, 278–286. [Google Scholar] [CrossRef]
- Schauser, N.S.; Nikolaev, A.; Richardson, P.M.; Xie, S.; Johnson, K.; Susca, E.M.; Wang, H.; Seshadri, R.; Clément, R.J.; Read De Alaniz, J.; et al. Glass Transition Temperature and Ion Binding Determine Conductivity and Lithium-Ion Transport in Polymer Electrolytes. ACS Macro Lett. 2021, 10, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Taberna, P.-L.; Fantini, S.; Presser, V.; Pérez, C.R.; Malbosc, F.; Rupesinghe, N.L.; Teo, K.B.K.; Gogotsi, Y.; Simon, P. Capacitive Energy Storage from −50 to 100 °C Using an Ionic Liquid Electrolyte. J. Phys. Chem. Lett. 2011, 2, 2396–2401. [Google Scholar] [CrossRef]
- Marcinek, M.; Syzdek, J.; Marczewski, M.; Piszcz, M.; Niedzicki, L.; Kalita, M.; Plewa-Marczewska, A.; Bitner, A.; Wieczorek, P.; Trzeciak, T.; et al. Electrolytes for Li-Ion Transport—Review. Solid State Ion. 2015, 276, 107–126. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte Selection for Supercapacitive Devices: A Critical Review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, J.; Wu, H.; Li, Q.; Fan, S.; Wang, J. Progress and Challenges of Flexible Lithium Ion Batteries. J. Power Sources 2020, 454, 227932. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, G.; Rawach, D.; Fu, C.; Wang, C.; Liu, X.; Dubois, M.; Lai, C.; Sun, S. Polymer Gel Electrolytes for Flexible Supercapacitors: Recent Progress, Challenges, and Perspectives. Energy Storage Mater. 2021, 34, 320–355. [Google Scholar] [CrossRef]
- Mohanta, J.; Kang, D.W.; Cho, J.S.; Jeong, S.M.; Kim, J.K. Stretchable Electrolytes for Stretchable/Flexible Energy Storage Systems—Recent Developments. Energy Storage Mater. 2020, 28, 315–324. [Google Scholar] [CrossRef]
- Swain, N.; Tripathy, A.; Thirumurugan, A.; Saravanakumar, B.; Schmidt-Mende, L.; Ramadoss, A. A Brief Review on Stretchable, Compressible, and Deformable Supercapacitor for Smart Devices. Chem. Eng. J. 2022, 446, 136876. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Gu, Y.; Xue, P.; Xu, X. Self-Healing and Highly Stretchable Hydrogel for Interfacial Compatible Flexible Paper-Based Micro-Supercapacitor. Materials 2021, 14, 1852. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, S.; Wang, D.; Yang, Q.; Mo, F.; Liang, G.; Li, N.; Zhang, H.; Zapien, J.A.; Zhi, C. Super-Stretchable Zinc–Air Batteries Based on an Alkaline-Tolerant Dual-Network Hydrogel Electrolyte. Adv. Energy Mater. 2019, 9, 1803046. [Google Scholar] [CrossRef]
- Tong, Y.; Xu, Y.; Chen, D.; Xie, Y.; Chen, L.; Que, M.; Hou, Y. Deformable and Flexible Electrospun Nanofiber-Supported Cross-Linked Gel Polymer Electrolyte Membranes for High Safety Lithium-Ion Batteries. RSC Adv. 2017, 7, 22728–22734. [Google Scholar] [CrossRef]
- Chen, D.; Wang, D.; Yang, Y.; Huang, Q.; Zhu, S.; Zheng, Z. Self-Healing Materials for next-Generation Energy Harvesting and Storage Devices. Adv. Energy Mater. 2017, 7, 27–31. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiao, X.; Pan, K.; Pang, H. Development and Application of Self-Healing Materials in Smart Batteries and Supercapacitors. Chem. Eng. J. 2020, 380, 122565. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Li, Y.; Hope, J.; Choo, W.L. Extrinsic Conditions for the Occurrence and Characterizations of Self-Healing Polyurea Coatings for Improved Medical Device Reliability: A Mini Review. ACS Omega 2023, 8, 26650–26662. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.A.; Hernández Santana, M. Evolution of Self-Healing Elastomers, from Extrinsic to Combined Intrinsic Mechanisms: A Review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Dahlke, J.; Zechel, S.; Hager, M.D.; Schubert, U.S. How to Design a Self-Healing Polymer: General Concepts of Dynamic Covalent Bonds and Their Application for Intrinsic Healable Materials. Adv. Mater. Interfaces 2018, 5, 1800051. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Liu, K.; Pan, X.; Chen, L.; Huang, L.; Ni, Y.; Liu, J.; Cao, S.; Wang, H. Ultrasoft Self-Healing Nanoparticle-Hydrogel Composites with Conductive and Magnetic Properties. ACS Sustain. Chem. Eng. 2018, 6, 6395–6403. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Jin, X.; Han, T.; Zhang, H.; Liu, J. A Flexible Self-Healing Zn3V2O7(OH)2·2H2O-Based Zn-Ion Battery under Continuous Folding and Twisting. Chem. Commun. 2022, 58, 8117–8120. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, J.; Wang, J.; Hu, M.; Mo, F.; Liang, G.; Zhi, C. An Intrinsically Self-Healing NiCo||Zn Rechargeable Battery with a Self-Healable Ferric-Ion-Crosslinking Sodium Polyacrylate Hydrogel Electrolyte. Angew. Chem. 2018, 130, 9958–9961. [Google Scholar] [CrossRef]
- Guo, P.; Su, A.; Wei, Y.; Liu, X.; Li, Y.; Guo, F.; Li, J.; Hu, Z.; Sun, J. Healable, Highly Conductive, Flexible, and Nonflammable Supramolecular Ionogel Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 19413–19420. [Google Scholar] [CrossRef]
- Chen, X.; Yi, L.; Zou, C.; Liu, J.; Yu, J.; Zang, Z.; Tao, X.; Luo, Z.; Guo, X.; Chen, G.; et al. High-Performance Gel Polymer Electrolyte with Self-Healing Capability for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 5267–5276. [Google Scholar] [CrossRef]
- Davino, S.; Callegari, D.; Pasini, D.; Thomas, M.; Nicotera, I.; Bonizzoni, S.; Mustarelli, P.; Quartarone, E. Cross-Linked Gel Electrolytes with Self-Healing Functionalities for Smart Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 51941–51953. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, X.; Sun, Z.; Zhang, B.; Bao, Y.; Liu, Z.; Han, D.; Niu, L. Self-Healing of a Covalently Cross-Linked Polymer Electrolyte Membrane by Diels-Alder Cycloaddition and Electrolyte Embedding for Lithium Ion Batteries. Polymers 2021, 13, 4155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, G.; Wang, C.; Geng, T.; Wang, J.; Liu, X.; Zhou, X.; Zhang, J. A Self-Healing Polymer Electrolyte Based on the Diels–Alder Reaction in Lithium-Ion Batteries. J. Appl. Polym. Sci. 2024, 141, e55473. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lu, Y.H.; Liu, Y.L. In Situ Self-Healing of Gel Polymer Electrolytes Enhancing the Cycling Stability of Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2024, 12, 7894–7902. [Google Scholar] [CrossRef]
- Cho, D.H.; Cho, K.G.; An, S.; Kim, M.M.S.; Oh, H.W.; Yeo, J.; Yoo, W.C.; Hong, K.; Kim, M.M.S.; Lee, K.H. Self-Healable, Stretchable, and Nonvolatile Solid Polymer Electrolytes for Sustainable Energy Storage and Sensing Applications. Energy Storage Mater. 2022, 45, 323–331. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Zhang, X.; Sun, Y.; Xie, H. Three-Dimensional Cross-Linked Network Deep Eutectic Gel Polymer Electrolyte with the Self-Healing Ability Enable by Hydrogen Bonds and Dynamic Disulfide Bonds. J. Colloid Interface Sci. 2024, 669, 529–536. [Google Scholar] [CrossRef]
- Wan, L.; Cao, X.; Xue, X.; Tong, Y.; Ci, S.; Huang, H.; Zhou, D. Self-Healing and Flexible Ionic Gel Polymer Electrolyte Based on Reversible Bond for High-Performance Lithium Metal Batteries. Energy Technol. 2022, 10, 2100749. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, K.; Xu, Z.; Xiao, M.; Meng, Y. Highly Conductive Self-Healing Polymer Electrolytes Based on Synergetic Dynamic Bonds for Highly Safe Lithium Metal Batteries. Chem. Eng. J. 2022, 442, 136083. [Google Scholar] [CrossRef]
- Deng, K.; Zhou, S.; Xu, Z.; Xiao, M.; Meng, Y. A High Ion-Conducting, Self-Healing and Nonflammable Polymer Electrolyte with Dynamic Imine Bonds for Dendrite-Free Lithium Metal Batteries. Chem. Eng. J. 2022, 428, 1385–8947. [Google Scholar] [CrossRef]
- Chandrasekar, J.; Venkatesan, M.; Sun, T.W.; Hsu, Y.C.; Huang, Y.H.; Chen, W.W.; Chen, M.H.; Tsai, M.L.; Chen, J.Y.; Lin, J.H.; et al. Recent Progress in Self-Healable Energy Harvesting and Storage Devices—A Future Direction for Reliable and Safe Electronics. Mater. Horiz. 2024, 11, 1395–1413. [Google Scholar] [CrossRef]
