An Up-to-Date Overview of Liquid Crystals and Liquid Crystal Polymers for Different Applications: A Review
Abstract
1. Introduction
2. Liquid Crystals: An Overview
2.1. Lyotropic Liquid Crystals
2.2. Thermotropic Liquid Crystals
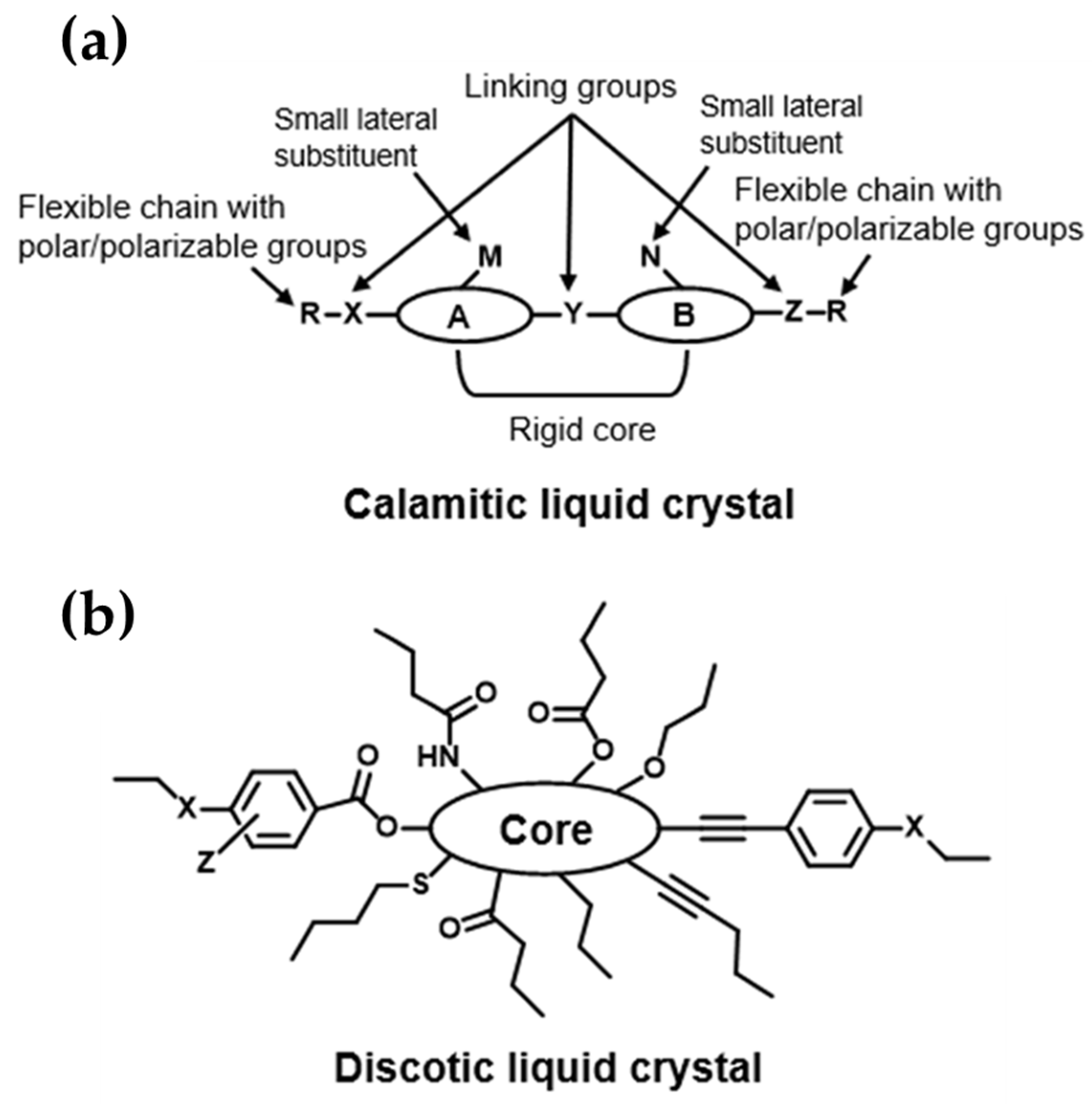
2.2.1. Calamitic Liquid Crystals
2.2.2. Discotic Liquid Crystals
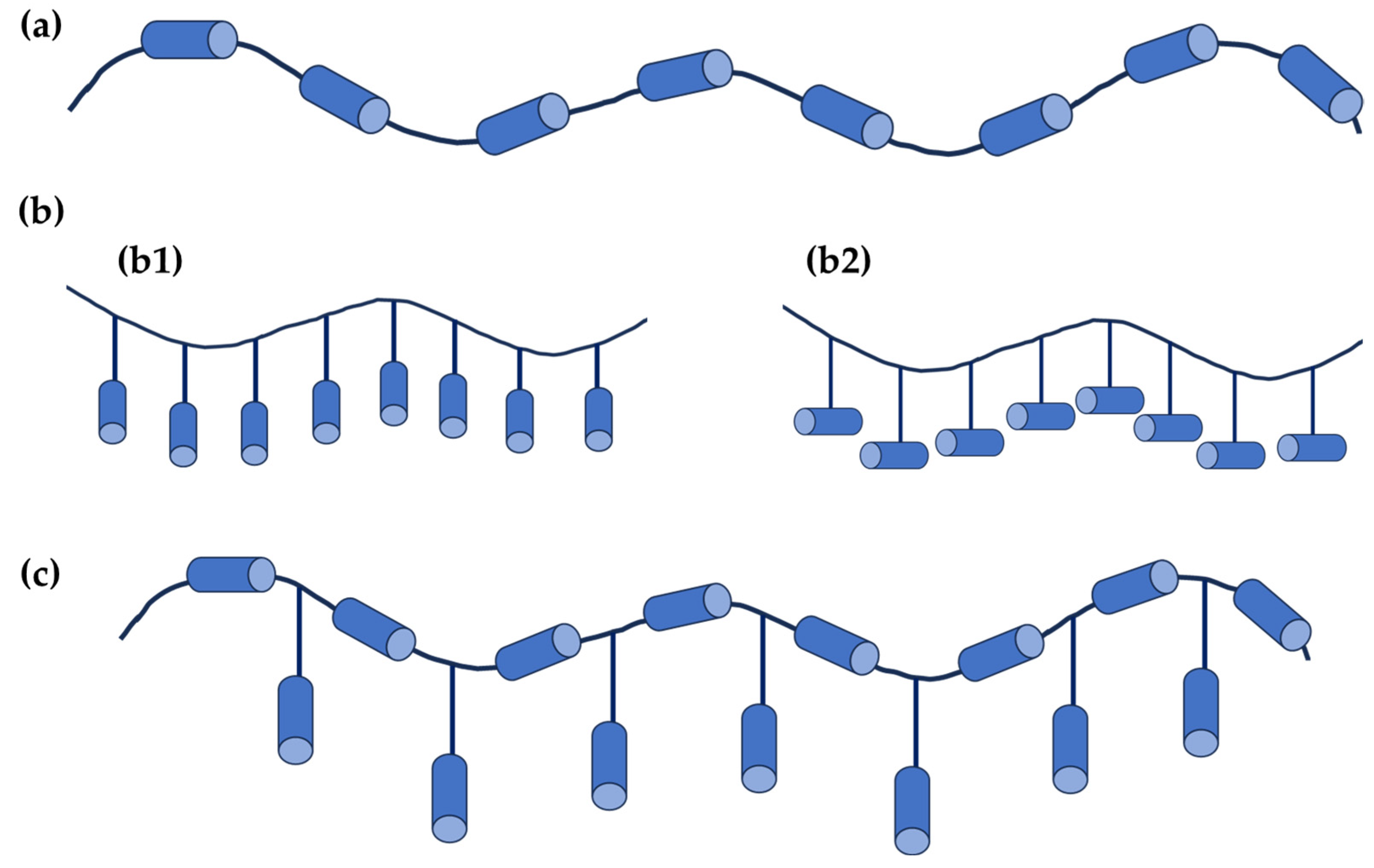
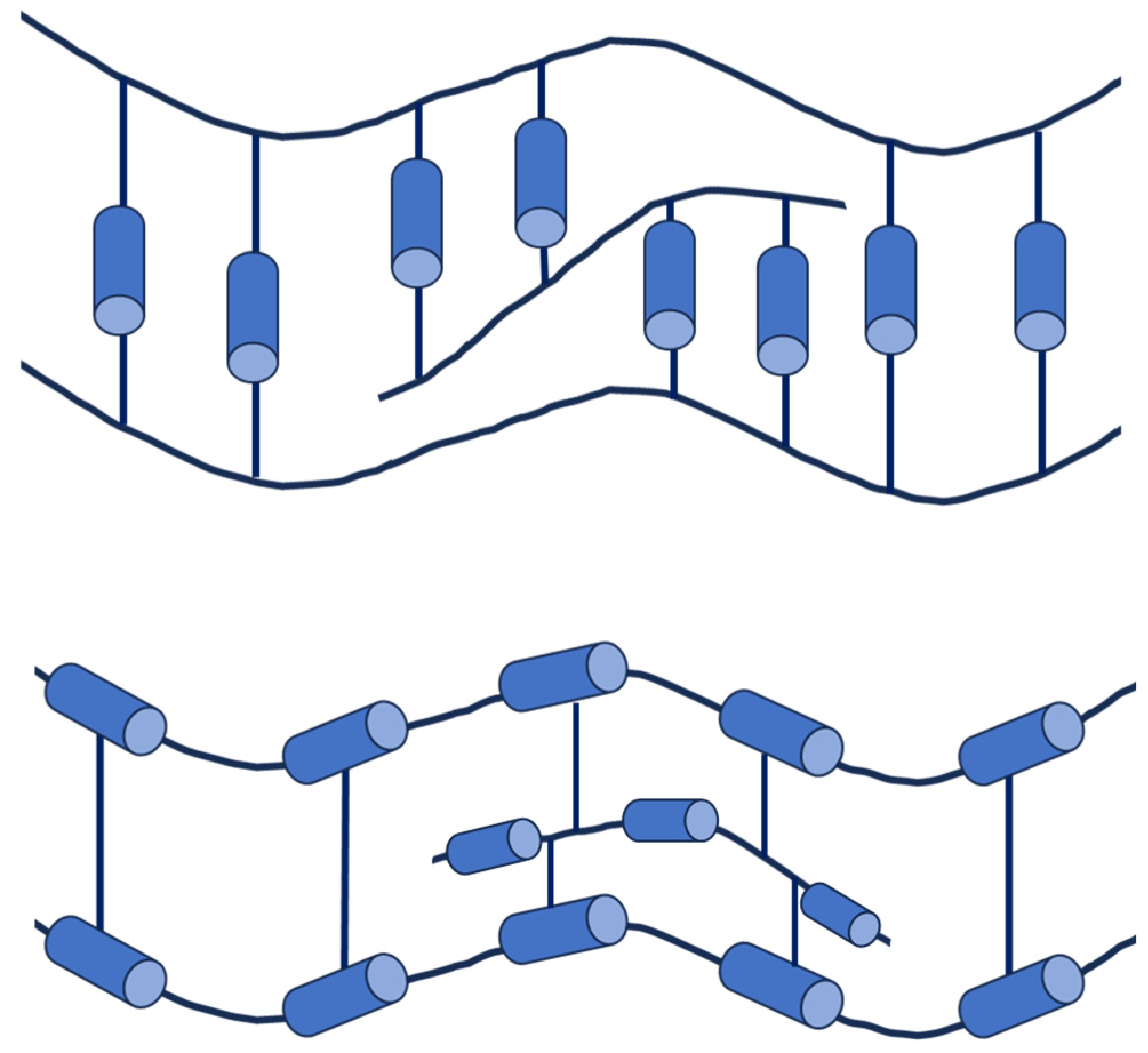
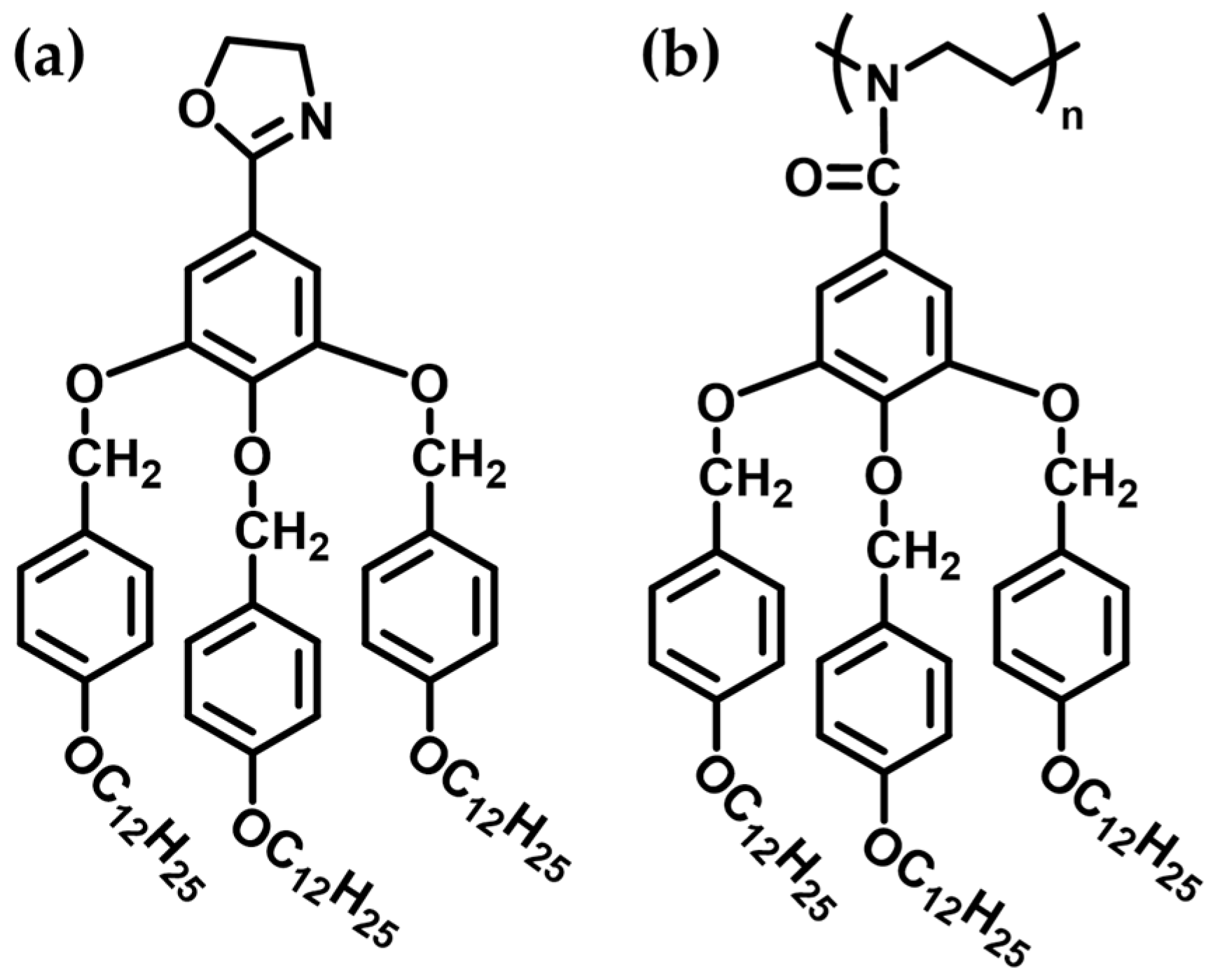
3. Liquid Crystal Polymers
4. Liquid Crystal Polymer-Based Membranes
4.1. Lyotropic Liquid Crystal Polymer-Based Membranes
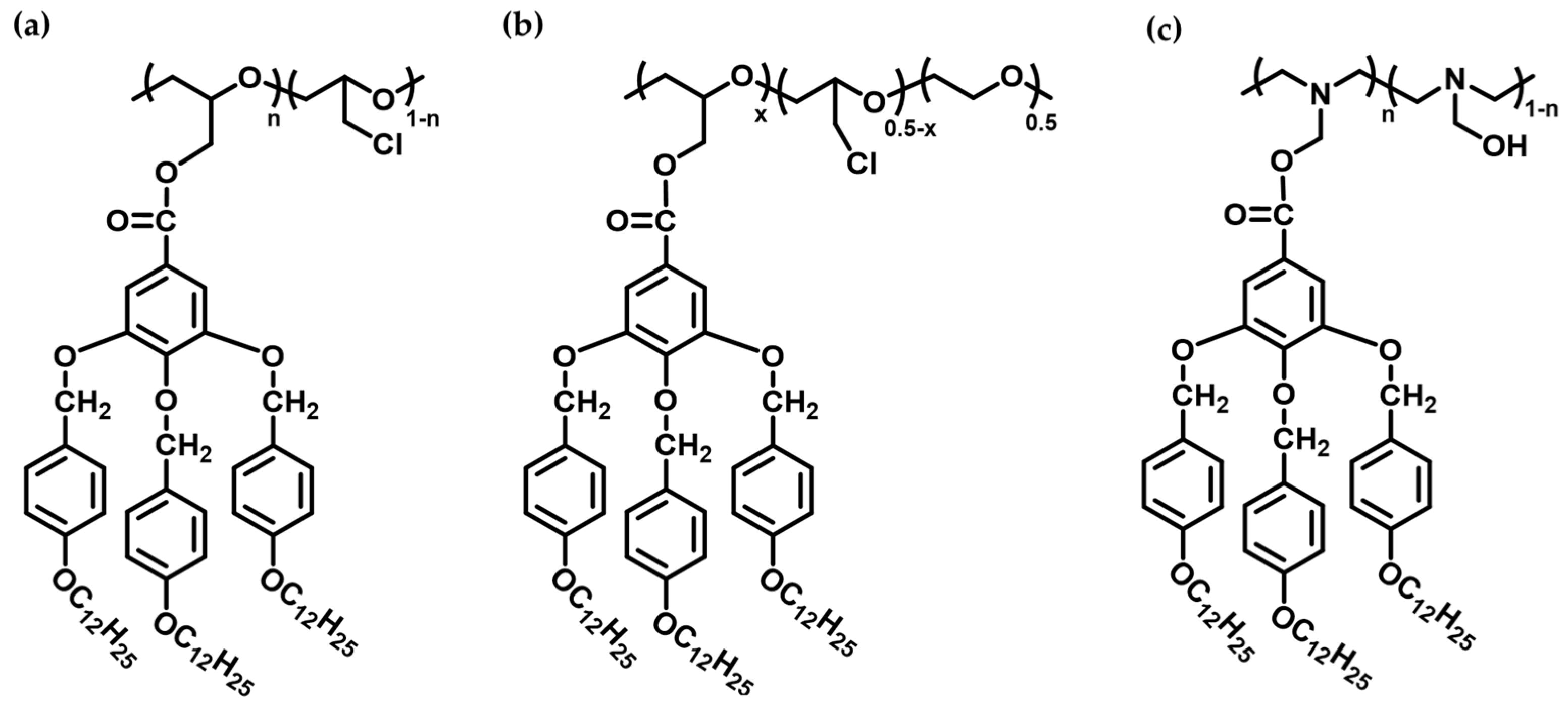
4.2. Thermotropic Liquid Crystal Polymer-Based Membranes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Höök, M.; Li, J.; Johansson, K.; Snowden, S. Growth Rates of Global Energy Systems and Future Outlooks. Nat. Resour. Res. 2012, 21, 23–41. [Google Scholar] [CrossRef]
- Wittmann, V.; Meissner, D. The Nexus of Global Challenges and Global Studies: A Trans-Disciplinary Global Sustainability Science Curriculum. Discov. Sustain. 2024, 5, 43. [Google Scholar] [CrossRef]
- Kweku, D.; Bismark, O.; Maxwell, A.; Desmond, K.; Danso, K.; Oti-Mensah, E.; Quachie, A.; Adormaa, B. Greenhouse Effect: Greenhouse Gases and Their Impact on Global Warming. J. Sci. Res. Rep. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Ramanathan, V.; Feng, Y. Air Pollution, Greenhouse Gases and Climate Change: Global and Regional Perspectives. Atmos. Environ. 2009, 43, 37–50. [Google Scholar] [CrossRef]
- Tranter, B.; Booth, K. Scepticism in a Changing Climate: A Cross-National Study. Glob. Environ. Chang. 2015, 33, 154–164. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Yeh, S.-C. The Trends of Major Issues Connecting Climate Change and the Sustainable Development Goals. Discov. Sustain. 2024, 5, 31. [Google Scholar] [CrossRef]
- Running, K. World Citizenship and Concern for Global Warming: Building the Case for a Strong International Civil Society. Soc. Forces 2013, 92, 377–399. [Google Scholar] [CrossRef]
- Deschênes, O.; Moretti, E. Extreme Weather Events, Mortality, and Migration. Rev. Econ. Stat. 2009, 91, 659–681. [Google Scholar] [CrossRef]
- Jatav, S.S. Farmers’ Perception of Climate Change and Livelihood Vulnerability: A Comparative Study of Bundelkhand and Central Regions of Uttar Pradesh, India. Discov. Sustain. 2024, 5, 11. [Google Scholar] [CrossRef]
- Chuong, H.N.; Loc, T.T.; Tuyen, T.L.T.; Ngoc, B.H. Livelihood Transitions in Rural Vietnam under Climate Change Effects in the Period of 2008–2018. Discov. Sustain. 2024, 5, 5. [Google Scholar] [CrossRef]
- Idawati, I.; Sasongko, N.A.; Santoso, A.D.; Septiani, M.; Handayani, T.; Sakti, A.Y.N.; Purnamasari, B.D. Cocoa Farmers’ Characteristics on Climate Variability and Its Effects on Climate Change Adaptation Strategy. Glob. J. Environ. Sci. Manag. 2024, 10, 337–354. [Google Scholar] [CrossRef]
- Dalei, N.N.; Gupta, A. Adoption of Renewable Energy to Phase down Fossil Fuel Energy Consumption and Mitigate Territorial Emissions: Evidence from BRICS Group Countries Using Panel FGLS and Panel GEE Models. Discov. Sustain. 2024, 5, 52. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An Overview of Fuel Cell Technology: Fundamentals and Applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Deluca, N.W.; Elabd, Y.A. Polymer Electrolyte Membranes for the Direct Methanol Fuel Cell: A Review. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2201–2225. [Google Scholar] [CrossRef]
