Gold Nanoparticles Modulate Excimer and Exciplex Dynamics of PDDCP-Conjugated Polymers
Abstract
1. Introduction
2. Materials and Methods
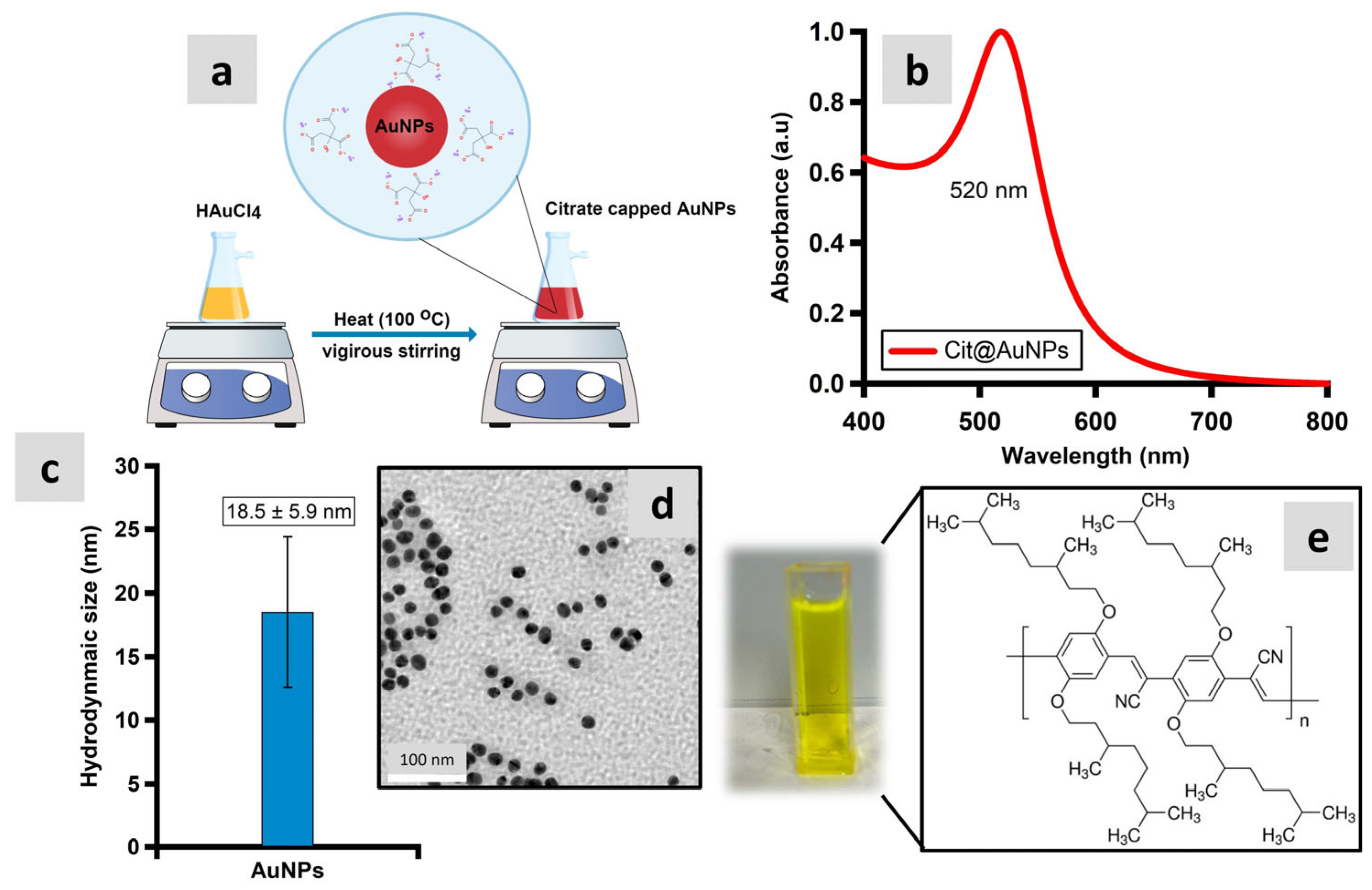
2.1. Synthesis of Colloidal AuNPs
2.2. Preparation of Polymer (PDDCP) Doped AuNPs
2.3. Characterization of Polymer PDDCP @AuNPs
3. Results and Discussion
3.1. Characterization of Synthesized AuNPs
3.2. Spectral Behavior of PDDCP@AuNPs
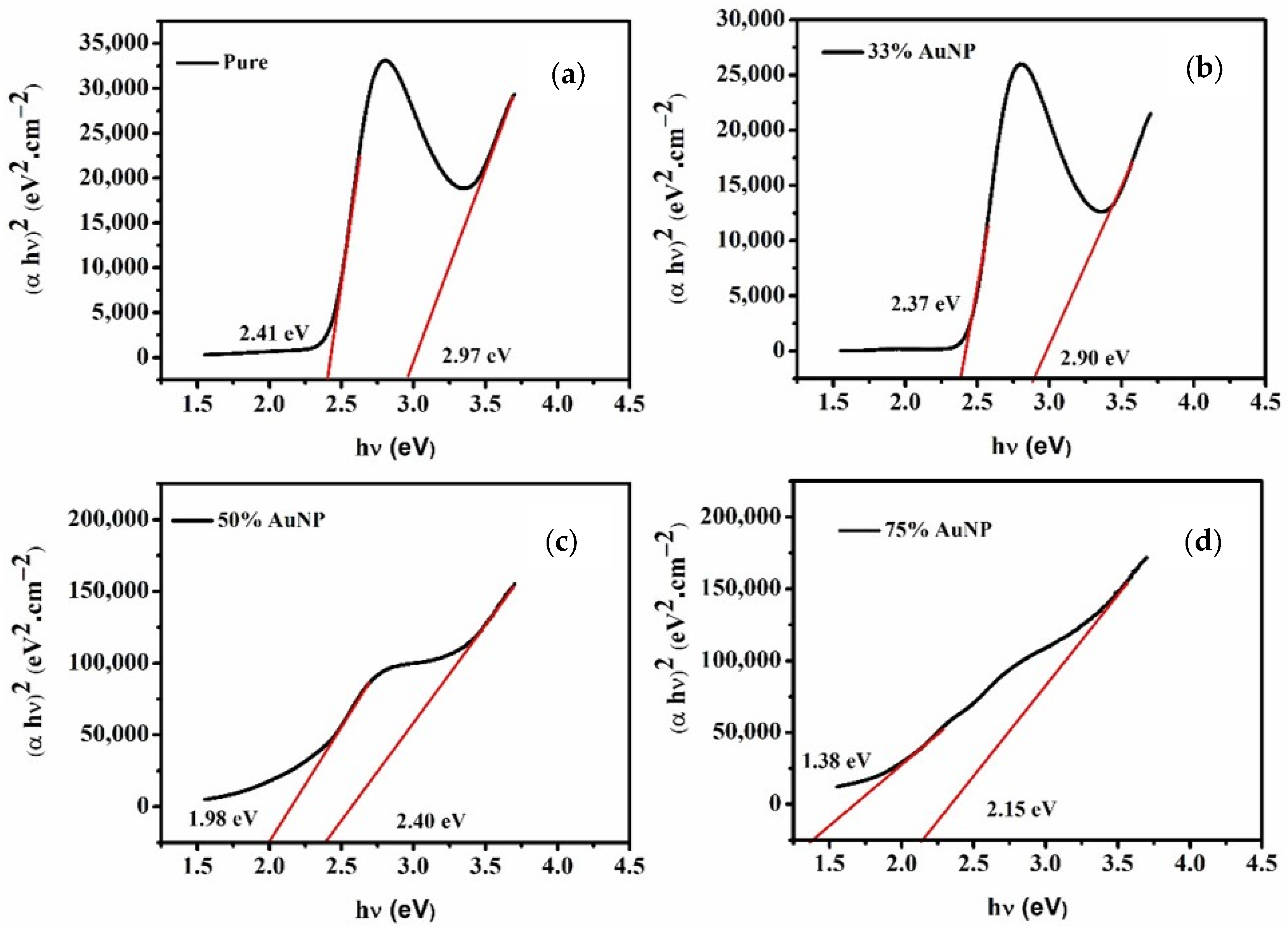
3.3. Energy Band Gap ()
| Samples | Refractive Index | |||
|---|---|---|---|---|
| PDDCP | 2.41 | 2.97 | 2.59 | 2.46 |
| PDDCP@AuNPs (33%) | 2.37 | 2.90 | 2.60 | 2.47 |
| PDDCP@AuNPs (50%) | 1.98 | 2.40 | 2.72 | 2.59 |
| PDDCP@AuNPs (75%) | 1.38 | 2.15 | 2.97 | 2.66 |
3.4. Fluorescence Spectra of PDDCP@AuNPs
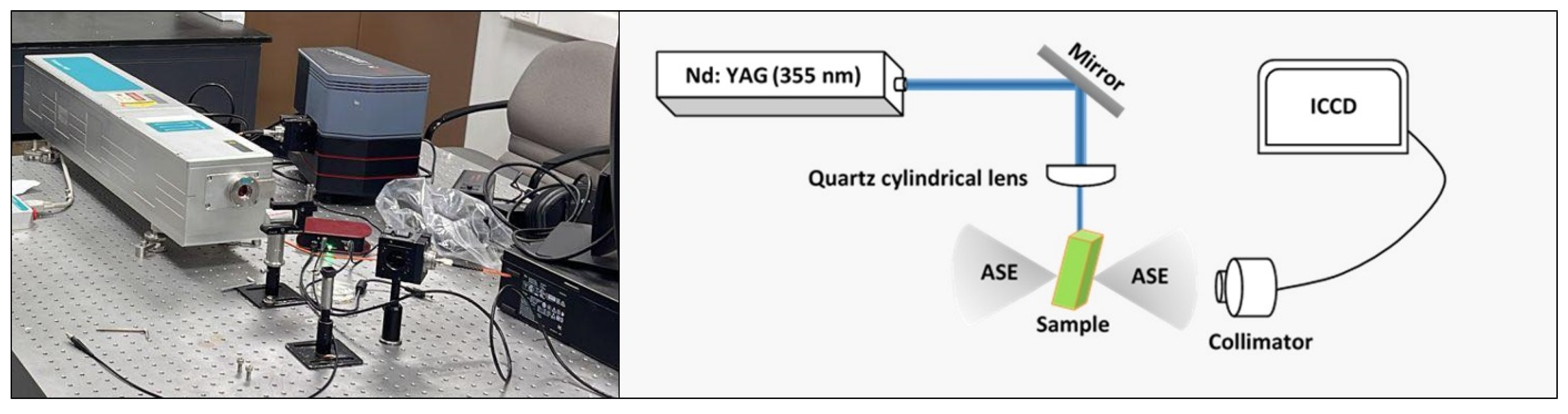
3.5. ASE and LIF Properties
3.6. Photochemical Stability of the PDDCP@AuNPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; He, H.; Xiao, D.; Yin, S.; Ji, W.; Jiang, S.; Luo, D.; Wang, B.; Liu, Y. Recent advances of plasmonic nanoparticles and their applications. Materials 2018, 11, 1833. [Google Scholar] [CrossRef] [PubMed]
- Stankus, D.P.; Lohse, S.E.; Hutchison, J.E.; Nason, J.A. Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ. Sci. Technol. 2011, 45, 3238–3244. [Google Scholar] [CrossRef]
- Wang, C.C.; Choy, W.C.; Duan, C.; Fung, D.D.; Wei, E.; Xie, F.-X.; Huang, F.; Cao, Y. Optical and electrical effects of gold nanoparticles in the active layer of polymer solar cells. J. Mater. Chem. 2012, 22, 1206–1211. [Google Scholar] [CrossRef]
