Free-Standing, Water-Resistant, and Conductivity-Enhanced PEDOT:PSS Films from In Situ Polymerization of 3-Hydroxymethyl-3-Methyl-Oxetane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PEDOT:PSS:HMO Free-Standing Films
2.3. Material Characterization
2.3.1. 1H and 13C Nuclear Magnetic Resonance (NMR)
2.3.2. Fourier-Transform Infrared Spectroscopy
2.3.3. Electrical Conductivity Measurements
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Atomic Force Microscopy (AFM)
2.3.6. Mechanical Properties
2.3.7. Electrocardiogram (EGC) Recording
3. Results and Discussion
3.1. Electrical Conductivity of PEDOT:PSS:HMO Free-Standing Films
3.2. Reactivity of HMO in PEDOT:PSS Medium
3.3. FTIR Spectroscopy Analysis
3.4. Thermal Behavior
3.5. Surface Morphology Analysis
3.6. Mechanical Properties
| Additive | σ (Scm−1) | Young’s Modulus (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) | Ref. |
|---|---|---|---|---|---|
| HMO | 106 | 300 | 11 | 4 | this work |
| PEG20K (50 wt.%) | 101 | Nr | ~5 | ~25 | [33] |
| PEO100K (44.4 wt.%) | 74.7 | Nr | ~13 | ~20 | [33] |
| PEO1000K (60.0 wt.%) | 57.7 | Nr | ~7 | 36.8 | [33] |
| PVA89K (66.7 wt.%) | 0.14 | Nr | ~40 | 54.7 | [33] |
| PEO100K (44.4 wt.%) +5 vol.% DMSO | 238 | Nr | Nr | ~20 | [33] |
| PEO100K (44.4 wt.%) +3 vol.% EG | 245 | Nr | Nr | ~20 | [33] |
| PVA89K (66.7 wt.%) +5 vol.% DMSO | 142 | Nr | Nr | 51 | [33] |
| PVA89K (66.7 wt.%) +3 vol.% EG | 172 | Nr | Nr | 47 | [33] |
| WPU (60 wt.%) | 185 | 434.7 | ~7 | 11.6 | [34] |
| PEG200 (4 vol.%) | 1415.7 | Nr | Nr | Nr | [35] |
| H2SO4 | 2500 | Nr | Nr | Nr | [37] |
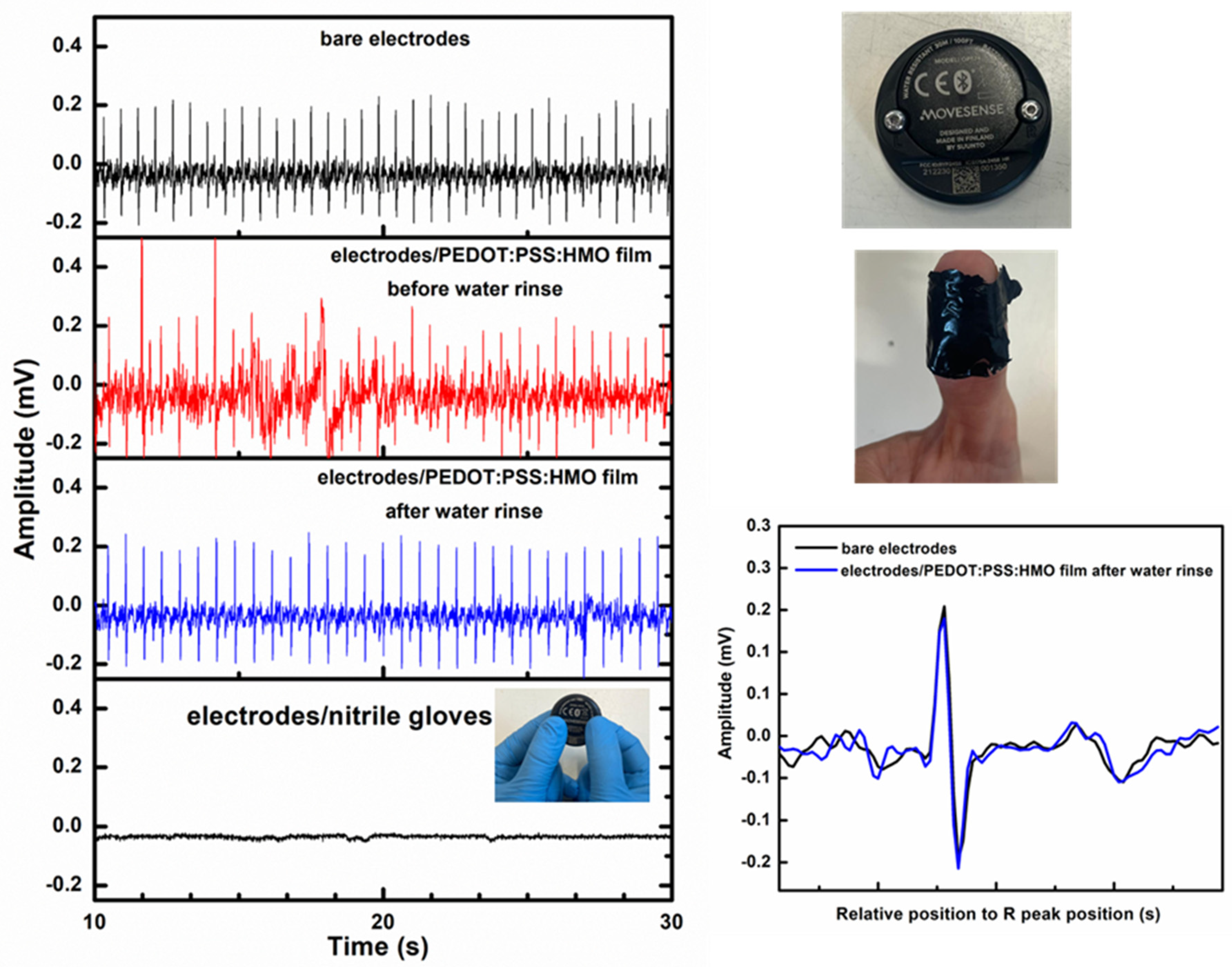
3.7. Application as Electrodes in ECG Biosensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Tang, L.; Jiang, Y. Chemical Strategies of Tailoring PEDOT:PSS for Bioelectronic Applications: Synthesis, Processing and Device Fabrication. CCS Chem. 2024, 6, 1844–1867. [Google Scholar] [CrossRef]
