A Wearable Electrochemical Sensor Based on a Molecularly Imprinted Polymer Integrated with a Copper Benzene-1,3,5-Tricarboxylate Metal-Organic Framework for the On-Body Monitoring of Cortisol in Sweat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Computational Studies and Energy Calculations
2.4. Preparation of GCE/MOF
2.5. Preparation of GCE/MOF/MIP
2.6. Experimental Design
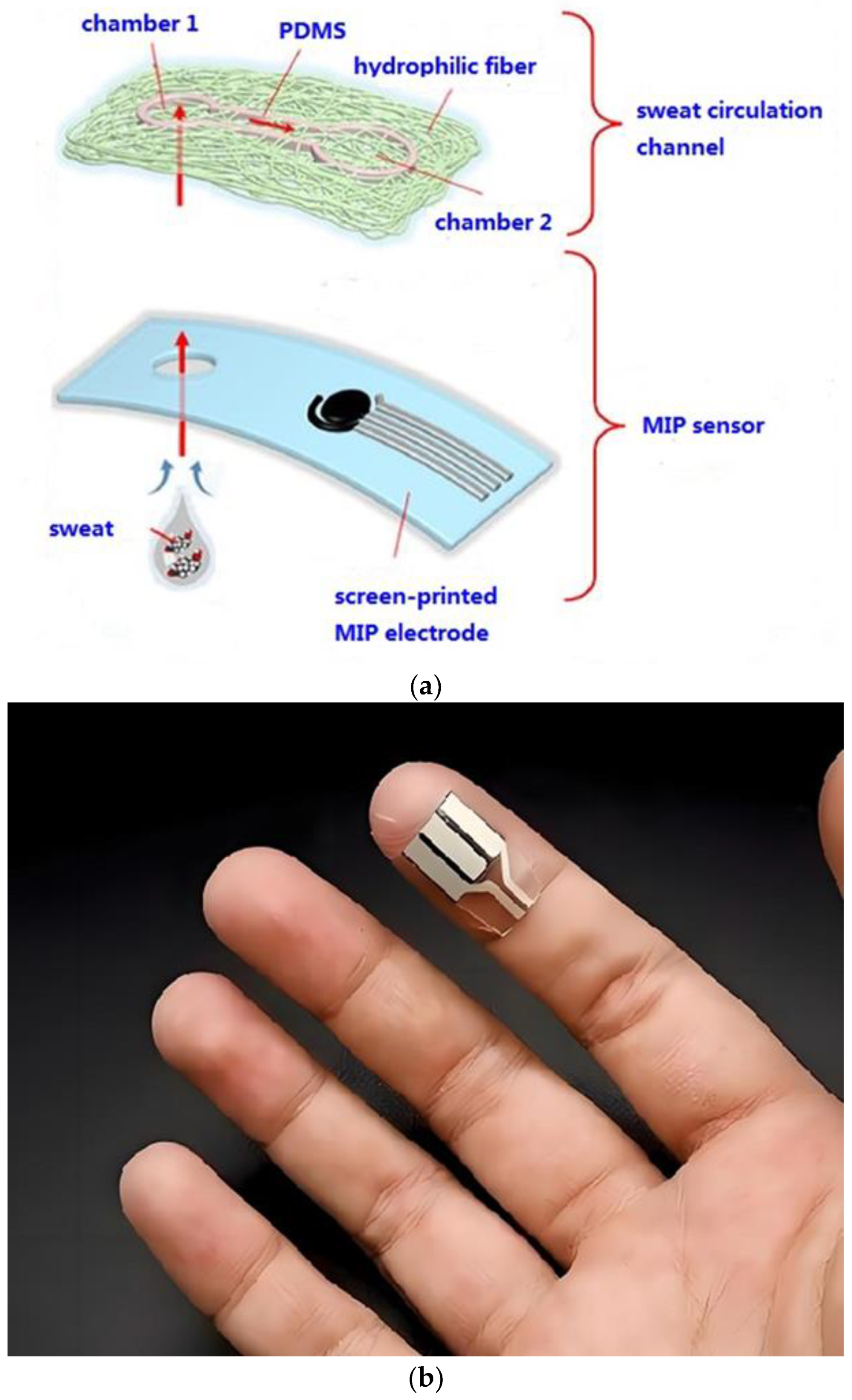
2.7. Fabrication of the Wearable Sensor
2.8. Electrochemical Detection and Performance Test
3. Results and Discussion
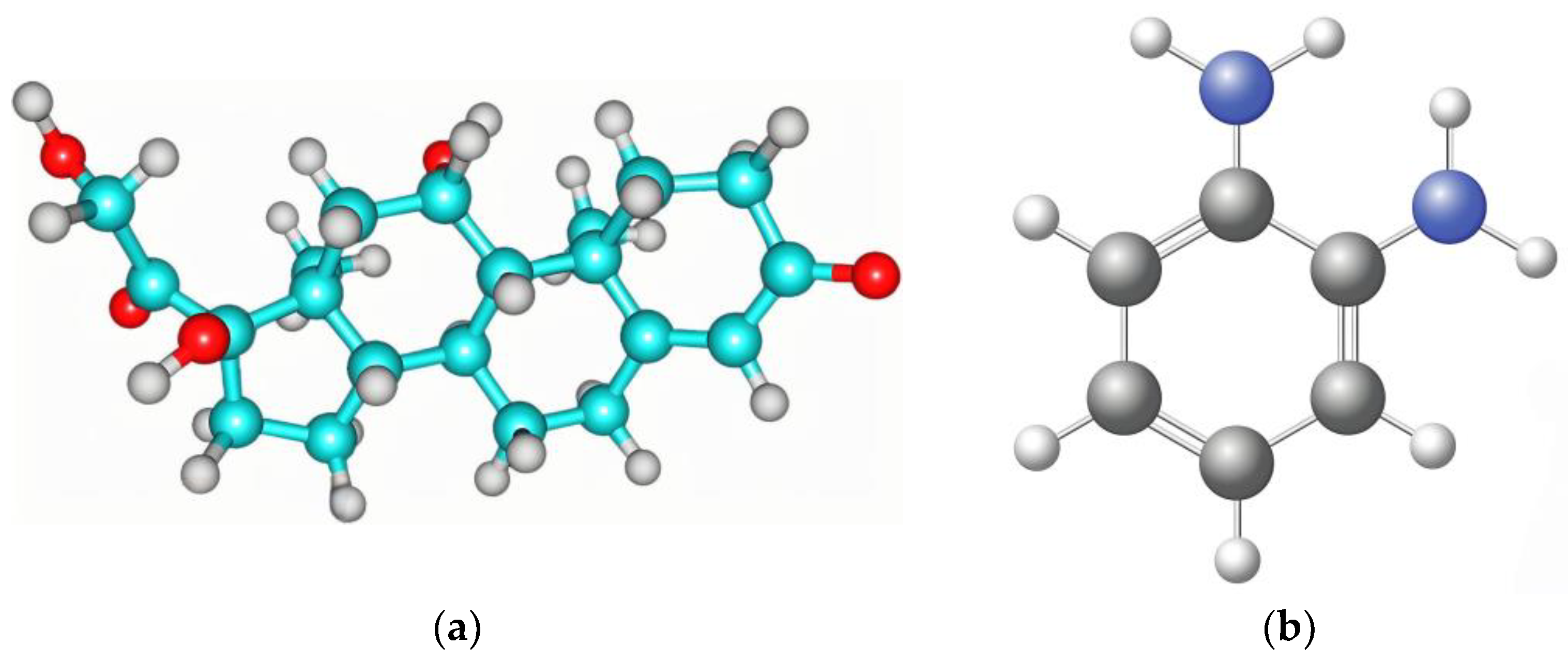
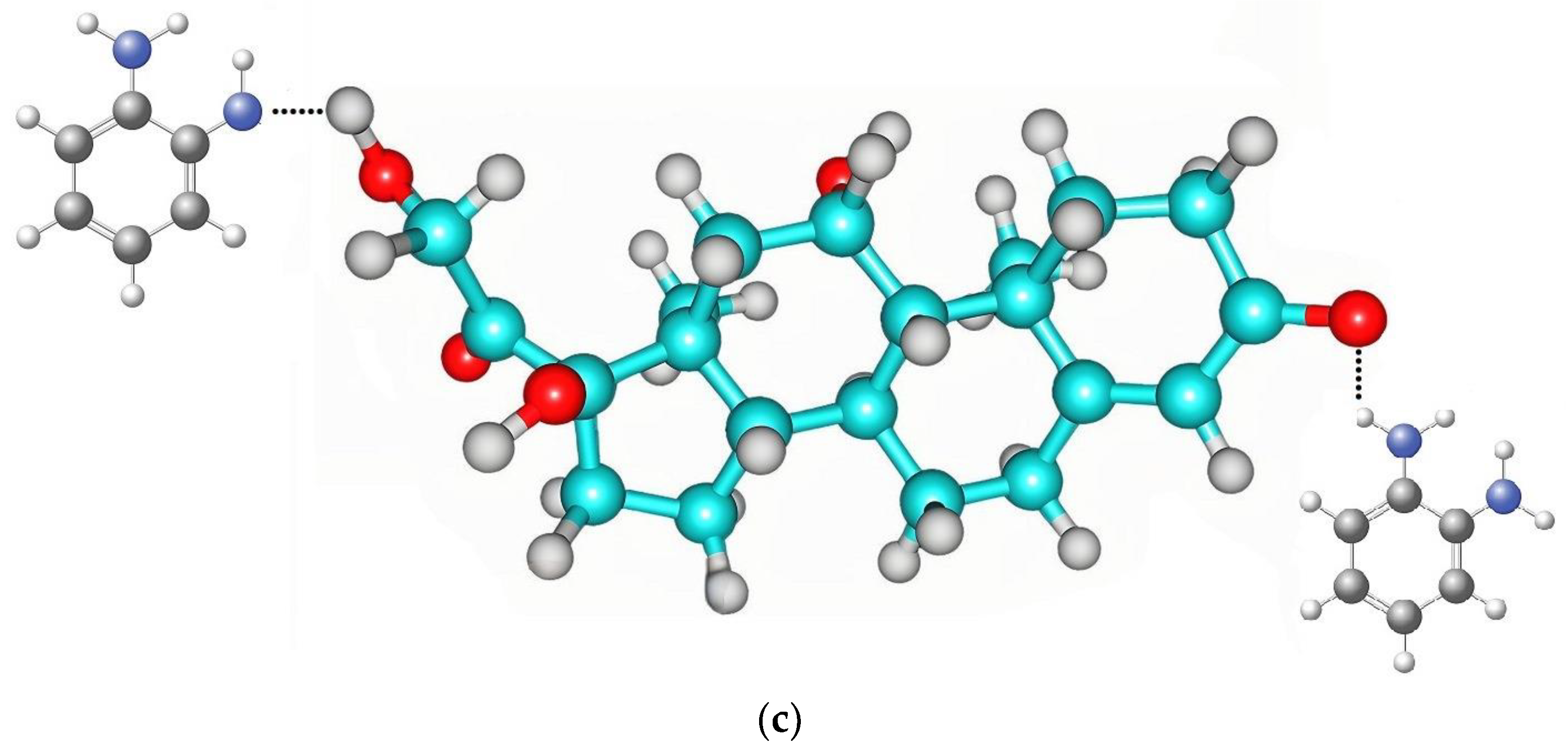
3.1. Computational Studies of the Interaction between Monomer and Cortisol
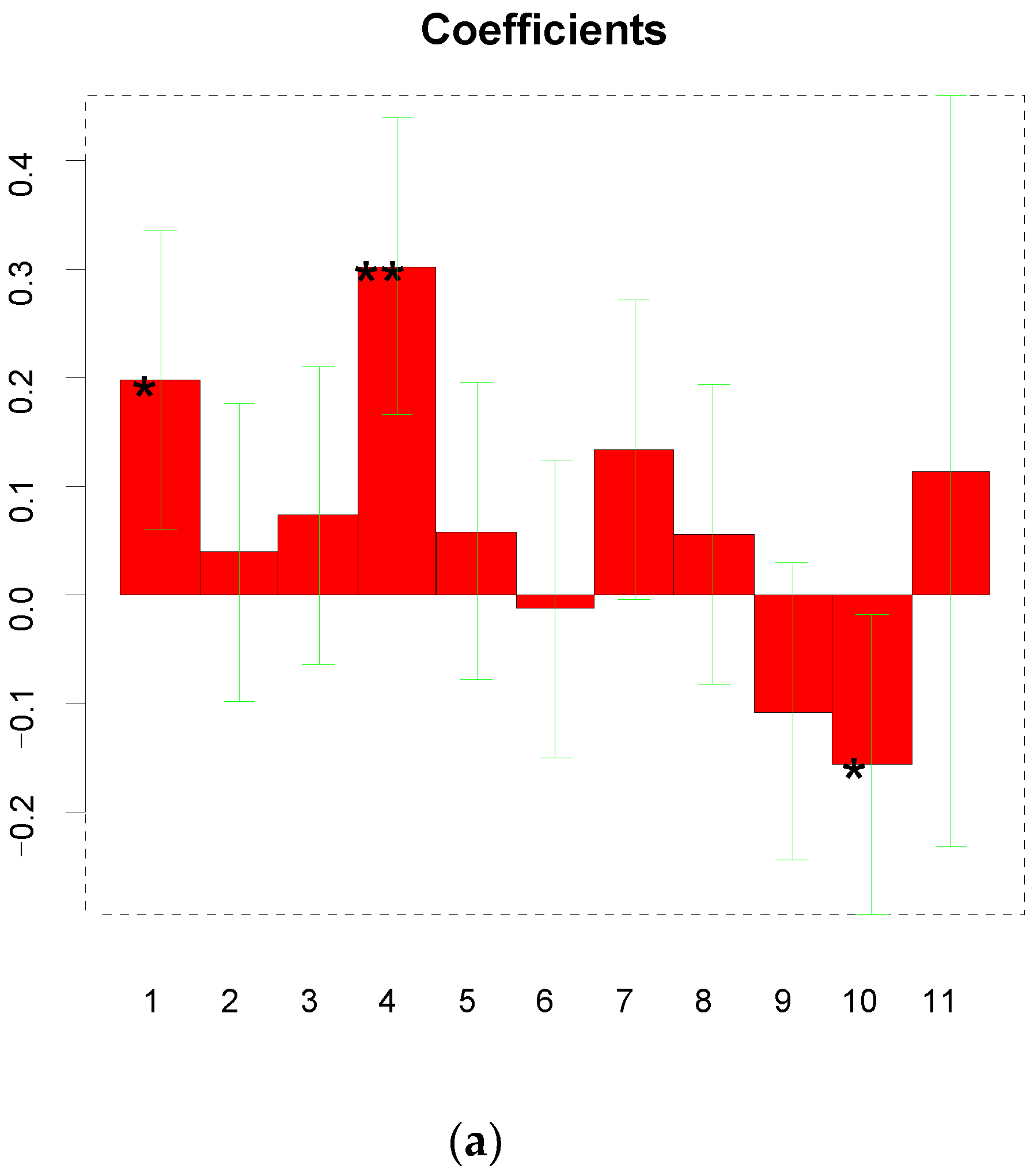
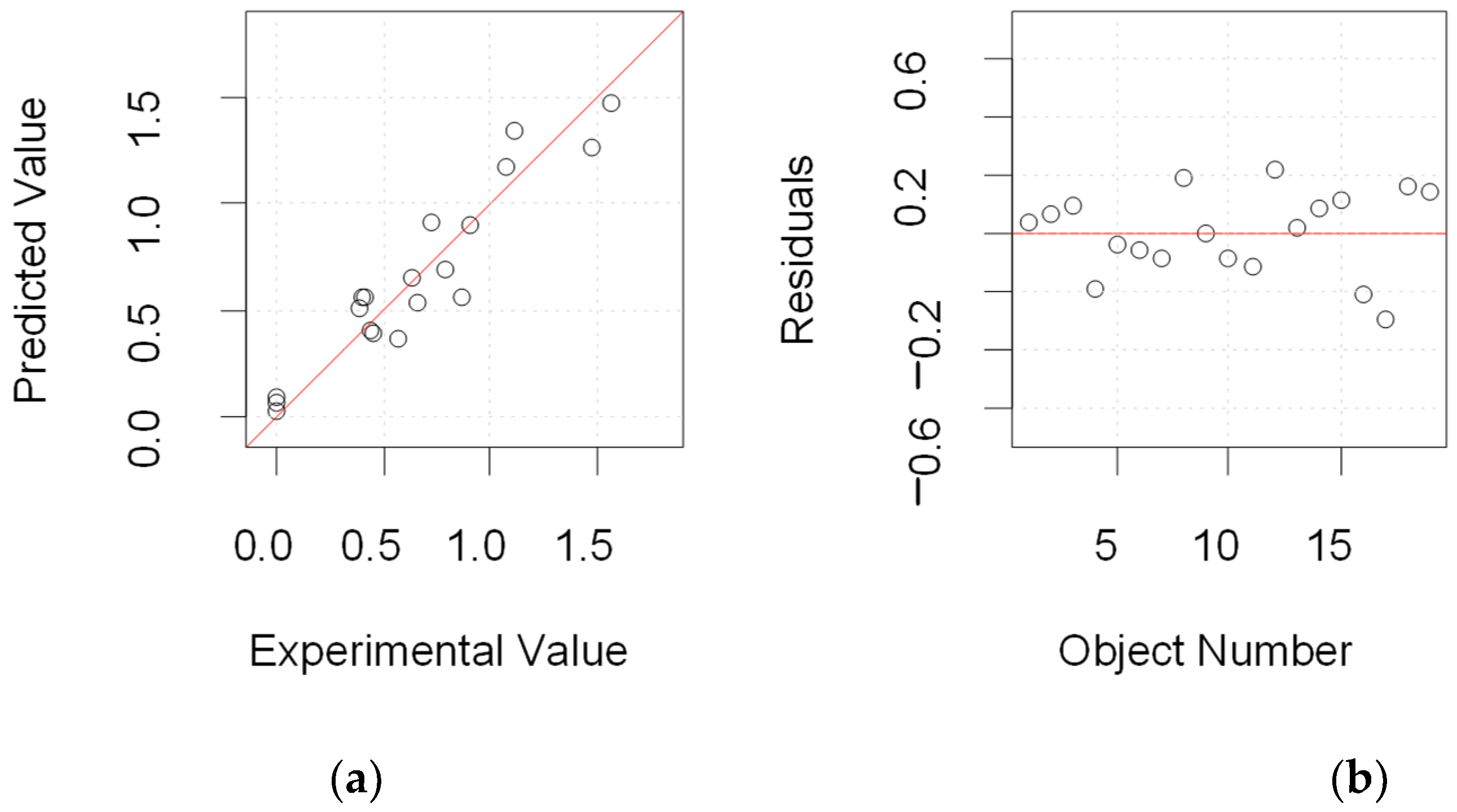
3.2. Interpretation of the Experimental Design
3.3. Characterization of the Proposed GCE/MOF/MIP
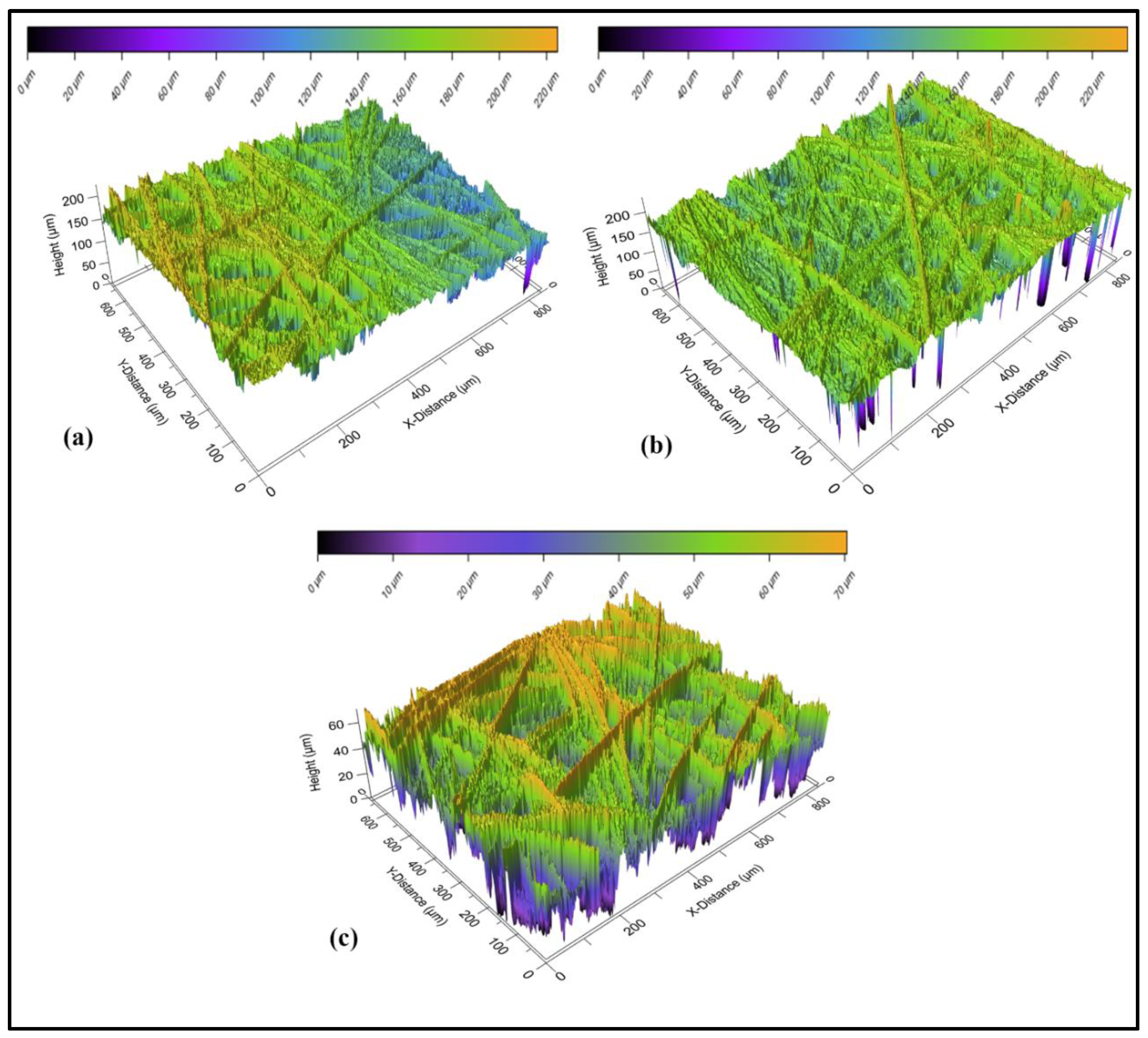
3.3.1. Optical Profilometry
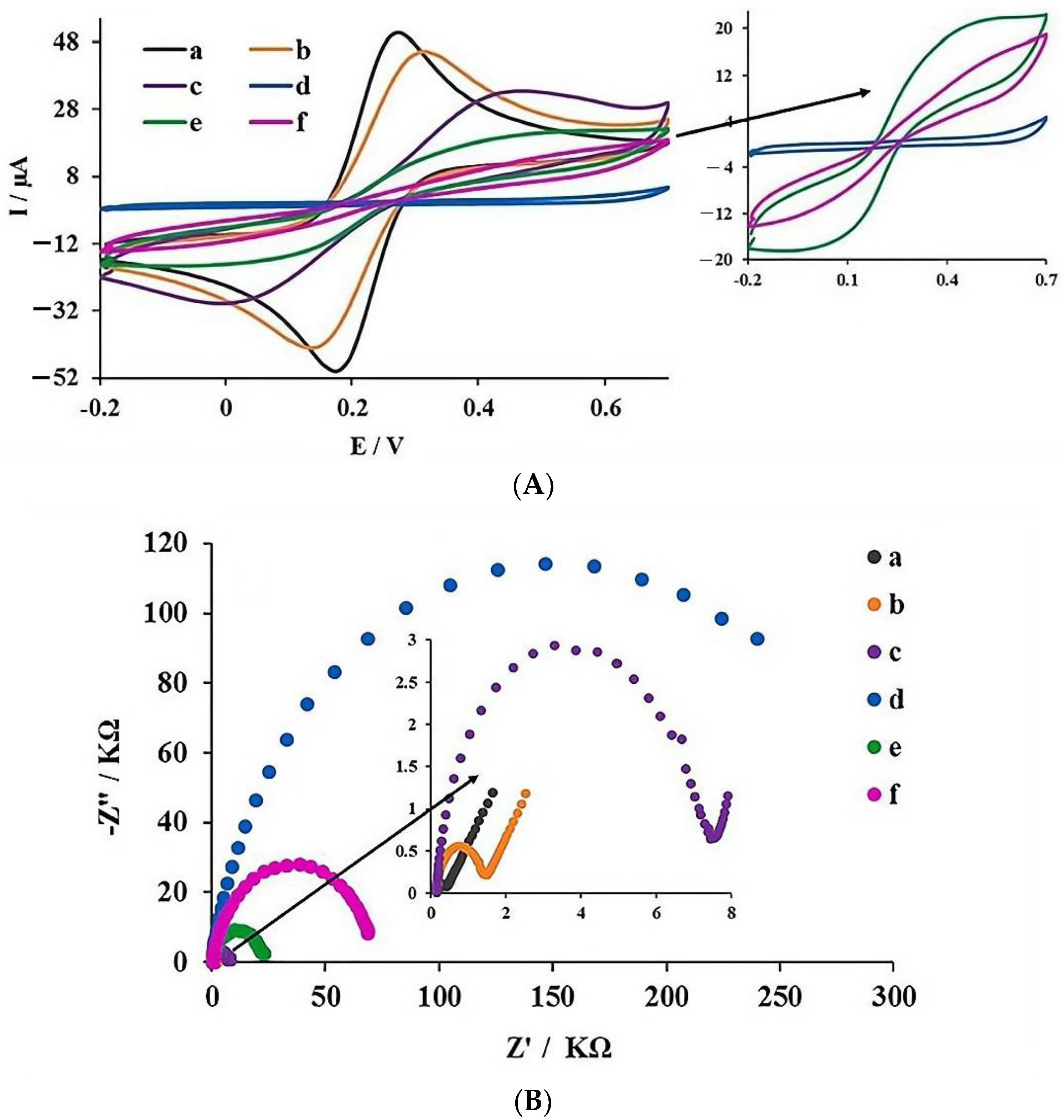
3.3.2. Electrochemical Characterization
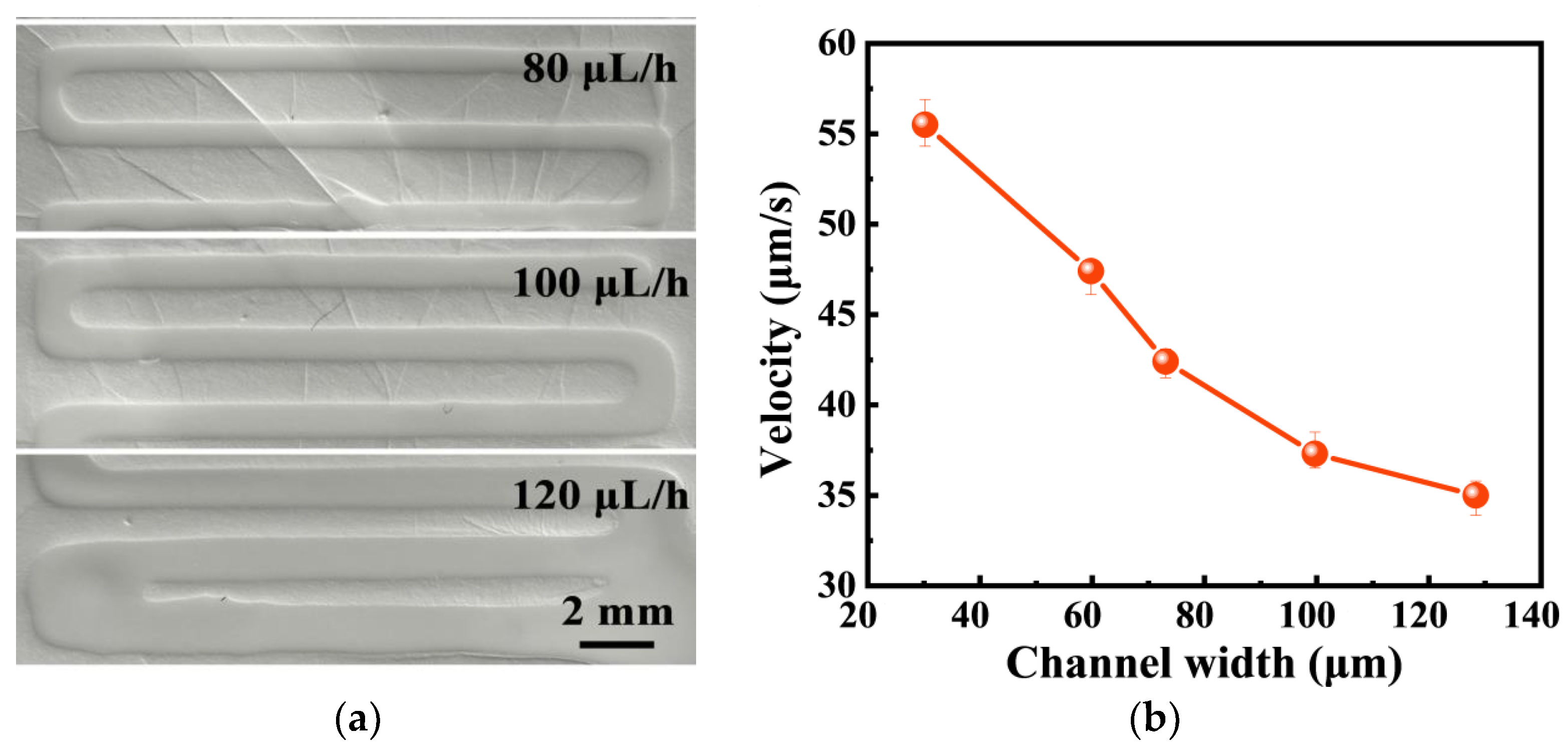
3.3.3. Characterization of Microfluidic Sample Acquisition
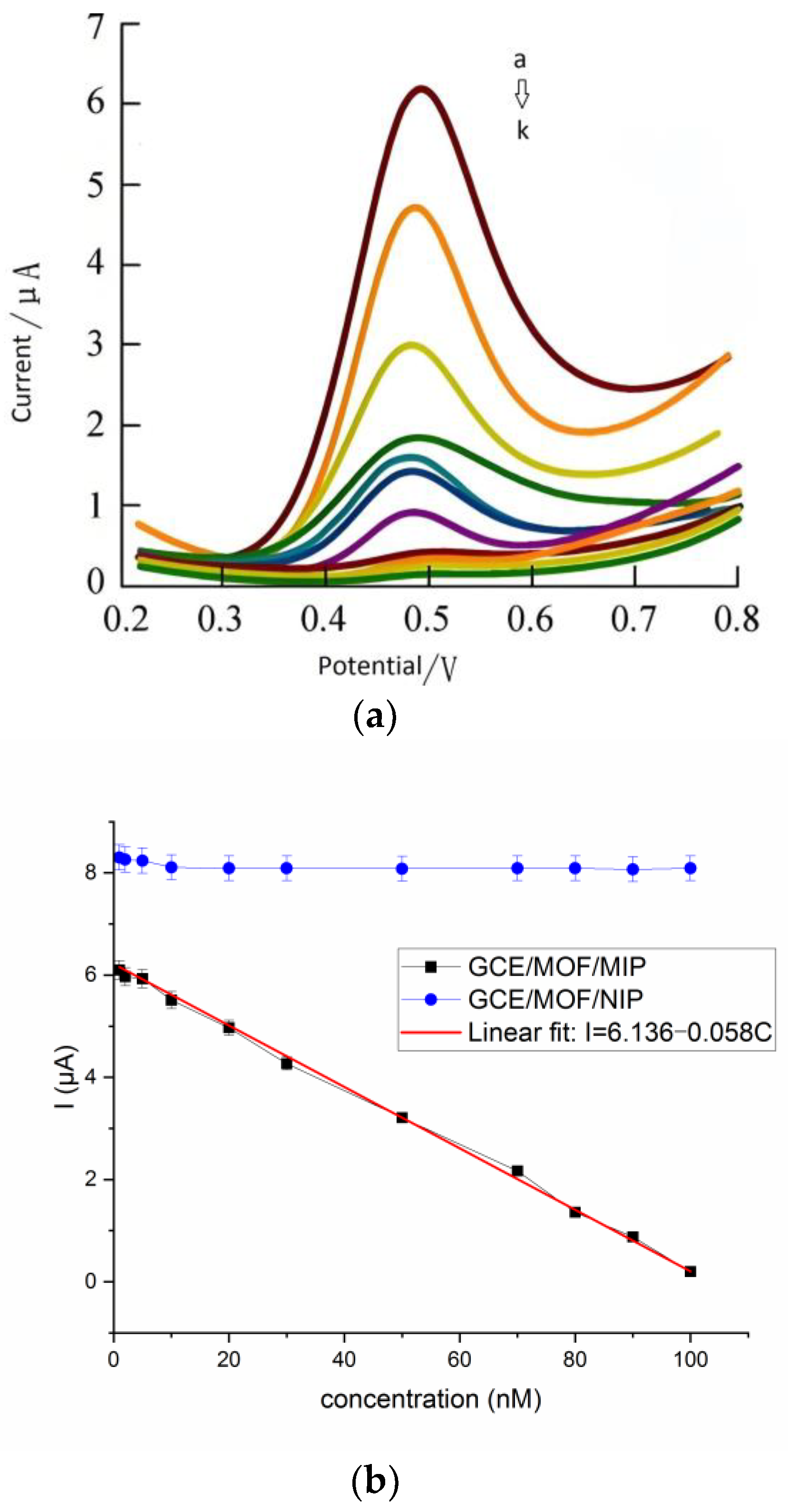
3.4. Real-Time Detection of Sweat Cortisol by Prepared Wearable GCE/MOF/MIP Sensor
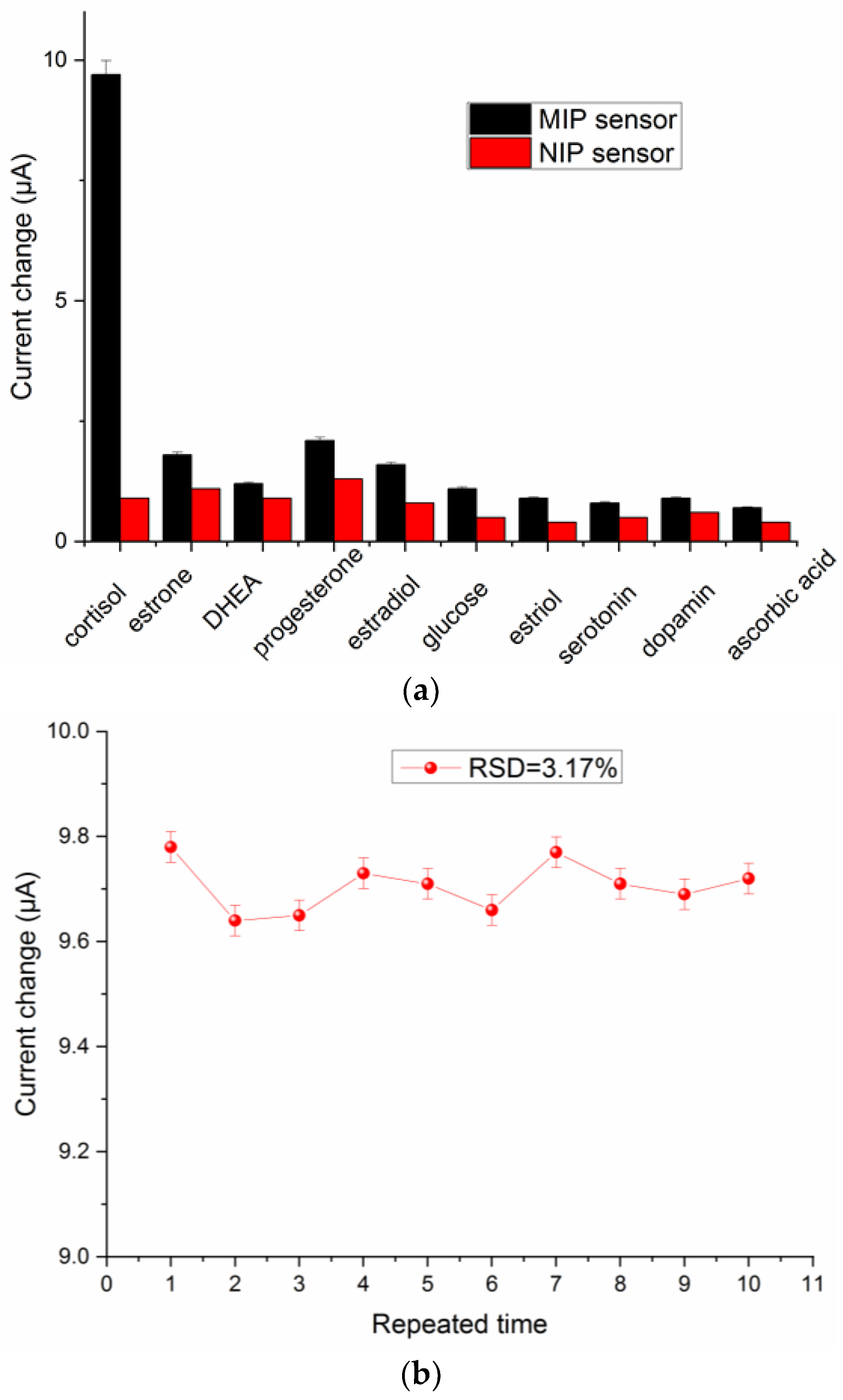
3.5. Selectivity and Durability of the Prosed GCE/MOF/MIP Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Kangas, M.; Henry, J.L.; Bryant, R.A. Posttraumatic stress disorder following cancer. A conceptual and empirical review. Clin. Psychol. Rev. 2002, 22, 499–524. [Google Scholar] [CrossRef] [PubMed]