- Tan, B.; Baycan, F. A new donor-acceptor conjugated polymer-gold nanoparticles biocomposite materials for enzymatic determination of glucose. Polymer 2020, 210, 123066. [Google Scholar] [CrossRef]
- Sanchis-Gual, R.; Coronado-Puchau, M.; Mallah, T.; Coronado, E. Hybrid nanostructures based on gold nanoparticles and functional coordination polymers: Chemistry, physics and applications in biomedicine, catalysis and magnetism. Coord. Chem. Rev. 2023, 480, 215025. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Kedem, O.; Tesler, A.B.; Vaskevich, A.; Rubinstein, I. Sensitivity and optimization of localized surface plasmon resonance transducers. ACS Nano 2011, 5, 748–760. [Google Scholar] [CrossRef]
- Stone, J.; Jackson, S.; Wright, D. Biological applications of gold nanorods. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Lee, S.-T.; Yang, S.; Kang, Z. Coupling surface plasmon resonance of gold nanoparticles with slow-photon-effect of TiO2 photonic crystals for synergistically enhanced photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 1409–1419. [Google Scholar] [CrossRef]
- Ibnaouf, K. Amplified spontaneous emission spectra of poly (9,9-dioctylfluorenyl-2,7-diyl) under pulsed laser excitation. Synth. Met. 2015, 209, 534–543. [Google Scholar] [CrossRef]
- Ibnaouf, K. Laser from external energy transfer of MEH–PPV conjugated polymer. Opt. Laser Technol. 2012, 44, 710–713. [Google Scholar] [CrossRef]
- Ismail, R.A.; Almashhadani, N.J.; Sadik, R.H. Preparation and properties of polystyrene incorporated with gold and silver nanoparticles for optoelectronic applications. Appl. Nanosci. 2017, 7, 109–116. [Google Scholar] [CrossRef]