- Li, Y.; Pang, Y.; Wang, L.; Li, Q.; Liu, B.; Li, J.; Liu, S.; Zha, Q. Boosting the Performance of PEDOT:PSS Based Electronics Via Ionic Liquids. Adv. Mater. 2024, 36, 2310973. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Song, J.; Yin, X.; Su, Z.; Li, Z. Research Progress on Polymer Solar Cells Based on PEDOT:PSS Electrodes. Polymers 2020, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Jewłoszewicz, B.; Bogdanowicz, K.A.; Przybył, W.; Iwan, A.; Plebankiewicz, I. PEDOT:PSS in Water and Toluene for Organic Devices—Technical Approach. Polymers 2020, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jung, D.; Lee, D.; Wang, H. Impedance Characterization and Modeling of Subcellular to Micro sized Electrodes with Varying Materials and PEDOT:PSS Coating for Bioelectrical Interfaces. ACS Appl. Electron. Mater. 2021, 3, 5226–5239. [Google Scholar] [CrossRef]
- Keene, S.T.; van der Pol, T.P.A.; Zakhidov, D.; Weijtens, C.H.L.; Janssen, R.A.J.; Salleo, A.; van de Burgt, Y. Enhancement-Mode PEDOT:PSS Organic Electrochemical Transistors Using Molecular De-Doping. Adv. Mater. 2020, 32, 2000270. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J. Solution-Processed PEDOT:PSS Films with Conductivities as Indium Tin Oxide through a Treatment with Mild and Weak Organic Acids. ACS Appl. Mater. Interfaces 2013, 5, 13082–13088. [Google Scholar] [CrossRef]
- Mengistie, D.A.; Ibrahem, M.A.; Wang, P.C.; Chu, C.W. Highly conductive PEDOT:PSS treated with formic acid for ITO-free polymer solar cells. ACS Appl. Mater. Interfaces 2014, 6, 2292–2299. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly Conductive PEDOT:PSS Electrode with Optimized Solvent and Thermal Post-Treatment for ITO-Free Organic Solar Cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Gao, C. On the mechanism of conductivity enhancement in PEDOT/PSS film doped with sorbitol. e-Polymers 2011, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, G.W.; Yang, M.; Kim, T.-S. Simultaneously Enhancing the Cohesion and Electrical Conductivity of PEDOT:PSS Conductive Polymer Films using DMSO Additives. ACS Appl. Mater. Int. 2016, 8, 302–310. [Google Scholar] [CrossRef]