- Aminudin, M.A.; Kamarudin, S.K.; Lim, B.H.; Majilan, E.H.; Masdar, M.S.; Shaari, N. An Overview: Current Progress on Hydrogen Fuel Cell Vehicles. Int. J. Hydrog. Energy 2023, 48, 4371–4388. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Recent Development of Polymer Electrolyte Membranes for Fuel Cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of Ions and Electrons in Nanostructured Liquid Crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Nie, Z.Z.; Zuo, B.; Liu, L.; Wang, M.; Huang, S.; Chen, X.M.; Yang, H. Nanoporous Supramolecular Liquid Crystal Polymeric Material for Specific and Selective Uptake of Melamine. Macromolecules 2020, 53, 4204–4213. [Google Scholar] [CrossRef]
- Carlton, R.J.; Hunter, J.T.; Miller, D.S.; Abbasi, R.; Mushenheim, P.C.; Tan, L.N.; Abbott, N.L. Chemical and Biological Sensing Using Liquid Crystals. Liq. Cryst. Rev. 2013, 1, 29–51. [Google Scholar] [CrossRef]
- Kaafarani, B.R. Discotic Liquid Crystals for Opto-Electronic Applications. Chem. Mater. 2011, 23, 378–396. [Google Scholar] [CrossRef]
- Šakalyte, A.; Reina, J.A.; Giamberini, M. Liquid Crystalline Polyamines Containing Side Dendrons: Toward the Building of Ion Channels Based on Polyamines. Polymer 2013, 54, 5133–5140. [Google Scholar] [CrossRef]
- Tylkowski, B.; Castelao, N.; Giamberini, M.; Garcia-Valls, R.; Reina, J.A.; Gumí, T. The Importance of Orientation in Proton Transport of a Polymer Film Based on an Oriented Self-Organized Columnar Liquid-Crystalline Polyether. Mater. Sci. Eng. C 2012, 32, 105–111. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Rapsilber, G.A.; Reina, J.A.; Giamberini, M. Liquid Crystalline Polymeric Wires for Selective Proton Transport, Part 1: Wires Preparation. Polymer 2016, 92, 50–57. [Google Scholar] [CrossRef]
- Zare, A.; Pascual-Jose, B.; De la Flor, S.; Ribes-Greus, A.; Montané, X.; Reina, J.A.; Giamberini, M. Membranes for Cation Transport Based on Dendronized Poly(Epichlorohydrin-Co-Ethylene Oxide). Part 1: The Effect of Dendron Amount and Column Orientation on Copolymer Mobility. Polymers 2021, 13, 3532. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Bhosale, S.V.; Li, Y.; Vankelecom, I.F.J.; Garcia-Valls, R.; Reina, J.A.; Giamberini, M. Mimicking Nature: Biomimetic Ionic Channels. J. Memb. Sci. 2016, 509, 10–18. [Google Scholar] [CrossRef]
- Montané, X.; Bogdanowicz, K.A.; Colace, G.; Reina, J.A.; Cerruti, P.; Lederer, A.; Giamberini, M. Advances in the Design of Self-Supported Ion-Conducting Membranes-New Family of Columnar Liquid Crystalline Polyamines. Part 1: Copolymer Synthesis and Membrane Preparation. Polymer 2016, 105, 298–309. [Google Scholar] [CrossRef]
- Montané, X.; Bogdanowicz, K.A.; Prats-Reig, J.; Colace, G.; Reina, J.A.; Giamberini, M. Advances in the Design of Self-Supported Ion-Conducting Membranes—New Family of Columnar Liquid Crystalline Polyamines. Part 2: Ion Transport Characterisation and Comparison to Hybrid Membranes. Polymer 2016, 105, 234–242. [Google Scholar] [CrossRef]
- Zare, A.; Montané, X.; Reina, J.A.; Giamberini, M. Membranes for Cation Transport Based on Dendronized Poly(Epichlorohydrin-Co-Ethylene Oxide). Part 2: Membrane Characterization and Transport Properties. Polymers 2021, 13, 3915. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Sistat, P.; Reina, J.A.; Giamberini, M. Liquid Crystalline Polymeric Wires for Selective Proton Transport, Part 2: Ion Transport in Solid-State. Polymer 2016, 92, 58–65. [Google Scholar] [CrossRef]
- Šakalyte, A.; Giamberini, M.; Reina, J.A. Synthesis and Characterisation of a Monotropic Dendritic Liquid Crystalline Aziridine Monomer. Liq. Cryst. 2014, 41, 153–162. [Google Scholar] [CrossRef]
- Guardià, J.; Zare, A.; Eleeza, J.; Giamberini, M.; Reina, J.A.; Montané, X. Synthesis and Characterization of Dendritic Compounds Containing Nitrogen: Monomer Precursors in the Construction of Biomimetic Membranes. Sci. Rep. 2022, 12, 1725. [Google Scholar] [CrossRef] [PubMed]