- Ibnaouf, K.; Prasad, S.; Masilamani, V.; Alsalhi, M.; Alaamer, A. Evidence for the double excimer state of conjugated polymer in a liquid solution. J. Eur. Opt. Soc. Rapid Publ. 2013, 8, 13001. [Google Scholar] [CrossRef]
- Idriss, H.; Taha, K.K.; Aldaghri, O.; Alhathlool, R.; AlSalhi, M.; Ibnaouf, K. Amplified spontaneous emission from the exciplex state of a conjugated polymer “PFO” in oleic acid. Opt. Laser Technol. 2016, 83, 148–152. [Google Scholar] [CrossRef]
- Alsadig, A.; Vondracek, H.; Pengo, P.; Pasquato, L.; Posocco, P.; Parisse, P.; Casalis, L. Label-free, rapid and facile gold-nanoparticles-based assay as a potential spectroscopic tool for trastuzumab quantification. Nanomaterials 2021, 11, 3181. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.; Zhu, H.; Zhu, Q.; Xia, Y. Three-in-one: Sensing, self-assembly, and cascade catalysis of cyclodextrin modified gold nanoparticles. J. Am. Chem. Soc. 2016, 138, 16645–16654. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Kim, M.S.; Joo, J. Hybrid nanostructures using π-conjugated polymers and nanoscale metals: Synthesis, characteristics, and optoelectronic applications. Chem. Soc. Rev. 2010, 39, 2439–2452. [Google Scholar] [CrossRef]
- Jana, B.; Bhattacharyya, S.; Patra, A. Conjugated polymer P3HT–Au hybrid nanostructures for enhancing photocatalytic activity. Phys. Chem. Chem. Phys. 2015, 17, 15392–15399. [Google Scholar] [CrossRef]
- Pankow, R.M.; Thompson, B.C. The development of conjugated polymers as the cornerstone of organic electronics. Polymer 2020, 207, 122874. [Google Scholar] [CrossRef]