- Nevrela, J.; Micjan, M.; Novota, M.; Kovacova, S.; Pavuk, M.; Juhasz, P.; Kovac, J., Jr.; Jakabovic, J.; Weis, M. Secondary doping in poly(3,4-ethylenedioxythiophene:Poly(4-styrenesulfonate) thin films. J. Polym. Sci. B Polym. Phys. 2015, 53, 1139–1146. [Google Scholar] [CrossRef]
- Jiang, K.; Hong, S.-H.; Tung, S.-H.; Liu, C.-L. Effects of cation size on thermoelectricity of PEDOT:PSS/ionic liquid hybrid films for wearable thermoelectric generator application. J. Mater. Chem. A 2022, 10, 18792–18802. [Google Scholar] [CrossRef]
- Huseynova, G.; Kim, Y.H.; Lee, J.H.; Lee, J. Rising advancements in the application of PEDOT:PSS as a prosperous transparent and flexible electrode material for solution-processed organic electronics. J. Inf. 2020, 21, 47–56. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, T.; Zhou, Y. Recent Advances of Synthesis, Properties, Film Fabrication Methods, Modifications of Poly(3,4-ethylenedioxythiophene), and Applications in Solution-Processed Photovoltaics. Adv. Funct. Mater. 2020, 30, 2006213. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.G.; Kwak, J.; Hong, J.I.; Kim, S.H. Enhanced Humid Reliability of Organic Thermoelectrics via Crosslinking with Glycerol. Nanomaterials 2019, 9, 1591. [Google Scholar] [CrossRef]
- Yi, C.; Wilhite, A.; Zhang, L.; Hu, R.; Chuang, S.S.C.; Zheng, J.; Gong, X. Enhanced Thermoelectric Properties of Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) by Binary Secondary Dopants. ACS Appl. Mater. Interfaces 2015, 7, 8984–8989. [Google Scholar] [CrossRef]
- del Agua, I.; Mantione, D.; Ismailov, U.; Sanchez-Sanchez, A.; Aramburu, N.; Malliaras, G.G.; Mecerreyes, D.; Ismailova, E. DVS-Crosslinked PEDOT:PSS Free-Standing and Textile Electrodes toward Wearable Health Monitoring. Adv. Mater. Technol. 2018, 3, 1700322. [Google Scholar] [CrossRef]
- Gunaydin, O.; Demir, A.; Demir, G.E.; Yücedağ, I.; Çoşut, B. The Preparation of Transparent Organic Field Effect Transistor Using a Novel EDOT Functional Styrene Copolymer Insulator With a PEDOT:PSS Gate Electrode. Macromol. Res. 2018, 26, 164–172. [Google Scholar] [CrossRef]
