From Molecular Design to Practical Applications: Strategies for Enhancing the Optical and Thermal Performance of Polyimide Films
Abstract
1. Introduction
2. Overview of PI Film Properties
2.1. Basic Characteristics
2.2. Application Fields
3. Strategies for Optimizing Optical and Thermal Performance of PI Films
3.1. Main Chain Optimization Strategies
3.1.1. Adjusting Main Chain Rigidity
3.1.2. Hydrogen Bond Regulation in the Main Chain
3.1.3. Incorporation of Alicyclic Structures into the Main Chain
3.2. Side-Chain Optimization Strategies
3.2.1. Fluorinated Substituents
3.2.2. Tert-Butyl
3.3. Non-Coplanar Optimization Strategies
3.3.1. Spiro Structure
3.3.2. Cardo Structure
3.4. End Group Optimization Strategies
4. Practical Applications and Future Development Directions
- (1)
- Nanoparticle doping and organic polymer blending modification: Nanoparticle doping modification to prepare PI hybrid materials can provide PI with superior comprehensive optical and thermal performance. Our research team introduced non-coplanar bulky alicyclic structural units with electron-donating effects to reduce the rigidity of PI molecules and increase the electron cloud density of the carbonyl and nitrogen atoms on the imide ring. We incorporated inorganic nanoparticles with low resistivity, corona resistance, and thermal stability such as TiO2 and BaTiO3. Meanwhile, organic polymers such as nanocellulose (CNC) [91], polyperfluoroethylene propylene (FEP) [92], and polymethylmethacrylate (PMMA) [93] can be blended with PIs to alter the interfacial polarization and molecular arrangement, suppress space charges, and expand the application of PIs in high-performance capacitors, optoelectronic devices, etc. We compared the modification principles, advantages, disadvantages, and applications of hybrid modification and molecular structure optimization, as shown in Table 2 [94,95,96,97,98].
- (2)
- Intrinsic microporous PI membranes: Extensive studies should be addressed to further explore intrinsic microporous PI polymers, study the relationship between the microstructure of PI films and gas permeability selectivity, design special diamine or dianhydride monomers to control micropore size, distribution, and gas interactions in PI films, decrease ordered aggregation states, improve H2 selectivity in PI gas separation membranes, extend the applications to hydrogen production fields, and achieve greater economic, social, and ecological benefits. Microporous PI membranes for bio-medical application deserve in-depth research, as PIs have exhibited desired bio-compatibility.
- (3)
- Photosensitive polyimides (PSPIs): PSPIs combine excellent thermal stability, mechanical properties, and photosensitivity, and are primarily used in photoresists and electronic packaging. PSPI production is based on photosensitive PAA, whose imidization could be properly accelerated by promoters under optical irradiation. Chang et al. [99] developed a series of negative-type PSPIs based on PAA and a photobase generator (PBG), finding that the rigidity and transparency of the PAA/PI backbone play an important role in the sensitivity and contrast of PSPI. Future research should focus on introducing suitable photosensitive groups, optimizing preparation processes, and re-pondering PSPI mechanisms in the revolutionary chip fabrication process, expanding PSPI applications in upgraded microelectronics packaging, optoelectronic packaging, and other fields.
- (4)
- Machine learning (ML) applications: Data-driven machine learning (ML) can quickly predict material properties, accelerating the screening of new materials and molecular structure design [100]. ML’s generative inverse design networks can take target functions or properties as input, searching corresponding molecular structures, conducting quickly high-throughput screening of PI films with multiple properties, and predicting new PI films with superior comprehensive optical and thermal performance.
- (5)
- Film surface metal-coating technology: Metal coating technology on film surfaces can provide electrical pathways for components packaged on the film surface, expanding applications in optics, electronics, solar energy, and micropatterning fields. Common methods include copper-clad laminate (CCL) and dense, adhesive metal films generated on PI films through sputtering and other dry processes. However, CCL technology is challenging to apply in fine wearable and portable devices, and sputtering processes are costly process. Zhong et al. [101] proposed a new technology for fabricating high-precision copper conductive micropatterns on PI films by directly preparing conductive copper micropatterns through electroless plating, without expensive lithography equipment and complex vacuum environments, reducing PI coating costs. Future research should explore PI film surface structure modification and metal-coating technology to meet the needs for miniaturization and multifunctionality in wearable and portable devices.