- Inal, S.; Rivnay, J.; Suiu, A.-O.; Malliaras, G.G.; McCulloch, I. Conjugated polymers in bioelectronics. Acc. Chem. Res. 2018, 51, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- AlSalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent advances in conjugated polymers for light emitting devices. Int. J. Mol. Sci. 2011, 12, 2036–2054. [Google Scholar] [CrossRef]
- Xie, R.; Weisen, A.R.; Lee, Y.; Aplan, M.A.; Fenton, A.M.; Masucci, A.E.; Kempe, F.; Sommer, M.; Pester, C.W.; Colby, R.H. Glass transition temperature from the chemical structure of conjugated polymers. Nat. Commun. 2020, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.V.; Aruchamy, K.; Kotrappanavar, N.S. Conjugated polymer-based smart composites for optoelectronics and energy applications. In Polymer-Based Advanced Functional Composites for Optoelectronic and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–49. [Google Scholar]
- Xiao, S.; Zhang, Q.; You, W. Molecular engineering of conjugated polymers for solar cells: An updated report. Adv. Mater. 2017, 29, 1601391. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Mustafa, M.; Ali, A.; Doh, Y.H.; Choi, K.H. Improvement of solution based conjugate polymer organic light emitting diode by ZnO–graphene quantum dots. J. Mater. Sci. Mater. Electron. 2015, 26, 3344–3351. [Google Scholar] [CrossRef]
- Mdluli, S.B.; Ramoroka, M.E.; Yussuf, S.T.; Modibane, K.D.; John-Denk, V.S.; Iwuoha, E.I. π-Conjugated polymers and their application in organic and hybrid organic-silicon solar cells. Polymers 2022, 14, 716. [Google Scholar] [CrossRef]
- Elzupir, A.O.; Hussein, R.K.; Ibnaouf, K.H. Intermolecular CH-π electrons interaction in poly (9,9-dioctylfluorenyl-2,7-diyl)(PFO): An experimental and theoretical study. Molecules 2022, 27, 1488. [Google Scholar] [CrossRef]
