Composite Mineralized Collagen/Polycaprolactone Scaffold-Loaded Microsphere System with Dual Osteogenesis and Antibacterial Functions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.2.1. PLGA(BMP-2) Microspheres
2.2.2. PLGA(BMP-2)/CS(Pac-525) Composite Microspheres
2.2.3. PLGA(BMP-2)/CS(Pac-525)@MC/PCL Scaffolds
2.3. Characterization of the Physical and Chemical Properties
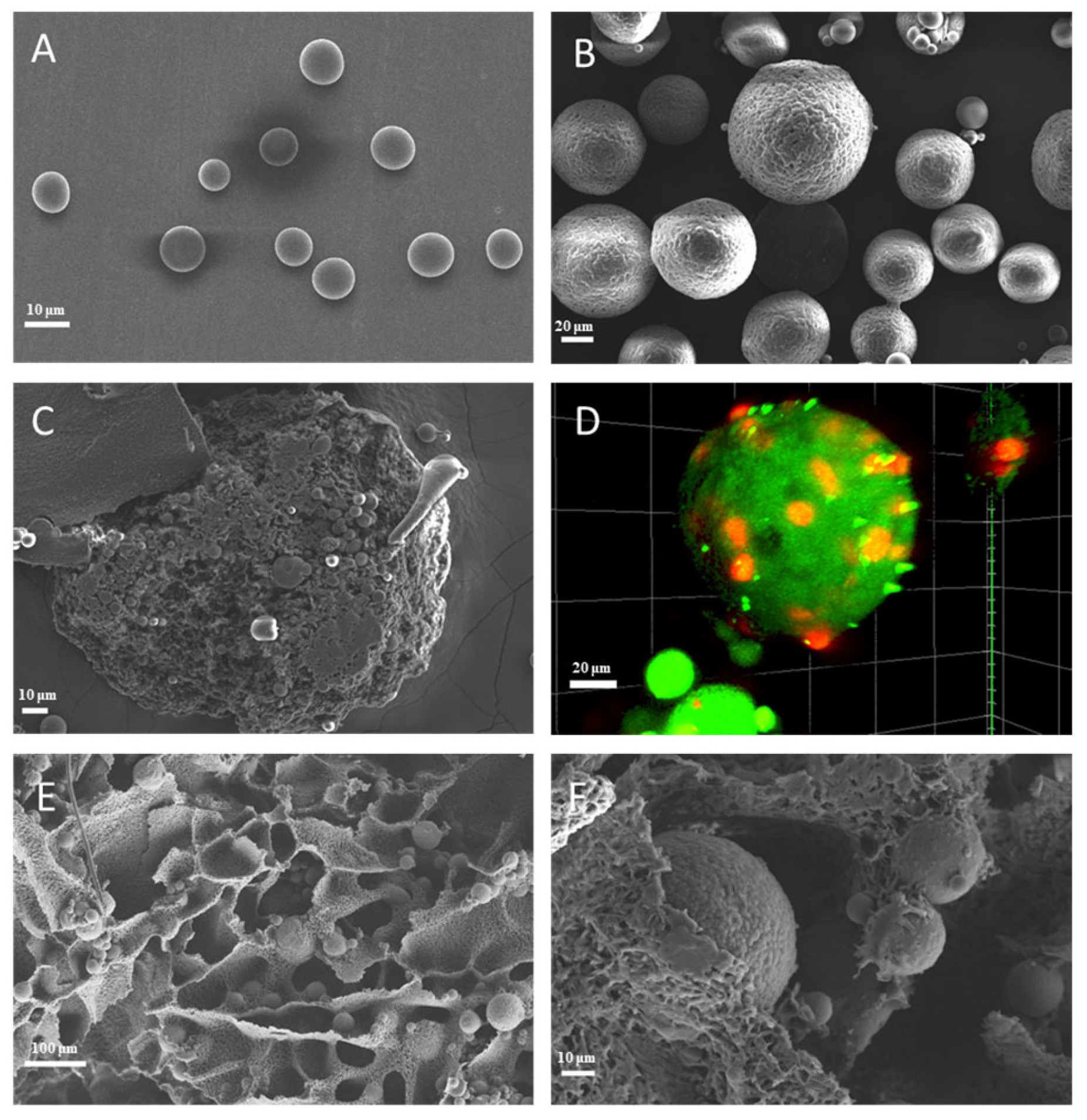
2.3.1. Morphology
2.3.2. Encapsulation Rates of Microspheres
2.3.3. Porosity of PLGA(BMP-2)/CS(Pac-525)@MC/PCL Scaffolds
2.3.4. Compressive Strength of PLGA(BMP-2)/CS(Pac-525)@MC/PCL Scaffolds
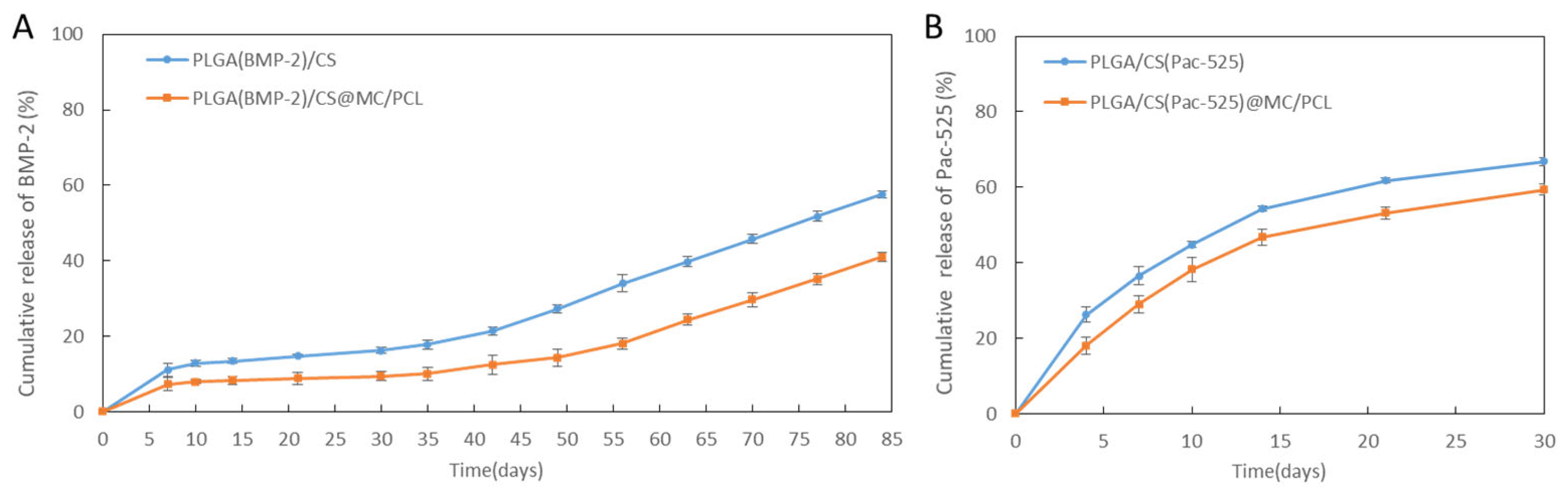
2.3.5. Drug Release Test in Vitro
2.4. Biological Properties of PLGA(BMP-2)/CS(Pac-525)@MC/PCL Scaffolds
2.4.1. Cell Culture
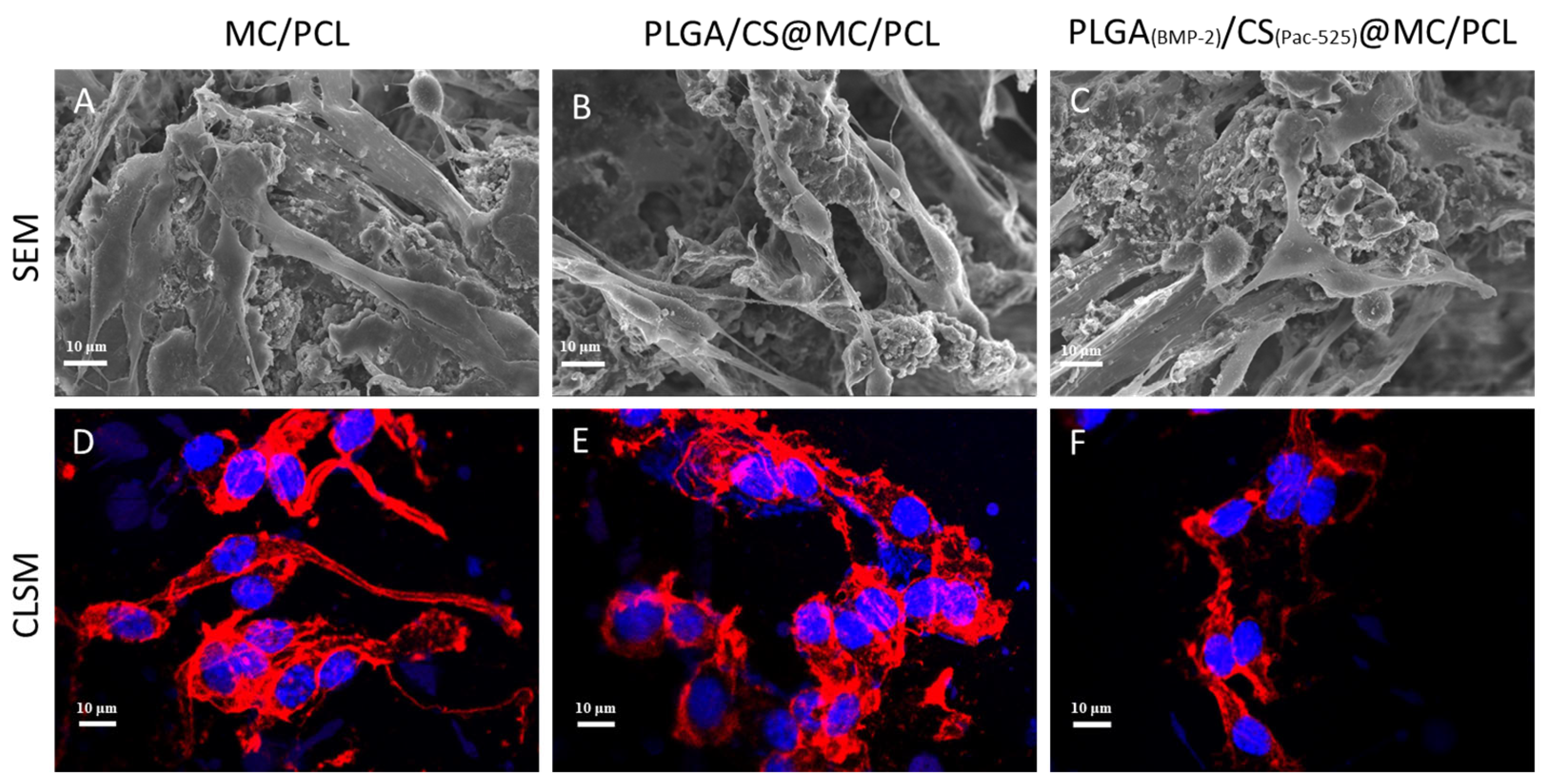
2.4.2. Cell Adhesion
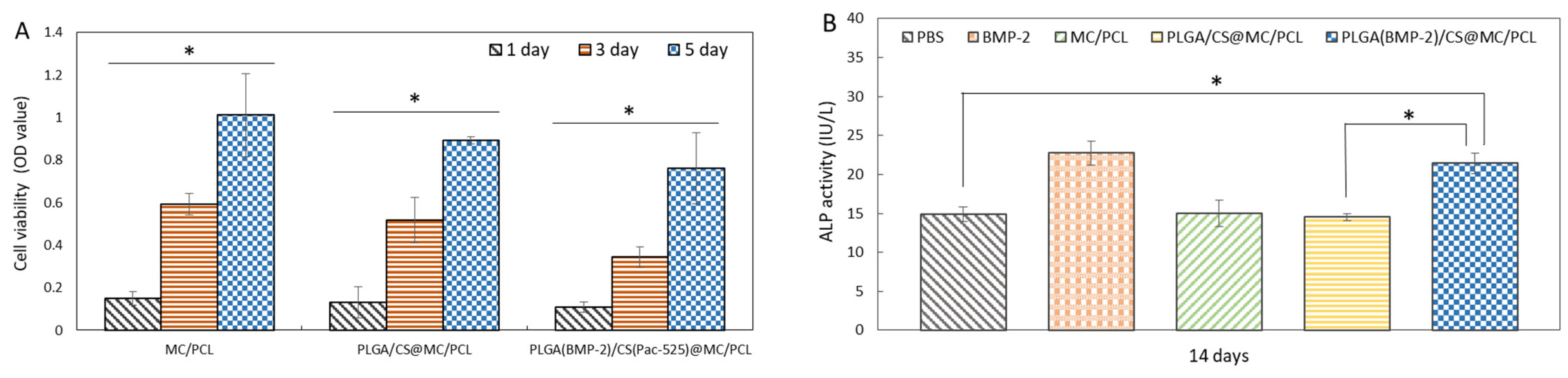
2.4.3. Cell Viability
2.4.4. Detection of Osteogenic Activity
2.5. Antibacterial Property of PLGA(BMP-2)/CS(Pac-525)@MC/PCL Scaffolds
2.6. Statistical Analysis
3. Results and Discussion
3.1. Morphology of Microspheres and Scaffolds
3.2. Physical and Chemical Characteristics
3.3. The Drug Release Behaviour in Vitro
3.4. Biocompatibility
3.5. Osteogenic Activity
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsukagoshi, Y.; Matsushita, Y. Bone regeneration: A message from clinical medicine and basic science. Clin. Anat. 2022, 35, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Janmohammadi, M.; Arab, S.; Talebi, A.; Nooshabadi, V.T.; Koohsarian, P.; Nourbakhsh, M.S. Bone Scaffolds: An Incorporation of Biomaterials, Cells, and Biofactors. ACS Biomater. Sci. Eng. 2021, 7, 5397–5431. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, B.; Matera, B.; Meglioli, M.; Rossi, F.; Duraccio, D.; Faga, M.G.; Zappettini, A.; Macaluso, G.M.; Lumetti, S. Composite PCL Scaffold with 70% β-TCP as Suitable Structure for Bone Replacement. Int. Dent. J. 2024, in press. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Gao, S.; Zhang, Y.-W.; Zhou, R.-B.; Zhou, F. Antibacterial biomaterials in bone tissue engineering. J. Mater. Chem. B 2021, 9, 2594–2612. [Google Scholar] [CrossRef] [PubMed]
- Ghaedamini, S.; Karbasi, S.; Hashemibeni, B.; Honarvar, A.; Rabiei, A. PCL/Agarose 3D-printed scaffold for tissue engineering applications: Fabrication, characterization, and cellular activities. Res. Pharm. Sci. 2023, 18, 566–579. [Google Scholar] [CrossRef]
- Xu, C.; Cheong, J.Y.; Mo, X.; Jérôme, V.; Freitag, R.; Agarwal, S.; Gharibi, R.; Greiner, A. Thoroughly Hydrophilized Electrospun Poly(L-Lactide)/Poly(ε-Caprolactone) Sponges for Tissue Engineering Application. Macromol. Biosci. 2023, 23, e2300143. [Google Scholar] [CrossRef] [PubMed]
- Ghalia, M.A.; Alhanish, A. Mechanical and biodegradability of porous PCL/PEG copolymer-reinforced cellulose nanofibers for soft tissue engineering applications. Med. Eng. Phys. 2023, 120, 104055. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.H.; Kim, H.W. Novel bone-mimetic nanohydroxyapatite/collagen porous scaffolds biomimetically mineralized from surface silanized mesoporous nanobioglass/collagen hybrid scaffold: Physicochemical, mechanical and in vivo evaluations. Mater. Sci. Eng. C 2020, 110, 110660. [Google Scholar] [CrossRef]
- Cui, F.Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Z.; Yang, Y.; Wang, S.; Zhao, Y.; Xiong, Y.; Wang, X. Biphasic mineralized collagen-based composite scaffold for cranial bone regeneration in developing sheep. Regen. Biomater. 2022, 9, rbac004. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.A.; Young, G.; Jones, J.R.; Rankin, S. Bioglass/carbonate apatite/collagen composite scaffold dissolution products promote human osteoblast differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111393. [Google Scholar] [CrossRef]
- Oosterlaken, B.M.; Vena, M.P.; de With, G. In Vitro Mineralization of Collagen. Adv. Mater. 2021, 33, e2004418. [Google Scholar] [CrossRef] [PubMed]
- Kolliopoulos, V.; Harley, B.A. Mineralized collagen scaffolds for regenerative engineering applications. Curr. Opin. Biotechnol. 2024, 86, 103080. [Google Scholar] [CrossRef]
- Grelewski, P.G.; Kwaśnicka, M.; Bar, J.K. Properties of scaffolds as carriers of mesenchymal stem cells for use in bone engineering. Polim. Med. 2023, 53, 129–139. [Google Scholar] [CrossRef]
- He, Y.Z.; Jin, Y.H.; Wang, X.M.; Yao, S.L.; Li, Y.Y.; Wu, Q.; Ma, G.W.; Cui, F.Z.; Liu, H.Y. An Antimicrobial Peptide-Loaded Gelatin/Chitosan Nanofibrous Membrane Fabricated by Sequential Layer-by-Layer Electrospinning and Electrospraying Techniques. Nanomaterials 2018, 8, 327. [Google Scholar] [CrossRef]
- He, Y.Z.; Li, Y.Y.; Zuo, E.J.; Chai, S.L.; Ren, X.; Fei, T.; Ma, G.W.; Wang, X.M.; Liu, H.Y. A Novel Antibacterial Titanium Modification with a Sustained Release of Pac-525. Nanomaterials 2021, 11, 3306. [Google Scholar] [CrossRef]
- He, Y.Z.; Jin, Y.H.; Ying, X.X.; Wu, Q.; Yao, S.L.; Li, Y.Y.; Liu, H.Y.; Ma, G.W.; Wang, X.M. Development of an antimicrobial peptide-loaded mineralized collagen bone scaffold for infective bone defect repair. Regen. Biomater. 2020, 7, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tan, B.; Bao, Z.; Wang, S.; Tang, R.; Wang, Z.; Peng, S. Enhanced bone regeneration via spatiotemporal and controlled delivery of a genetically engineered BMP-2 in a composite Hydrogel. Biomaterials 2021, 277, 121117. [Google Scholar] [CrossRef]