- Guardià, J.; Giamberini, M.; Reina, J.A.; Montané, X. Synthesis and Characterization of Dendronized Side Chain Liquid Crystalline Poly(2-Oxazoline)s towards Biomimetic Ion Channels. Eur. Polym. J. 2023, 196, 112273. [Google Scholar] [CrossRef]
- Montané, X.; Bhosale, S.V.; Reina, J.A.; Giamberini, M. Columnar Liquid Crystalline Polyglycidol Derivatives: A Novel Alternative for Proton-Conducting Membranes. Polymer 2015, 66, 100–109. [Google Scholar] [CrossRef]
- Giamberini, M.; Ronda, J.C.; Reina, J.A. Poly(Epichlorohydrin) Modified with 3,4,5-Tris(Dodecyloxy)Benzoate: The Structure and Dynamics of the Aliphatic Side Chains in the Columnar Mesophase. J. Polym. Sci. A Polym. Chem. 2005, 43, 2099–2111. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Rasool, M.A.; Reina, J.A.; Giamberini, M. New Liquid Crystalline Columnar Poly(Epichlorohydrin-Co-Ethylene Oxide) Derivatives Leading to Biomimetic Ion Channels. Polym. Eng. Sci. 2013, 53, 159–167. [Google Scholar] [CrossRef]
- Giamberini, M.; Reina, J.A.; Ronda, J.C. Influence of the Side Group Shape on the Arrangement of Liquid-Crystalline Polyethers Obtained by Ring Opening Polymerization of Oxiranes. J. Polym. Sci. A Polym. Chem. 2006, 44, 1722–1733. [Google Scholar] [CrossRef]
- Percec, V.; Holerca, M.N.; Magonov, S.N.; Yeardley, D.J.P.; Ungar, G.; Duan, H.; Hudson, S.D. Poly(Oxazolines)s with Tapered Minidendritic Side Groups. The Simplest Cylindrical Models to Investigate the Formation of Two-Dimensional and Three-Dimensional Order by Direct Visualization. Biomacromolecules 2001, 2, 706–728. [Google Scholar] [CrossRef]
- Rapp, A.; Schnell, I.; Sebastiani, D.; Brown, S.P.; Percec, V.; Spiess, H.W. Supramolecular Assembly of Dendritic Polymers Elucidated by 1 H and 13 C Solid-State MAS NMR Spectroscopy. J. Am. Chem. Soc. 2003, 125, 13284–13297. [Google Scholar] [CrossRef]
- Percec, V.; Glodde, M.; Bera, T.K.; Miura, Y.; Shiyanovskaya, I.; Singer, K.D.; Balagurusamy, V.S.K.; Heiney, P.A.; Schnell, I.; Rapp, A.; et al. Self-Organization of Supramolecular Helical Dendrimers into Complex Electronic Materials. Nature 2002, 417, 384–387. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Han, J.; Yang, L.; Li, J.; Song, Z.; Wang, T.; Zhu, J. Advanced Liquid Crystal-Based Switchable Optical Devices for Light Protection Applications: Principles and Strategies. Light Sci. Appl. 2023, 12, 11. [Google Scholar] [CrossRef]
- Surampudi, A.; Zhang, G.; Singh, R.; Faulkner, G.; O’Brien, D.C.; Booth, M.J.; Morris, S.M. Liquid Crystals for Luminescent Concentrators: A Review. Crystals 2023, 13, 1615. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, D.; Choi, J.K.; Oh, K.S.; Cho, E.; Im, J.H.; Singh, D.P.; Kim, Y.K. Stimuli-Responsive Materials from Liquid Crystals. ACS Appl. Opt. Mater. 2023, 1, 1879–1897. [Google Scholar] [CrossRef]
- Tsuei, M.; Shivrayan, M.; Kim, Y.K.; Thayumanavan, S.; Abbott, N.L. Optical “Blinking” Triggered by Collisions of Single Supramolecular Assemblies of Amphiphilic Molecules with Interfaces of Liquid Crystals. J. Am. Chem. Soc. 2020, 142, 6139–6148. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Huang, Y.; Tsuei, M.; Wang, X.; Gianneschi, N.C.; Abbott, N.L. Multi-Scale Responses of Liquid Crystals Triggered by Interfacial Assemblies of Cleavable Homopolymers. ChemPhysChem 2018, 19, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Deng, Y.; Shi, Y. Cellulose-Based Stimuli-Responsive Anisotropic Hydrogel for Sensor Applications. ACS Appl. Nano Mater. 2023, 6, 11524–11530. [Google Scholar] [CrossRef]