- Ibnaouf, K. Photodynamic properties of poly [2-methoxy-5-(3′,7′-dimethyloctyloxy)-1,4-phenylenevinylene] under pulsed laser excitation. Opt. Laser Technol. 2020, 130, 106369. [Google Scholar] [CrossRef]
- Aldaghri, O. Spectral Characteristics and Molecular Structure of (E)-1-(4-Chlorophenyl)-3-(4-(Dimethylamino) Phenyl) Prop-2-en-1-One (DAP). Materials 2021, 14, 2766. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Osaheni, J.A. Excimers and exciplexes of conjugated polymers. Science 1994, 265, 765–768. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Zhu, S.; Peng, J.; Li, L. Preparation of exciplex-based fluorescent organic nanoparticles and their application in cell imaging. RSC Adv. 2017, 7, 40842–40848. [Google Scholar] [CrossRef]
- Al-Bati, S.; Jumali, M.H.; Al-Asbahi, B.A.; Ibtehaj, K.; Yap, C.C.; Qaid, S.M.; Ghaithan, H.M.; Farooq, W. Improving photophysical properties of white emitting ternary conjugated polymer blend thin film via additions of TiO2 nanoparticles. Polymers 2020, 12, 2154. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Jana, B.; Maiti, S.; Bera, R.; Ghosh, H.N.; Patra, A. Light harvesting and photocurrent generation in a conjugated polymer nanoparticle–reduced graphene oxide composite. ChemPhysChem 2017, 18, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, D. π-Conjugated nanostructured materials: Preparation, properties and photonic applications. Nanoscale Adv. 2019, 1, 19–33. [Google Scholar] [CrossRef]
- Rodrigues, R.d.R.; Pellosi, D.S.; Louarn, G.; Péres, L.O. Nanocomposite films of silver nanoparticles and conjugated copolymer in natural and nano-form: Structural and morphological studies. Materials 2023, 16, 3663. [Google Scholar] [CrossRef]