- Keitel, A.; Ringleb, M.; Schwartges, I.; Weik, U.; Picker, O.; Stockhorst, U.; Deinzer, R. Endocrine and psychological stress responses in a simulated emergency situation. Psychoneuroendocrinology 2011, 36, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Lovallo, W.R. Cardiovascular responses to stress and disease outcomes A test of the reactivity hypothesis. Hypertension 2010, 55, 842–843. [Google Scholar] [CrossRef] [PubMed]
- Hadar, R.; Edemann-Callesen, H.; Hlusicka, E.B.; Wieske, F.; Vogel, M.; Günther, L.; Vollmayr, B.; Hellweg, R.; Heinz, A.; Garthe, A.; et al. Recurrent stress across life may improve cognitive performance in individual rats, suggesting the induction of resilience. Transl. Psychiatry 2019, 9, 185. [Google Scholar] [CrossRef]
- Uy, J.P.; Galván, A. Acute stress increases risky decisions and dampens prefrontal activation among adolescent boys. Neuroimage 2017, 146, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, C.Z.; Wang, Z.Q.; Yang, K.A.; Cheng, X.B.; Liu, W.F.; Yu, W.Z.; Lin, S.Y.; Zhao, Y.C.; Cheung, K.M.; et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 2022, 8, eabk0967. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Li, Y.Y.; Lin, H.Y.; Liu, Q.Q.; Lu, J.J.; Wang, X.L. A new three-dimensional zinc(ii) metal-organic framework as a fluorescence sensor for sensing the biomarker 3-nitrotyrosine. Dalton Trans. 2022, 51, 11390–11396. [Google Scholar] [CrossRef] [PubMed]
- Benfield, R.D.; Newton, E.R.; Tanner, C.J.; Heitkemper, M.M. Cortisol as a biomarker of stress in term human labor: Physiological and Methodological Issues. Biol. Res. Nurs. 2014, 16, 64–71. [Google Scholar] [CrossRef]
- Stelly, C.E.; Tritley, S.C.; Rafati, Y.; Wanat, M.J. Acute stress enhances associative learning via dopamine signaling in the ventral lateral striatum. J. Neurosci. 2020, 40, 4391–4400. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.A.; McCord, J.L.; Ely, M.R.; Sieck, D.C.; Buck, T.M.; Luttrell, M.J.; MacLean, D.A.; Halliwill, J.R. Mast cell degranulation and de novo histamine formation contribute to sustained postexercise vasodilation in humans. J. Appl. Physiol. 2017, 122, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Iob, E.; Steptoe, A. Cardiovascular disease and hair cortisol: A novel biomarker of chronic stress. Curr. Cardiol. Rep. 2019, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Y.Z.; Breeding, T.; Hejl, C.; Guleria, R.S.; Nelson, S.M.; Zambrano-Vazquez, L. Cortisol as a biomarker of alcohol use in combat veterans: A Literature Review and Framework for Future Research. J. Dual. Diagn. 2020, 16, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Safarian, S.M.; Kusov, P.A.; Kosolobov, S.S.; Borzenkova, O.V.; Khakimov, A.V.; Kotelevtsev, Y.V.; Drachev, V.P. Surface-specific washing-free immunosensor for time-resolved cortisol monitoring. Talanta 2021, 225, 122070. [Google Scholar] [CrossRef] [PubMed]
- Khumngern, S.; Jeerapan, I. Advances in wearable electrochemical antibody-based sensors for cortisol sensing. Anal. Bioanal. Chem. 2023, 415, 3863–3877. [Google Scholar] [CrossRef]
- Mugo, S.M.; Lu, W.H.; Robertson, S. A wearable, textile-based polyacrylate imprinted electrochemical sensor for cortisol detection in sweat. Biosensors 2022, 12, 854. [Google Scholar] [CrossRef] [PubMed]
- Sesay, A.M.; Micheli, L.; Tervo, P.; Palleschi, G.; Virtanen, V. Development of a competitive immunoassay for the determination of cortisol in human saliva. Anal. Biochem. 2013, 434, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, E.V.; Temerdashev, A.Z.; Zorina, M.O.; Feng, Y.Q.; Nesterenko, P.N. Solid-phase analytical derivatization as a tool for the quantification of steroid hormones in human urine with HPLC-Q-ToF detection. J. Pharm. Biomed. Anal. 2022, 214, 114736. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.L.; Guo, M.; Chen, Y.; Mo, X.H.; Cao, J.L.; Hu, F.D. Ultrasensitive cortisol electrochemical immunosensor amplifying by Au single-atom nanozymes and HRP enzymes. Anal. Chim. Acta 2024, 1303, 342462. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wulff, A.; Thomas, A.; Sonkusale, S. Ultrasensitive electrochemical sensor for detection of salivary cortisol in stress conditions. Mikrochim. Acta 2024, 191, 103. [Google Scholar] [CrossRef] [PubMed]
- Janghorban, M.; Aradanas, I.; Malaeb, K.; Abuelazm, H.; Nittala, A.; Hu, J.G.; Murari, K.; Pandey, R. Redox-Concatenated Aptamer Integrated Skin Mimicking Electrochemical Patch for Noninvasive Detection of Cortisol. ACS Sens. 2023, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Nguyen, K.N.; Malo, S.; Banerjee, I.; Wu, D.H.; Du-Thumm, L.; Dauphin-Ducharme, P. Electrochemical aptamer-based biosensors for measurements in undiluted human saliva. ACS Sens. 2023, 8, 4625–4635. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Wang, X.Y.; Lu, W.H.; Wu, X.Q.; Li, J.H. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Vlatakis, G.; Andersson, L.I.; Muller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef]
- Qronfla, M.M.; Jamoussi, B.; Chakroun, R.; Al-Mur, B.A.; Halawani, R.F.; Aloufi, F.A. Synthesis of a new molecularly imprinted polymer and optimisation of phenylglyoxylic acid extraction from human urine samples using a central composite design within the response surface methodology. Polymers 2023, 15, 3279. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Liu, Y.; Zhong, M.; Yang, J.Y.; Lin, Z.M.; Liang, Y. Preparation and properties of boron affinity molecularly imprinted mesoporous polymers based on Mn-doped ZnS quantum dots. Anal. Sci. 2023, 39, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Pataer, P.; Muhammad, T.; Turahun, Y.; Yang, W.W.; Aihebaier, S.; Wubulikasimu, M.; Chen, L.X. Preparation of a stoichiometric molecularly imprinted polymer for auramine O and application in solid-phase extraction. J. Sep. Sci. 2019, 42, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Liu, G.Q. Carbon nanomaterial-based molecularly imprinted polymer sensors for detection of hazardous substances in food: Recent progress and future trends. Food Chem. 2023, 420, 136100. [Google Scholar] [CrossRef] [PubMed]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly imprinted polymer micro- and nano-particles: A review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.H.; Pan, M.F.; Fang, G.Z.; Wang, S. Carbon dots embedded metal-organic framework@molecularly imprinted nanoparticles for highly sensitive and selective detection of quercetin. Sens. Actuators B Chem. 2019, 286, 321–327. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y. An overview of the most common lab-made coating materials in solid phase microextraction. Talanta 2019, 191, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Tang, S.S.; Zhao, P.F.; Tang, K.L.; Chen, Y.; Fu, J.L.; Zhou, S.; Yang, Z.X.; Zhang, Z.H. One-pot synthesis of ternary-emission molecularly imprinted fluorescence sensor based on metal-organic framework for visual detection of chloramphenicol. Food Chem. 2023, 402, 134256. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.X.; Ni, K.Y.; You, E.; Wang, M.L.; Culbert, A.; Jiang, X.M.; Lin, W.B. Multifunctional nanoscale metal-organic layers for ratiometric pH and oxygen sensing. J. Am. Chem. Soc. 2019, 141, 18964–18969. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.F.; Liu, Y.Y.; Zhang, Y.J.; Zhang, C.Y.; Li, Y.J.; Wang, Z.Y.; Wang, P.; Zheng, Z.K.; Cheng, H.F.; Dai, Y.; et al. Extended Light Absorption and improved charge separation by protonation of the organic ligand in a bismuth-based metal-organic framework. Chem. Eur. J. 2023, 29, 843. [Google Scholar] [CrossRef] [PubMed]
- Sutar, P.; Bakuru, V.R.; Yadav, P.; Laha, S.; Kalidindi, S.B.; Maji, T.K. Nanocomposite hydrogel of Pd@ZIF-8 and Laponite®: Size-selective hydrogenation catalyst under mild conditions. Chem. Eur. J. 2021, 27, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Tao, Y.; Du, Y.X.; Ding, W.; Chen, C.; Ma, X.F. Metal organic framework HKUST-1 modified with carboxymethyl--cyclodextrin for use in improved open tubular capillary electrochromatographic enantioseparation of five basic drugs. Mikrochim. Acta 2019, 186, 462. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kwon, Y.U.; de Castro, B.; Cunha-Silva, L. Novel Mn(II)-based metal-organic frameworks isolated in ionic liquids. Cryst. Growth Des. 2013, 13, 1260–1266. [Google Scholar] [CrossRef]
- Takeuchi, T.; Mukawa, T.; Shinmori, H. Signaling molecularly imprinted polymers: Molecular recognition-based sensing materials. Chem. Rec. 2005, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.D.; Chen, J.Q.; Xu, G.F.; Yu, T.; Du, Y.X. Enantiomeric analysis of chiral phenyl aromatic compounds by coated capillary electrochromatography based on a MOF-on-MOF stationary phase. Mikrochim. Acta 2024, 191, 160. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.S. Terthiophene as a model sensor for some atmospheric gases: Theoretical study. Mol. Phys. 2016, 114, 584–591. [Google Scholar] [CrossRef]