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.Q.; Long, Z.; Zhang, D. Research progress of high-performance polyimide electromagnetic shielding composites. Fine Chem. 2023, 40, 10–20+43. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.X.; Wei, X.Y.; Qi, H.R.; Wu, H.; Zhang, Y.; Liu, J.G.; Liu, Y.G. Preparation and properties of polyimide alignment films containing alkyl-substituted cyclobutene ring. J. Funct. Mater. 2020, 51, 12184–12191. [Google Scholar] [CrossRef]
- Dutta, A.; Bisoi, S.; Mukherjee, R.; Chatterjee, R.; Das, R.K.; Banerjee, S. Soluble polyimides with propeller shape triphenyl core for membrane based gas separation. J. Appl. Polym. Sci. 2018, 135, 46658. [Google Scholar] [CrossRef]
- Chen, H.S. Research on application of polyimide composite in microelectronics field. Plast. Sci. Technol. 2020, 48, 48–51. [Google Scholar] [CrossRef]
- Analysis of the Development Status of the Chinese Polyimide (PI) Film Industry in 2022. 2022. Available online: https://www.vzkoo.com (accessed on 8 January 2024).
- Li, J.X. Synthesis of High Heat-Resistant Polyimide. Master’s Thesis, Changchun University of Technology, Changchun, China, 2020. [Google Scholar]
- Zhou, D.R.; Yuan, L.L.; Hong, W.J.; Zhang, H.Y.; Hu, A.J.; Yang, S.Y. Molecular design of interpenetrating fluorinated polyimide network with enhanced high performance for heat-resistant matrix. Polymers 2019, 173, 66–79. [Google Scholar] [CrossRef]
- Li, L.R.; Mi, Z.C.; Chen, Z.J.; Peng, Y.; Peng, J.; Liang, J.; Ai, S.; Zhang, Z.H. Preparation and properties of polyimide/nano-titanium particles hybrid films. Eng. Plast. Appl. 2022, 50, 43–48. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhou, X.; Feng, X.; Yu, X.L.; Li, S.H.; Zhan, J.; Sun, X.Y.; Zhang, P.B.; Cui, J.; Guo, M.J.; et al. Polyimide films containing trifluoromethoxy groups with high comprehensive performance for flexible circuitry substrates. ACS Appl. Polym. Mater. 2022, 4, 5831–5839. [Google Scholar] [CrossRef]
- Bao, F.; Lei, H.; Zou, B.; Peng, W.; Qiu, L.H.; Ye, F.; Song, Y.H.; Qi, F.L.; Qiu, X.P.; Huang, M.J. Colorless polyimides derived from rigid trifluoromethyl-substituted triphenylenediamines. Polymer 2023, 273, 125883. [Google Scholar] [CrossRef]
- Bui, V.T.; Huynh, N.D.; Chau, N.M.; Kim, W.; Kim, H.; Oh, I.K.; Huynh, D.P.; Choi, D. High-temperature operatable triboelectric nanogenerator using microdome-patterned polyimide for self-powered sensors. Nano Energy 2022, 101, 107612. [Google Scholar] [CrossRef]
- Li, H.J.; Wang, S.X.; Kong, X.Y.; Yan, B.; Gong, M.; Lin, X.; Zhang, L.; Wang, D.R. Polyphenol-functionalized boron nitride nanosheet-reinforced sustainable polyimide dielectric films for antenna-in-package applications. ACS Appl. Electron. Mater. 2024, 6, 2667–2676. [Google Scholar] [CrossRef]
- Li, Y.L.; Jiang, S.R.; Li, F.; Chen, C. Research of aging test on high Tg colorless polyimide. J. Appl. Polym. Sci. 2024, 141, e55286. [Google Scholar] [CrossRef]
- Shu, C.; Wu, X.M.; Zhong, M.; Yan, D.Y.; Huang, W. The atomic oxygen resistant study of a transparent polyimide film containing phosphorus and fluorine. Appl. Surf. Sci. 2023, 631, 157562. [Google Scholar] [CrossRef]
- Okamoto, T.; Uehara, H. Partial discharge current measurements with small discharge gaps over polyimide film. IEEE Trans. Dielectr. Electr. Insul. 2022, 30, 158–164. [Google Scholar] [CrossRef]
- Wang, C.O.; Zhai, L.; Gao, M.Y.; Jia, Y.; Mo, S.; He, M.H.; Fan, L. Research progress in thermal expansion behavior of polyimide films. Sci. Sin. Chim. 2022, 52, 437–451. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, S.J.; Guo, J.Y.; Zhang, L.L.; Jiang, Y.H.; Liu, F. Synthesis and research of oxygen heterocyclic/cyclohexyl dianhydride with bulky structure. New Chem. Mater. 2018, 46, 112–114. [Google Scholar]
- Wang, K.J. Synthesis and Properties of Heat-Resistant Polyimide Materials. Master’s Thesis, Tianjin University, Tianjin, China, 2020. [Google Scholar]
- Wang, Z.K.; Li, Y.J.; Zhu, T.S.; Xiong, L.; Liu, F.; Qi, H.X. Conversion of renewable vanillin into high performance polyimides via an asymmetric aromatic diamine derivation. Polym. Degrad. Stab. 2019, 167, 67–76. [Google Scholar] [CrossRef]