- Devadiga, D.; Tantri Nagaraja, A.; Devadiga, D.; Selvakumar, M. Minireview and Perspectives of Liquid Crystals in Perovskite Solar Cells. Energy Fuels 2024, 38, 854–868. [Google Scholar] [CrossRef]
- Chavda, V.P.; Dyawanapelly, S.; Dawre, S.; Ferreira-Faria, I.; Bezbaruah, R.; Rani Gogoi, N.; Kolimi, P.; Dave, D.J.; Paiva-Santos, A.C.; Vora, L.K. Lyotropic Liquid Crystalline Phases: Drug Delivery and Biomedical Applications. Int. J. Pharm. 2023, 647, 123546. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Pispas, S.; Tseti, I.K.; Demetzos, C. Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms. Pharmaceuticals 2022, 15, 429. [Google Scholar] [CrossRef] [PubMed]
- Zatloukalova, M.; Poltorak, L.; Bilewicz, R.; Vacek, J. Lipid-Based Liquid Crystalline Materials in Electrochemical Sensing and Nanocarrier Technology. Microchim. Acta 2023, 190, 187. [Google Scholar] [CrossRef]
- Akram, A.; Shahzady, T.G.; Hussain, S.; Saad, N.A.; Islam, M.T.; Ikram, M. Liquid Crystal Polymers: Overview of Characteristics and Applications in Communication and Biomedical Technologies. Russ. J. Appl. Chem. 2021, 94, 1585–1593. [Google Scholar] [CrossRef]
- Gin, D.L.; Pecinovsky, C.S.; Bara, J.E.; Kerr, R.L. Functional Lyotropic Liquid Crystal Materials. Struct. Bond. 2007, 128, 181–222. [Google Scholar] [CrossRef]
- Mezzenga, R.; Seddon, J.M.; Drummond, C.J.; Boyd, B.J.; Schröder-Turk, G.E.; Sagalowicz, L. Nature-Inspired Design and Application of Lipidic Lyotropic Liquid Crystals. Adv. Mater. 2019, 31, 1900818. [Google Scholar] [CrossRef] [PubMed]
- Dierking, I.; Neto, A.M.F. Novel Trends in Lyotropic Liquid Crystals. Crystals 2020, 10, 604. [Google Scholar] [CrossRef]
- Stepulane, A.; Ahlgren, K.; Rodriguez-Palomo, A.; Rajasekharan, A.K.; Andersson, M. Lyotropic Liquid Crystal Elastomers for Drug Delivery. Colloids Surf. B Biointerfaces 2023, 226, 113304. [Google Scholar] [CrossRef]
- Chavda, V.P.; Jogi, G.; Paiva-Santos, A.C.; Kaushik, A. Biodegradable and Removable Implants for Controlled Drug Delivery and Release Application. Expert Opin. Drug Deliv. 2022, 19, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cheng, D.; Lu, Y.; Rong, R.; Kong, Y.; Wang, X.; Niu, B. A Liquid Crystal in Situ Gel Based on Rotigotine for the Treatment of Parkinson’s Disease. Drug Deliv. Transl. Res. 2024, 14, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.; Wang, H.; Wen, P.; Xu, G.; Tian, F.; Bian, F.; Zeng, X. The Development of an Artificial Skin Membrane Mimicking the Lipid Bilayer Structure for in Vitro Permeation Study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128609. [Google Scholar] [CrossRef]
- Araz, O.U.; Kemiklioglu, E.; Gurboga, B. Modeling of the Lyotropic Cholesteric Liquid Crystal Based Toxic Gas Sensor Using Adaptive Neuro-Fuzzy Inference Systems. Expert Syst. Appl. 2024, 240, 122326. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Li, J.; Zeng, X. Stable Graphene Oxide-Based Lyotropic Liquid Crystals for Interfacial Lubrication. Friction 2024, 12, 954–967. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J.; Yang, S. Liquid Crystalline Reduced Graphene Oxide Composite Fibers as Artificial Muscles. Mater. Today 2023, 69, 19–30. [Google Scholar] [CrossRef]
- Wu, X.; Xu, C.; Zhang, Z. Preparation and Optimization of Si3N4 Ceramic Slurry for Low-Cost LCD Mask Stereolithography. Ceram. Int. 2021, 47, 9400–9408. [Google Scholar] [CrossRef]
- Gong, X.; Lv, G.Q.; Li, J.J.; Hu, Z.A.; Wang, Z.; Feng, Q. Bin. Analyzing the Effect of Temperature on Optical Performance of Dual-Layer Liquid Crystal Displays. J. Soc. Inf. Disp. 2021, 29, 537–546. [Google Scholar] [CrossRef]