- Liu, S.; Ma, S.; Zhang, Q.; Xu, X. Thermal-Responsive Electrolytes for Reversible Self-Protection of Electrochemical Storage Devices at Excessive Temperature. Ionics 2022, 28, 5119–5128. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, L.; Luo, S.; Zhao, E.; Saito, N. A Non-Flammable, Flexible and UV-Cured Gel Polymer Electrolyte with Crosslinked Polymer Network for Dendrite-Suppressing Lithium Metal Batteries. Ionics 2022, 28, 3743–3759. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, K.; Liu, Y.; Ye, L.; Gao, Y.; Lin, W.; Xu, H.; Wang, X.; Bai, Y.; Wu, C. Flame-Retardant Gel Polymer Electrolyte and Interface for Quasi-Solid-State Sodium Ion Batteries. Chem. Eng. J. 2020, 401, 126065. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Y.; Xiao, Y.; Xi, C.; Xu, G.; Li, B.; Yang, C.; Yu, Y. Flame-Retardant Composite Gel Polymer Electrolyte with a Dual Acceleration Conduction Mechanism for Lithium Ion Batteries. Chem. Eng. J. 2021, 422, 1385–8947. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Feng, Y.; Feng, W. Thermally Responsive Polymers for Overcoming Thermal Runaway in High-Safety Electrochemical Storage Devices. Mater. Chem. Front. 2023, 7, 1562–1590. [Google Scholar] [CrossRef]
- Wei, Z.; Yu, L.; Lu, S.; Zhao, Y. Reversibly Thermo-Responsive Materials Applied in Lithium Batteries. Energy Storage Mater. 2023, 61, 2405–8297. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Peng, M.; Shen, D.; Zhu, C.; Qian, S.; Liu, J.; Cao, Y.; Yan, C.; Zhou, J.; et al. Developing Thermoregulatory Hydrogel Electrolyte to Overcome Thermal Runaway in Zinc-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2206653. [Google Scholar] [CrossRef]
- Qian, Y.; Chai, C.; Qi, P.; Ma, L.; Hao, J. Integrated Thermoelectric Design Inspired by Ionic Liquid Microemulsion-Based Gel with Regulatable Dual-Temperature Responsiveness. ACS Appl. Polym. Mater. 2023, 5, 2983–2994. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Q.; Guo, S.; Yu, L.; Hu, X. Thermoregulating Separators Based on Phase-Change Materials for Safe Lithium-Ion Batteries. Adv. Mater. 2021, 33, e2008088. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yao, M.; Huang, S.; Tian, J.; Niu, Z. Thermal-Gated Polymer Electrolytes for Smart Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 16480–16484. [Google Scholar] [CrossRef]
- Xu, T.; Yang, D.; Zhang, S.; Zhao, T.; Zhang, M.; Yu, Z.Z. Antifreezing and Stretchable All-Gel-State Supercapacitor with Enhanced Capacitances Established by Graphene/PEDOT-Polyvinyl Alcohol Hydrogel Fibers with Dual Networks. Carbon N. Y. 2021, 171, 201–210. [Google Scholar] [CrossRef]
- Gomez, I.; Alesanco, Y.; Colmenares, L.C.; Blázquez, J.A.; Viñuales, A. Room-Temperature Self-Standing Cellulose-Based Hydrogel Electrolytes for Electrochemical Devices. Polymers 2020, 12, 2686. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ha, H.; Al-Sudani, A.; Ellison, C.J.; Yu, G. Thermoplastic Elastomer-Enabled Smart Electrolyte for Thermoresponsive Self-Protection of Electrochemical Energy Storage Devices. Adv. Mater. 2016, 28, 7921–7928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, S.; Zhang, Q.; Cao, M.; Wang, Y.; Gu, Y.; Xu, X. Thermoreversible and Self-Protective Sol-Gel Transition Electrolytes for All-Printed Transferable Microsupercapacitors as Safer Micro-Energy Storage Devices. ACS Appl. Mater. Interfaces 2020, 12, 41819–41831. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, S.; Lv, Z.; Xu, G.; Huang, L.; Wang, Q.; Cui, Z.; Cui, G. A Temperature-Responsive Electrolyte Endowing Superior Safety Characteristic of Lithium Metal Batteries. Adv. Energy Mater. 2020, 10, 1903441. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, P.; Liu, J.; Xu, X. Thermal-Switching and Repeatable Self-Protective Hydrogel Polyelectrolytes for Energy Storage Applications of Flexible Electronics. ACS Appl. Energy Mater. 2021, 4, 6116–6124. [Google Scholar] [CrossRef]
- Kimizuka, N.; Nakashima, T. Spontaneous Self-Assembly of Glycolipid Bilayer Membranes in Sugar-Philic Ionic Liquids and Formation of Ionogels. Langmuir 2001, 17, 6759–6761. [Google Scholar] [CrossRef]
- Susan, M.A.B.H.; Kaneko, T.; Noda, A.; Watanabe, M. Ion Gels Prepared by in Situ Radical Polymerization of Vinyl Monomers in an Ionic Liquid and Their Characterization as Polymer Electrolytes. J. Am. Chem. Soc. 2005, 127, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Néouze, M.A.; Le Bideau, J.; Leroux, F.; Vioux, A. A Route to Heat Resistant Solid Membranes with Performances of Liquid Electrolytes. Chem. Commun. 2005, 8, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wu, P. Underwater Communication and Optical Camouflage Ionogels. Adv. Mater. 2021, 33, 2008479. [Google Scholar] [CrossRef]
- Long, T.; Li, Y.; Fang, X.; Sun, J. Salt-Mediated Polyampholyte Hydrogels with High Mechanical Strength, Excellent Self-Healing Property, and Satisfactory Electrical Conductivity. Adv. Funct. Mater. 2018, 28, 1804416. [Google Scholar] [CrossRef]
- Wu, A.; Lu, F.; Sun, P.; Qiao, X.; Gao, X.; Zheng, L. Low-Molecular-Weight Supramolecular Ionogel Based on Host-Guest Interaction. Langmuir 2017, 33, 13982–13989. [Google Scholar] [CrossRef]
- Vioux, A.; Coasne, B. From Ionogels to Biredox Ionic Liquids: Some Emerging Opportunities for Electrochemical Energy Storage and Conversion Devices. Adv. Energy Mater. 2017, 7, 1700883. [Google Scholar] [CrossRef]
- Singh, A.; Vedarajan, R.; Matsumi, N. Modified Metal Organic Frameworks (MOFs)/Ionic Liquid Matrices for Efficient Charge Storage. J. Electrochem. Soc. 2017, 164, H5169–H5174. [Google Scholar] [CrossRef]
- Pont, A.L.; Marcilla, R.; De Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-Based Polymeric Ionic Liquids as Mechanically and Electrochemically Stable Polymer Electrolytes. J. Power Sources 2009, 188, 558–563. [Google Scholar] [CrossRef]
- Balo, L.; Shalu; Gupta, H.; Kumar Singh, V.; Kumar Singh, R. Flexible Gel Polymer Electrolyte Based on Ionic Liquid EMIMTFSI for Rechargeable Battery Application. Electrochim. Acta 2017, 230, 123–131. [Google Scholar] [CrossRef]
- Li, Q.; Ardebili, H. Flexible Thin-Film Battery Based on Solid-like Ionic Liquid-Polymer Electrolyte. J. Power Sources 2016, 303, 17–21. [Google Scholar] [CrossRef]
- Ravi, M.; Kim, S.; Ran, F.; Kim, D.S.; Lee, Y.M.; Ryou, M.H. Hybrid Gel Polymer Electrolyte Based on 1-Methyl−1-Propylpyrrolidinium Bis(Trifluoromethanesulfonyl) Imide for Flexible and Shape-Variant Lithium Secondary Batteries. J. Memb. Sci. 2021, 621, 119018. [Google Scholar] [CrossRef]
- Swiderska-Mocek, A.; Gabryelczyk, A. Interfacial Stabilizing Effect of Lithium Borates and Pyrrolidinium Ionic Liquid in Gel Polymer Electrolytes for Lithium-Metal Batteries. J. Phys. Chem. C 2023, 127, 18875–18890. [Google Scholar] [CrossRef]
- Chen, L.; Fu, J.; Lu, Q.; Shi, L.; Li, M.; Dong, L.; Xu, Y.; Jia, R. Cross-Linked Polymeric Ionic Liquids Ion Gel Electrolytes by in Situ Radical Polymerization. Chem. Eng. J. 2019, 378, 122245. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Fan, W.; Wang, J.; Li, C. Long Cycling, Thermal Stable, Dendrites Free Gel Polymer Electrolyte for Flexible Lithium Metal Batteries. Electrochim. Acta 2019, 301, 304–311. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Y.; Montazami, R. Ionic Liquid-Doped Gel Polymer Electrolyte for Flexible Lithium-Ion Polymer Batteries. Materials 2015, 8, 2735–2748. [Google Scholar] [CrossRef]
- Su, Q.; Huang, S.; Liao, J.; Song, D.; Yuan, W.; Li, C.; He, J. A Flame Retardant and Flexible Gel Polymer Electrolytes for High Temperature Lithium Metal Batteries. J. Electroanal. Chem. 2023, 945, 1572–6657. [Google Scholar] [CrossRef]
- Sen, S.; Goodwin, S.E.; Barbará, P.V.; Rance, G.A.; Wales, D.; Cameron, J.M.; Sans, V.; Mamlouk, M.; Scott, K.; Walsh, D.A. Gel-Polymer Electrolytes Based on Poly(Ionic Liquid)/Ionic Liquid Networks. ACS Appl. Polym. Mater. 2021, 3, 200–208. [Google Scholar] [CrossRef]