- Khadir, S.; Diallo, A.; Chakaroun, M.; Boudrioua, A. Exciton enhancement and exciplex quenching by plasmonic effect of Aluminum nanoparticle arrays in a blue organic light emitting diode. Opt. Express 2017, 25, 9812–9822. [Google Scholar]
- Abdelaziz, B.B.; Mustapha, N.; Bedja, I.M.; Aldaghri, O.; Idriss, H.; Ibrahem, M.; Ibnaouf, K.H. Spectral Behavior of a Conjugated Polymer MDMO-PPV Doped with ZnO Nanoparticles: Thin Films. Nanomaterials 2023, 13, 2405. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Han, S.G.; Mun, J.; Yang, K.; Kim, S.H.; Rho, J.; Cho, K.; Oh, D.X.; Jeong, M.S. Elucidating the photoluminescence-enhancement mechanism in a push-pull conjugated polymer induced by hot-electron injection from gold nanoparticles. Photonics Res. 2021, 9, 131–141. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, S.; Jana, B.; Patra, A. Ultrafast Relaxation Processes of Conjugated Polymer Nanoparticles in the Presence of Au Nanoparticles. Chem. Asian J. 2019, 14, 4681–4687. [Google Scholar] [CrossRef]
- Park, J.H.; Lim, Y.T.; Park, O.O.; Kim, J.K.; Yu, J.-W.; Kim, Y.C. Polymer/gold nanoparticle nanocomposite light-emitting diodes: Enhancement of electroluminescence stability and quantum efficiency of blue-light-emitting polymers. Chem. Mater. 2004, 16, 688–692. [Google Scholar] [CrossRef]
- Shen, Z.; O’Carroll, D.M. Nanoporous silver thin films: Multifunctional platforms for influencing chain morphology and optical properties of conjugated polymers. Adv. Funct. Mater. 2015, 25, 3302–3313. [Google Scholar] [CrossRef]