- Qi, H.X.; Wang, X.L.; Zhu, T.; Li, J.; Xiong, L.; Liu, F. Low dielectric poly(imide siloxane) films enabled by a well-defined disiloxane-linked alkyl diamine. ACS Omega 2019, 4, 22143–22151. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.X.; Liu, Q.M.; Zhang, A.R.; Liu, F.; Zhang, N. Comparative studies on structure-property relationships of the polyimide films derived from the dianhydride containing alicyclic cis-ethylenic double bond. Macromol. Res. 2019, 27, 703–712. [Google Scholar] [CrossRef]
- Ma, P.C.; Dai, C.T.; Liu, H. High performance polyimide films containing benzimidazole moieties for thin film solar cells. e-Polym. 2019, 19, 555–562. [Google Scholar] [CrossRef]
- Liu, T.Q.; Zheng, F.; Ding, T.M.; Zhang, S.Y.; Lu, Q.H. Design and synthesis of a novel quinoxaline diamine and its polyimides with high-Tg and red color. Polymer 2019, 179, 121612. [Google Scholar] [CrossRef]
- Jeong, K.M.; Tapaswi, P.K.; Kambara, T.; Ishige, R.; Ando, S.; Ha, C.S. Photoconductive polyimides derived from a novel imidazole-containing diamine. High Perform. Polym. 2020, 32, 620–630. [Google Scholar] [CrossRef]
- Luo, F.; Lin, C.J.; Jiao, L.; Du, Z.J.; Dong, Z.X.; Dai, X.M.; Duan, X.Z.; Qiu, X.P. High glass transition temperature and ultra-low thermal expansion coefficient polyimide films containing rigid pyridine and bisbenzoxazole units. J. Polym. Sci. 2023, 61, 1289–1297. [Google Scholar] [CrossRef]
- Yang, Z.H.; Guo, H.Q.; Kang, C.; Gao, L.X. Synthesis and characterization of amide-bridged colorless polyimide films with low CTE and high optical performance for flexible OLED displays. Polym. Chem. 2021, 12, 5364–5376. [Google Scholar] [CrossRef]
- Zuo, H.T.; Qian, G.T.; Li, H.B.; Gan, F.; Fang, Y.T.; Li, X.T.; Dong, J.; Zhao, X.; Zhang, Q.H. Reduced coefficient of linear thermal expansion for colorless and transparent polyimide by introducing rigid-rod amide units: Synthesis and properties. Polym. Chem. 2022, 13, 2999–3008. [Google Scholar] [CrossRef]
- Lao, H.J.; Mushtaq, N.; Chen, G.; Wang, B.Y.; Ba, Y.X.; Fang, X.Z. Synthesis and properties of transparent random and multi-block polyamide-imide films with high modulus and low CTE. Eur. Polym. J. 2021, 153, 110512. [Google Scholar] [CrossRef]
- Cao, T.M.; Wang, X.; Zhang, R.B.; Chen, S.C.; Qi, H.; Yao, Y.Q.; Liu, F. Cooperative synergistic effects of multiple functional groups in amide-containing polyimides with pyridine ring and pendent tert-butyl. J. Polym. Sci. 2023, 61, 1584–1595. [Google Scholar] [CrossRef]
- Wang, Z.A.; Guo, L.Y.; Han, S.Y.; Qi, H.X.; Cheng, Y.S.; Liu, F. Polyimides from an asymmetric hydroxyl-containing aliphatic-aromatic diamine synthesized via henry reaction. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3413–3423. [Google Scholar] [CrossRef]
- Yang, Z.H.; Ma, P.C.; Li, F.R.; Guo, H.Q.; Kang, C.Q.; Gao, L.X. Ultrahigh thermal-stability polyimides with low CTE and required flexibility by formation of hydrogen bonds between poly (amic acid)s. Eur. Polym. J. 2021, 148, 110369. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Luo, L.B.; Yang, Q.Q.; Zhang, L.J.; Wang, M.; Wu, D.F.; Wang, X.; Liu, X.Y. Construction of stable hydrogen bonds at high temperature for preparation of polyimide films with ultralow coefficient of thermal expansion and high Tg. Polymer 2020, 188, 122100. [Google Scholar] [CrossRef]
- Lian, M.; Lu, X.M.; Lu, Q.H. Synthesis of superheat-resistant polyimides with high Tg and low coefficient of thermal expansion by introduction of strong intermolecular interaction. Macromolecules 2018, 51, 10127–10135. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent trends on transparent colorless polyimides with balanced thermal and optical properties: Design and synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Lee, B.; Kim, S.D.; Park, J.; Byun, T.; Kim, S.J.; Seo, M.; Kim, S.Y. Transparent poly(amide-imide)s containing trifluoromethyl groups with high glass transition temperature. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1782–1786. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, X.L.; Qi, H.X.; Liu, F. Synthesis of a new siloxane-containing alicyclic dianhydride and the derived polyimides with improved solubility and hydrophobicity. J. Appl. Polym. Sci. 2012, 127, 1493–1501. [Google Scholar] [CrossRef]
- Qi, H.X.; Liu, F.; Zhang, N.; Chen, Y.W.; Yang, H.L.; Wang, Z. Studies on high performance nonvolatile polyimides coating: Gamma ray initiated bulk copolymerization of vinyl polar monomer and maleimide-terminated polyimides with flexible backbone and the modifications. Prog. Org. Coat. 2012, 73, 33–41. [Google Scholar] [CrossRef]
- Zou, L.N.; Ge, Y.D.; Wang, G.R.; Fu, J.W.; Liu, F. Effect of annealing treatment on refractive index and optical propagation in fluorinated polyimide film waveguide. Optik 2015, 126, 2470–2473. [Google Scholar] [CrossRef]