- da Silva, E.R.; Hinojosa, A.R.C.; Eccher, J.; Tonet, M.D.; Brondani, D.; Zapp, E.; Curcio, S.F.; Postacchini, B.B.; Cazati, T.; Vieira, A.A. Strongly Luminescent and Liquid-Crystalline π-Conjugated 2-Methyl[1,2,3]Benzotriazoles with a Linear Donor-Acceptor-Donor Structure. J. Mol. Liq. 2020, 314, 113616. [Google Scholar] [CrossRef]
- Kuczyński, W.; Hoffmann, J.; Dardas, D.; Nowicka, K.; Bielejewska, N. Flexo- and Piezo-Electric Polarization of Smectic Layers in Ferroelectric and Antiferroelectric Liquid Crystals. Appl. Phys. Lett. 2015, 107, 191908. [Google Scholar] [CrossRef]
- Hoffmann, J.; Nowicka, K.; Kuczyński, W.; Bielejewska, N. Experimental Evidence of Soft Mode in the Smectic Cα∗ Phase of Chiral Ferroelectric Liquid Crystals. Soft Matter 2014, 10, 8548–8557. [Google Scholar] [CrossRef]
- Pozhidaev, E.P.; Torgova, S.I.; Budynina, E.M.; Tkachenko, T.P.; Kuznetsov, A.V.; Barbashov, V.A. Ferroelectric Smectic C* Phase with Sub-Wavelength Helix Pitch Induced in a Nematic Liquid Crystal by Chiral Non-Mesogenic Dopants. Zhidkie Krist. Ikh Prakt. Ispol’zovanie 2020, 20, 26–33. [Google Scholar] [CrossRef]
- Pozhidaev, E.P.; Budynina, E.M.; Kuznetsov, A.V.; Torgova, S.I.; Tkachenko, T.P.; Barbashov, V.A. Short Helix Pitch Ferroelectric Liquid Crystals Induced in Nematic Matrix by Chiral Non-Mesogenic Dopants. J. Mol. Liq. 2023, 391, 123351. [Google Scholar] [CrossRef]
- Pote, N.; Hinge, S.; Ganguly, P.; Banpurkar, A. Improvement of Optical Properties and Memory Effect in Ferroelectric Liquid Crystal by Incorporating Core/Shell CoFe2O4/ZnO Nanocrystals. J. Mater. Sci. Mater. Electron. 2024, 35, 320. [Google Scholar] [CrossRef]
- Węgłowska, D.; Chen, Y.; Ladouceur, F.; Silvestri, L.; Węgłowski, R.; Czerwiński, M. Single Ferroelectric Liquid Crystal Compounds Targeted for Optical Voltage Sensing. J. Mol. Liq. 2024, 399, 124454. [Google Scholar] [CrossRef]
- Tang, J.; Li, Z.; Xie, M.; Luo, Y.; Yu, J.; Chen, G.; Chen, Z. Liquid Crystal Based Label-Free Optical Sensors for Biochemical Application. Photonic Sens. 2024, 14, 240203. [Google Scholar] [CrossRef]
- Wu, P.C.; Pai, C.P.; Lee, M.J.; Lee, W. A Single-Substrate Biosensor with Spin-Coated Liquid Crystal Film for Simple, Sensitive and Label-Free Protein Detection. Biosensors 2021, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, I.; Ehni, P.; Ebert, M.; Dumait, N.; Taupier, G.; Amela-Cortes, M.; Roiland, C.; Cordier, S.; Knöller, J.A.; Jacques, E.; et al. Game of Crowns: Na+ Is Coming! Red NIR-Emissive Hybrid Liquid Crystals Containing Discotic Crown Ethers and Na2Mo6X8iCl6 (Xi = Cl or Br). ACS Appl. Mater. Interfaces 2023, 15, 39752–39764. [Google Scholar] [CrossRef]
- Lambov, M.; Hensiek, N.; Pöppler, A.C.; Lehmann, M. Columnar Liquid Crystals from Star-Shaped Conjugated Mesogens as Nano-Reservoirs for Small Acceptors. Chempluschem 2020, 85, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Chen, F.R.; Siddiqui, I.; Gupta, S.P.; Wang, C.W.; Jou, J.H.; Pal, S.K. Room Temperature Tri-Alkynyl Benzene Based Discotic Nematic Mesophase Enabling High-Efficiency Deep Blue OLEDs. J. Mol. Liq. 2023, 390, 122984. [Google Scholar] [CrossRef]
- Gupta, M.; Ashy. Solar Thermal Energy Storage Systems Based on Discotic Nematic Liquid Crystals That Can Efficiently Charge and Discharge below 0 °C. Adv. Energy Mater. 2024, 14, 2303845. [Google Scholar] [CrossRef]