- Zhang, S.; Kumar, P.; Nouas, A.S.; Fontaine, L.; Tang, H.; Cicoira, F. Solvent-induced changes in PEDOT:PSS films for organic electrochemical transistors. APL Mater. 2015, 3, 014911. [Google Scholar] [CrossRef]
- Song, J.; Ma, Q.; Qin, F.; Hu, L.; Luo, B.; Liu, T.; Yin, X.; Su, Z.; Zeng, Z.; Jiang, Y.; et al. High-Conductivity, Flexible and Transparent PEDOT:PSS Electrodes for High Performance Semi-Transparent Supercapacitors. Polymers 2020, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, H.R.; Kim, B.J.; Lee, K. Highly Conductive PEDOT:PSS Nanofibrils Induced by Solution-Processed Crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef]
- Stadermann, M.; Baxamusa, S.H.; Aracne-Ruddle, C.; Chea, M.; Li, S.; Youngblood, K.; Suratwala, T. Fabrication of Large-area Free-standing Ultrathin Polymer Films. J. Vis. Exp. 2015, 100, 52832. [Google Scholar]
- Taccola, S.; Greco, F.; Zucca, A.; Innocenti, C.; Fernández, C.J.; Campo, G.; Sangregorio, C.; Mazzolai, B.; Mattoli, V. Characterization of Free-Standing PEDOT:PSS/Iron Oxide Nanoparticle Composite Thin Films and Application As Conformable Humidity Sensors. ACS Appl. Mater. Interfaces 2013, 5, 6324–6332. [Google Scholar] [CrossRef]
- He, H.; Ouyang, J. Enhancements in the Mechanical Stretchability and Thermoelectric Properties of PEDOT:PSS for Flexible Electronics Applications. Acc. Mater. Res. 2020, 1, 146–157. [Google Scholar] [CrossRef]
- Yamato, H.; Kai, K.; Ohwa, M.; Asakura, T.; Koshiba, T.; Wernet, W. Synthesis of free-standing poly (3,4-ethylenedioxythiophene) conducting polymer films on a pilot scale. Synth. Met. 1996, 83, 125–130. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2018, 31, 1806133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, E.Q.; Li, R.; Xu, X.; Ventura, I.A.; Moussawi, A.; Anjum, D.H.; Hedhili, M.N.; Smilgies, D.-M.; Lubineau, G.; et al. Semi-metallic, Strong and Stretchable Wet-spun Conjugated Polymer Microfibers. J. Mater. Chem. C 2015, 3, 2528. [Google Scholar] [CrossRef]
- He, H.; Zhang, L.; Guan, X.; Cheng, H.; Liu, X.; Yu, S.; Wei, J.; Ouyang, J. Biocompatible Conductive Polymers with High Conductivity and High Stretchability. ACS Appl. Mater. Interfaces 2019, 11, 26185–26193. [Google Scholar] [CrossRef]