- Wang, X.M.; Liu, F.; Lai, J.C.; Fu, Z.Q.; You, X.Z. Comparative investigations on the effects of pendent trifluoromethyl group to the properties of the polyimides containing diphenyl-substituted cyclopentyl cardo-structure. J. Fluorine Chem. 2014, 164, 27–37. [Google Scholar] [CrossRef]
- Xiong, L.; Huang, J.; Chen, X.T.; Yu, C.L.; Li, S.; Li, L.R. Research progress on synthesis and application of wholly aromatic soluble polyimides. Plast. Sci. Technol. 2022, 50, 123–128. [Google Scholar] [CrossRef]
- Xiong, L.; Li, S.; Chen, X.T.; Xiong, B.; Huang, J.; Hu, H.M. Research progress in synthesis and application of semi-alicyclic polyimides. Plast. Sci. Technol. 2022, 50, 117–122. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, X.T.; Huang, J.; Wu, M.L.; Liu, F.; Li, L.R. Synthesis and research of a novel hydrophobic polyimide film derived from diamine containing 7-oxonorbornene fused norcamphane. New Chem. Mater. 2022, 50, 102–105. [Google Scholar] [CrossRef]
- Xiong, L.; Xiong, B.; Li, S.; Liu, H.; Li, L.R. Synthesis and research of a series of novel polyimides copolymer derived from dianhydride containing. New Chem. Mater. 2023, 51, 100–103. [Google Scholar] [CrossRef]
- Zhang, H.S.; Li, J.; Tian, Z.L.; Liu, F. Synthesis and properties of novel alicyclic-functionalized polyimides prepared from natural-(d)-camphor. J. Appl. Polym. Sci. 2013, 129, 3333–3340. [Google Scholar] [CrossRef]
- Yi, L.; Huang, W.; Yan, D.Y. Polyimides with side groups: Synthesis and effects of side groups on their properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 533–559. [Google Scholar] [CrossRef]
- Wu, Z.F.; Yan, G.M.; Lu, J.H.; Zhang, G.; Yang, J. Thermal plastic and optical transparent polyimide derived from isophorone diamine and sulfhydryl compounds. Ind. Eng. Chem. Res. 2019, 58, 6992–7000. [Google Scholar] [CrossRef]
- Caldona, E.B.; Borrego, E.I.; Shelar, K.E.; Mukeba, K.M.; Smith, D.W., Jr. Ring-forming polymerization toward perfluorocyclobutyl and ortho-diynylarene-derived materials: From synthesis to practical applications. Materials 2021, 14, 1486. [Google Scholar] [CrossRef]
- Yi, L.; Li, C.Y.; Huang, W.; Yan, D.Y. Soluble and transparent polyimides with high tg from a new diamine containing tert-butyl and fluorene units. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 976–984. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jiang, C.R.; Yu, B.; Zhao, X.Y.; Cui, Z.L.; Li, J.; Ren, Q. Highly soluble polyimides containing di-tert-butylbenzene and dimethyl groups with good gas separation properties and optical transparency. Chin. J. Polym. Sci. 2020, 38, 759–768. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.K.; Wu, D.Y. Synthetic strategies for highly transparent and colorless polyimide film. J. Appl. Polym. Sci. 2022, 139, e52604. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.S.; Liu, F.; Lai, J.C.; Qi, H.X.; You, X.Z. A new series of fluorinated alicyclic-functionalized polyimides derivated from natural-(d)-camphor: Synthesis, structureeproperties relationships and dynamic dielectric analyses. Polymer 2013, 54, 5673–5683. [Google Scholar] [CrossRef]
- Min, H.G.; Kang, B.; Shin, Y.S.; Kim, B.S.; Lee, S.W.; Cho, J.H. Transparent and colorless polyimides containing multiple trifluoromethyl groups as gate insulators for flexible organic transistors with superior electrical stability. ACS Appl. Mater. Interfaces 2020, 12, 18739–18747. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhai, L.; Bai, L.; He, M.H.; Wang, C.G.; Mo, S.; Fan, L. Synthesis and characterization of optically transparent semi-aromatic polyimide films with low fluorine content. Polymer 2019, 163, 106–114. [Google Scholar] [CrossRef]
- Zhang, X.L.; Song, C.; Wei, M.H.; Huang, Z.Z.; Sheng, S.R. Organosoluble and transparent cardo polyimides with high tg derived from 9,9-bis(4-aminophenyl)xanthene. High Perform. Polym. 2019, 31, 909–918. [Google Scholar] [CrossRef]
- Rojas-Rodriguez, M.; Aguilar-Lugo, C.; Lozano, A.E.; Hernández, A.; Mancilla-Cetina, E.; Alexandrova, L. Synthesis and properties of highly processable asymmetric polyimides with bulky phenoxy groups. High Perform. Polym. 2020, 32, 455–468. [Google Scholar] [CrossRef]
- Jiang, C.R.; Wang, C.Y.; Yu, B.; Zhao, X.Y.; Li, J.; Ren, Q. New soluble polyamides with high transparence and improved gas separation properties. J. Macromol. Sci. Part A Pure Appl.Chem. 2020, 58, 44–51. [Google Scholar] [CrossRef]
- Bermejo, L.A.; Alvarez, C.; Maya, E.M.; Garcia, C.; de la Campa, J.G.; Lozano, A.E. Synthesis, characterization and gas separation properties of novel polyimides containing cardo and tert-butyl-m-terphenyl moieties. eXPRESS Polym. Lett. 2018, 12, 479–489. [Google Scholar] [CrossRef]