- Mu, B.; Hao, X.; Luo, X.; Yang, Z.; Lu, H.; Tian, W. Bioinspired Polymeric Supramolecular Columns as Efficient yet Controllable Artificial Light-Harvesting Platform. Nat. Commun. 2024, 15, 903. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.S.; Sharma, A.S.; Rathod, S.L.; Mali, H.A.; Suthar, D.; Dhaka, M.S.; Shrivastav, P.S. A New Conformationally Symmetrical Calix[4]Pyrrole Based Supramolecular System for Liquid Crystalline and Window Layer Solar Cell Applications. ChemPhysChem 2023, 24, e202200760. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Maity, M.; Joseph, A.; Gupta, S.P.; Nailwal, Y.; Namboothiry, M.A.G.; Pal, S.K. High Electrical Conductivity and Hole Transport in an Insightfully Engineered Columnar Liquid Crystal for Solution-Processable Nanoelectronics. Small 2024, 20, 2308983. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cao, S.; Yoshio, M. Ion-Conducting Non-Flammable Liquid Crystal–Polymer Composites for High-Frequency Soft Actuators. Adv. Funct. Mater. 2023, 33, 2300538. [Google Scholar] [CrossRef]
- Suwa, S.; Liu, C.; Yoshio, M. Anisotropic Ion-Conductive Supramolecular Columnar Liquid Crystals Composed of a Benzonitrile Derivative and Ionic Liquids. Mater. Today Chem. 2023, 34, 101829. [Google Scholar] [CrossRef]
- Behera, P.K.; Rao, S.; Popoola, L.T.; Swamirayachar, S.A.; AlFalah, M.G.K.; Kandemirli, F.; Kodange, S.; Prashanth, G.K.; Achalkumar, A.S. Room Temperature Columnar Liquid Crystalline Perylene Bisimide as a Novel Corrosion Resistant Surface Film for Mild Steel Surface. J. Bio Tribo-Corros. 2023, 9, 18. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Chen, X.; Fan, Y.; Huang, J.; Yu, H.; Yang, S.; Chen, E.Q. Precisely Controllable Artificial Muscle with Continuous Morphing Based on “Breathing” of Supramolecular Columns. Adv. Mater. 2023, 35, 2211648. [Google Scholar] [CrossRef] [PubMed]
- Demus, D. One Century Liquid Crystal Chemistry: From Vorländer’s Rods to Disks, Stars and Dendrites. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 2001, 364, 25–91. [Google Scholar] [CrossRef]
- Lyu, X.; Xiao, A.; Shi, D.; Li, Y.; Shen, Z.; Chen, E.Q.; Zheng, S.; Fan, X.H.; Zhou, Q.F. Liquid Crystalline Polymers: Discovery, Development, and the Future. Polymer 2020, 202, 122740. [Google Scholar] [CrossRef]
- Vita, F.; Adamo, F.C.; Pisani, M.; Heist, L.M.; Li, M.; Hegde, M.; Dingemans, T.J.; Samulski, E.T.; Francescangeli, O. Liquid Crystal Thermosets. A New Class of High-Performance Materials. Liq. Cryst. 2020, 47, 2016–2026. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, Y.; Chen, Q.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable Liquid-Crystalline Elastomer Actuators with Exchangeable Covalent Bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Orodepo, G.O.; Gowd, E.B.; Ramakrishnan, S. Main-Chain Liquid Crystalline Polymers Bearing Periodically Grafted Folding Elements. Polym. Chem. 2021, 12, 1050–1059. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Q.; Cao, S.; Sun, C.; Zhang, Y.; Qiu, Y.; Wang, H.; Yin, G.; Liao, Y.; Xie, X. Z/E Effect on Phase Behavior of Main-Chain Liquid Crystalline Polymers Bearing AIEgens. Macromolecules 2021, 54, 10740–10749. [Google Scholar] [CrossRef]
- Imanishi, R.; Nagashima, Y.; Takishima, K.; Hara, M.; Nagano, S.; Seki, T. Induction of Highly Ordered Smectic Phases in Side Chain Liquid Crystalline Polymers by Means of Random Copolymerization. Macromolecules 2020, 53, 1942–1949. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, P.; Li, Z.; Yuan, Y.; Zhang, H. Side-Chain Chiral Fluorescent Liquid Crystal Polymers with Highly Efficient Circularly Polarized Luminescence Emission in a Glassy-State SmC∗ Film. Polym. Chem. 2021, 12, 2572–2579. [Google Scholar] [CrossRef]
- Alauddin, S.M.; Aripin, N.F.K.; Velayutham, T.S.; Martinez-Felipe, A. Liquid Crystalline Copolymers Containing Sulfonic and Light-Responsive Groups: From Molecular Design to Conductivity. Molecules 2020, 25, 2579. [Google Scholar] [CrossRef] [PubMed]