- Teo, M.T.; Kim, N.; Kee, S.; Kim, B.S.; Kim, G.; Hong, S.; Jung, S.; Lee, K. Highly Stretchable and Highly Conductive PEDOT:PSS/Ionic Liquid Composite Transparent Electrodes for Solution-Processed Stretchable Electronics. ACS Appl. Mater. Interfaces 2017, 9, 819–826. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017, 3, e1602076. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sun, K.; Ouyang, J. Stretchable and Conductive Polymer Films Prepared by Solution Blending. ACS Appl. Mater. Interfaces 2015, 7, 18415–18423. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Du, D.; Guo, L.; Guob, Y.; Ouyang, J. Stretchable and Conductive Polymer Films for High-Performance Electromagnetic Interference Shielding. J. Mater. Chem. C 2016, 4, 6525–6532. [Google Scholar] [CrossRef]
- Sun, Z.; Shu, M.; Li, W.; Li, P.; Zhang, Y.; Yao, H.; Guan, S. Enhanced thermoelectric performance of PEDOT:PSS self-supporting thick films through a binary treatment with polyethylene glycol and water. Polymer 2020, 192, 122328. [Google Scholar] [CrossRef]
- Li, Z.; Ma, G.; Ge, R.; Qin, F.; Dong, X.; Meng, W.; Liu, T.; Tong, J.; Jiang, F.; Zhou, Y.; et al. Free-Standing Conducting Polymer Films for High-Performance Energy Devices. Angew. Chem. Int. Ed. 2016, 55, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, H.; Hsiao, G.L.; Yao, Y.; Xiao, Y.; Shahi, M.; Jin, Y.; Cruce, A.; Liu, X.; Jiang, Y.; et al. A Free-Standing High-Output Power Density Thermoelectric Device Based on Structure-Ordered PEDOT:PSS. Adv. Electron. Mater. 2018, 4, 1700496. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Z.; Leiqiang, Q.; Liu, X.; Mao, L.; Wang, Y.; Qin, F.; Liu, Y.; Zhou, Y.; Zhang, F. Laminated Free Standing PEDOT:PSS Electrode for Solution Processed Integrated Photocapacitors via Hydrogen-Bond Interaction. Adv. Mater. Interfaces 2017, 4, 1700704. [Google Scholar] [CrossRef]