- Wu, X.M.; Shu, C.; He, X.Q.; Wang, S.B.; Fan, X.; Yu, Z.H.; Yan, D.Y.; Huang, W. Optically transparent and thermal-stable polyimide films derived from a semi-aliphatic diamine: Synthesis and properties. Macromol. Chem. Phys. 2020, 221, 1900506. [Google Scholar] [CrossRef]
- Sulub-Sulub, R.; Loría-Bastarrachea, M.I.; Vázquez-Torres, H.; Santiago-García, J.L.; Aguilar-Vega, M. Highly permeable polyimide membranes with a structural pyrene containing tert-butyl groups: Synthesis, characterization and gas transport. J. Membr. Sci. 2018, 563, 134–141. [Google Scholar] [CrossRef]
- Santiago-García, J.L.; Álvarez, C.; Sánchez, F.; de la Campa, J.G. Gas transport properties of new aromatic polyimides based on 3,8-diphenylpyrene-1,2,6,7-tetracarboxylic dianhydride. J. Membr. Sci. 2015, 476, 442–448. [Google Scholar] [CrossRef]
- Li, L.R.; Liu, F.H.; Ni, Z.C.; Lin, W.Q.; Jiang, W.D.; Xiong, L. Research progress on solubility of polyimide modified by molecular structure. Eng. Plast. Appl. 2022, 50, 136–142+148. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Amooghin, A.E.; Bandehali, S.; Moghadassi, A.; Matsuura, T.; Van der Bruggen, B. Polyimides in membrane gas separation: Monomer’s molecular design and structural engineering. Prog. Polym. Sci. 2019, 91, 80–125. [Google Scholar] [CrossRef]
- Butnaru, I.; Constantin, C.P.; Asandulesa, M.; Wolińska-Grabczyk, A.; Jankowski, A.; Szeluga, U.; Damaceanu, M.D. Insights into molecular engineering of membranes based on fluorinated polyimide-polyamide miscible blends which do not obey the trade-off rule. Sep. Purif. Technol. 2020, 233, 116031. [Google Scholar] [CrossRef]
- Sim, S.J.; Kang, J.H.; Lee, J.H.; Suh, D.H. Ultrafast biodegradation pathway of polyimides using aromatic diamine with two spiro moieties derived from camphor. J. Polym. Environ. 2023, 31, 825–831. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wei, J.N.; Chi, Y.; Zhang, X.; Zhang, W.X.; Xi, Z.F. Spiro metallaaromatics of Pd, Pt, Rh: Synthesis and characterization. J. Am. Chem. Soc. 2017, 139, 5039–5042. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Lu, Y.H.; Hao, J.C.; Wang, W.; Xaio, G.Y.; Dong, Y.; Hu, Z.Z.; Wang, T.H. Synthesis and properties of polyimides containing spiro structure. Polym. Mater. Sci. Eng. 2017, 33, 1–6. [Google Scholar] [CrossRef]
- Yu, H.C.; Kumar, S.V.; Lee, J.H.; Oh, S.Y.; Chung, C.M. Preparation of robust, flexible, transparent films from partially aliphatic copolyimides. Macromol. Res. 2015, 23, 566–573. [Google Scholar] [CrossRef]
- Yu, H.C.; Kim, M.Y.; Hong, M.; Nam, K.; Choi, J.Y.; Lee, K.H.; Baeck, K.K.; Kim, K.K.; Cho, S.; Chung, C.M. Fully transparent, non-volatile bipolar resistive memory based on flexible copolyimide films. Electron. Mater. Lett. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Fan, B.; Sun, D. Synthesis and properties of novel polyimides based on 2′,7′-bis(4- aminophenoxy)-spiro(4,5-diazafluorene-9,9′-xanthene). J. Macromol. Sci. Part A Pure Appl. Chem. 2021, 58, 811–819. [Google Scholar] [CrossRef]
- Korshak, V.V.; Vinogradova, S.V.; Vygodskii, Y.S. Cardo polymers. J. Macromol. Sci. Part C Polym. Rev. 1974, 11, 45–142. [Google Scholar] [CrossRef]
- Li, F.; Wan, W.J.; Lai, J.C.; Liu, F.; Qi, H.X.; Li, X.S.; You, X.Z. Investigations on the polyimides derived from unfunctionalized symmetric cyclopentyl-containing alicyclic cardo-type dianhydride. J. Appl. Polym. Sci. 2015, 132, 42670. [Google Scholar] [CrossRef]
- Tang, Y.M.; Li, L.; Ma, K.; Chen, G.F.; Wang, W.; Fang, X.Z. Transparent and organosoluble cardo polyimides with different trans/cis ratios of 1,4-diaminocyclohexane via aromatic nucleophilic substitution polymerization. Polym. Int. 2018, 67, 598–605. [Google Scholar] [CrossRef]
- Wu, Y.C.; Liu, S.M.; Chen, Z.G.; Zhao, J.Q. Synthesis and properties of cardo-type polyimides containing hydroxyl groups for application in specific detection of fluoride ion. Dyes Pigment. 2020, 173, 107924. [Google Scholar] [CrossRef]
- Yue, J.Y.; Huan, H.J.; Su, C.X.; Gao, Z.F.; Zhao, J.Y.; Gao, L.X. The optical properties of polyimide films improved by two-step end-capping method. Acta Polym. Sin. 2023, 54, 1229–1240. [Google Scholar] [CrossRef]
- Su, C.X.; Liu, P.J.; Yue, J.Y.; Huan, H.J.; Yang, Z.H.; Yang, K.; Guo, H.Q.; Zhao, J.Y. High-transparency and colorless polyimide film prepared by inhibiting the formation of chromophores. Polymers 2022, 14, 4242. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Q.; Ruan, K.P.; Shi, X.T.; Yang, X.T.; Gu, J.W. Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]