- Wasalathanthri, D.P.; Feroz, H.; Puri, N.; Hung, J.; Lane, G.; Holstein, M.; Chemmalil, L.; Both, D.; Ghose, S.; Ding, J.; et al. Real-time monitoring of quality attributes by in-line Fourier transform infrared spectroscopic sensors at ultrafiltration and diafiltration of bioprocess. Biotechnol. Bioeng. 2020, 117, 3766–3774. [Google Scholar] [CrossRef]
- Li, H.H.; Zhang, W.C.; Wu, Z.Y.; Huang, X.S.; Hui, A.L.; He, Y.W.; Wang, H.Y. Theoretical design, preparation, and evaluation of Ginkgolide B molecularly imprinted polymers. J. Sep. Sci. 2020, 43, 514–523. [Google Scholar] [CrossRef]
- Van der Jeught, S.; Dirckx, J.J.J. Real-time structured light profilometry: A review. Opt. Lasers Eng. 2016, 87, 18–31. [Google Scholar] [CrossRef]
- Arhab, S.; Soriano, G.; Ruan, Y.; Maire, G.; Talneau, A.; Sentenac, D.; Chaumet, P.C.; Belkebir, K.; Giovannini, H. Nanometric resolution with far-field optical profilometry. Phys. Rev. Lett. 2013, 111, 053902. [Google Scholar] [CrossRef] [PubMed]
- Bosserdt, M.; Gajovic-Eichelman, N.; Scheller, F.W. Modulation of direct electron transfer of cytochrome c by use of a molecularly imprinted thin film. Anal. Bioanal. Chem. 2013, 405, 6437–6444. [Google Scholar] [CrossRef]
- Ting, W.T.; Wang, M.J.; Howlader, M.M.R. Interleukin-6 electrochemical sensor using poly (o-phenylenediamine)-based molecularly imprinted polymer. Sens. Actuators B Chem. 2024, 404, 135282. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wei, X.Z.; Dai, H.F. Estimation of state of health of lithium-ion batteries based on charge transfer resistance considering different temperature and state of charge. J. Energy Storage 2019, 21, 618–631. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Tanyi, E.K.; Yeasmin, S.; Cheng, L.J. Label-free sensitive detection of steroid hormone cortisol based on target-induced fluorescence quenching of quantum dots. Langmuir 2020, 36, 7781–7788. [Google Scholar] [CrossRef]