- Jorge, S.M.; Santos, L.F.; Galvão, A.; Morgado, J.; Charas, A. Concurrent Enhancement of Conductivity and Stability in Water of Poly(3,4-Ethylenedioxythiophene):Poly(Styrenesulfonate) Films Using an Oxetane Additive. Adv. Mater. Interfaces 2021, 8, 2100517. [Google Scholar] [CrossRef]
- Jorge, S.M.; Ablú, A.; Garrudo, F.; Galvão, A.; Santos, L.F.; Morgado, J.; Charas, A. Oxetanes as polymerizable additives to PEDOT:PSS for water-resistant and transparent electrodes. Polymer 2023, 283, 126250. [Google Scholar] [CrossRef]
- Príncipe, C.; Jorge, S.M.; Matos, M.; Santos, L.; Morgado, J.; Charas, A. 3-oxetanylmethanol as additive to PEDOT:PSS for improved conductivity and water-resistance and applications in ink-jet printed electrochemical transistors. Org. Electron. 2024, 125, 106987. [Google Scholar] [CrossRef]
- Malapit, C.A.; Howell, A.R. Recent Applications of Oxetanes in the Synthesis of Heterocyclic Compounds. J. Org. Chem. 2015, 80, 8489–8495. [Google Scholar] [CrossRef] [PubMed]
- Charas, A.; Morgado, J. Oxetane-functionalized Conjugated Polymers in Organic (Opto)Electronic Devices. Curr. Phys. Chem. 2012, 2, 241–264. [Google Scholar] [CrossRef]
- DeLongchamp, D.M.; Vogt, B.D.; Brooks, C.M.; Kano, K.; Obrzut, J.; Richter, C.A.; Kirillov, O.A.; Lin, E.K. Influence of a Water Rinse on the Structure and Properties of Poly(3,4-ethylene dioxythiophene):Poly(styrene sulfonate) Films. Langmuir 2005, 21, 11480–11483. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ouyang, J. PEDOT:PSS films with significantly enhanced conductivities induced by preferential solvation with cosolvents and their application in polymer photovoltaic cells. J. Mater. Chem. 2011, 21, 4927–4936. [Google Scholar] [CrossRef]
- Parzuchowski, P.; Mamiński, M.Ł. Poly-(3-ethyl-3-hydroxymethyl)oxetanes—Synthesis and Adhesive Interactions with Polar Substrates. Polymers 2020, 12, 222. [Google Scholar] [CrossRef]
- Huang, T.M.; Batra, S.; Hu, J.; Miyoshi, T.; Cakmak, M. Chemical crosslinking of conducting poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) using poly(ethylene oxide) (PEO). Polymer 2013, 54, 6455–6462. [Google Scholar] [CrossRef]