- Ruan, K.P.; Guo, Y.Q.; Gu, J.W. Liquid crystalline polyimide films with high intrinsic thermal conductivities and robust toughness. Macromolecules 2021, 54, 4934–4944. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhai, J.F.; Song, L.X.; Du, P.F.; Xiong, J. Research on the construction and properties of flexible light-transmitting nanofiber film electrode for solar cells. J. Silk 2021, 58, 27–31. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, D.J. Preparation and properties of a new colorless transparent polyimide film. Mod. Chem. Res. 2021, 1, 155–156. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, U.; Yoo, Y.; Byeon, J.; Lee, S.K.; Nam, J.S.; Kim, K.; Zhang, Q.; Kauppinen, E.I.; Maruyama, S.; et al. Foldable Perovskite solar cells using carbon nanotube-embedded ultrathin polyimide conductor. Adv. Sci. 2021, 8, 2004092. [Google Scholar] [CrossRef]
- O’ Harra, K.E.; Kammakakam, I.; Devriese, E.M.; Noll, D.M.; Bara, J.E.; Jackson, E.M. Synthesis and performance of 6 FDA-based polyimide-ionenes and composites with ionic liquids as gas separation membranes. Membranes 2019, 9, 79. [Google Scholar] [CrossRef]
- Ma, X.H.; Yang, S.Y. Polyimide gas separation membranes. Adv. Polyimide Mater. 2018, 6, 257–322. [Google Scholar] [CrossRef]
- Wang, Z.G.; Ren, H.T.; Zhang, S.X.; Zhang, F.; Jin, J. Carbon molecular sieve membranes derived from tröger’s base-based microporous polyimide for gas separation. ChemSusChem 2018, 11, 916–923. [Google Scholar] [CrossRef]
- Nimmy, J.V.; Cherumannil, K.S.; Varghese, S. Nano P(VDF-TrFE) doped polyimide alignment layers for twisted nematic liquid crystal devices. Liq. Cryst. 2020, 47, 36–41. [Google Scholar] [CrossRef]
- Epure, E.L.; Stoica, I.; Albu, R.M.; Hulubei, C.; Barzic, A.I. New strategy for inducing surface anisotropy in polyimide films for nematics orientation in display applications. Nanomaterials 2021, 11, 3107. [Google Scholar] [CrossRef]
- Lin, M.G. Ruihuatai: Holding the “Golden Film” Localized chips. Manager 2021, 8, 42–45. [Google Scholar]
- Chen, L.L.; Yu, H.; Dirican, M.; Fang, D.J.; Tian, Y.; Yan, C.Y.; Xie, J.Y.; Jia, D.M.; Liu, H.; Wang, J.S.; et al. Highly transparent and colorless nanocellulose/polyimide substrates with enhanced thermal and mechanical properties for flexible OLED displays. Adv. Mater. Interfaces 2020, 7, 2000928. [Google Scholar] [CrossRef]
- Cheng, T.J.; Lv, G.P.; Li, Y.T.; Yun, H.; Zhang, F.L.; Deng, Y.M.; Lin, L.P.; Lou, X.J.; Nan, J.M. Low dielectric polyimide/fluorinated ethylene propylene (PI/FEP) nanocomposite film for high-frequency flexible circuit board application. Macromol. Mater. Eng. 2021, 306, 2100086. [Google Scholar] [CrossRef]
- Vural, S.; Seckin, T. Brush-type surface modification of kapton with a new approach. Adv. Polym. Technol. 2018, 37, 1703–1711. [Google Scholar] [CrossRef]
- Shin, H.I.; Chang, J.H. Transparent polyimide/organoclay nanocomposite films containing different diamine monomers. Polymers 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ding, Y.; Huang, R.; Zhu, Z.; Fong, H.; Hou, H. High-performance polyimide nanofibers reinforced polyimide nanocomposite films fabricated by co-electrospinning followed by hot-pressing. J. Appl. Polym. Sci. 2018, 135, 46849. [Google Scholar] [CrossRef]
- Yin, X.L. Study on Flexible Organic Photovoltaic and Light-Emitting Device Based on Sliver Nanowires/Polyimide Electrode. Master’s Thesis, Jinan University, Guangzhou, China, 2019. [Google Scholar]
- Nguyen, T.H.L.; Cortes, L.Q.; Lonjon, A.; Dantras, E.; Lacabanne, C. High conductive ag nanowire–polyimide composites: Charge transport mechanism in thermoplastic thermostable materials. J. Non-Cryst. Solids 2014, 385, 34–39. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, X.W.; Li, X.; Jia, Y.J.; Zhi, X.X.; Yang, C.X.; Wang, X.L.; Liu, J.G. Preparation and properties of colorless and transparent polyimide-silica nanocomposite films. Insul. Mater. 2023, 56, 54–62. [Google Scholar] [CrossRef]
- Chang, E.C.; Tseng, L.Y.; Liu, Y.; Chen, C.K.; Kuo, C.C.; Ueda, M.; Lin, Y.C.; Chen, W.C. Investigating the structure–sensitivity relationship of photosensitive polyimide formulated by using a photobase generator. J. Polym. Sci. 2023, 61, 2122–2132. [Google Scholar] [CrossRef]
- Qi, X.Y.; Hu, Y.F.; Wang, R.Y.; Yang, Y.Q.; Zhao, Y.F. Recent advance of machine learning in selecting new materials. Acta Chim. Sinica 2023, 81, 158–174. [Google Scholar] [CrossRef]
- Zhong, S.L.; Zhou, B.Y.; Gu, X.R.; Yu, D.S.; Chen, X.D. Chin Palladium-assisted metal patterning on polyimide surfaces. Chin. J. Polym. Sci. 2022, 40, 1287–1296. [Google Scholar] [CrossRef]

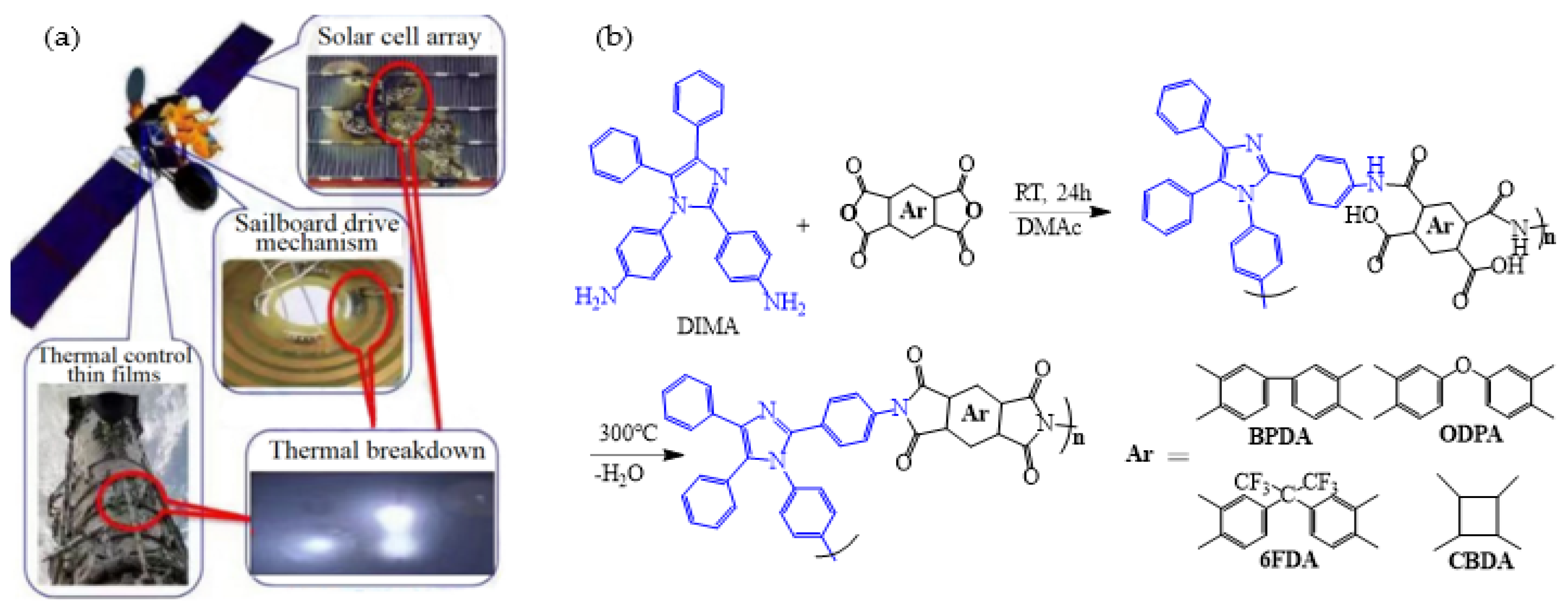
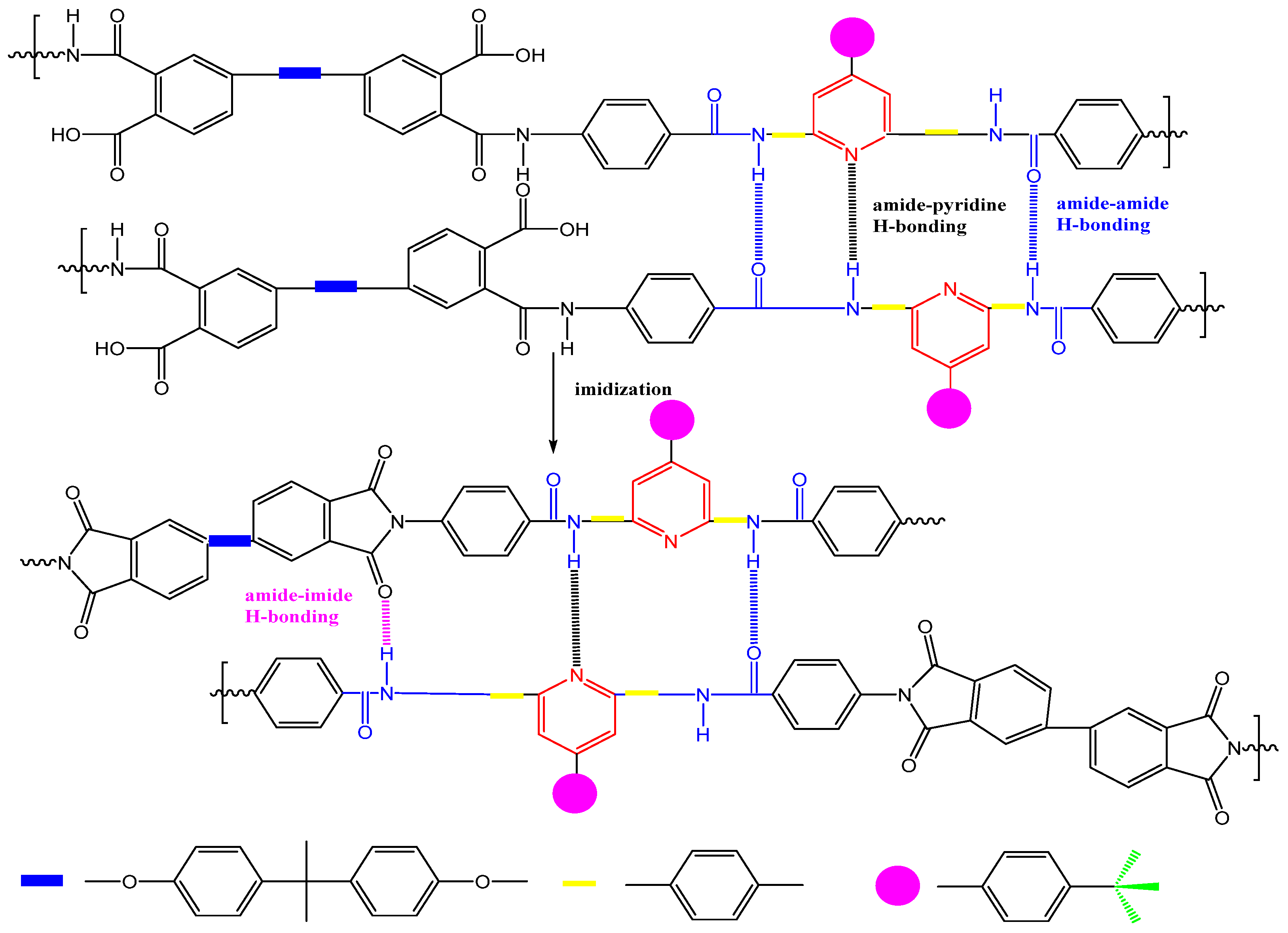
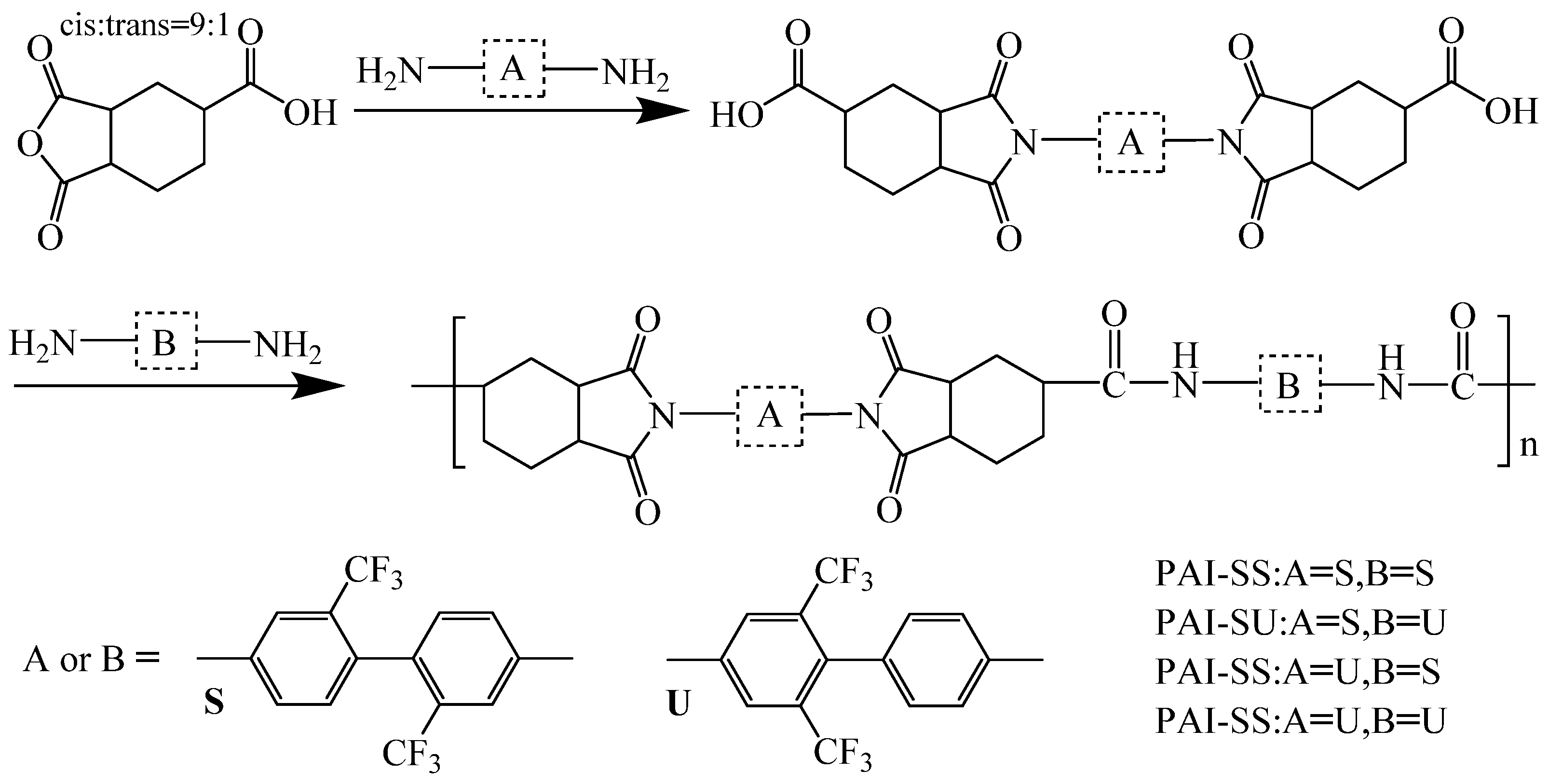
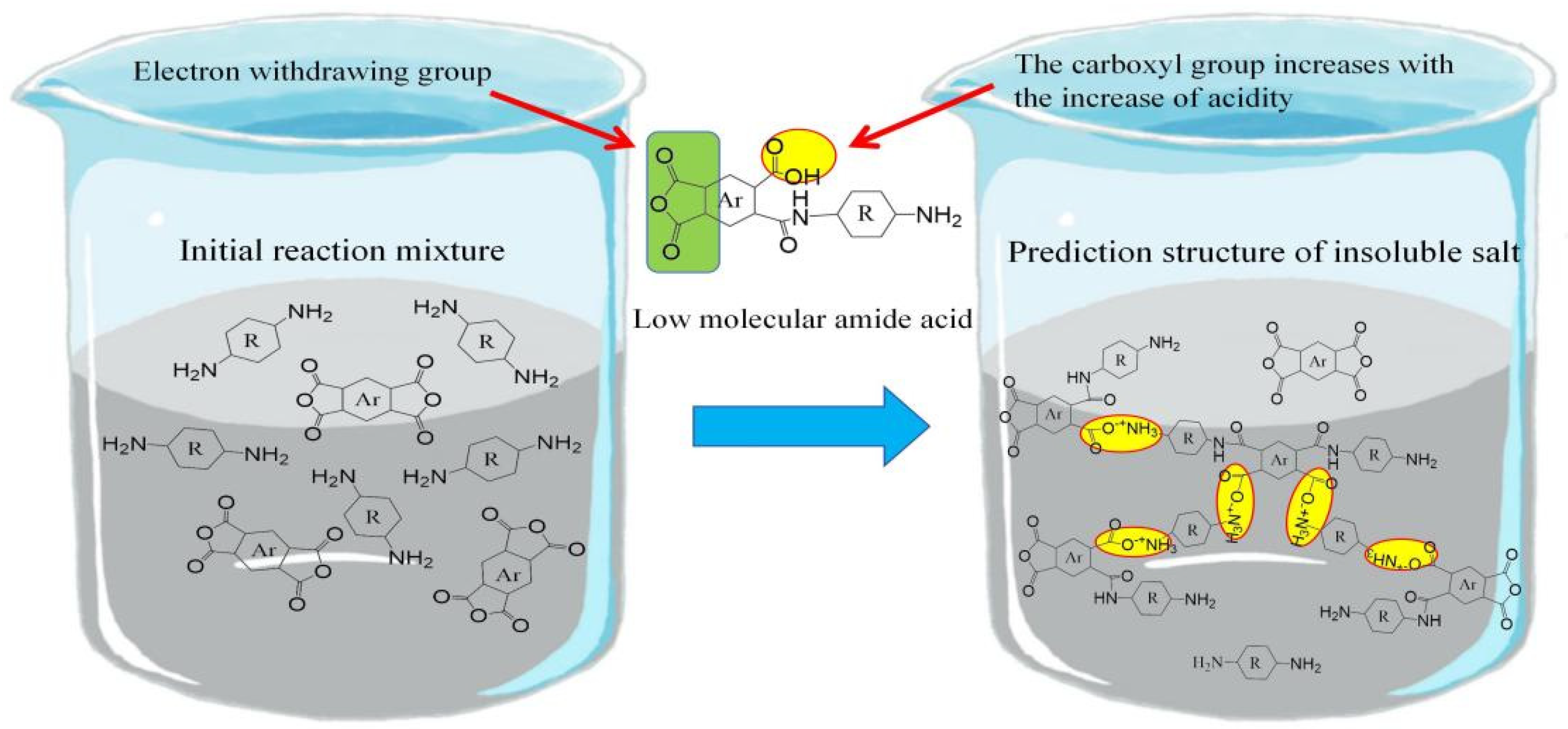
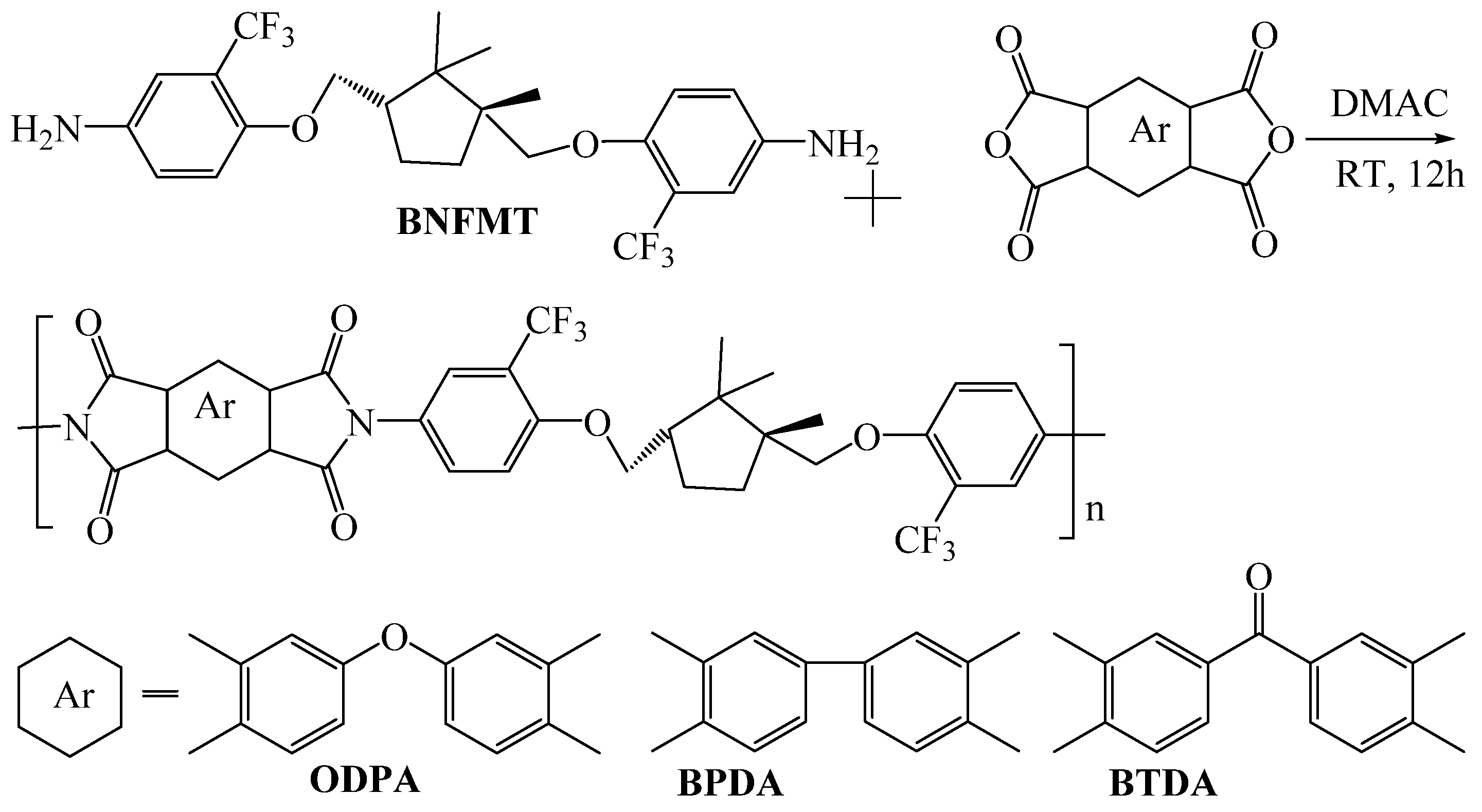

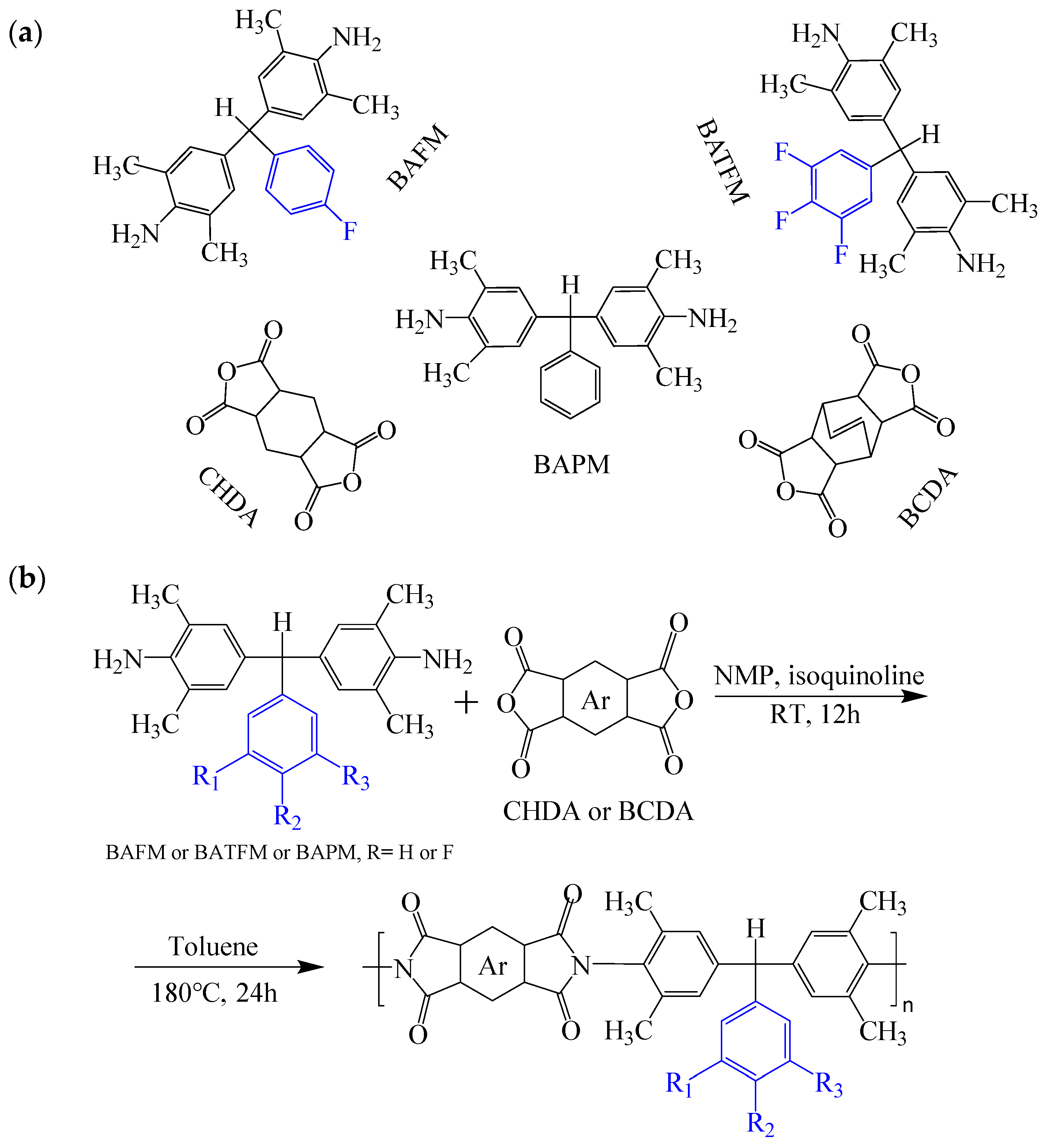


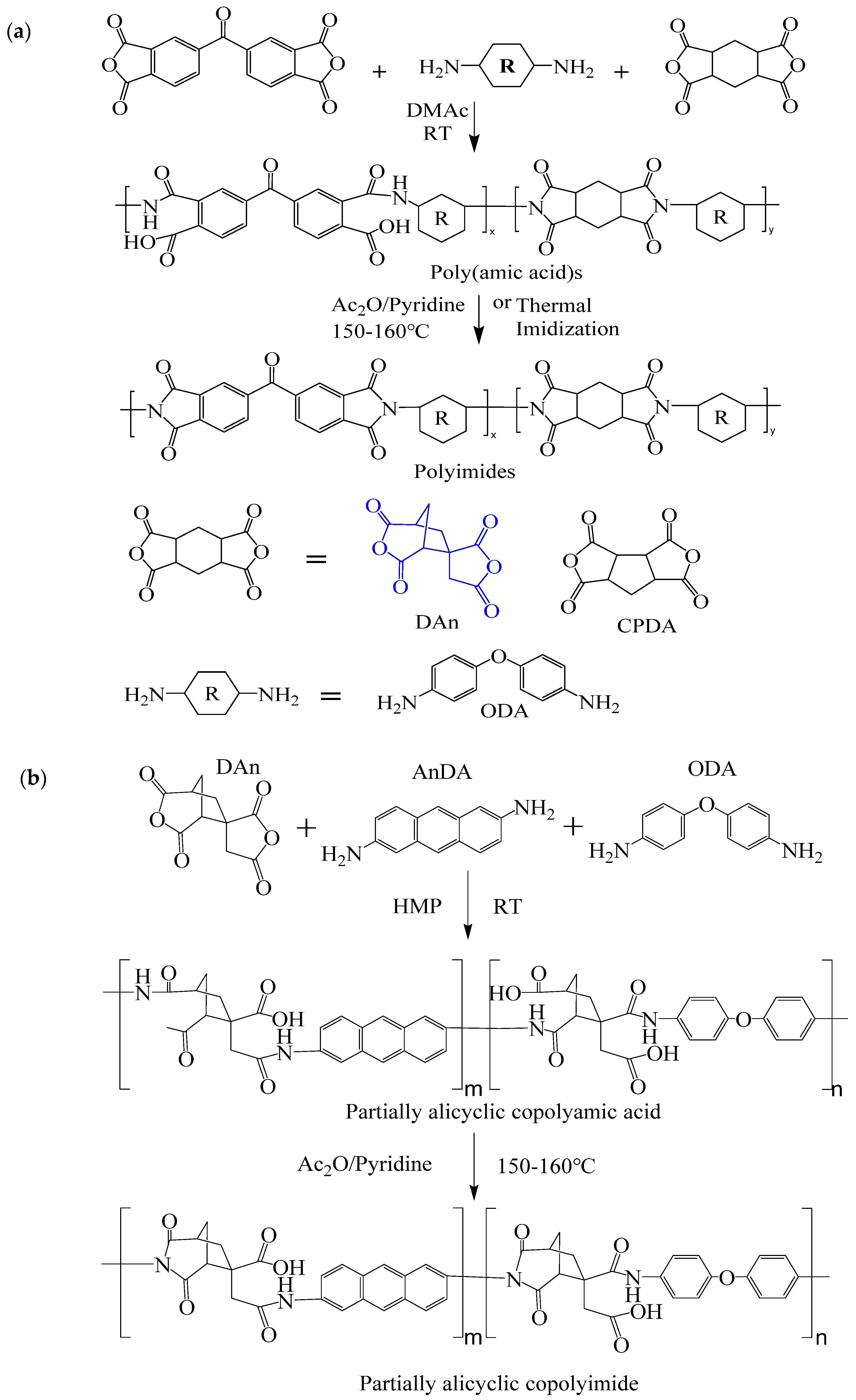
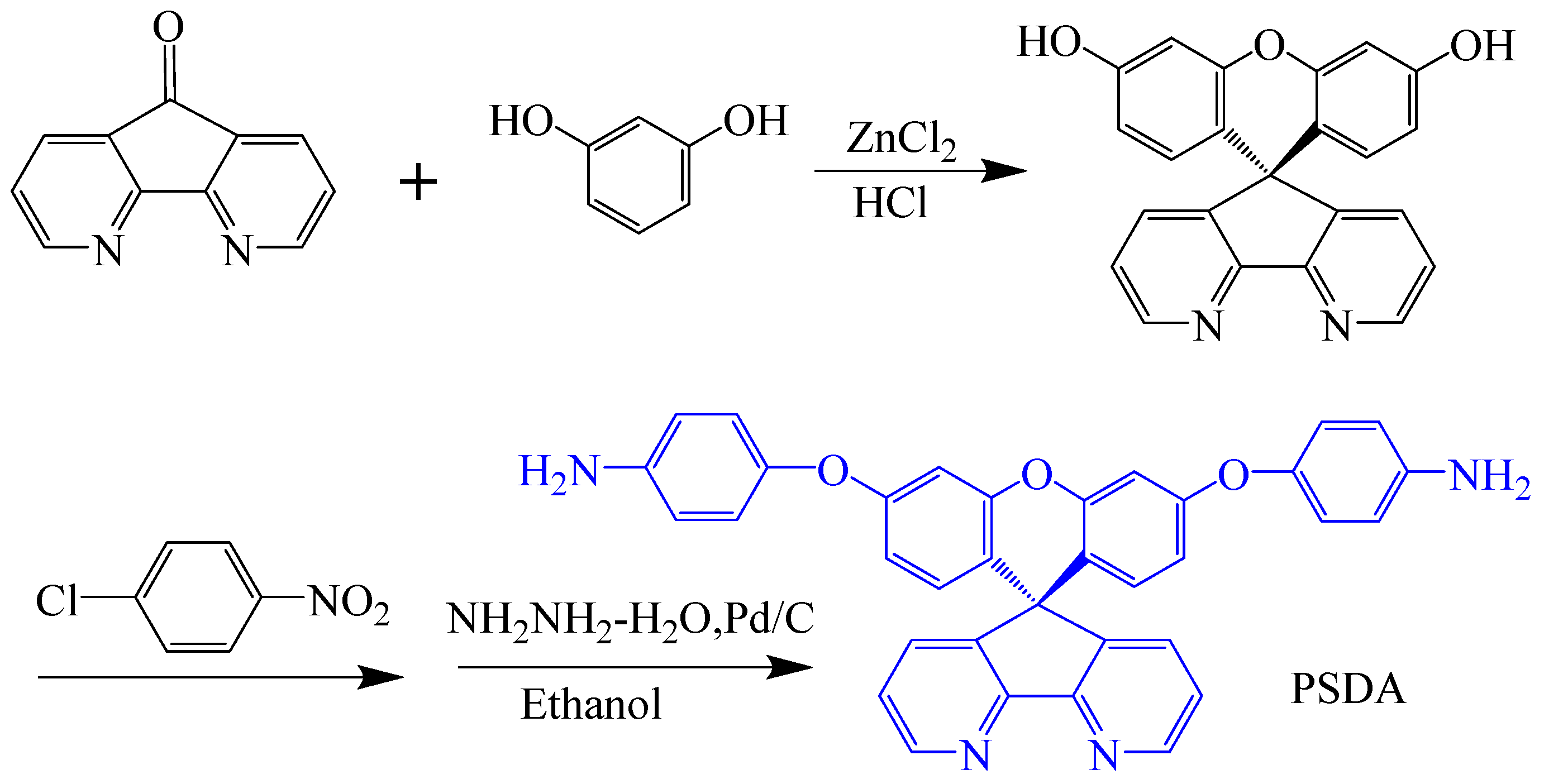
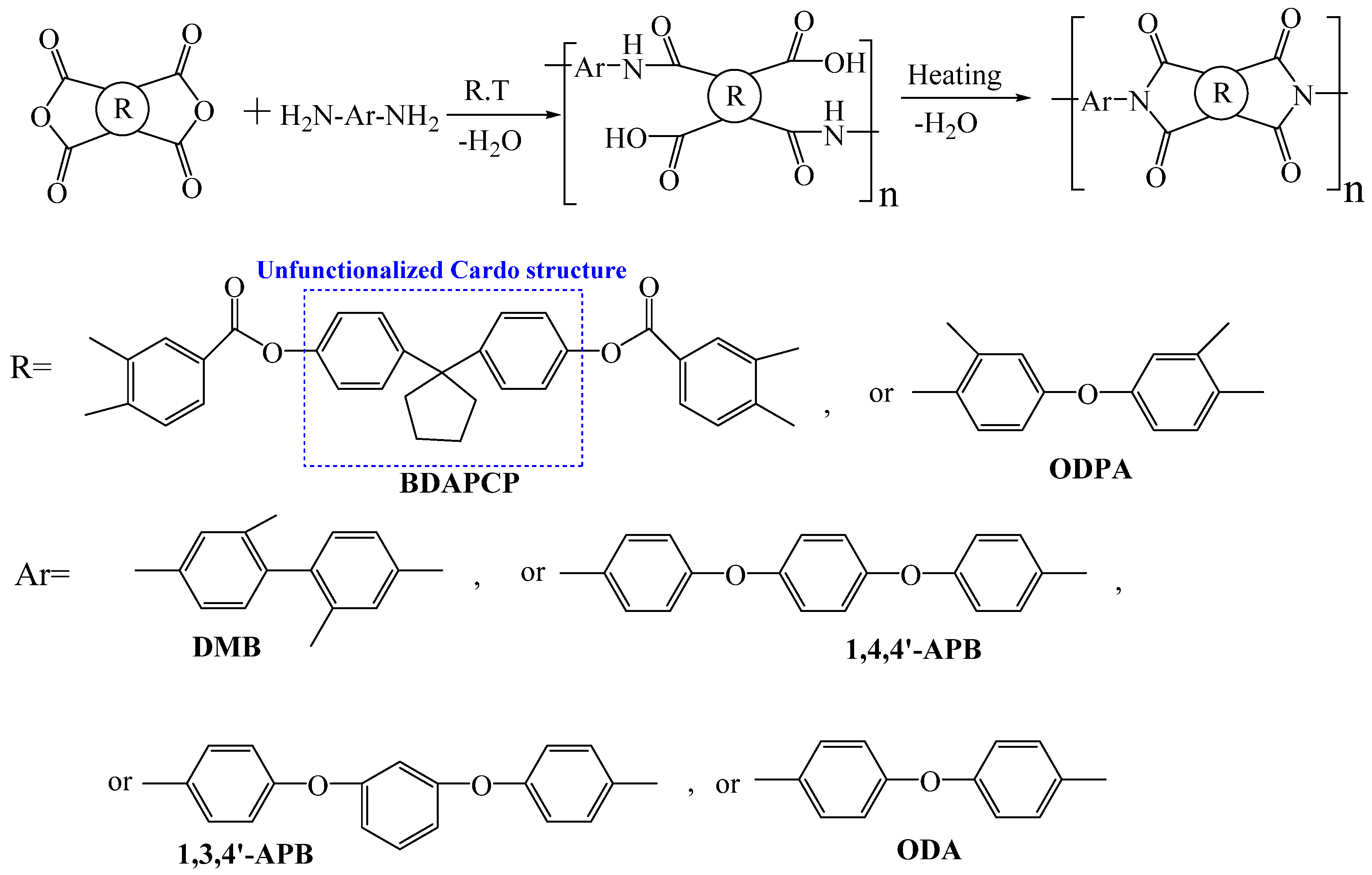