- Teruel-Juanes, R.; Bogdanowicz, K.A.; Badia, J.D.; de Juano-Arbona, V.S.; Graf, R.; Reina, J.A.; Giamberini, M.; Ribes-Greus, A. Molecular Mobility in Oriented and Unoriented Membranes Based on Poly[2-(Aziridin-1-Yl)Ethanol]. Polymers 2021, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Huang, L.; Guo, P.; Zhou, B.; Tao, Y.; Yang, C. Design, Synthesis and Performance Control of Dendritic Fluorescent Liquid Crystal Polymers with Aggregation-Induced Emission Properties. Eur. Polym. J. 2023, 186, 111855. [Google Scholar] [CrossRef]
- Bi, W.; Zheng, Z.; Wang, Y.; Shi, Z.; Wang, K.; Liu, Y.; Xu, S.; Cao, S. Design, Synthesis and Properties of Mesogen Jacketed Liquid Crystalline Polymers with Fluorinated Triphenylene Pendants. J. Mol. Liq. 2023, 391, 123225. [Google Scholar] [CrossRef]
- Hinokimoto, A.; Ono, T.; Fujiwara, M.; Mori, H.; Akiyoshi, R.; Nakamura, S.; Tsutsumi, O.; Saeki, A.; Kitagawa, Y.; Horike, S.; et al. Synthesis and Strong π–π Interaction of Hexaazatriphenylene Derivatives with Alternating Electron-Withdrawing and -Donating Groups. Chem. Asian J. 2022, 17, e202200225. [Google Scholar] [CrossRef]
- Xiong, X.P.; Yang, Q.; Wang, R.J.; Zeng, L.Y.; Yu, W.H.; Chen, H.M.; Ni, H.L.; Feng, C.; Zhao, K.Q.; Hu, P. Fast Thermally-Responsive Azatriphenylene Ionic Discotic Liquid Crystalline Polymers with Shape-Memory Properties. Polym. Chem. 2023, 14, 4521–4529. [Google Scholar] [CrossRef]
- Li, M.; Guo, J.; Zhang, C.; Che, Y.; Yi, Y.; Liu, B. Uniform Colloidal Polymer Rods by Stabilizer-Assisted Liquid-Crystallization-Driven Self-Assembly. Angew. Chem.—Int. Ed. 2023, 62, e202309914. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J. Once upon a Time the Cell Membranes: 175 Years of Cell Boundary Research. Biol. Direct 2014, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Saeed, B.; AlMohamadi, H.; Lee, M.; Gilani, M.A.; Nawaz, R.; Khan, A.L.; Yasin, M. Sustainable and Green Membranes for Chemical Separations: A Review. Sep. Purif. Technol. 2024, 336, 126271. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, J.; Wang, F.; Wu, H.; Zhou, J.; Dai, Z.; Guo, H. Recent Advances in Membranes Modified with Plant Polyphenols in Wastewater Treatment: A Review. Sep. Purif. Technol. 2024, 334, 125861. [Google Scholar] [CrossRef]
- Yu, W.; Xiong, L.; Teng, J.; Chen, C.; Li, B.; Zhao, L.; Lin, H.; Shen, L. Advances in Synthesis and Application of Amphoteric Polymer-Based Water Treatment Agents. Desalination 2024, 574, 117280. [Google Scholar] [CrossRef]
- Zubair, M.; Yasir, M.; Ponnamma, D.; Mazhar, H.; Sedlarik, V.; Hawari, A.H.; Al-Harthi, M.A.; Al-Ejji, M. Recent Advances in Nanocellulose-Based Two-Dimensional Nanostructured Membranes for Sustainable Water Purification: A Review. Carbohydr. Polym. 2024, 329, 121775. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Hazarika, G.; Yadav, D.; Ingole, P.G. Polymeric Membranes for Industrial Applications: Recent Progress, Challenges and Perspectives. Desalination 2024, 573, 117200. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Zhu, X. Poly(Ionic Liquids) Membranes Preparation and Its Application. J. Mol. Struct. 2024, 1304, 137683. [Google Scholar] [CrossRef]
- Kang, J.; Deng, N.; Cheng, B.; Kang, W. Progress in the Application of Polymer Fibers in Solid Electrolytes for Lithium Metal Batteries. J. Energy Chem. 2024, 92, 26–42. [Google Scholar] [CrossRef]
- Mishra, A.; Park, H.; El-Mellouhi, F.; Suk Han, D. Seawater Electrolysis for Hydrogen Production: Technological Advancements and Future Perspectives. Fuel 2024, 361, 130636. [Google Scholar] [CrossRef]
- Wei, X.; Reddy, V.S.; Gao, S.; Zhai, X.; Li, Z.; Shi, J.; Niu, L.; Zhang, D.; Ramakrishna, S.; Zou, X. Recent Advances in Electrochemical Cell-Based Biosensors for Food Analysis: Strategies for Sensor Construction. Biosens. Bioelectron. 2024, 248, 115947. [Google Scholar] [CrossRef] [PubMed]