- Nykaza, J.R.; Savage, A.M.; Pan, Q.; Wang, S.; Beyer, F.L.; Tang, M.H.; Li, C.Y.; Elabd, Y.A. Polymerized Ionic Liquid Diblock Copolymer as Solid-State Electrolyte and Separator in Lithium-Ion Battery. Polymers 2016, 101, 311–318. [Google Scholar] [CrossRef]
- Gouverneur, M.; Jeremias, S.; Schönhoff, M. 7Li Nuclear Magnetic Resonance Studies of Dynamics in a Ternary Gel Polymer Electrolyte Based on Polymeric Ionic Liquids. Electrochim. Acta 2015, 175, 35–41. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Qian, Q.; Huang, H.; Chen, Y.; Wang, Z.; Chen, Q.; Yang, J.; Li, J.; Mai, Y.W. Electrospinning-Based Strategies for Battery Materials. Adv. Energy Mater. 2021, 11, 2000845. [Google Scholar] [CrossRef]
- Que, M.; Tong, Y.; Wei, G.; Yuan, K.; Wei, J.; Jiang, Y.; Zhu, H.; Chen, Y. Safe and Flexible Ion Gel Based Composite Electrolyte for Lithium Batteries. J. Mater. Chem. A 2016, 4, 14132–14140. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, Y.; Que, M.; Xiao, Y.; Jiang, Y.; Yuan, K.; Chen, Y. A Facile: In Situ Approach to Ion Gel Based Polymer Electrolytes for Flexible Lithium Batteries. RSC Adv. 2017, 7, 54391–54398. [Google Scholar] [CrossRef]
- Wu, J.; Xia, G.; Li, S.; Wang, L.; Ma, J. A Flexible and Self-Healable Gelled Polymer Electrolyte Based on a Dynamically Cross-Linked PVA Ionogel for High-Performance Supercapacitors. Ind. Eng. Chem. Res. 2020, 59, 22509–22519. [Google Scholar] [CrossRef]
- Kim, D.; Lee, G.; Kim, D.; Ha, J.S. Air-Stable, High-Performance, Flexible Microsupercapacitor with Patterned Ionogel Electrolyte. ACS Appl. Mater. Interfaces 2015, 7, 4608–4615. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, C.; Zhou, F.; Dong, Y.; Shi, X.; Nicolosi, V.; Wu, Z.S.; Bao, X. Ionic Liquid Pre-Intercalated MXene Films for Ionogel-Based Flexible Micro-Supercapacitors with High Volumetric Energy Density. J. Mater. Chem. A 2019, 7, 9478–9485. [Google Scholar] [CrossRef]
- Lee, K.S.; Jeong, H.T. Development and Optimization of Ionic Liquid Based Gel Polymer Electrolyte for All Solid-State Supercapacitor. J. Energy Storage 2021, 42, 103001. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, Ionic Liquid Based Hybrid Materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.P.; Hashmi, S.A. Performance of Solid-State Supercapacitors with Ionic Liquid 1-Ethyl−3-Methylimidazolium Tris(Pentafluoroethyl) Trifluorophosphate Based Gel Polymer Electrolyte and Modified MWCNT Electrodes. Electrochim. Acta 2013, 105, 333–341. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Gu, Y.; Zheng, L.; Ma, S.; Xu, X. Self-Healable and Stretchable Ionogels Serve as Electrolytes and Substrates for Integrated All-in-One Micro-Supercapacitors. Chem. Eng. J. 2020, 392, 123645. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Ji, T.; Bai, R.; Zhu, H. Highly Ionic Conductive, Stretchable, and Tough Ionogel for Flexible Solid-State Supercapacitor. Small 2024, 20, 2307019. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhang, Y.; Yan, C.; Fu, Y.; Guo, Y.; Ma, X. High-Performance Ionic Liquid-Based Gel Polymer Electrolyte Incorporating Anion-Trapping Boron Sites for All-Solid-State Supercapacitor Application. ACS Appl. Mater. Interfaces 2018, 10, 39570–39580. [Google Scholar] [CrossRef] [PubMed]
- Caillon-Caravanier, M.; Claude-Montigny, B.; Lemordant, D.; Bosser, G. Conductivity Study of Diacrylate-Based Gels: Part III. Influence of the Nature of the Constituents and Improvement of a Theoretical Model of Ionic Transport. Solid State Ion. 2003, 156, 113–127. [Google Scholar] [CrossRef]
- Kim, S.K.; Koo, H.J.; Lee, A.; Braun, P.V. Selective Wetting-Induced Micro-Electrode Patterning for Flexible Micro-Supercapacitors. Adv. Mater. 2014, 26, 5108–5112. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X. In Situ Synthesized PEO/NBR Composite Ionogels for High-Performance All-Solid-State Supercapacitors. Chem. Commun. 2019, 55, 8470–8473. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(Ionic Liquid)s: An Update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Biswas, Y.; Banerjee, P.; Mandal, T.K. From Polymerizable Ionic Liquids to Poly(Ionic Liquid)s: Structure-Dependent Thermal, Crystalline, Conductivity, and Solution Thermoresponsive Behaviors. Macromolecules 2019, 52, 945–958. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.O.; Vygodskii, Y.S. Poly(Ionic Liquid)s: Synthesis, Properties, and Application. Polym. Sci. Ser. B 2016, 58, 73–142. [Google Scholar] [CrossRef]
- Ye, Y.; Elabd, Y.A. Anion Exchanged Polymerized Ionic Liquids: High Free Volume Single Ion Conductors. Polymers 2011, 52, 1309–1317. [Google Scholar] [CrossRef]
- Mao, T.; Wang, S.; Yong, Z.; Wang, X.; Wang, X.; Chen, H.; Liu, G.; Wang, D.; Wang, Z. High-Stable, Outstanding Heat Resistance Ionogel Electrolyte and the Poly(3,4-Ethylenedioxythiophene) Electrodes with Excellent Long-Term Stability for All-Solid-State Supercapacitor. Chem. Eng. J. 2021, 417, 129269. [Google Scholar] [CrossRef]
- Ma, G.; Pan, F.; Zhou, X.; Yong, Z.; Wang, X.; Li, C.; Bai, W.; Wang, S. A Flexible Supercapacitor Based on Deep Eutectic Solvent/[EMIM][TFSI] Ionogel with High Energy Density and Wide Temperature Range. ACS Appl. Electron. Mater. 2024, 6, 1434–1443. [Google Scholar] [CrossRef]
- Dong, K.; Liu, Y.; Chen, Z.; Lv, T.; Tang, W.; Cao, S.; Chen, T. A Novel Bilayer Heterogeneous Poly(Ionic Liquid) Electrolyte for High-Performance Flexible Supercapacitors with Ultraslow Self-Discharge. Mater. Horiz. 2023, 10, 2618–2626. [Google Scholar] [CrossRef]
- Taghavikish, M.; Subianto, S.; Gu, Y.; Sun, X.; Zhao, X.S.; Choudhury, N.R. A Poly(Ionic Liquid) Gel Electrolyte for Efficient All Solid Electrochemical Double-Layer Capacitor. Sci. Rep. 2018, 8, 10918. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of Hydrogels: Linking the Nano to the Microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zong, S.; Li, T.; Zhao, Q.; Xu, Z.; Duan, J. Room Temperature Ca2+-Initiated Free Radical Polymerization for the Preparation of Conductive, Adhesive, Anti-Freezing and UV-Blocking Hydrogels for Monitoring Human Movement. ACS Omega 2023, 8, 9434–9444. [Google Scholar] [CrossRef]
- Seidi, F.; Zhao, W.; Xiao, H.; Jin, Y.; Saeb, M.R.; Zhao, C. Radical Polymerization as a Versatile Tool for Surface Grafting of Thin Hydrogel Films. Polym. Chem. 2020, 11, 4355–4381. [Google Scholar] [CrossRef]
- Mohammed, J.S.; Murphy, W.L. Bioinspired Design of Dynamic Materials. Adv. Mater. 2009, 21, 2361–2374. [Google Scholar] [CrossRef]
- Fu, D.; Lu, Y.; Peng, Z.; Zhong, W. A Zwitterionic Hydrogel with a Surprising Function of Increasing the Ionic Conductivity of Alkali Metal Chloride or Sulfuric Acid Water-Soluble Electrolyte. J. Mater. Chem. A 2023, 11, 13543–13551. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Hou, P.; Gao, Z.; Liu, Y.; Zhao, J.; Huo, P. Preparation of Dual Cross-Linked Hydrogel Electrolytes Containing Modified Lignin for Supercapacitors and Sensors. Chem. Eng. J. 2024, 480, 148259. [Google Scholar] [CrossRef]
- Li, H.; Lv, T.; Sun, H.; Qian, G.; Li, N.; Yao, Y.; Chen, T. Ultrastretchable and Superior Healable Supercapacitors Based on a Double Cross-Linked Hydrogel Electrolyte. Nat. Commun. 2019, 10, 536. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, N.; Liu, F.; Na, R.; Wang, G.; Guan, S.; Liu, F. Highly Strong and Tough Double-Crosslinked Hydrogel Electrolyte for Flexible Supercapacitors. ChemElectroChem 2020, 7, 1007–1015. [Google Scholar] [CrossRef]
- Guo, L.; Ma, W.B.; Wang, Y.; Song, X.Z.; Ma, J.; Han, X.D.; Tao, X.Y.; Guo, L.T.; Fan, H.L.; Liu, Z.S.; et al. A Chemically Crosslinked Hydrogel Electrolyte Based All-in-One Flexible Supercapacitor with Superior Performance. J. Alloys Compd. 2020, 843, 155895. [Google Scholar] [CrossRef]