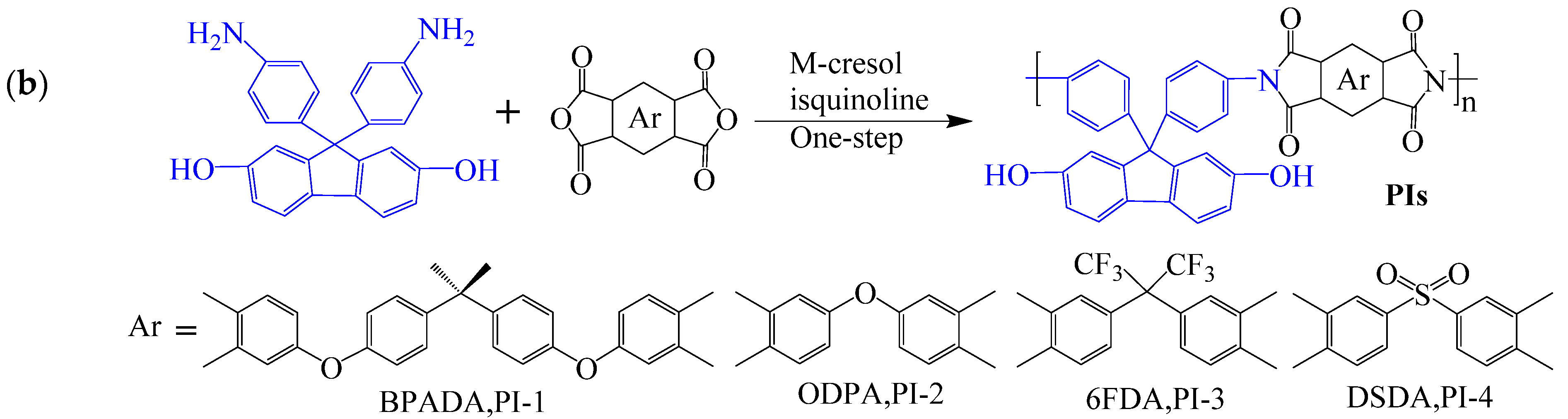
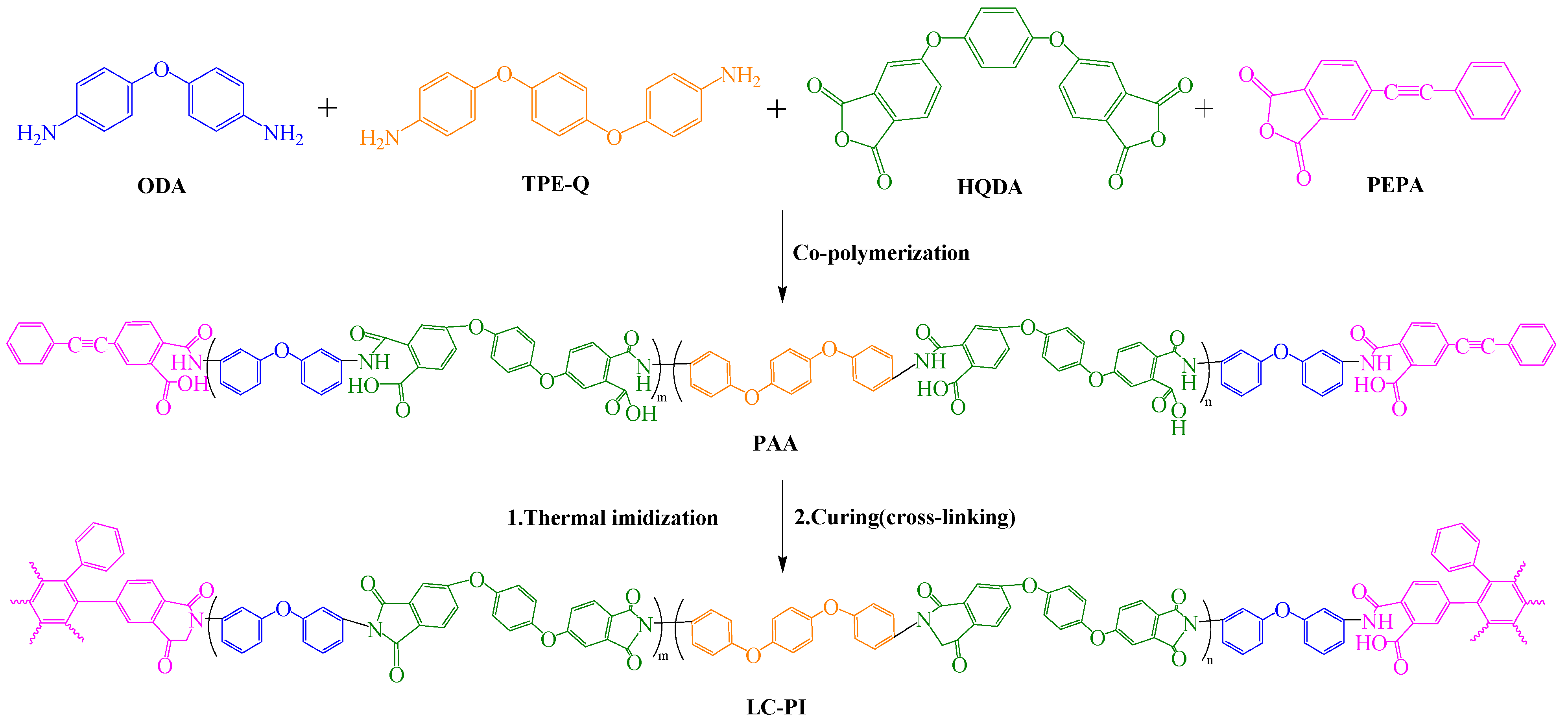
| Optimization Strategy | Optimized Structure | Strengths | Weaknesses | Representative Application | Ref. |
|---|---|---|---|---|---|
| Main-chain optimization | Rigid structure | Resonance of coplanar imide ring with aromatic rings, strong electron delocalization and interchain CT, excellent thermal and mechanical properties | Poor processability, low optical transparency | Solar cell flexible substrate, flexible copper clad laminate, electronic device heat dissipation | [23,24,25] |
| Hydrogen bond | Non-covalent interaction, amide and hydroxyl as two main hydrogen bonding groups, increasing the chain rigidity while maintaining the optical transparency. | Tend to dissociate under harsh working condition, potentially leading to instability in the performance of PI films | Substrate for flexible organic light-emitting diode (OLED) displays, FPC matrix film | [26,27,28,29,30,31,32] | |
| Flexible structure | Decreased mainchain coplanarity, weakened interchain CT, increased processibility, and improved optical transparency | Formation of insoluble nylon salt for alicyclic diamine, reduced thermal and mechanical performance | Plastic substrates for color filters, optical compensating films, optical fibers, wave guide, and optical lens | [35,36,37,38,39,40,41,42,43,44,45,46,47] | |
| Side-chain optimization | Fluorinated groups | Steric bulkiness and electron-withdrawing capability, reduced interchain CT, hydrophobic and nonpolarized properties leading to low water affinity and low dielectric constant | Difficult to achieve fluorinated PI monomers, high cost of fluorinated monomers | Flexible display panel, optical wave communication device, image display device, liquid crystal orientation layer | [51,52,53] |
| Tert-butyl | Increased steric hindrance, reduced chain packing, inhibited electron flow in the molecular chain, improved processibility and optical transparency | Complicated synthesis of new dianhydride and diamine monomer containing pendent t-butyl | Liquid crystal display, gas separation membrane | [53,54,55,56,57,58,59,60,61,62] | |
| Functional backbone structural optimization | Spiro structure | Highly twisted structure, deformed polymer chain, enlarged interchain space, improved optical transparency, improved gas permeability, maintained thermal properties | Easy to plasticize and age | High-temperature-resistant gas separation membrane | [68,69,70,71,72,73] |
| Cardo structure | Non-coplanar structure, weakened interchain CT, improved optical transparency, maintained thermal performances | Reduced chemical stability | Thin-film optoelectronic devices, chemical sensors | [75,76,77] | |
| Terminal group optimization | Functional capping structure | Strong electron-withdrawing groups capping structure leading to improved optical transparency, reactive capping agent leading to cross-linked and ordered macromolecular chains | Bringing new chromophore, reduced CT energy gap, needing to balance curing temperature and liquid crystal transition temperature | Flexible display substrate, high-temperature flexible electronics field | [78,79,80,81] |
| Strategy | Hybridization | Structural Optimization | |||||
|---|---|---|---|---|---|---|---|
| Classification | Organic Polymer | Inorganic Particle | Organic- Inorganic Hybrid Particles | Main-Chain Optimization | Side-Chain Optimization | Non-Coplanar Optimization | Terminal Groups Optimization |
| Strengths | Good compatibility with PI, robust mechanical properties of composites | Low resistivity, good corona resistance, and thermal stability | Rigidity, excellent thermal stability, and adjustable dielectric properties | Regulating the properties of PIs by modifying the rigidity and flexibility of polymer backbone | Enhancing steric hindrance, inhibiting electron delocation | Reducing the degree of coplanarity of PI molecular chains and optical absorption | Adjusting thermal stability and optical transparency of PI by controlling chain packing |
| Weaknesses | Reduced thermal stability when excessive doping | Poor compatibility and dispersion | Complicated preparation process and high cost | Difficult to balance the optical and thermal properties | Delicacy in balanced structural design | Lack of novel non-coplanar structure design | Introducing new chromophore, unmatched curing temperature |
| Application |  OLED |  Flexible device |  Integrated circuit package |  Solar cell |  Flexible substrate |  Gas separation membrane |  Flexible display devices |
| Monomer Structure | Full Name | Abbreviation | Ref. |
|---|---|---|---|
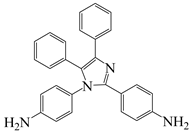 | 4,4′-(4,5-diphenyl-1 H-imidazole-1,2-diyl)dianiline | DIMA | [25] |