- Mahmoud, M.; Poncheri, A.; El-Sayed, M. Properties of π-conjugated fluorescence polymer–plasmonic nanoparticles hybrid materials. J. Phys. Chem. C 2012, 116, 13336–13342. [Google Scholar] [CrossRef]
- Aldaghri, O.; Alsadig, A.; Idriss, H.; Ali, M.; Ibrahem, M.; Ibnaouf, K. Exploring the photodynamic profile of laser-generated exciplex from a conjugated polymer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 300, 122929. [Google Scholar] [CrossRef]
- Raju, B.B.; Varadarajan, T. Photophysical Properties and Energy Transfer Dye Laser Characteristics of 7-Diethylamino-3-Heteroaryl Coumarin in Solution. Laser Chem. 1995, 16, 109–120. [Google Scholar] [CrossRef]
- Al-Shamiri, H.A.S.; Badr, Y.; Abou Kana, M.T. Optical, Photo-physical properties and photostability of laser dyes impregnated in Sol-Gel matrix. In Proceedings of the 2011 Saudi International Electronics, Communications and Photonics Conference (SIECPC), Riyadh, Saudi Arabia, 24–26 April 2011; pp. 1–6. [Google Scholar]
- Ravindra, N.; Srivastava, V. Variation of refractive index with energy gap in semiconductors. Infrared Phys. 1979, 19, 603–604. [Google Scholar] [CrossRef]
- Sharma, E.; Sharma, P. Applicability of different models of energy bandgap and refractive index for chalcogenide thin films. Mater. Today Proc. 2020, 28, 92–95. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Intense quenching of fluorescence intensity of poly (vinyl pyrrolidone) molecules in presence of gold nanoparticles. Appl. Nanosci. 2013, 3, 543–548. [Google Scholar] [CrossRef]