- Jiang, F.; Qi, X.; Wu, X.; Lin, S.; Shi, J.; Zhang, W.; Jiang, X. Regulating macrophage-MSC interaction to optimize BMP-2-induced osteogenesis in the local microenvironment. Bioact. Mater. 2023, 25, 307–318. [Google Scholar] [CrossRef]
- Bal, Z.; Kushioka, J.; Kodama, J.; Kaito, T.; Yoshikawa, H.; Korkusuz, P.; Korkusuz, F. BMP and TGFβ use and release in bone regeneration. Turk. J. Med. Sci. 2020, 50, 1707–1722. [Google Scholar] [CrossRef]
- Costa, J.V.; Portugal, J.; Neves, C.B.; Bettencourt, A.F. Should local drug delivery systems be used in dentistry? Drug Deliv. Transl. Res. 2022, 12, 1395–1407. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Meng, F.; Yin, Z.; Ren, X.; Geng, Z.; Su, J. Construction of Local Drug Delivery System on Titanium-Based Implants to Improve Osseointegration. Pharmaceutics 2022, 14, 1069. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Go, E.J.; Ko, K.W.; Oh, H.J.; Han, J.; Han, D.K.; Park, W. PLGA Microspheres Containing Hydrophobically Modified Magnesium Hydroxide Particles for Acid Neutralization-Mediated Anti-Inflammation. Tissue Eng. Regen. Med. 2021, 18, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Mirzaei, H. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Sang, S.; Wang, S.; Meng, F.; Li, Z.; Zhu, S.; Cui, Z.; Jing, Y.; Wang, C.; Su, J. Optimizing the strontium content to achieve an ideal osseointegration through balancing apatite-forming ability and osteogenic activity. Biomater. Adv. 2022, 133, 112647. [Google Scholar] [CrossRef]
- Wu, X.; Ni, S.; Dai, T.; Li, J.; Shao, F.; Liu, C.; Wang, J.; Fan, S.; Tan, Y.; Zhang, L.; et al. Biomineralized tetramethylpyrazine-loaded PCL/gelatin nanofibrous membrane promotes vascularization and bone regeneration of rat cranium defects. J. Nanobiotechnology 2023, 21, 423. [Google Scholar] [CrossRef]