- Wu, S.; Tang, L.; Xu, Y.; Yao, J.; Tang, G.; Dai, B.; Wang, W.; Tang, J.; Gong, L. A Self-Powered Flexible Sensing System Based on a Super-Tough, High Ionic Conductivity Supercapacitor and a Rapid Self-Recovering Fully Physically Crosslinked Double Network Hydrogel. J. Mater. Chem. C 2022, 10, 3027–3035. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Dhand, A.P.; Galarraga, J.H.; Burdick, J.A. Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol. 2021, 39, 519–538. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly Stretchable and Tough Hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Lin, T.; Shi, M.; Huang, F.; Peng, J.; Bai, Q.; Li, J.; Zhai, M. One-Pot Synthesis of a Double-Network Hydrogel Electrolyte with Extraordinarily Excellent Mechanical Properties for a Highly Compressible and Bendable Flexible Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 29684–29693. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, D.; Liu, Y.; Shen, L.; Zhu, T.; Xu, X.; Zheng, J.; Gong, X. Solid-State Double-Network Hydrogel Redox Electrolytes for High-Performance Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 34168–34177. [Google Scholar] [CrossRef]
- Xu, Y.; Rong, Q.; Zhao, T.; Liu, M. Anti-Freezing Multiphase Gel Materials: Bioinspired Design Strategies and Applications. Giant 2020, 2, 100014. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2018, 28, 1704195. [Google Scholar] [CrossRef]
- Bai, G.; Gao, D.; Liu, Z.; Zhou, X.; Wang, J. Probing the Critical Nucleus Size for Ice Formation with Graphene Oxide Nanosheets. Nature 2019, 576, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, L.; Wang, S.; Zhang, L.; Chen, L.; Xu, X.; Song, Z.; Liu, H.; Chen, C. Strong, Tough, Ionic Conductive, and Freezing-Tolerant All-Natural Hydrogel Enabled by Cellulose-Bentonite Coordination Interactions. Nat. Commun. 2022, 13, 3408. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Kim, H.J. Ultra-Stretchable Dual-Network Ionic Hydrogel Strain Sensor with Moistening and Anti-Freezing Ability. Prog. Org. Coat. 2022, 166, 106784. [Google Scholar] [CrossRef]
- Morelle, X.P.; Illeperuma, W.R.; Tian, K.; Bai, R.; Suo, Z.; Vlassak, J.J. Highly Stretchable and Tough Hydrogels below Water Freezing Temperature. Adv. Mater. 2018, 30, 1801541. [Google Scholar] [CrossRef]
- Wu, S.; Hua, M.; Alsaid, Y.; Du, Y.; Ma, Y.; Zhao, Y.; Lo, C.Y.; Wang, C.; Wu, D.; Yao, B.; et al. Poly(Vinyl Alcohol) Hydrogels with Broad-Range Tunable Mechanical Properties via the Hofmeister Effect. Adv. Mater. 2021, 33, 2007829. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhou, Y.; Shu, H.; Yu, C.; Zhong, W. Ultrahigh-Ionic-Conductivity, Antifreezing Poly(Amidoxime)-Grafted Polyzwitterion Hydrogel for Facile Integrated into High-Performance Stretchable Flexible Supercapacitor. ACS Omega 2024, 9, 2234–2249. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Huang, J.; Liu, M. Low Temperature Tolerant Organohydrogel Electrolytes for Flexible Solid-State Supercapacitors. Adv. Energy Mater. 2018, 8, 1801967. [Google Scholar] [CrossRef]
- Feng, E.; Li, J.; Zheng, G.; Yan, Z.; Li, X.; Gao, W.; Ma, X.; Yang, Z. Long-Term Anti-Freezing Active Organohydrogel Based Superior Flexible Supercapacitor and Strain Sensor. ACS Sustain. Chem. Eng. 2021, 9, 7267–7276. [Google Scholar] [CrossRef]
- Zheng, H.; Guan, R.; Liu, Q.; Ou, K.T.; Li, D.S.; Fang, J.; Fu, Q.; Sun, Y. A Flexible Supercapacitor with High Capacitance Retention at an Ultra-Low Temperature of −65.0 °C. Electrochim. Acta 2022, 424, 140644. [Google Scholar] [CrossRef]
- Lu, N.; Na, R.; Li, L.; Zhang, C.; Chen, Z.; Zhang, S.; Luan, J.; Wang, G. Rational Design of Antifreezing Organohydrogel Electrolytes for Flexible Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 1944–1951. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Q.; Shi, D.; Yang, Z.; Dong, K.; Kaneko, D.; Dong, W.; Chen, M. Tough and Antifreezing Organohydrogel Electrolyte for Flexible Supercapacitors with Wide Temperature Stability. ACS Appl. Energy Mater. 2021, 4, 9353–9361. [Google Scholar] [CrossRef]
- Lu, X.; Jiménez-Riobóo, R.J.; Leech, D.; Gutiérrez, M.C.; Ferrer, M.L.; Del Monte, F. Aqueous-Eutectic-in-Salt Electrolytes for High-Energy-Density Supercapacitors with an Operational Temperature Window of 100 °c, from−35 to +65 °c. ACS Appl. Mater. Interfaces 2020, 12, 29181–29193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.; Liu, Y.; Peng, Y.; Tang, Y.; Xiong, L.; Gong, X.; Zheng, J. A General Crosslinker Strategy to Realize Intrinsic Frozen Resistance of Hydrogels. Adv. Mater. 2021, 33, 2104006. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Tang, Y.; Gong, X.; Zheng, J. Development of a Radical Polymerization Algorithm for Molecular Dynamics Simulations of Antifreezing Hydrogels with Double-Network Structures. npj Comput. Mater. 2023, 9, 209. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, Z.; Xue, Y.; Zhao, J.; Ji, J.; Wang, C.; Wu, Y. Ionic Liquid Cross-Linked Poly(N-Isopropylacrylamide) Hydrogel Electrolytes for Self-Protective Flexible Separator-Free Supercapacitors. Ind. Eng. Chem. Res. 2023, 62, 2741–2751. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-Isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Elashnikov, R.; Trelin, A.; Tulupova, A.; Miliutina, E.; Zahorjanová, K.; Ulbrich, P.; Tomeček, D.; Fitl, P.; Švorčík, V.; Lyutakov, O. Switchable PNIPAm/PPyNT Hydrogel for Smart Supercapacitors: External Control of Capacitance for Pulsed Energy Generation or Prolongation of Discharge Time. ACS Appl. Mater. Interfaces 2021, 13, 48030–48039. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Chi, X.; Zhang, S.; Liu, Y.; Zhou, B.; Yang, J.; Liu, Y. Durable, Flexible Self-Standing Hydrogel Electrolytes Enabling High-Safety Rechargeable Solid-State Zinc Metal Batteries. J. Mater. Chem. A 2018, 6, 23046–23054. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Wang, J.; Hu, M.; Feng, Y.; Wang, P.; Wang, Y.; Nie, N.; Zhang, J.; Chen, H.; et al. Concentrated Hydrogel Electrolyte-Enabled Aqueous Rechargeable NiCo//Zn Battery Working from −20 to 50 °C. ACS Appl. Mater. Interfaces 2019, 11, 49–55. [Google Scholar] [CrossRef]
- Liu, J.; Hu, M.; Wang, J.; Nie, N.; Wang, Y.; Wang, Y.; Zhang, J.; Huang, Y. An Intrinsically 400% Stretchable and 50% Compressible NiCo//Zn Battery. Nano Energy 2019, 58, 338–346. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Pei, Z.; Liu, Z.; Li, H.; Zhu, M.; Fan, J.; Dai, Q.; Zhang, M.; Dai, L.; et al. Solid-State Rechargeable Zn//NiCo and Zn–Air Batteries with Ultralong Lifetime and High Capacity: The Role of a Sodium Polyacrylate Hydrogel Electrolyte. Adv. Energy Mater. 2018, 8, 1802288. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y. A Flexible Zinc-Ion Battery Based on the Optimized Concentrated Hydrogel Electrolyte for Enhanced Performance at Subzero Temperature. Electrochim. Acta 2021, 395, 139178. [Google Scholar] [CrossRef]
- Wang, Z.; Mo, F.; Ma, L.; Yang, Q.; Liang, G.; Liu, Z.; Li, H.; Li, N.; Zhang, H.; Zhi, C. Highly Compressible Cross-Linked Polyacrylamide Hydrogel-Enabled Compressible Zn-MnO 2 Battery and a Flexible Battery-Sensor System. ACS Appl. Mater. Interfaces 2018, 10, 44527–44534. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Tang, Z.; Liang, G.; Yang, Q.; Li, H.; Ma, L.; Mo, F.; Zhi, C. A Mechanically Durable and Device-Level Tough Zn-MnO2 Battery with High Flexibility. Energy Storage Mater. 2019, 23, 636–645. [Google Scholar] [CrossRef]
- Huang, J.; Chi, X.; Yang, J.; Liu, Y. An Ultrastable Na-Zn Solid-State Hybrid Battery Enabled by a Robust Dual-Cross-Linked Polymer Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 17583–17591. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.J.; Weiss, R.G. Organogels and Low Molecular Mass Organic Gelators. Adv. Mater. 2000, 12, 1237–1247. [Google Scholar] [CrossRef]
- Fasciani, C.; Panero, S.; Hassoun, J.; Scrosati, B. Novel Configuration of Poly(Vinylidenedifluoride)-Based Gel Polymer Electrolyte for Application in Lithium-Ion Batteries. J. Power Sources 2015, 294, 180–186. [Google Scholar] [CrossRef]