 | N,N-((4-(4-(tertbutyl)phenyl)pyridine-2,6-diyl)bis(1,4-phenylene))bis(4-aminobenzamide) | NTPA | [30] |
 | 2,5-bis(4-aminophenyl)pyridine | PRD | [32] |
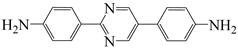 | 2,5-bis(4-aminophenyl)pyrimidine | PRM | [32] |
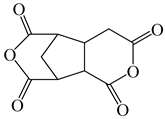 | 2,3,5-Tricarboxycyclopentylacetic dianhydride | TCA-AH | [35] |
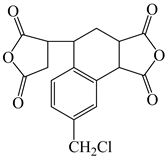 | 3,4-Dicarboxy-1,2,3,4-tetrahydro-6-chloro-methyl-1-naphthalene succinic dianhydride | CMTDA | [35] |
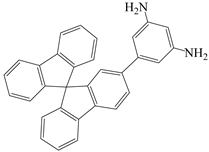 | 2-(3,5-diaminobenzene)-9,9′-spirobifluorene | 35 DABSBF | [36] |
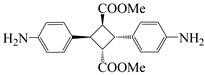 | 4,4′-diamino-α-truxilic acid | 4 ATA | [36] |
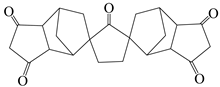 | Norbornane-2-spiro-α-cyclopentanone-α′-spiro-2′-norbornane-5,5′,6,6′-tetracarboxylicdianhy-dride | CPODA | [36] |
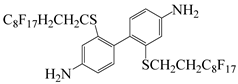 | 2,2′bis((1 H,1 H,2 H,2 Hperfluorodecyl)thio)[1,1′-biphenyl]4,4′-diamine | BPFBD | [36] |
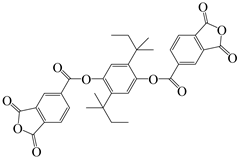 | 2,5-di-tert-butylhydroquinone | 25 DBHQ | [36] |
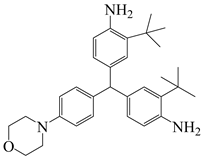 | 3,3′-di(tert-butyl)-4,4′-diaminodiphenyl-4′-morpholinophenylmethane | TAMPM | [36] |
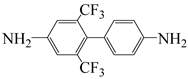 | 2,6-bis(trifluoromethyl)benzidine | TFMT | [37] |
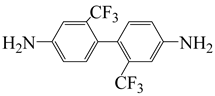 | 2,2′-bis(trifluoromethyl)benzidine | TFDB | [37] |
 | (+)-cis-1,3-bis(4-amino-2(trifluoromethyl)phenoxylmethylene)-1,2,2-trimethylcyclopentane | BAFMT | [54] |
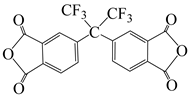 | 2,2′-bis-(3,4-dicarboxyphenyl)hexafluoro-propane dianhydride | 6 FDA | [55] |
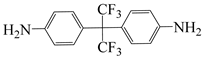 | 4,4′-(hexafluoroisopropylidene)dianiline | 6 FDAM | [55] |
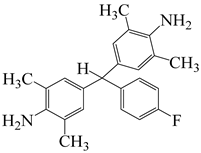 | α,α-bis(4-amino-3,5-dimethylphenyl)-1-(4′-fluorophenyl)methane | BAFM | [56] |
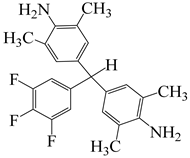 | α,α-bis(4-amino-3,5-dimethyl-phenyl)-1-(3′,4′,5′-trifluorophenyl)methane | BATFM | [56] |
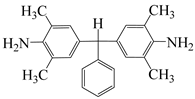 | α,α-bis(4-amino-3,5-dimethylphenyl)-1-phenylmethane | BAPM | [56] |
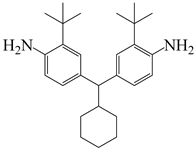 | 4,4′-(cyclohexylmethylene)bis(2-(tert-butyl)aniline) | CHMBTBA | [61] |
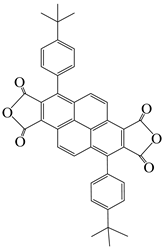 | 3,8-di(4-tert-butylphenyl)pyrene-1,2,6,7-tetracarboxylic dianhydride | DPt | [62] |
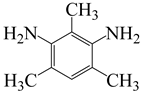 | 2,4,6-trimethyl-m-phenylenediamine | TMPD | [62] |
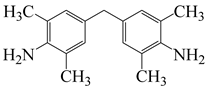 | 4,4′-methylenebis(2,6-dimethylaniline) | MBDAM | [62] |
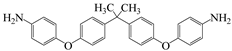 | 4,4′-((propane-2,2-diylbis(4,1-phenylene))bis(oxy))dianiline | BAPHF | [62] |
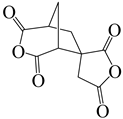 | (1′R,3 S,5′S)-spiro[furan-3(2 H),6′-[3]oxabicyclo [3.2.1]octane]-2,2′,4′,5(4 H)-tetrone | DAn | [71] |
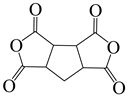 | 1,2,3,4-cyclopentanetetra carboxylic dianhydride | CPDA | [71] |
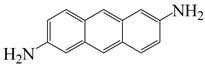 | 2,6-diaminoanthracene | AnDA | [72] |
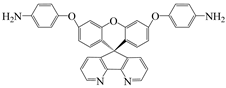 | 2′,7′-bis(4-aminophenoxy)-spiro(4,5-diazafluoren-9,9′-xanthene) | PSDA | [73] |
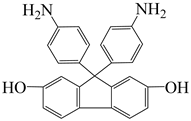 | 9,9-bis(4-aminophenyl)-2,7-dihydroxy-fluorene | AHF | [77] |
 | 4,4′-(4,4′-Isopropylidenediphenoxy) diphthalic anhydride | BPADA | [77] |
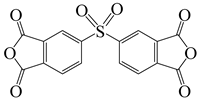 | 3,3′,4,4′-diphenylsulfonetetracarboxylic dianhydride | DSDA | [77] |
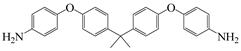 | 4,4′-((propane-2,2-diylbis(4,1-phenylene))bis(oxy))dianiline | BAPP | [79] |
 | 3,3′-sulfonyldianiline | APS | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Jiang, W.; Yang, X.; Meng, Y.; Hu, P.; Huang, C.; Liu, F. From Molecular Design to Practical Applications: Strategies for Enhancing the Optical and Thermal Performance of Polyimide Films. Polymers 2024, 16, 2315. https://doi.org/10.3390/polym16162315
Li L, Jiang W, Yang X, Meng Y, Hu P, Huang C, Liu F. From Molecular Design to Practical Applications: Strategies for Enhancing the Optical and Thermal Performance of Polyimide Films. Polymers. 2024; 16(16):2315. https://doi.org/10.3390/polym16162315
Chicago/Turabian StyleLi, Liangrong, Wendan Jiang, Xiaozhe Yang, Yundong Meng, Peng Hu, Cheng Huang, and Feng Liu. 2024. "From Molecular Design to Practical Applications: Strategies for Enhancing the Optical and Thermal Performance of Polyimide Films" Polymers 16, no. 16: 2315. https://doi.org/10.3390/polym16162315
APA StyleLi, L., Jiang, W., Yang, X., Meng, Y., Hu, P., Huang, C., & Liu, F. (2024). From Molecular Design to Practical Applications: Strategies for Enhancing the Optical and Thermal Performance of Polyimide Films. Polymers, 16(16), 2315. https://doi.org/10.3390/polym16162315