- Sang, S.; Wang, S.; Yang, C.; Geng, Z.; Zhang, X. Sponge-inspired sulfonated polyetheretherketone loaded with polydopamine-protected osthole nanoparticles and berberine enhances osteogenic activity and prevents implant-related infections. Chem. Eng. J. 2022, 437, 135255. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, M.; Li, W.; Li, W.; Zhang, F. Controlled-release of fluazinam from biodegradable PLGA-based microspheres. J. Environ. Sci. Health B 2019, 54, 810–816. [Google Scholar] [CrossRef]
- Yu, H.; Xu, M.; Duan, Q.; Li, Y.; Liu, Y.; Song, L.; Cheng, L.; Ying, J.; Zhao, D. 3D-printed porous tantalum artificial bone scaffolds: Fabrication, properties, and applications. Biomed. Mater. 2024, 19, 042002. [Google Scholar] [CrossRef] [PubMed]
- Callens, S.J.P.; Tourolle Né Betts, D.C.; Müller, R.; Zadpoor, A.A. The local and global geometry of trabecular bone. Acta Biomater. 2021, 130, 343–361. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, W.; Zheng, J.; Wang, Q.; Ma, G.; Liu, H.; Wang, X. Hierarchically electrospraying a PLGA@chitosan sphere-in-sphere composite microsphere for multi-drug-controlled release. Regen. Biomater. 2020, 7, 381–390. [Google Scholar] [CrossRef]
- Seidi, F.; Khodadadi Yazdi, M.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-based blends for biomedical applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Wan, B.; Bao, Q.; Burgess, D.J. Influence of PLGA molecular weight distribution on leuprolide release from microspheres. Int. J. Pharm. 2021, 599, 120450. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Teixeira, B.I.B.; Monteiro, F.J.M. Biomimetic Composite Scaffold with Phosphoserine Signaling for Bone Tissue Engineering Application. Front. Bioeng. Biotechnol. 2019, 7, 206. [Google Scholar] [CrossRef]