- Ma, X.; Huang, X.; Gao, J.; Zhang, S.; Deng, Z.; Suo, J. Compliant Gel Polymer Electrolyte Based on Poly(Methyl Acrylate-Co-Acrylonitrile)/Poly(Vinyl Alcohol) for Flexible Lithium-Ion Batteries. Electrochim. Acta 2014, 115, 216–222. [Google Scholar] [CrossRef]
- Choudhury, S.; Saha, T.; Naskar, K.; Stamm, M.; Heinrich, G.; Das, A. A Highly Stretchable Gel-Polymer Electrolyte for Lithium-Sulfur Batteries. Polymers 2017, 112, 447–456. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Wang, W.; Li, W.; Lou, J. Carboxymethylated Nanocellulose-Based Gel Polymer Electrolyte with a High Lithium Ion Transfer Number for Flexible Lithium-Ion Batteries Application. Chem. Eng. J. 2022, 428, 1385–8947. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, H.; Zhao, L.; Sun, Z.; Li, Y.; Mo, Y.; Chen, Y. A Flexible Cellulose/Methylcellulose Gel Polymer Electrolyte Endowing Superior Li+ Conducting Property for Lithium Ion Battery. Carbohydr. Polym. 2020, 246, 116622. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, N.; Mishra, K.; Shahid, R.; Arif, T.; Kanchan, D.K. Sodium Ion Conducting Flame-Retardant Gel Polymer Electrolyte for Sodium Batteries and Electric Double Layer Capacitors (EDLCs). J. Energy Storage 2022, 46, 103899. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, H.; Liu, J.; Qian, T.; Yan, C. A New High Ionic Conductive Gel Polymer Electrolyte Enables Highly Stable Quasi-Solid-State Lithium Sulfur Battery. Energy Storage Mater. 2019, 22, 256–264. [Google Scholar] [CrossRef]
- Kil, E.H.; Ha, H.J.; Lee, S.Y. A Facile Approach to Fabricate Self-Standing Gel-Polymer Electrolytes for Flexible Lithium-Ion Batteries by Exploitation of UV-Cured Trivalent/Monovalent Acrylate Polymer Matrices. Macromol. Chem. Phys. 2011, 212, 2217–2223. [Google Scholar] [CrossRef]
- George, S.M.; Sampath, S.; Bhattacharyya, A.J. A Self-Standing Flexible Gel Polymer Electrolyte for Dendrite-Free Lithium-Metal Batteries. Batter. Supercaps 2022, 5, e202200252. [Google Scholar] [CrossRef]
- Gao, X.; Yuan, W.; Yang, Y.; Wu, Y.; Wang, C.; Wu, X.; Zhang, X.; Yuan, Y.; Tang, Y.; Chen, Y.; et al. High-Performance and Highly Safe Solvate Ionic Liquid-Based Gel Polymer Electrolyte by Rapid UV-Curing for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 43397–43406. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, J.; Guo, Y.; Zhu, J.; Qu, X.; Niu, W.; Liu, X. Fire-Resistant, High-Performance Gel Polymer Electrolytes Derived from Poly (Ionic Liquid)/P(VDF-HFP) Composite Membranes for Lithium Ion Batteries. J. Memb. Sci. 2020, 599, 117827. [Google Scholar] [CrossRef]
- Long, M.C.; Wu, G.; Wang, X.L.; Wang, Y.Z. Self-Adaptable Gel Polymer Electrolytes Enable High-Performance and All-Round Safety Lithium Ion Batteries. Energy Storage Mater. 2022, 53, 62–71. [Google Scholar] [CrossRef]
- Mu, X.; Li, X.; Liao, C.; Yu, H.; Jin, Y.; Yu, B.; Han, L.; Chen, L.K.; Kan, Y.; Song, L.; et al. Phosphorus-Fixed Stable Interfacial Nonflammable Gel Polymer Electrolyte for Safe Flexible Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2203006. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, X.; Yang, Q.; Li, C.; Liu, G.; Wang, Y.; Sun, P.; Tian, H.; Wang, C.; Chen, X.; et al. High Performance Solid-State Supercapacitors Based on Highly Conductive Organogel Electrolyte at Low Temperature. J. Power Sources 2022, 524, 231102. [Google Scholar] [CrossRef]
- Yang, C.; Sun, M.; Wang, X.; Wang, G. A Novel Flexible Supercapacitor Based on Cross-Linked PVDF-HFP Porous Organogel Electrolyte and Carbon Nanotube Paper-Conjugated Polymer Film Electrodes. ACS Sustain. Chem. Eng. 2015, 3, 2067–2076. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Tang, J.; Ren, T.; Wei, J.; Liang, Y.; Feng, E. A Supercapacitor with Large Capacitance and Pressure Resistance Based on Multifunctional Organogel. J. Mater. Sci. Mater. Electron. 2024, 35, 435. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium Metal Anodes for Rechargeable Batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Li, Y.; Yu, H.; Fei, B.; Xin, J.H. Single-Ion Conducting Double-Network Hydrogel Electrolytes for Long Cycling Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 30594–30602. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Lopez, G.; Iojoiu, C.; Bouchet, R.; Ameduri, B. Novel Single-Ion Conducting Electrolytes Based on Vinylidene Fluoride Copolymer for Lithium Metal Batteries. J. Power Sources 2021, 498, 229920. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Liang, X.; Yu, M.; Liu, B.; Sun, Z.; Hu, W.; Zhu, G. A Single-Ion Gel Polymer Electrolyte Based on Polyimide Grafted with Lithium 3-Chloropropanesulfonyl (Trifluoromethanesulfonyl) Imide for High Performance Lithium Ion Batteries. J. Mater. Chem. A 2022, 11, 1766–1773. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, G.; Liu, X.; Pan, Q.; Zhang, Y.; Zeng, D.; Sun, Y.; Ke, H.; Cheng, H. A Gel Single Ion Conducting Polymer Electrolyte Enables Durable and Safe Lithium Ion Batteries via Graft Polymerization. RSC Adv. 2018, 8, 39967–39975. [Google Scholar] [CrossRef]
- Shen, X.; Hua, H.; Li, H.; Li, R.; Hu, T.; Wu, D.; Zhang, P.; Zhao, J. Synthesis and Molecular Dynamic Simulation of a Novel Single Ion Conducting Gel Polymer Electrolyte for Lithium-Ion Batteries. Polymer 2020, 201, 122568. [Google Scholar] [CrossRef]
- Guan, X.; Wu, Q.; Zhang, X.; Guo, X.; Li, C.; Xu, J. In-Situ Crosslinked Single Ion Gel Polymer Electrolyte with Superior Performances for Lithium Metal Batteries. Chem. Eng. J. 2020, 382M, 122935. [Google Scholar] [CrossRef]
- Zhang, P.; Li, R.; Huang, J.; Liu, B.; Zhou, M.; Wen, B.; Xia, Y.; Okada, S. Flexible Poly(Vinylidene Fluoride-Co-Hexafluoropropylene)-Based Gel Polymer Electrolyte for High-Performance Lithium-Ion Batteries. RSC Adv. 2021, 11, 11943–11951. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Zhang, Z.; Huang, J.; Yang, L.; Hirano, S.I. High-Performance Polymeric Ionic Liquid-Silica Hybrid Ionogel Electrolytes for Lithium Metal Batteries. J. Mater. Chem. A 2016, 4, 13822–13829. [Google Scholar] [CrossRef]
- Candhadai Murali, S.P.; Samuel, A.S. Zinc Ion Conducting Blended Polymer Electrolytes Based on Room Temperature Ionic Liquid and Ceramic Filler. J. Appl. Polym. Sci. 2019, 136, 47654. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, M.; Xie, B.; Wang, X.; Jiang, M.; Guan, P.; Han, P.; Cui, G. A Rigid-Flexible Coupling Gel Polymer Electrolyte towards High Safety Flexible Li-Ion Battery. J. Power Sources 2021, 499, 229944. [Google Scholar] [CrossRef]
- Chen, X.; Liang, L.; Hu, W.; Liao, H.; Zhang, Y. POSS Hybrid Poly(Ionic Liquid) Ionogel Solid Electrolyte for Flexible Lithium Batteries. J. Power Sources 2022, 542, 231766. [Google Scholar] [CrossRef]
- Kuo, T.R.; Lin, L.Y.; Lin, K.Y.; Yougbaré, S. Effects of Size and Phase of TiO2 in Poly (Vinyl Alcohol)-Based Gel Electrolyte on Energy Storage Ability of Flexible Capacitive Supercapacitors. J. Energy Storage 2022, 52, 104773. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, P.; Yao, H.; Hu, L.; Fan, H.J. Inert Filler Selection Strategies in Li-Ion Gel Polymer Electrolytes. ACS Appl. Mater. Interfaces 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, L.; Qing, Y.; Zhang, Z.; Yan, N.; Wu, Y.; Tian, C. Stretchable Alkaline Poly(Acrylic Acid) Electrolyte with High Ionic Conductivity Enhanced by Cellulose Nanofibrils. Electrochim. Acta 2018, 270, 302–309. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Liu, Z.; Tang, Z.; Liang, G.; Mo, F.; Yang, Q.; Ma, L.; Zhi, C. A Nanofibrillated Cellulose/Polyacrylamide Electrolyte-Based Flexible and Sewable High-Performance Zn–MnO2 Battery with Superior Shear Resistance. Small 2018, 14, 1803978. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, J.; Du, Z.; Jia, X.; Qu, Y.; Yu, F.; Du, J.; Chen, Y. High-Strength and Flexible Cellulose/PEG Based Gel Polymer Electrolyte with High Performance for Lithium Ion Batteries. J. Memb. Sci. 2020, 593, 117428. [Google Scholar] [CrossRef]