- Borràs-Brull, M.; Blondeau, P.; Riu, J. Characterization and Validation of a Platinum Paper-Based Potentiometric Sensor for Glucose Detection in Saliva. Electroanalysis 2021, 33, 181–187. [Google Scholar] [CrossRef]
- Cánovas, R.; Parrilla, M.; Blondeau, P.; Andrade, F.J. A Novel Wireless Paper-Based Potentiometric Platform for Monitoring Glucose in Blood. Lab. Chip 2017, 17, 2500–2507. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Lysova, A.A.; Voropaeva, D.Y.; Yaroslavtsev, A.B. Approaches to the Modification of Perfluorosulfonic Acid Membranes. Membranes 2023, 13, 721. [Google Scholar] [CrossRef]
- Deng, Y.; Hussain, A.; Raza, W.; Cai, X.; Liu, D.; Shen, J. Review on Current Development of Polybenzimidazole Membrane for Lithium Battery. J. Energy Chem. 2024, 91, 579–608. [Google Scholar] [CrossRef]
- Kloos, J.; Joosten, N.; Schenning, A.; Nijmeijer, K. Self-Assembling Liquid Crystals as Building Blocks to Design Nanoporous Membranes Suitable for Molecular Separations. J. Membr. Sci. 2021, 620, 118849. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sakamoto, T.; Yoshio, M.; Kato, T. Development of Functional Nanoporous Membranes Based on Photocleavable Columnar Liquid Crystals—Selective Adsorption of Ionic Dyes. Eur. Polym. J. 2020, 134, 109859. [Google Scholar] [CrossRef]
- Lugger, J.A.M.; Marín San Román, P.P.; Kroonen, C.C.E.; Sijbesma, R.P. Nanoporous Films with Photoswitchable Absorption Kinetics Based on Polymerizable Columnar Discotic Liquid Crystals. ACS Appl. Mater. Interfaces 2021, 13, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhong, Z.; Wang, C.; Zhao, Z.; Zhang, X.; Wang, G.; Wang, W.; Xia, X.; Zhou, Z.; Cui, Y.; et al. Antimicrobial Peptide Dendrimers Assisted Nanocomposite-Loaded Lyotropic Liquid Crystalline for Multimodal Surgical Site Infection Management. Chem. Eng. J. 2024, 479, 147812. [Google Scholar] [CrossRef]
- Saadat, Y.; Tabatabaei, S.M.; Kim, K.; Foudazi, R. Thermoresponsive Antifouling Ultrafiltration Membranes from Mesophase Templating. J. Memb. Sci. 2023, 684, 121861. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, G.; Du, S.; Chen, R.; Chen, P.; Weng, Q.; Gao, A.; Chen, X.; An, Z. Preparation and Properties of Novel Liquid Crystal Ionomers Bearing Benzoxazole-Based Mesogenic Side-Chains and Their Membranes. J. Mol. Liq. 2024, 394, 123677. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Pirone, D.; Prats-Reig, J.; Ambrogi, V.; Reina, J.A.; Giamberini, M. In Situ Raman Spectroscopy as a Tool for Structural Insight into Cation Non-Ionomeric Polymer Interactions during Ion Transport. Polymers 2018, 10, 416. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guardià, J.; Reina, J.A.; Giamberini, M.; Montané, X. An Up-to-Date Overview of Liquid Crystals and Liquid Crystal Polymers for Different Applications: A Review. Polymers 2024, 16, 2293. https://doi.org/10.3390/polym16162293
Guardià J, Reina JA, Giamberini M, Montané X. An Up-to-Date Overview of Liquid Crystals and Liquid Crystal Polymers for Different Applications: A Review. Polymers. 2024; 16(16):2293. https://doi.org/10.3390/polym16162293
Chicago/Turabian StyleGuardià, Jordi, José Antonio Reina, Marta Giamberini, and Xavier Montané. 2024. "An Up-to-Date Overview of Liquid Crystals and Liquid Crystal Polymers for Different Applications: A Review" Polymers 16, no. 16: 2293. https://doi.org/10.3390/polym16162293
APA StyleGuardià, J., Reina, J. A., Giamberini, M., & Montané, X. (2024). An Up-to-Date Overview of Liquid Crystals and Liquid Crystal Polymers for Different Applications: A Review. Polymers, 16(16), 2293. https://doi.org/10.3390/polym16162293