- Li, J.-Y.; Wang, X.-J.; Wang, L.-N.; Ying, X.-X.; Ren, X.; Liu, H.-Y.; Xu, L.; Ma, G.-W. High in vitro antibacterial activity of Pac-525 against Porphyromonas gingivalis biofilms cultured on titanium. Biomed. Res. Int. 2015, 2015, 909870. [Google Scholar]
- Zhang, Q.; Zhou, H.; Jiang, P.; Xiao, X. Metal-based nanomaterials as antimicrobial agents: A novel driveway to accelerate the aggravation of antibiotic resistance. J. Hazard. Mater. 2023, 455, 131658. [Google Scholar] [CrossRef]
- Coelho, C.C.; Padrão, T.; Costa, L.; Pinto, M.T.; Costa, P.C.; Domingues, V.F.; Quadros, P.A.; Monteiro, F.J.; Sousa, S.R. The antibacterial and angiogenic effect of magnesium oxide in a hydroxyapatite bone substitute. Sci. Rep. 2020, 10, 19098. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Melo, L.D.; Poeta, P.; Igrejas, G.; Ferraz, M.P.; Azeredo, J.; Monteiro, F.J. Lytic bacteriophages against multidrug-resistant Staphylococcus aureus, Enterococcus faecalis and Escherichia coli isolates from orthopaedic implant-associated infections. Int. J. Antimicrob. Agents 2019, 54, 329–337. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, C. Antimicrobial peptides: Structure, mechanism, and modification. Eur. J. Med. Chem. 2023, 255, 115377. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]


| Name | Mean Diameter (µm) | Encapsulation Ratio (%) |
|---|---|---|
| PLGA(BMP-2) | 8.9 ± 1.0 | 83.75 ± 3.25 |
| PLGA/CS(Pac-525) | -- | 66.21 ± 2.29 |
| PLGA(BMP-2)/CS(Pac-525) | 61.3 ± 15.3 | -- |
| Name | Average Pore Size (µm) | Porosity (%) | Compressive Strength (Mpa) |
|---|---|---|---|
| PLGA(BMP-2)/CS(Pac-525)@MC/PCL | 97.7 ± 3.4 | 72.75 ± 2.61 | 0.42 ± 0.02 |