- Duc, C.; Vlandas, A.; Malliarasb, G.G.; Senez, V. Wettability of PEDOT:PSS films. Soft Matter 2016, 23, 5146. [Google Scholar] [CrossRef]
- Mengistie, D.A.; Wang, P.C.; Chu, C.W. Effect of molecular weight of additives on the conductivity of PEDOT:PSS and efficiency for ITO-free organic solar cells. J. Mater. Chem. A 2013, 34, 9907–9916. [Google Scholar] [CrossRef]
- Rodríguez, A.B.; Voigt, M.M.; Martin, S.J.; Whittle, T.J.; Dalgliesh, R.M.; Thompson, R.L.; Lidzey, D.G.; Geoghegan, M. Structure of films of poly(3,4-ethylene dioxythiophene)-poly(styrene sulfonate) crosslinked with glycerol. J. Mater. Chem. 2011, 21, 19324–19331. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Q.; Lessner, P. Thermal Stability Investigation of PEDOT Films from Chemical Oxidation and Prepolymerized Dispersion. Electrochemistry 2013, 81, 801–803. [Google Scholar] [CrossRef]
- Yemata, T.A.; Zheng, Y.; Kyaw, A.K.K.; Wang, X.; Song, J.; Chin, W.S.; Xu, J. Improved Thermoelectric Properties and Environmental Stability of Conducting PEDOT:PSS Films Post-treated With Imidazolium Ionic Liquids. Front. Chem. 2020, 7, 870–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Chen, D.; Ma, Y.; Wang, L.; Zhao, C.; Yang, W. Preparation of a hybrid core–shell structured BaTiO3@PEDOT nanocomposite and its applications in dielectric and electrode materials. Appl. Surf. Sci. 2015, 356, 232–239. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, R.; Liu, F.; Zheng, X.; Zhu, D. Effect of poly(sodium 4-styrene-sulfonate) on the crystal growth of hydroxyapatite prepared by hydrothermal method. Mater. Chem. Phys. 2010, 120, 603–607. [Google Scholar] [CrossRef]
- Kiebooms, R.; Aleshin, A.; Hutchison, K.; Wudl, F.; Heeger, A. Doped poly(3,4-ethylenedioxythiophene) films: Thermal, electromagnetical and morphological analysis. Synth. Met. 1999, 101, 436–437. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, S.; Liu, L.; Hamad, N.; Bobbara, S.R.; Pasini, D.; Cicoira, F. Autonomic Self-Healing of PEDOT:PSS Achieved Via Polyethylene Glycol Addition. Adv. Funct. Mater. 2020, 30, 2002853. [Google Scholar] [CrossRef]

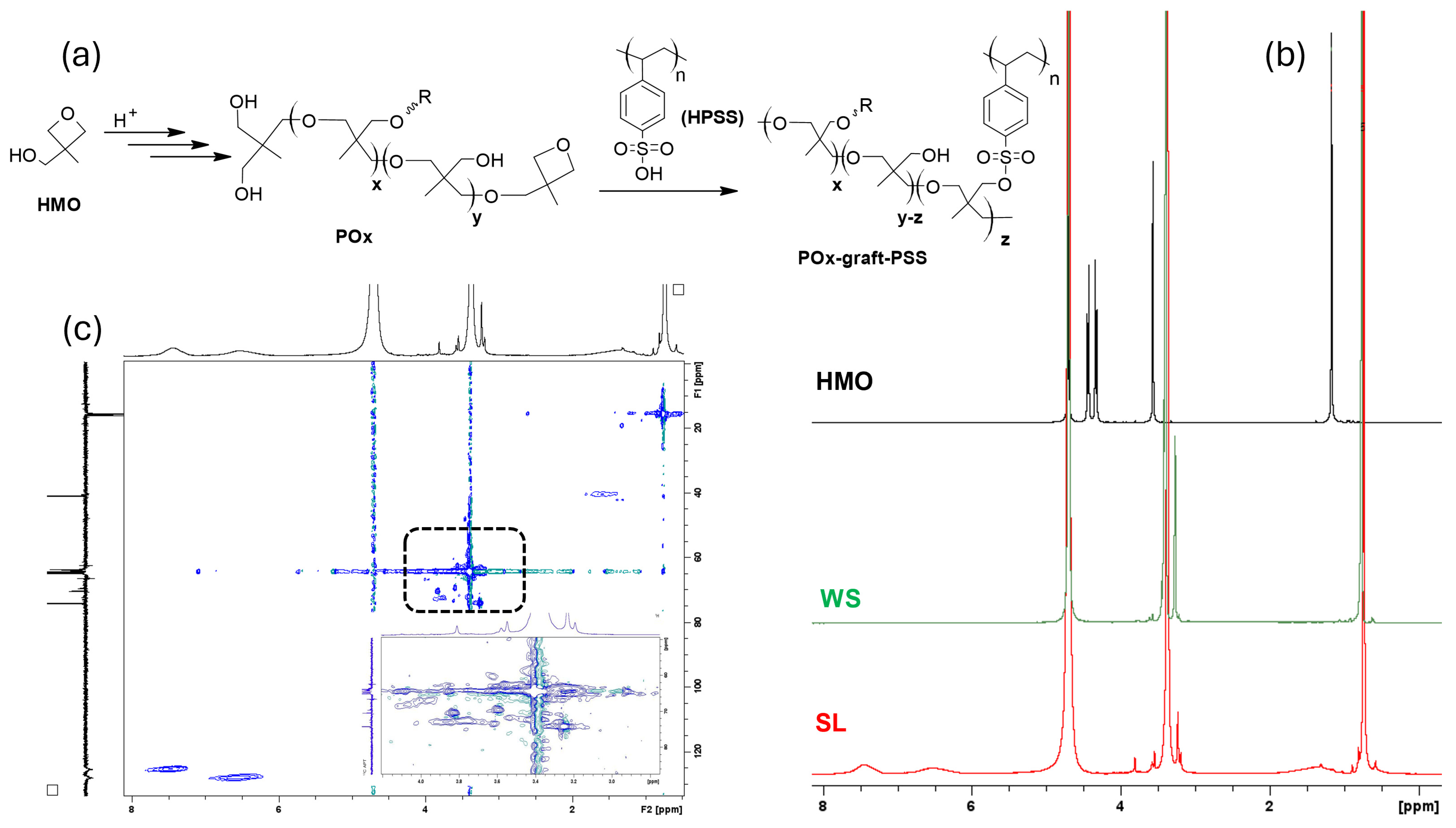