- Lee, H.; Erwin, A.; Buxton, M.L.; Kim, M.; Stryutsky, A.V.; Shevchenko, V.V.; Sokolov, A.P.; Tsukruk, V.V. Shape Persistent, Highly Conductive Ionogels from Ionic Liquids Reinforced with Cellulose Nanocrystal Network. Adv. Funct. Mater. 2021, 31, 2103083. [Google Scholar] [CrossRef]
- Flouda, P.; Bukharina, D.; Pierce, K.J.; Stryutsky, A.V.; Shevchenko, V.V.; Tsukruk, V.V. Flexible Sustained Ionogels with Ionic Hyperbranched Polymers for Enhanced Ion-Conduction and Energy Storage. ACS Appl. Mater. Interfaces 2022, 14, 27028–27039. [Google Scholar] [CrossRef] [PubMed]
- Porthault, H.; Calberg, C.; Amiran, J.; Martin, S.; Páez, C.; Job, N.; Heinrichs, B.; Liquet, D.; Salot, R. Development of a Thin Flexible Li Battery Design with a New Gel Polymer Electrolyte Operating at Room Temperature. J. Power Sources 2021, 482, 4480. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, W.; Sun, W.; Zhao, L.; Yuan, W. Covalent Organic Frameworks-Enhanced Ionic Conductivity of Polymeric Ionic Liquid-Based Ionic Gel Electrolyte for Lithium Metal Battery. ACS Appl. Energy Mater. 2021, 4, 2808–2819. [Google Scholar] [CrossRef]
- Shen, W.; Li, K.; Lv, Y.; Xu, T.; Wei, D.; Liu, Z. Highly-Safe and Ultra-Stable All-Flexible Gel Polymer Lithium Ion Batteries Aiming for Scalable Applications. Adv. Energy Mater. 2020, 10, 1904281. [Google Scholar] [CrossRef]
- Li, K.; Shen, W.; Xu, T.; Yang, L.; Xu, X.; Yang, F.; Zhang, L.; Wang, Y.; Zhou, Y.; Zhong, M.; et al. Fibrous Gel Polymer Electrolyte for an Ultrastable and Highly Safe Flexible Lithium-Ion Battery in a Wide Temperature Range. Carbon Energy 2021, 3, 916–928. [Google Scholar] [CrossRef]
- Zhao, T.; Gai, Q.; Deng, X.; Ma, J.; Gao, H. A New Type of LATP Doped PVDF-HFP Based Electrolyte Membrane with Flame Retardancy and Long Cycle Stability for Solid State Batteries. J. Energy Storage 2023, 73, 108576. [Google Scholar] [CrossRef]
- Wei, D.; Shen, W.; Xu, T.; Li, K.; Yang, L.; Zhou, Y.; Zhong, M.; Yang, F.; Xu, X.; Wang, Y.; et al. Ultra-Flexible and Foldable Gel Polymer Lithium–Ion Batteries Enabling Scalable Production. Mater. Today Energy 2022, 23, 100889. [Google Scholar] [CrossRef]
- Bae, J.; Li, Y.; Zhang, J.; Zhou, X.; Zhao, F.; Shi, Y.; Goodenough, J.B.; Yu, G. A 3D Nanostructured Hydrogel-Framework-Derived High-Performance Composite Polymer Lithium-Ion Electrolyte. Angew. Chem. 2018, 130, 2118–2122. [Google Scholar] [CrossRef]
- Bae, J.; Li, Y.; Zhao, F.; Zhou, X.; Ding, Y.; Yu, G. Designing 3D Nanostructured Garnet Frameworks for Enhancing Ionic Conductivity and Flexibility in Composite Polymer Electrolytes for Lithium Batteries. Energy Storage Mater. 2018, 15, 46–52. [Google Scholar] [CrossRef]
- Jamalpour, S.; Ghahramani, M.; Ghaffarian, S.R.; Javanbakht, M. The Effect of Poly(Hydroxyl Ethyl Methacrylate) on the Performance of PVDF/P(MMA-Co-HEMA) Hybrid Gel Polymer Electrolytes for Lithium Ion Battery Application. Polymers 2020, 195, 122427. [Google Scholar] [CrossRef]
- Ahn, J.H.; You, T.S.; Lee, S.M.; Esken, D.; Dehe, D.; Huang, Y.C.; Kim, D.W. Hybrid Separator Containing Reactive, Nanostructured Alumina Promoting in-Situ Gel Electrolyte Formation for Lithium-Ion Batteries with Good Cycling Stability and Enhanced Safety. J. Power Sources 2020, 472, 228519. [Google Scholar] [CrossRef]
- Ma, W.; Wu, H.; Cai, Y.; Yu, Z.; Wang, Y.; Zhang, J.H.; Zhang, Q.; Jia, X. A Flexible Single-Ion Gel Electrolyte with a Multiscale Channel for the High-Performance Lithium Metal Batteries. ACS Mater. Lett. 2022, 4, 944–952. [Google Scholar] [CrossRef]
- Zou, X.; Lu, Q.; Zhong, Y.; Liao, K.; Zhou, W.; Shao, Z. Flexible, Flame-Resistant, and Dendrite-Impermeable Gel-Polymer Electrolyte for Li–O2/Air Batteries Workable Under Hurdle Conditions. Small 2018, 14, 1801798. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Guo, J.; Lai, F.; Zhao, F.; Jiao, Y.; Brett, D.J.L.; Liu, T.; He, G.; Parkin, I.P. Insights on Flexible Zinc-Ion Batteries from Lab Research to Commercialization. Adv. Mater. 2021, 33, 2007548. [Google Scholar] [CrossRef]
- Jie, L.; Ningyuan, N.; Hua, W.; Zhe, C.; Zhenyuan, J.; Xinfeng, D.; Yan, H. A Zinc Ion Yarn Battery with High Capacity and Fire Retardancy Based on a SiO2 Nanoparticle Doped Ionogel Electrolyte. Soft Matter 2020, 16, 7432. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Chen, L.; Yin, S.; Xie, Y.; Li, A.; Liang, X.; Luo, Y.; Wu, F.; Mei, Y.; et al. 3D Hierarchical Fireproof Gel Polymer Electrolyte towards High-Performance and Comprehensive Safety Lithium-Ion Batteries. Chem. Eng. J. 2023, 476, 1385–8947. [Google Scholar] [CrossRef]
- Du, Y.; Xie, Y.; Liu, X.; Jiang, H.; Wu, F.; Wu, H.; Mei, Y.; Xie, D. In-Situ Formed Phosphorus Modified Gel Polymer Electrolyte with Good Flame Retardancy and Cycling Stability for Rechargeable Lithium Batteries. ACS Sustain. Chem. Eng. 2023, 11, 4498–4508. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Huang, Y.; Zhu, M.; Pei, Z.; Wang, Z.; Xue, Q.; Xie, X.; Zhi, C. A Self-Healable and Highly Stretchable Supercapacitor Based on a Dual Crosslinked Polyelectrolyte. Nat. Commun. 2015, 6, 10310. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, B.; Brandon, N.; Wang, Q. Tough Ionogel-in-Mask Hybrid Gel Electrolytes in Supercapacitors with Durable Pressure and Thermal Tolerances. Energy Technol. 2017, 5, 220–224. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Sang, M.; Shu, Q.; Zhang, J.; Xuan, S.; Gong, X. A Safeguarding and High Temperature Tolerant Organogel Electrolyte for Flexible Solid-State Supercapacitors. J. Power Sources 2021, 505, 230083. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, J. Preparation of High Strain Porous Polyvinyl Alcohol/Polyaniline Composite and Its Applications in All-Solid-State Supercapacitor. J. Power Sources 2017, 364, 200–207. [Google Scholar] [CrossRef]
- Peng, Y.; Yuan, W.; Liu, X.; Xie, P.; Yang, F.; Zhao, H.; Lu, D.; Yin, Y.; Wu, Z. All-in-One Integration of Polyaniline-Polyvinyl Alcohol Electrode/Electrolyte Interface for Tailorable Solid-State Supercapacitors. J. Energy Storage 2023, 61, 106701. [Google Scholar] [CrossRef]
- Han, L.; Li, Y.; Chen, C.; Liu, L.; Lu, Z. Multifunctional Enhanced Energy Density of Flexible Wide-Temperature Supercapacitors Based on MXene/PANI Conductive Hydrogel. Chem. Eng. J. 2024, 485, 149951. [Google Scholar] [CrossRef]
- Dai, J.; Qin, H.; Dong, W.X.; Cong, H.P.; Yu, S.H. Autonomous Self-Healing of Highly Stretchable Supercapacitors at All Climates. Nano Lett. 2022, 22, 6444–6453. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Ajaj, Y.; Ghadir, G.K.; Al-Tmimi, H.M.; Alani, Z.K.; Almulla, A.A.; Hussein, M.A.; Al-Tameemi, A.R.; Mahmoud, Z.H.; Ahmed mustafa, M.; et al. Rechargeable Batteries for Energy Storage: A Review. e-Prime Adv. Electr. Eng. Electron. Energy 2024, 8, 100510. [Google Scholar] [CrossRef]



| Applications | Gel Electrolytes Composition | Gel Properties | Energy Storage Devices | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gel Polymer Network | Electrolyte | Crosslinker and Others | Mechanical Properties | Ionic Conductivity (mS cm−1) at RT | Electrode Material | Capacitance /Capacity | |||||
| Flexible | Stretchable | Self-Healable | Thermo Responsive | ||||||||
| Ionogel | |||||||||||
| BATTERIES | PEGMA/PVdF-co-HFP electrospun | 1 M LiTFSI [EMI+][BF4−] | PTA | √ | x | x | x | 1.7 | LiFePO4 Li metal | 160 mA h g−1 @0.1 C | [23] |
| PIL-UPy copolymer | [DEIM+][TFSI−]/ LiTFSI | √ | √ | √ | x | 1.57 | LiFePO4 Li metal | 147.5 mA h g−1 @0.2 C | [33] | ||
| PEG-IP−2SS | [EMI+][TFSI−] | 4-arm PEG HPDS/IPDI | √ | √ | √ | x | 1.75 | 3DMC | 42.6 mF cm−2 @0.4 mA cm−2 | [39] | |
| PEO | LiTFSI (20 wt.%) [EMI+][TFSI−] (12.5 wt.%) | √ | x | x | x | 2.08 | LiMnO2 Li metal | 120 mA h g−1 @0.1 C | [70] | ||