| Cumulative Release Rate (%) | Microsphere | Scaffold | ||
|---|---|---|---|---|
| PLGA(BMP-2)/CS | PLGA/CS(Pac-525) | PLGA(BMP-2)/CS@MC/PCL | PLGA/CS(Pac-525)@MC/PCL | |
| BMP-2 | 16.26 ± 0.84 | -- | 9.45 ± 1.11 | -- |
| Pac-525 | -- | 66.85 ± 1.04 | -- | 59.42 ± 1.46 |
| Name | PBS | 10 Days # | 20 Days # | 30 Days # |
|---|---|---|---|---|
| E. coli | 1 ± 0.02 | 1.57 ± 0.10 | 1.36 ± 0.04 | 1.30 ± 0.04 |
| S. aureus | 1 ± 0.02 | 1.35 ± 0.01 | 1.14 ± 0.02 | 1.15 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wang, Q.; Liu, Y.; Zhang, Z.; Cao, Z.; Wang, S.; Ying, X.; Ma, G.; Wang, X.; Liu, H. Composite Mineralized Collagen/Polycaprolactone Scaffold-Loaded Microsphere System with Dual Osteogenesis and Antibacterial Functions. Polymers 2024, 16, 2394. https://doi.org/10.3390/polym16172394
He Y, Wang Q, Liu Y, Zhang Z, Cao Z, Wang S, Ying X, Ma G, Wang X, Liu H. Composite Mineralized Collagen/Polycaprolactone Scaffold-Loaded Microsphere System with Dual Osteogenesis and Antibacterial Functions. Polymers. 2024; 16(17):2394. https://doi.org/10.3390/polym16172394
Chicago/Turabian StyleHe, Yuzhu, Qindong Wang, Yuqi Liu, Zijiao Zhang, Zheng Cao, Shuo Wang, Xiaoxia Ying, Guowu Ma, Xiumei Wang, and Huiying Liu. 2024. "Composite Mineralized Collagen/Polycaprolactone Scaffold-Loaded Microsphere System with Dual Osteogenesis and Antibacterial Functions" Polymers 16, no. 17: 2394. https://doi.org/10.3390/polym16172394
APA StyleHe, Y., Wang, Q., Liu, Y., Zhang, Z., Cao, Z., Wang, S., Ying, X., Ma, G., Wang, X., & Liu, H. (2024). Composite Mineralized Collagen/Polycaprolactone Scaffold-Loaded Microsphere System with Dual Osteogenesis and Antibacterial Functions. Polymers, 16(17), 2394. https://doi.org/10.3390/polym16172394