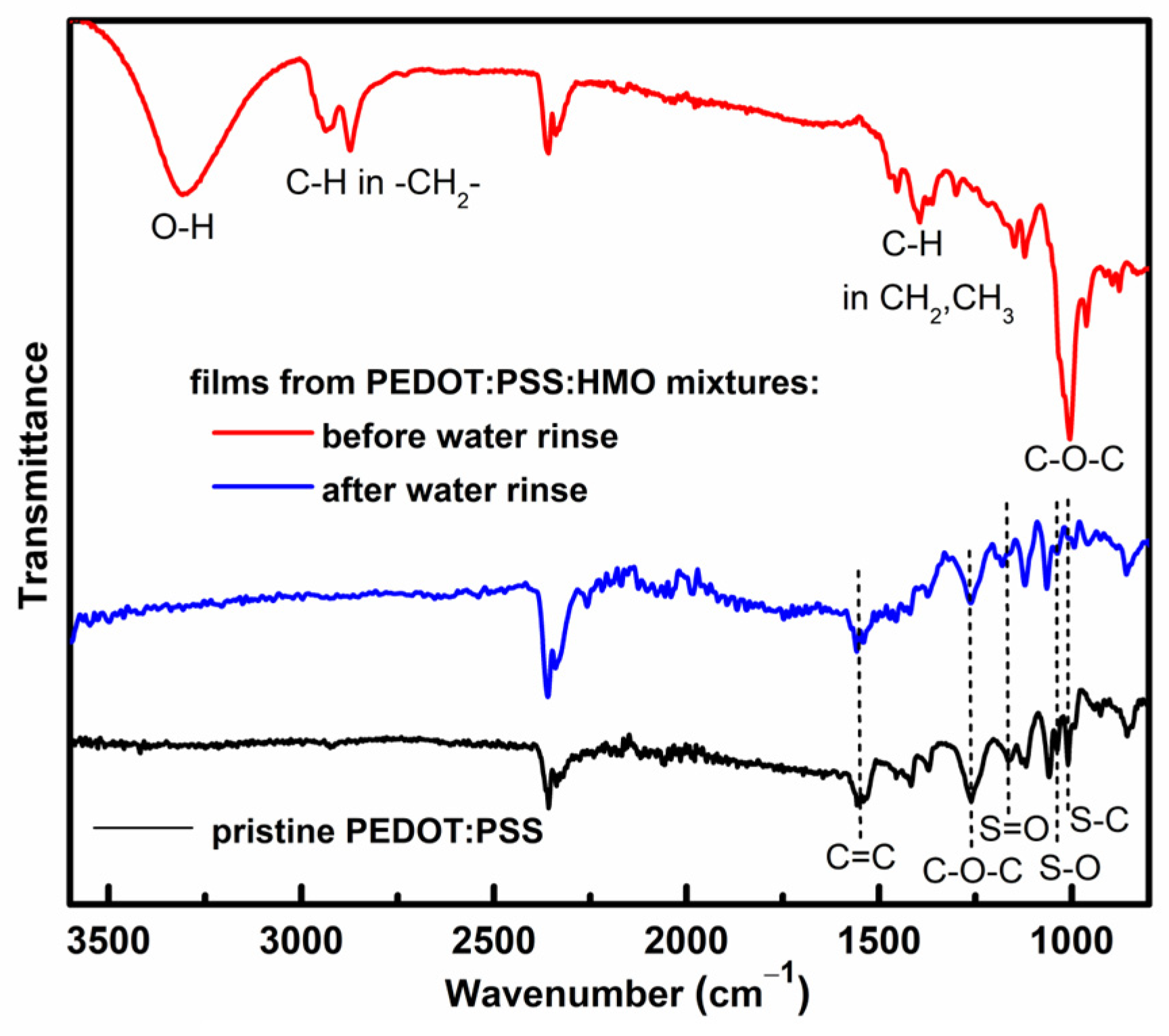
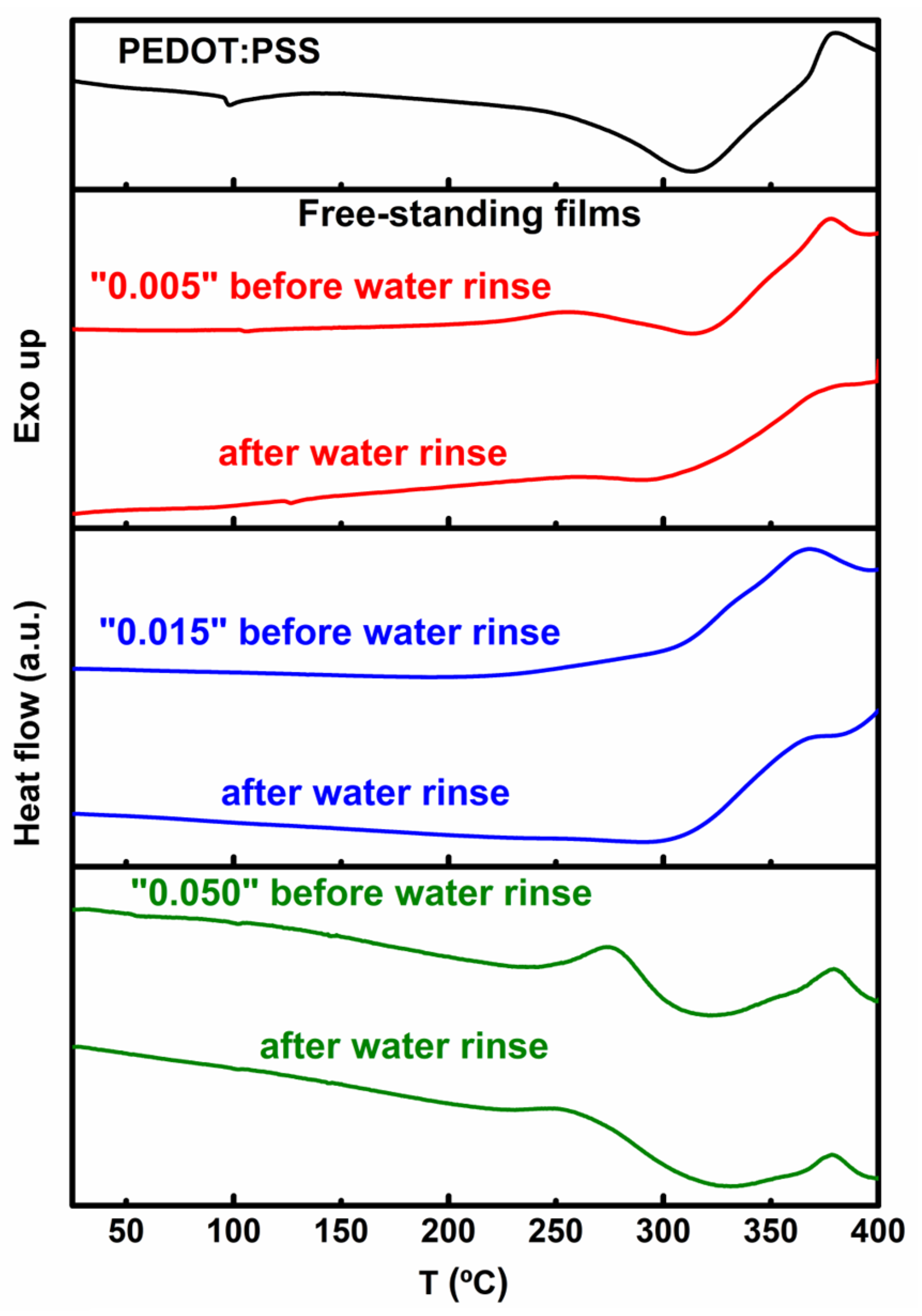
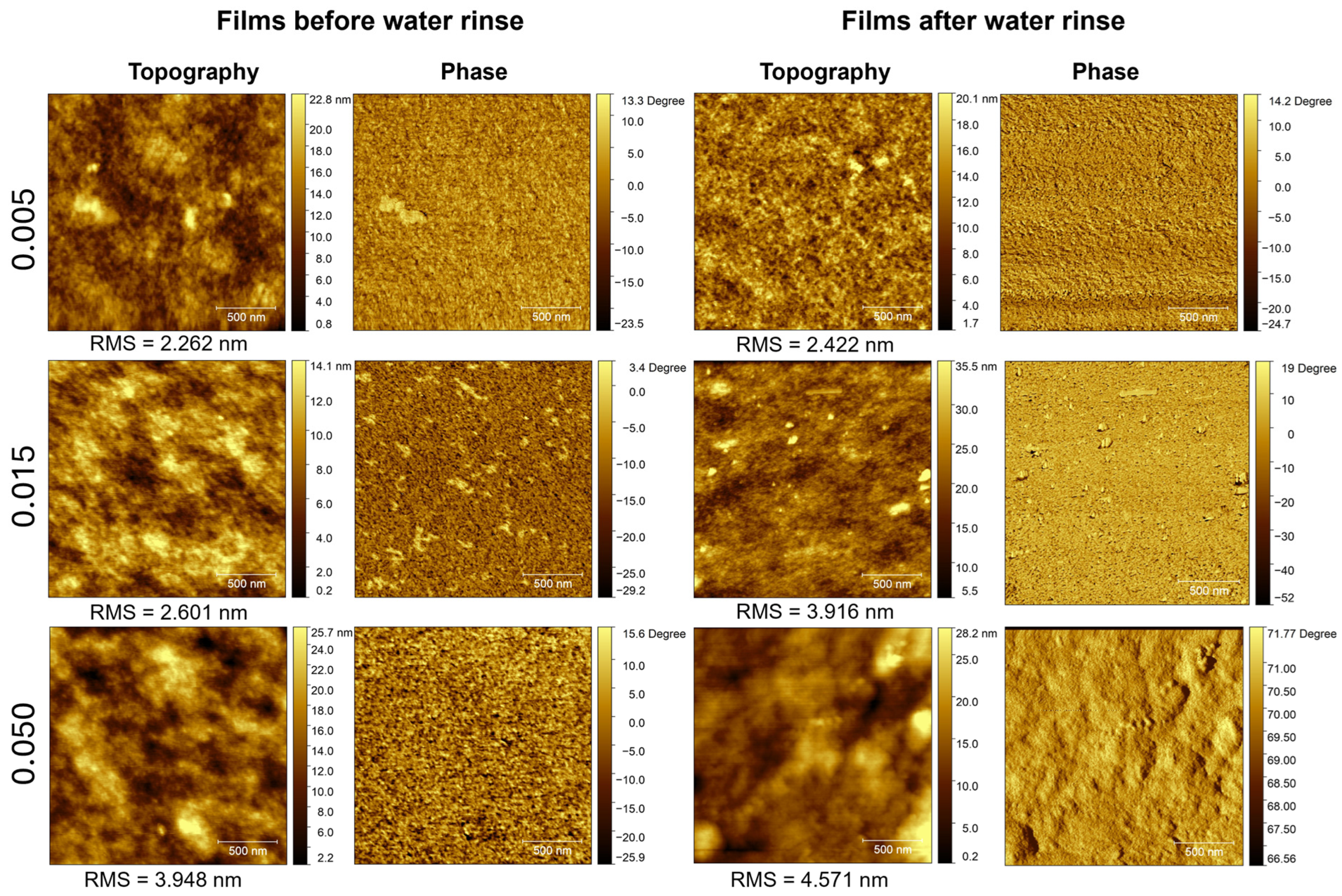
| HMO/PEDOT:PSSaq.(v/v) | Conductivity(Scm−1) | Thickness(μm) | ||
|---|---|---|---|---|
| Before rinse | After rinse | Before rinse | After rinse | |
| 0.005 | 49 ± 6 | 73 ± 10 | 45 ± 17 | 33 ± 5 |
| 0.015 | 32 ± 3 | 72 ± 8 | 42 ± 4 | 40 ± 9 |
| 0.050 | 8 ± 1 | 106 ± 15 | 202 ± 11 | 55 ± 10 |
| 0 (pristine PEDOT:PSS) | 1.505 ± 0.003 | - | 3.3 ± 0.5 | - |
| HMO/PEDOT:PSSaq (v/v) | Young’s Modulus (GPa) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| 0.005 | 0.4 ± 0.1 | 7.8 ± 0.9 | 2.3 ± 0.8 |
| 0.015 | 0.5 ± 0.1 | 12 ± 3 | 3 ± 1 |
| 0.050 | 0.3 ± 0.3 | 11 ± 7 | 4 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge, S.M.; Santos, L.F.; Ferreira, M.J.; Marto-Costa, C.; Serro, A.P.; Galvão, A.M.; Morgado, J.; Charas, A. Free-Standing, Water-Resistant, and Conductivity-Enhanced PEDOT:PSS Films from In Situ Polymerization of 3-Hydroxymethyl-3-Methyl-Oxetane. Polymers 2024, 16, 2292. https://doi.org/10.3390/polym16162292
Jorge SM, Santos LF, Ferreira MJ, Marto-Costa C, Serro AP, Galvão AM, Morgado J, Charas A. Free-Standing, Water-Resistant, and Conductivity-Enhanced PEDOT:PSS Films from In Situ Polymerization of 3-Hydroxymethyl-3-Methyl-Oxetane. Polymers. 2024; 16(16):2292. https://doi.org/10.3390/polym16162292
Chicago/Turabian StyleJorge, Sara M., Luís F. Santos, Maria João Ferreira, Carolina Marto-Costa, Ana Paula Serro, Adelino M. Galvão, Jorge Morgado, and Ana Charas. 2024. "Free-Standing, Water-Resistant, and Conductivity-Enhanced PEDOT:PSS Films from In Situ Polymerization of 3-Hydroxymethyl-3-Methyl-Oxetane" Polymers 16, no. 16: 2292. https://doi.org/10.3390/polym16162292
APA StyleJorge, S. M., Santos, L. F., Ferreira, M. J., Marto-Costa, C., Serro, A. P., Galvão, A. M., Morgado, J., & Charas, A. (2024). Free-Standing, Water-Resistant, and Conductivity-Enhanced PEDOT:PSS Films from In Situ Polymerization of 3-Hydroxymethyl-3-Methyl-Oxetane. Polymers, 16(16), 2292. https://doi.org/10.3390/polym16162292