- Yang, P.-J.; Chu, H.-C.; Lee, Y.-H.; Kobayashi, T.; Chen, T.-C.; Lin, H.-C. Quenching effects of gold nanoparticles in nanocomposites formed in water-soluble conjugated polymer nanoreactors. Polymer 2012, 53, 939–946. [Google Scholar] [CrossRef]
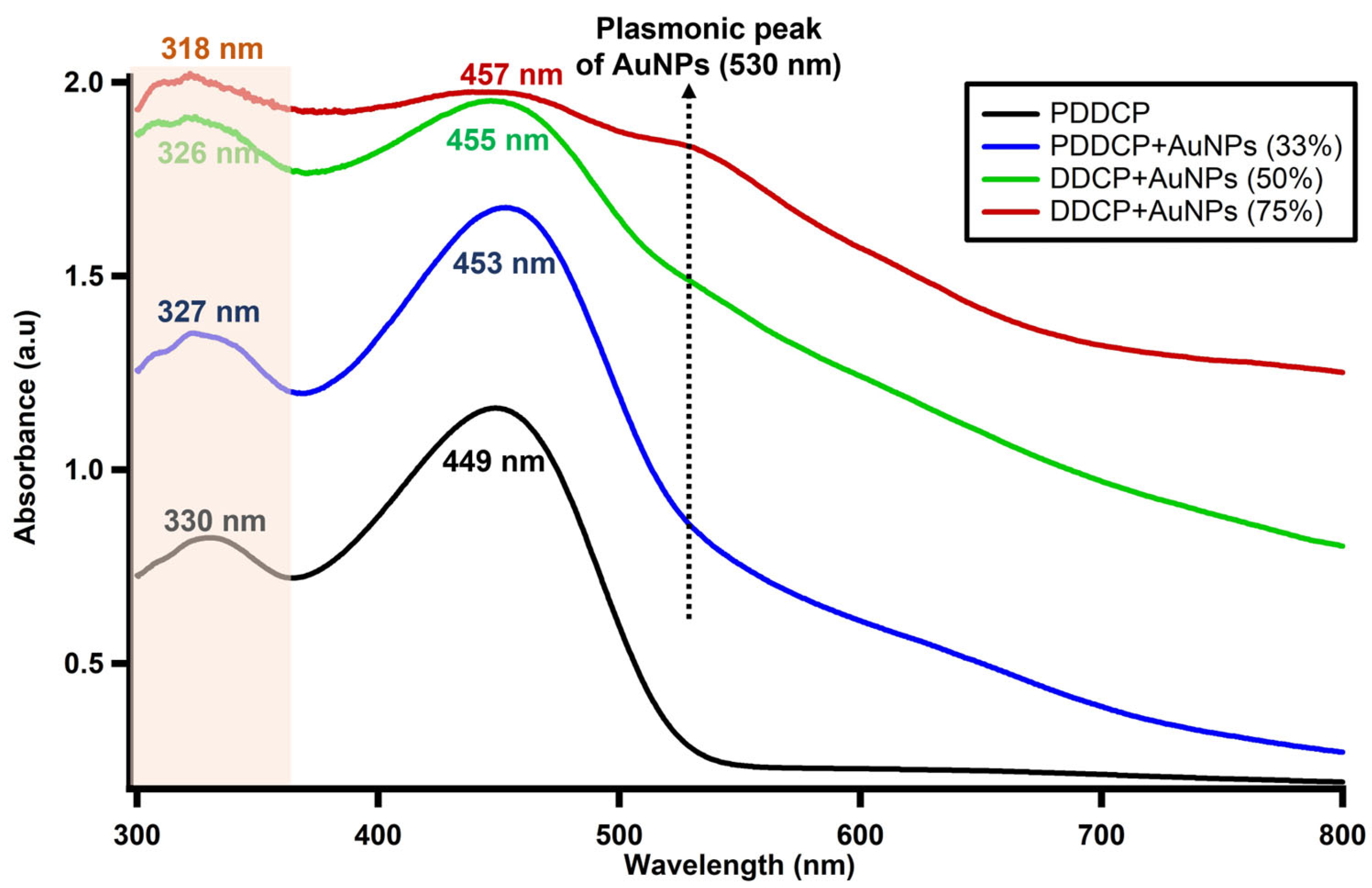
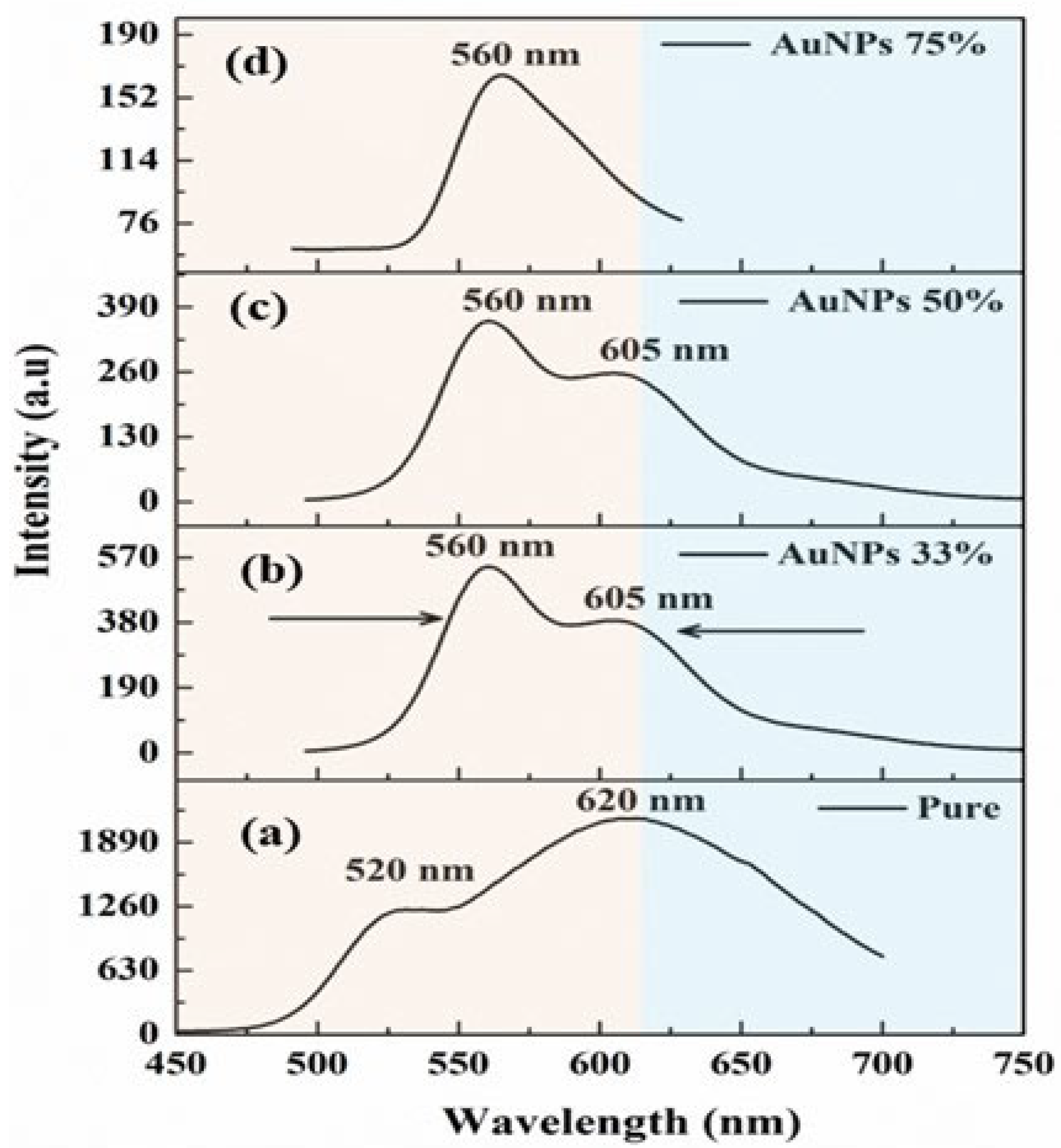
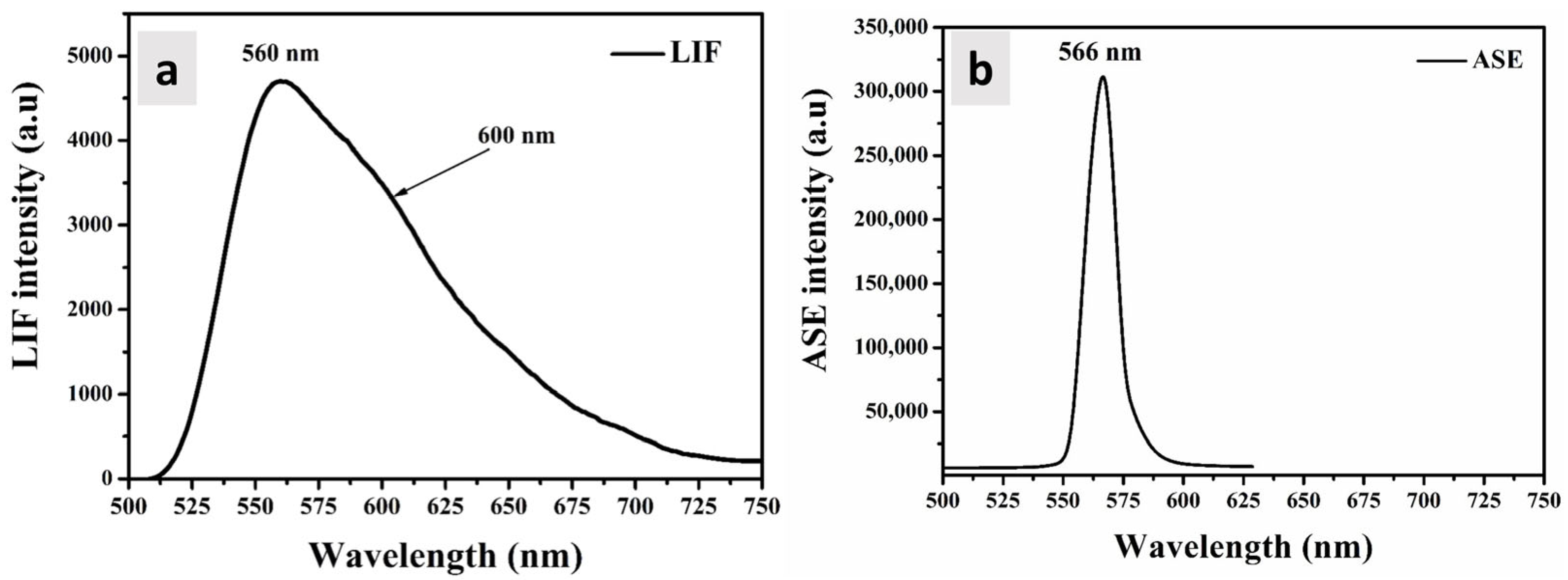
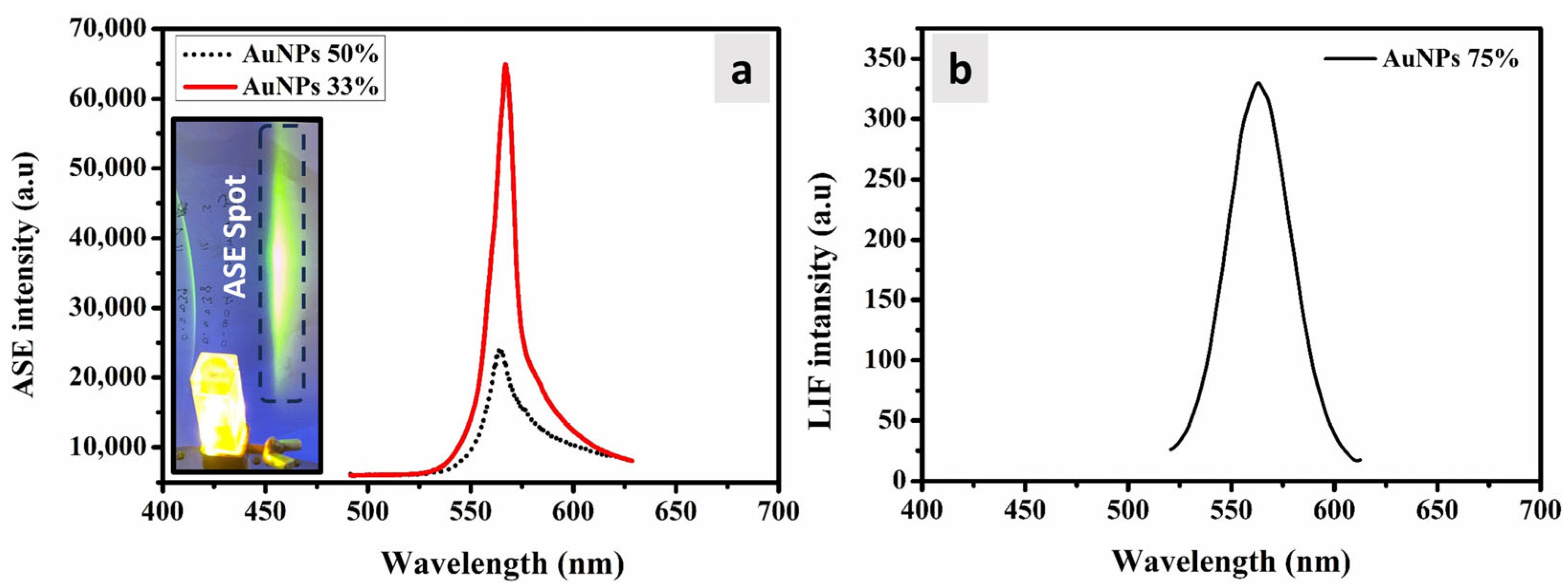
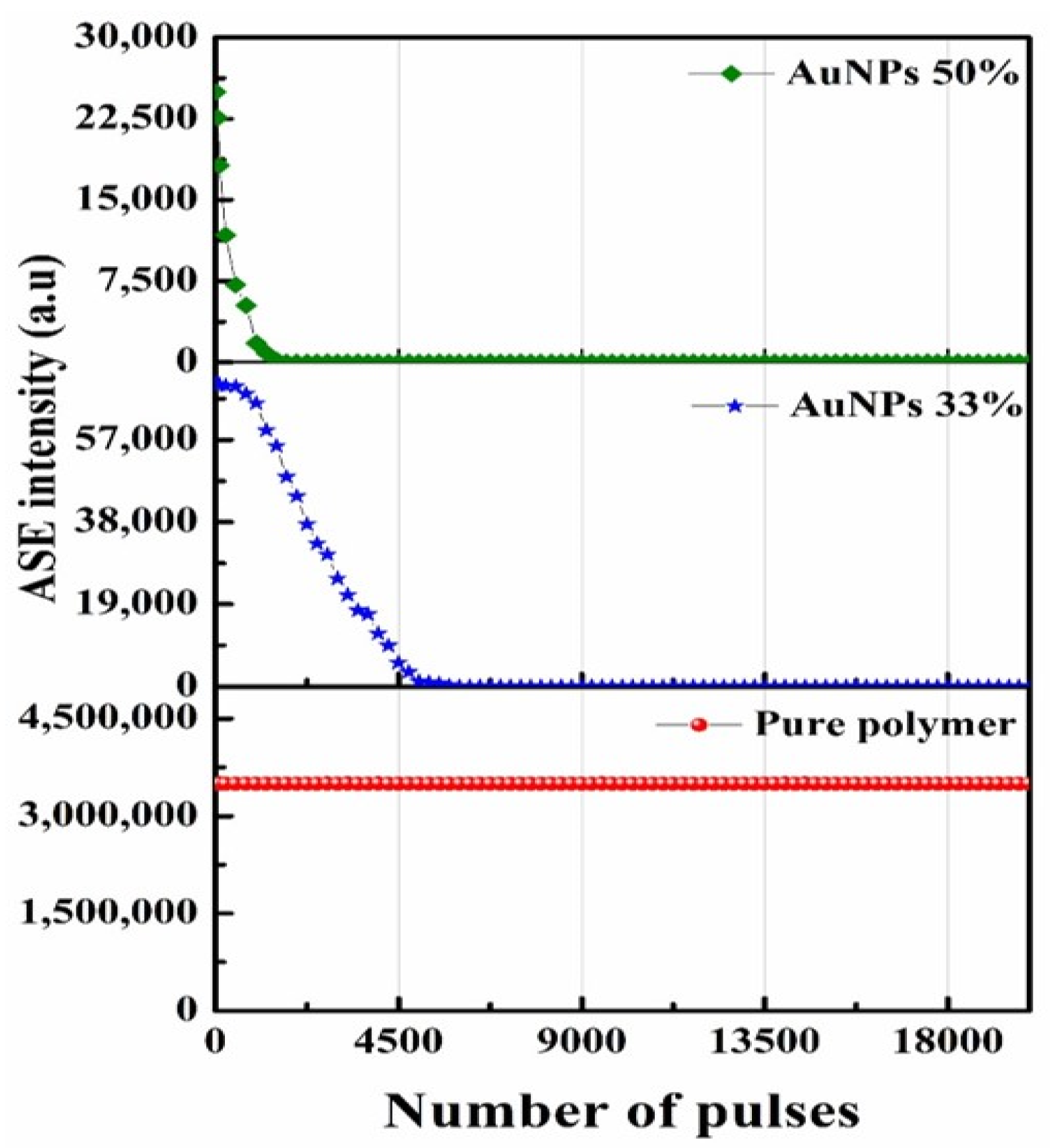
| Volume Ratio of AuNPs (%) | Absorption Band () (nm) | Absorption Band () (nm) | Extinction | Absorption Cross | Absorption Cross
|
|---|---|---|---|---|---|
| 0 | 330 | 449 | 3.34 × | 4.94 × | |
| 33 | 327 | 453 | 4.18 × | 4.86 × | |
| 50 | 326 | 455 | 4.60 × | 4.56 × | |
| 75 | 318 | 457 | 4.70 × | 4.23× |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibnaouf, K.H.; Alsadig, A.; Idriss, H.; Ibrahem, M.A.; Cabrera, H. Gold Nanoparticles Modulate Excimer and Exciplex Dynamics of PDDCP-Conjugated Polymers. Polymers 2024, 16, 2420. https://doi.org/10.3390/polym16172420
Ibnaouf KH, Alsadig A, Idriss H, Ibrahem MA, Cabrera H. Gold Nanoparticles Modulate Excimer and Exciplex Dynamics of PDDCP-Conjugated Polymers. Polymers. 2024; 16(17):2420. https://doi.org/10.3390/polym16172420
Chicago/Turabian StyleIbnaouf, Khalid H., Ahmed Alsadig, Hajo Idriss, Moez A. Ibrahem, and Humberto Cabrera. 2024. "Gold Nanoparticles Modulate Excimer and Exciplex Dynamics of PDDCP-Conjugated Polymers" Polymers 16, no. 17: 2420. https://doi.org/10.3390/polym16172420
APA StyleIbnaouf, K. H., Alsadig, A., Idriss, H., Ibrahem, M. A., & Cabrera, H. (2024). Gold Nanoparticles Modulate Excimer and Exciplex Dynamics of PDDCP-Conjugated Polymers. Polymers, 16(17), 2420. https://doi.org/10.3390/polym16172420