| PVdF-HFP | LiClO4 (10 wt.%) [EMI+][DCA−] (60 wt.%) | √ | x | x | x | 0.6 | LiCoO2 Li4Ti5O12 | 300 µAh cm−2 @0.25 C | [71] | ||
| PVdF-HFP | LiTFSI (30 wt.%) [PMPyrr+][TFSI−] | √ | x | x | x | 0.69 | LiFePO4 Li metal | 151 mA h g−1 @0.1 C | [72] | ||
| PVdF-HFP | 0.4 LiBOB [MePrPyr+][NTf2−] | TMS | √ | √ | x | x | 1.77 | LiFePO4 Li metal | 135.15 mA h g−1 @0.1 C | [73] | |
| PVdF | 1 M LiPF6 [EMI+][Tf−] EC/PC | √ | x | x | x | 2.34 | LiMnO2 Graphite | 91.8 mA h g−1 @125 mA cm−2 | [76] | ||
| PVdF-HFP PEO | LiTFSI [Pyr13+] [TFSI−] | CA | √ | √ | x | √ | 1.06 | LiFePO4 Li metal | 162.9 mA h g−1 @0.2 C | [77] | |
| Poly(MMA-b-MUBI-TFSI) | 100 mM LiTFSI/ [EMI+][TFSI−] | √ | x | x | x | 10 | LiCoO2 Li4Ti5O12 | 112 mA h g−1 @0.1 C | [79] | ||
| PVdF/3P(MPBI-TFSI) | LiTFSI [EMI+][TFSI−] | √ | √ | x | √ | ~0.78 | LiFePO4 Li metal | 151 mA h g−1 @0.1 C | [82] | ||
| PVdF/PMHBI-TFSI electrospun | 0.5 M LiTFSI [EMI+][TFSI−] | PEGDA | √ | √ | x | x | 1.3 | LiFePO4 Li metal | 152 mA h g−1 @0.1 C @40 °C | [83] | |
| P(DADMA)TFSI | LiTFSI [PYR1,2O1+][TFSI−] | TEOS (precursor of SiO2 NPs) | √ | √ | x | x | 0.58 | LiFePO4 Li metal | 150 mA h g−1 @0.5C | [171] | |
| Poly ([VI-NH2+] [TFSI−])-co-PBA | [BMI+] [TFSI−] | POSS PEGDA | √ | √ | x | √ | 2.5 | LiFePO4 Li metal | 160.5 mA h g−1 @0.5C | [174] | |
| PVdF-HFP | LiTFSI/ [PYR1,3+] [FSI−] | Li-MMT clay | √ | x | x | x | 0.48 | LiCoO2 Li metal | 1 mA h cm−2 @0.4 mA cm−2 | [181] | |
| PDADMATFSI | LiTFSI/ [PYR14+] [TFSI−] | TTB-DMTP-COF | √ | x | x | x | 0.28 | LiFePO4 Li metal | 160 mA h g−1 @0.5 C | [183] | |
| SUPERCAPACITORS | Pluronic F127 | [EMI+][BF4−] LiBF4 | x | x | x | √ | Not provided | AC | 40 F g−1@ 100 °C 17.4 F g−1@ 14 °C | [45] | |
| PVA | [EMI+] [Cl−] | Boric acid | √ | √ | √ | x | 2.43 | AC electrodes | 90 F g−1 @0.1 A g−1 | [84] | |
| PEGDA | [EMI+][TFSI−] | PEGDA | √ | x | x | x | Not provided | MWCNT electrode (µSC) | 5.3 F cm−3 @10 mV s−1 | [85] | |
| PVdF-HFP | [EMI+][BF4−] | MXene (all-in-one) No MXene | √ | x | x | x | 2.2 × 106 25 | MXene (all-in-one) (µSC) | 44 F cm−2 @0.1 mA cm−2 | [86] | |
| PVA | KI EC [EMI+][BF4−] | √ | x | x | x | Not provided | AC | 160 F g−1 @5 mV s−1 | [87] | ||
| PVdF-HFP | [EMI+][FAP−] | √ | x | x | x | 2 | MWCNTs | 90 F g−1 @1 mA cm−2 | [89] | ||
| PEO/Pluronic | 1.2 M LiTFSI [EMI+][TFSI−] | √ | √ | √ | x | 4.07 | MWCNTs | 65.5 mF cm−2 @0.5 mA cm−2 (µSC) | [90] | ||
| PVA/PAA | LiCl KOH [EMI+][DCA−] | √ | √ | x | x | 3180 | PAN derived porous carbon fibers | 615 mF cm−2 @1 mA cm−2 | [91] | ||
| PEO | 1 M LiClO4 [EMI+][BF4−] | BEM (15 wt.%) | √ | x | √ | √ | 5.13 | rGO | 34.35 F g−1 @1 A g−1 (total mass of electrodes) | [92] | |
| PEO/NBR | [EMI+][TFSI−] | PEGDM | √ | √ | x | x | 2.4 | Graphene | 208 F g−1 @1 A g−1 | [95] | |
| PAAm/PVMITFSI | [EMI+][TFSI−] | MBAA | √ | √ | x | x | 13 | PEDOT | 157.8 F g−1 @0.4 A g−1 | [100] | |
| PAAm/PVMITFSI | EG/ChCl [EMI+][TFSI−] | MBAA | √ | √ | x | x | ~4.5@RT 13.3@90 °C | AC | 32 F g−1@RT 43.8 F g−1@90 °C @0.25 A g−1 (total mass of electrodes) | [101] | |
| PMT/PES bilayer | [EMI+][TFSI−] | PEGDA | √ | x | x | x | 0.18 | AC | 32.1 F g−1 @0.5 mA g−1 (total mass of electrodes) Self-discharge time of 23.2 h | [102] | |
| PHEMA | [BMI+][PF6−] | DVIMBr | √ | x | x | x | 0.3 | CNTs | 25 mF cm−2 @0.2 mA cm−2 | [103] | |
| PDMAA On mask | [BMI+] [BF4−] | MBAA TiO2 NPs | √ | √ | x | x | 10@RT | AC | 143 F g−1@RT 177 F g−1@200 °C @1 A g−1 | [199] | |
| Hydrogel | |||||||||||
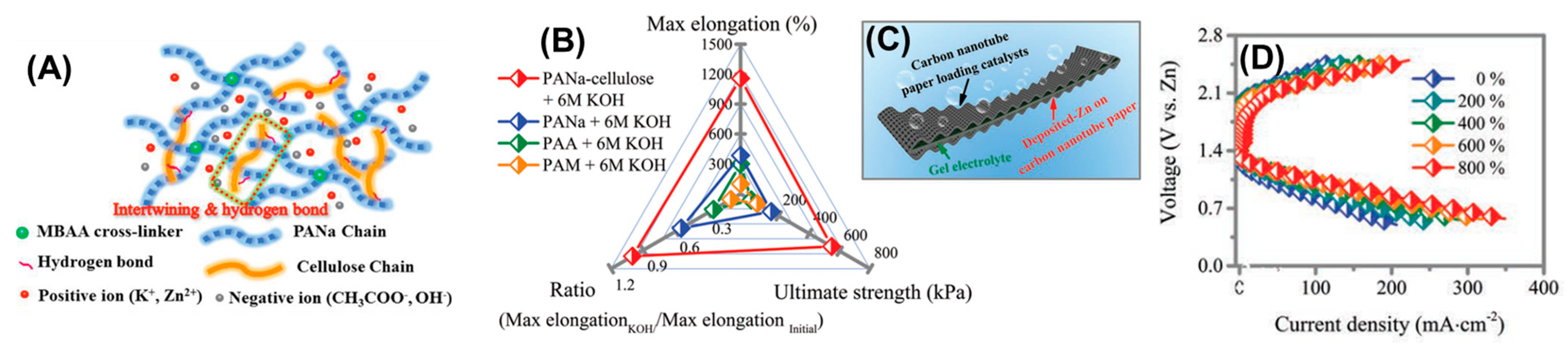
| BATTERIES | PANa-Cellulose | 6M KOH/ H2O | MBAA 0.2 M Zn2+ | √ | √ | x | x | 280 | CNTs@CC Zn metal | 800 mA h g−1 @5 mA cm−2 | [22] |
| PVA | H2O | Zn2+/Mn2+ | √ | x | √ | x | 170 | ZVO/CC Zn | 96 mA h g−1 @0.1 A g−1 | [31] | |
| PANa | H2O 6 M KOH | Fe3+/Zn2+ | √ | √ | √ | x | Not provided | NiCoO/CC Zn/CC | 247 mA h g−1 @1 C | [32] | |
| CBH | 20 mM KOH/ H2O | DVS | √ | x | x | x | 1.63 | Zn/MnO2 | 11 mA h g−1 @0.5 mA g−1 | [56] | |
| Pluronic/PNIPAM/AM-grafted copolymer | 0.5 M H3PO4/ H2O | x | x | x | √ | 7.3 (sol) 0.07 (gel) | GO/CNT/AC | 73 F g−1@RT 3.6 F g−1@80 °C (at 10 mV s−1) | [58] | ||
| P(ICZn-AAm) | H2O | MBAA 0.1 M Zn2+ | √ | √ | x | x | 2.15 | V2O5/CNT Zn metal | 322.3 mA h g−1 @0.5 C | [159] | |
| NFC/PAM | H2O | MBAA 2M Zn2+ 0.2 M Mn2+ | √ | √ | x | x | 22.8 | MnO2 Zn metal | 200 mA h g−1 @4 C | [178] | |
| PEG/ Cellulose | H2O | epichlorohydrin | √ | √ | x | x | 3.31 | NMC532 Li metal | 159.3 mA h g−1 @0.2 C | [179] | |
| SUPERCAPACITORS | Pluronic/PVA | 1 M KCl/ H2O | Borax | √ | √ | √ | x | 25.6 | MWCNTs | 801.9 mF cm−3@20 µA cm−2 | [21] |
| PVA | EG/ Glycerol H2SO4 H2O | PEDOT:PSS Graphene for electrode | √ | √ | x | x | 182 × 103 | All-in-gel | 281.2 F g−1 @0.1 A g−1 | [55] | |
| Pluronic MW 2800 Da | H2SO4/H2O | x | x | x | √ | 3.9 (sol) 0.027 (gel) | PPy@carbon paper AC@carbon paper | 110 F g−1@RT 10 F g−1@70 °C (at 10 mV s−1) | [57] | ||
| PNIPAM-co-NMAM | 0.5 M LiTFSI/ H2O | TEMED | √ | x | x | √ | Not provided | PPy@CC | 168 mF cm−2@RT 34 mF cm−2@70 °C @0.5 mA cm−2 | [60] | |
| PVA | H3PO4 H2O | √ | x | x | x | Not provided | MWCNTs (µSC) | 5.5 F cm−3 @ 10 mV s−1 (total volume of electrodes) | [94] | ||
| PAA/PAM | 1 M LiClO4 H2O | 0.1 M Fe3+ | √ | √ | x | x | 40.1 | AC | 178.5 F g−1 @ 0.25 A g−1 | [111] | |
| PVA/ PHEAA | NaCl H2O | √ | √ | x | x | 1300 | MWCNTs | 2.89 mF cm−2 @0.05 mA cm−2 | [113] | ||
| PAM/ Lignin | 6 M KOH H2O | MBAA Fe3+ | √ | √ | √ | x | 345@RT 44@−40 °C | AC | 210 F g−1@RT 176 F g−1@−40 °C @1 A g−1 | [109] | |
| poly(AMPS-co-DMAAm) | H2O | Laponite GO | √ | √ | √ | x | 5 | CNT/PANI | 180 F g−1 @10 mV s−1 | [110] | |
| PVA/PEO | H2SO4 H2O | GA | √ | √ | x | x | 67.1 | PPy-co-PANI | 773 mF cm−2 @0.2 mA cm−2 | [112] | |
| Agarose/ PAM | Li2SO4 H2O | MBAA | √ | √ | x | x | 41 | AC | 84.7 F g−1 @0.2 A g−1 | [117] | |
| Gelatin/ PHEAA | HCl H2O | Chitosan Na2MoO4 | √ | √ | x | x | 300 | AC | 84 mF cm−2 @1 mA cm−2 | [118] | |
| PAO-g-PSBMA | 7 M LiCl H2O | AGE | √ | √ | x | x | 29.8 × 103 @RT 3.4 × 103 @−30 °C | mSTi3C2Tx MXene | 519 F cm−3@RT 317 F cm−3@−30 °C @1 A g−1 | [126] | |
| PVA | LiCl EG/H2O | √ | √ | x | x | 22@RT 2.38@−40 °C | CNTs | 16 mF cm−2@RT 11 mF cm−2@−40 °C @1 mA cm−2 | [127] | ||
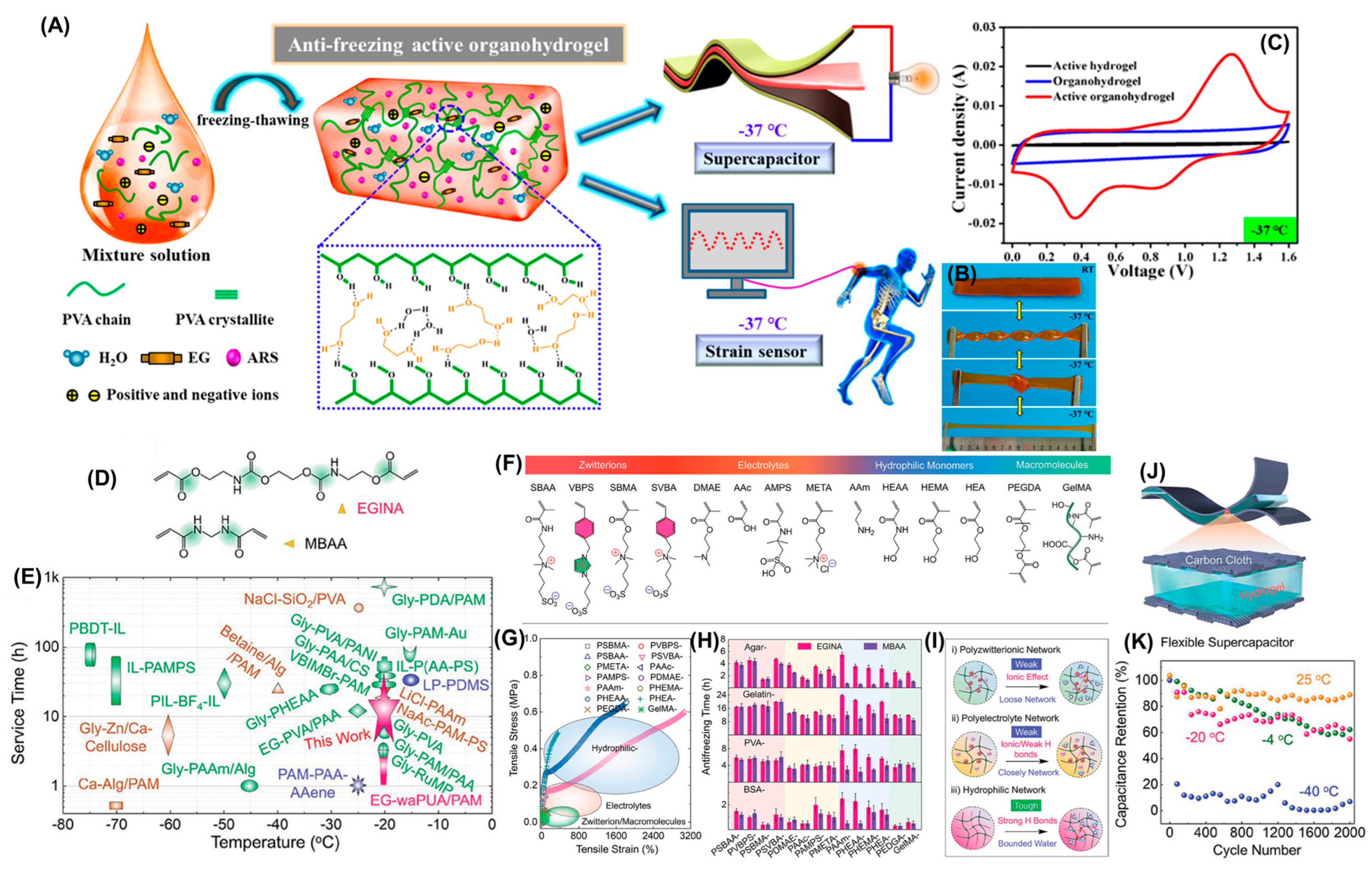
| PVA | H2SO4 EG/H2O | ARS | √ | √ | x | x | 25.2@RT 8.5@−37 °C | AC | 345 F g−1@RT 240 F g−1@−37 °C @0.5 A g−1 | [128] | |
| PVA | H2SO4 DMSO/H2O | Graphene | √ | √ | x | x | 390@RT 100@−65 °C | PANI | 261.2 F g−1@RT 225 F g−1@−65 °C @0.33 A g−1 | [129] | |
| HPC/PVA | LiClO4 Glycerol/ H2O | √ | √ | x | x | 27.3@RT 5.7@−40 °C | AC | 194.7 F g−1@RT 143.6 F g−1@−40 °C @2 A g−1 | [130] | ||
| PVA/ Alginate | Glycerol/ H2O | CaCl2 | √ | √ | x | x | 169@RT 135@−20 °C | PANI | 141 mF cm−2@RT 129 mF cm−2 @−20 °C @1 mA cm−2 | [131] | |
| PVA/ PHEAA | LiCl H2O | EGINA | √ | √ | x | x | ~500@RT ~150@−40 °C | CC | 20 mF cm−2@RT 8.1 mF cm−2 @−40 °C @5 mA cm−2 | [133] | |
| Poly NIPAM/ PAM | LiOTf H2O | Bis[C6VI+][Br−] | √ | √ | x | √ | 1.75@RT 0.1@60 °C | MnO2 | 100 F g−1@RT 0.03 F g−1@60 °C @5 mV s−1 | [135] | |
| Poly NIPAM | H2O | MBAA | √ | √ | x | √ | Not provided | PPy NTs All-in-one And rGO/CNT | 360 F g−1@RT 35 F g−1@40 °C @100 mV s−1 | [137] | |
| PVA | H3PO4 H2O | TiO2 | √ | x | x | x | 64.9 | Graphene | 69.2 F g−1 @20 mV s−1 | [175] | |
| PAA | H3PO4 H2O | Vinyl-SiO2 NPs | √ | √ | √ | x | 8 | PPy@CNTs | 220 F g−1 @5 mV s−1 | [198] | |
| PVA | H2SO4 H2O | PANI | √ | √ | x | x | Not provided R = 2.2 Ω | PVA/PANI All-in-one | 300 mF cm−2 @2 mV s−1 | [201] | |
| PVA | H2SO4 H2O | PANI | √ | √ | x | x | 0.12 | PVA/PANI All-in-one AC | 1.88 F cm−2 @0.1 mA g−1 | [202] | |
| PAAM | H2O | MBAA MXene/PANI 0.3 M Fe3+ PA | √ | √ | x | x | Not provided R = 50.9 Ω @−20 °C R = 20Ω @RT | MXene/PANI/PAAM All-in-one | 645 mF cm−2@RT 355 mF cm−2 @−20 °C @5 mA g−1 | [203] | |
| PAM | 1 M LiCl EG/H2O | AgNW@Ag-CNTs BACA-Au NPs | √ | √ | √ | x | ~965 × 103@ RT to −35 °C Not provided | AgNW@Ag-CNTs/PAM All-in-one | ~168 mF cm−2 @RT to −35 °C @0.5 mA g−1 | [204] | |
| Organogel | |||||||||||
| BATTERIES | PEG-UPy/PEO/PVdF-HFP | Liquid electrolyte Not precise | √ | √ | √ | x | 0.75 | LiFePO4 Li metal | 147.1 mA h g−1 @1 C | [34] | |
| PEGDA-UPy | 1 M LiPF6 /EC/DMC | PEGDA Dipenta-erythritol penta-/hexa-acrylate | x | x | √ | x | 0.5 | Li metal | 150 mA h g−1 @0.5 C | [35] | |
| P(MMA-BA-(F-MA)) | LiBOB EC:DMC (10 µL) | BMI | √ | √ | √ | x | 2.56 | LiCoO2 Li metal | 149 mA h g−1 @0.1 C | [36] | |
| DASHPE | 1 M LiPF6 EC/DEC/DMC | BMI | √ | √ | √ | x | 0.16 | LiFePO4 Li metal | 133.1 mA h g−1 @0.1 C | [37] | |
| TF/TMI-based GPEs | LiTFSI (10 wt.%) EC/DEC | x | x | √ | x | 1.07 | Li metal | 140 mA h g−1 @0.2 C | [38] | ||
| CNGPE | LiTFSI/ NMA | LiDFOB LiNO3 POSS-SH PDMS-DMA JDMA DSDA-DMA | √ | x | √ | x | 0.77 | LiFePO4 Li metal | 150 mA h g−1 @0.5 C (118.3 mA h g−1 @0.1 C for pouch cell) | [40] | |
| IBshPE | 0.3 M LiDFOB/ 0.02 M LiPF6/ 0.8 M LiTFSI FEC/EC | NH2-PEG-NH2 2-FBA | √ | √ | √ | x | 5.08 | LiFePO4 Li metal | 156.6 mA h g−1 @0.1 C | [42] | |
| PBPE | 0.3 M LiDFOB/ 0.02 M LiPF6/ 0.8 M LiTFSI | NH2-PEG-NH2 BTC | √ | √ | √ | x | 4.79 | LiFePO4 Li metal | 157.8 mA h g−1 @0.1 C | [43] | |
| PETPTA | 0.2 M LiDFOB/ 1 M LiTFSI/ FEC/FEPE | √ | x | x | √ | 1.19 | Li metal | 220 mA h g−1 @ 0.1 C | [46] | ||
| PPEGMA on Whatman substrate | NaTFSI | TEP FEC | √ | x | x | √ | 0.91 | Na3V2(PO4)3 Na metal | 117.2 mA h g−1 @0.1 C | [47] | |
| PEGDA | LiTFSI | PEGDA TEP TiO2 NPs | x | x | x | √ | 0.07 | LiFePO4 Li metal | 132.3 mA h g−1 @0.2 C | [48] | |
| PAN@ Paraffin Electrospun | 1 M LiPF6 EC/DEC/DMC | x | x | x | √ | 1.4 | LiFePO4 Li metal | 150 mA h g−1 @0.2 C | [53] | ||
| PFE/PEGA | 1 M LiBOB LiNO3 (3 wt.% of polymer) EC/DMC | VC | x | x | x | √ | 2.28@RT 0.14@−10 °C | LiFePO4 Li metal | 170 mA h g−1 @0.1 C | [59] | |
| MOF/AMImTFSI | LiTFSI | x | x | x | x | 2.3 | Li metal Si anode | 3250 mA h g−1 @0.1 C | [68] | ||
| PMMA/PAN | 1 M LiTFSI TEGDME | ETPTA | √ | x | x | x | 0.33 | LiFePO4 Li metal | 156.8 mA h g−1 @0.2 C | [75] | |
| Gelatin | Li2SO4 H2O | ZnSO4 | √ | √ | x | x | 61 | LiMn2O4 Zn metal | 110 mA h g−1 @25 mA g−1 | [138] | |
| PANa | 6 M KOH H2O | 0.2 Zn2+ | √ | √ | x | x | 126@RT 163@50 °C 57@−20 °C | NiCo Zn metal | 110 mA h g−1 @−20 °C@9 C 157 mA h g−1 @50 °C@8 C | [139] | |
| PANa | 6M KOH H2O | 0.2 M Zn2+ | √ | √ | x | x | 200 | NiCo Zn metal | 87 mA h g−1 @11.3 C | [140] | |
| PANa | 6M KOH H2O | 0.2 M Zn2+ | √ | √ | x | x | 170 | NiCo@CC Zn@CC | 259 mA h g−1 @5.8 C | [141] | |
| Xanthan gum | H2O | 4 M Zn2+ | √ | x | x | x | 12.08@RT 2.54@−20 °C | NH4V3O8. 1.9H2O Zn metal | 426 mA h g−1@RT 201 mA h g−1 @−20 °C @0.2 A g−1 | [142] | |
| PAAm | H2O | MBAA 1 M Zn2+ 1 M Mn2+ | √ | √ | x | x | Not provided (R = 36.8 Ω) | MnO2 Zn metal | 230.5 mA h g−1 @1 C | [143] | |
| PAAm/ Alginate | H2O | 2 M Zn2+ 0.1 M Mn2+ | √ | √ | x | x | 43.2 | MnO2 Zn metal | 300.4 mA h g−1 @0.11 A g−1 | [144] | |
| PAM/ Alginate | H2O | MBAA 2 M Zn2+ 1 M Na+ | √ | √ | x | x | 19.74 | Prussian blue@CNTs Zn metal | 136.4 mA h g−1 @0.1 C | [145] | |
| PVdF | 1 M LiPF6 EC/DMC | √ | x | x | x | 0.5 | LiFePO4 Graphite | 150 mA h g−1 @C/7 | [147] | ||
| P(MA-co-AN)/PVA | 1 M LiPF6 EC/DEC/DMC | MBAA | √ | √ | x | x | 0.98 | LiCoO2 Graphite | 140 mA h g−1 @0.1 C | [148] | |
| GECO | 1 M LiTFSI DOL/DME | ETU MgO | √ | √ | x | x | 0.24 | Carbon—sulfur cathode Li metal | 700 mA h gSulfur−1 @0.1 C | [149] | |
| CMNC | 1 M LiPF6 EC/DEC/ DMC | Epichlorohydrin | √ | √ | x | x | 3.93 | NMC532 Li metal | 151.4 mA h g−1 @0.2 C | [150] | |
| Allyl-modified cellulose | 1 M LiPF6 EC/ DMC | √ | √ | x | x | 4.36 | LiFePO4 Li metal | 150.6 mA h g−1 @0.2 C | [151] | ||
| PVdF-HFP | 0.5 M NaPF6 TMP | √ | x | x | √ | 1.4 | Na battery Red P/NaHg SC AC | 225 mA h g−1 @31 mA g−1 100 F g−1 @10 mV s−1 | [152] | ||
| PEI | 1 M LiTFSI DOL/DME | PEGDE | √ | x | x | x | 0.75 | Carbon–sulfur cathode Li metal | 1100 mA h gSulfur−1 @0.1 C | [153] | |
| PEGMEA | 1 M LiPF6 EC/DEC | ETPTA | √ | √ | x | x | 1.66 | LiCoO2 Li metal | 140 mA h g−1 @0.1 C | [154] | |
| PDEI/ PVdF-HFP | 1 M LiPF6 EC/DMC | √ | √ | x | √ | 1.78 | LiFePO4 Li metal | 155.5 mA h g−1 @0.1 C | [157] | ||
| P(AN-DEVP) | 1 M LiPF6 EC/DEC/DMC | √ | x | x | √ | 1.4 | LiFePO4 Li metal | 145.4 mA h g−1 @0.5 C | [158] | ||
| Poly(VDF-co-VEPFSIS) | EC/PC | Nota bene Single ion GPE | √ | x | x | x | 0.5 | NMC Li metal | 143 mA h g−1 @0.05 C | [165] | |
| PI-LiCPSI/ PVdF-HFP | EC/DMC | Nota bene Single ion GPE | √ | x | x | x | 0.14 | LiFePO4 Li metal | 141 mA h g−1 @0.2 C | [166] | |
| PE-co-PVA-graft-LiCPSI PVdF-HFP | EC/DMC | Nota bene Single ion GPE | √ | x | x | x | 0.057 | LiFePO4 Li metal | 100 mA h g−1 @1 C | [167] | |
| P(MPEGA-AMPSLi) | EC/DMC | Nota bene Single ion GPE | √ | x | x | x | 0.028 | LiFePO4 Li metal | 146.2 mA h g−1 @0.2 C | [168] | |
| P(LiSTFSI) | DME/DOL | PEGDMA Nota bene Single ion GPE | √ | x | x | x | 0.027 | LiFePO4 Li metal | 81.1 mA h g−1 @0.1 C | [169] | |
| PVdF-HFP /PEO | 1 M LiTFSI EC/DMC | SiO2 NPs | √ | √ | x | x | 1.12 | LiFePO4 Li metal | 152.5 mA h g−1 @0.1 C | [170] | |
| PVAc/ PVdF-HFP | 1 M LiPF6 EC/EMC/ DMC | SiO2 NPs | √ | x | x | x | 1.3 | NMC532 C µbeads | 159.8 mA h g−1 @0.5 C | [173] | |
| PTC | 1 M LiPF6 EC/DEC | Graphene oxide | √ | x | x | x | 2.28 | LiCoO2 Li4Ti5O12 | 143 mA h g−1 @1 C | [184] | |
| PTC | 1 M LiPF6 EC/DEC | Graphene oxide | √ | x | x | x | 5.5 | LiCoO2 Graphite | 161.3 mA h g−1 @0.2 C | [185] | |
| PVdF-HFP /PEG | LiTFSI | LATP | √ | x | x | x | 0.81@40 °C | LiFePO4 Li metal | 144.8 mA h g−1 @0.1 C | [186] | |
| PTC | LiTFSI 1 M LiPF6 EC/DEC | LLZTO | √ | x | x | x | 1.8 | LiCoO2 Li4Ti5O12 | 151.9 mA h g−1 @0.2 C | [187] | |
| PVA | Glutaraldehyde LLZO | √ | x | x | x | 0.09 | LiFePO4 Li metal | 158 mA h g−1 @0.1 C | [189] | ||
| PVdF-HFP P(MMA-co-HEMA) | 1 M LiClO4 /PC | SiO2 | √ | x | x | x | 2.63 | LiMn2O4 Li metal | 139.6 mA h g−1 @0.1 C | [190] | |
| PV-CD/ BSIP | SiO2 Li-MMT PEGDA | √ | √ | x | x | 0.25 | LiFePO4 Li metal | ~150 mA h g−1 @0.2 C | [192] | ||
| Fabric/TPU | LiTFSI G4 | SiO2 aerogel | √ | √ | x | √ | 1.02 | MWCNTs Li metal | 930 mA h g−1 @0.5 A g−1 | [193] | |
| SUPERCAPACITORS | PVdF-HFP | NaPF6 TMP | √ | x | x | √ | 1.4 | AC | 100 F g−1@10 mV s−1 | [152] | |
| PVdF-HFP | ACN/MF PC | [EMI+][BF4−] | √ | √ | x | x | 9.21@RT 2.95@−60 °C | AC | 120.4 F g−1@RT 118.9 F g−1@−60 °C @0.5 A g−1 | [160] | |
| PVA | Trimesic acid DMSO | Phenylene diamine | √ | √ | x | x | 45.45 | AC | 322.5 F g−1@0.1 A g−1 | [162] | |
| PVA | LiCl EG | SiO2 STF | √ | √ | x | x | 2.3@RT ~7@80 °C | AC | 21F g−1@RT 40 F g−1@80 °C @1 A g−1 | [200] | |
| Item | Cost | Item | Cost |
|---|---|---|---|
| Acrylic acid | USD ~1500 per ton | EMITFSI | USD ~50,000 per ton |
| MBAA | USD ~37,000 per ton | NaOH | USD ~500 per ton |
| Ethylene glycol | USD ~580 per ton | Potassium persulfate | USD ~2000 per ton |
| PEO | USD 1200–1800 per ton | PEGDM | USD ~30,000 per ton |
| Pluronic | USD ~4000 per ton | ||
| Cellulose | USD 2000–5000 per ton |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.K.L.; Pham-Truong, T.-N. Recent Advancements in Gel Polymer Electrolytes for Flexible Energy Storage Applications. Polymers 2024, 16, 2506. https://doi.org/10.3390/polym16172506
Nguyen TKL, Pham-Truong T-N. Recent Advancements in Gel Polymer Electrolytes for Flexible Energy Storage Applications. Polymers. 2024; 16(17):2506. https://doi.org/10.3390/polym16172506
Chicago/Turabian StyleNguyen, Thi Khanh Ly, and Thuan-Nguyen Pham-Truong. 2024. "Recent Advancements in Gel Polymer Electrolytes for Flexible Energy Storage Applications" Polymers 16, no. 17: 2506. https://doi.org/10.3390/polym16172506
APA StyleNguyen, T. K. L., & Pham-Truong, T.-N. (2024). Recent Advancements in Gel Polymer Electrolytes for Flexible Energy Storage Applications. Polymers, 16(17), 2506. https://doi.org/10.3390/polym16172506