- Yu, X.H.; Meng, W.C.; Li, Y.; Luo, X.L. A low-fouling electrochemical biosensor based on BSA hydrogel doped with carbon black for the detection of cortisol in human serum. Anal. Chim. Acta 2024, 1307, 342645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Lai, Q.T.; Chen, W.; Zhang, C.; Mo, L.; Liu, Z.C. Recent advance in cortisol immunosensing technologies and devices. Chemosensors 2023, 11, 90. [Google Scholar] [CrossRef]
- Abdulsattar, J.O.; Greenway, G.M.; Wadhawan, J.D. Electrochemical immunoassay for the detection of stress biomarkers. Heliyon 2020, 6, e03558. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, S.K.; Ormsby, C.; Neethirajan, S. Noninvasive label-free detection of cortisol and lactate using graphene embedded screen-printed electrode. Nano-Micro Lett. 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Manickam, P.; Fernandez, R.E.; Umasankar, Y.; Gurusamy, M.; Arizaleta, F.; Urizar, G.; Bhansali, S. Salivary cortisol analysis using metalloporphyrins and multi-walled carbon nanotubes nanocomposite functionalized electrodes. Sensor. Actuators B Chem. 2018, 274, 47–53. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Moore, J.A.; Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N.; Chou, C.-F.; Swami, N.S. Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens. Bioelectron. 2016, 78, 244–252. [Google Scholar] [CrossRef] [PubMed]

| Factors | Units | Low | High |
|---|---|---|---|
| Monomer concentration (X1) | mmol L−1 | 0.05 | 3 |
| Cortisol concentration (X2) | mmol L−1 | 0.1 | 6 |
| Number of CV cycles (X3) | 7 | 39 | |
| Elution time (X4) | min | 3 | 13 |
| Molecule | Energy (a.u.) | Energy (eV) | ΔE (a.u) | ΔE (eV) |
|---|---|---|---|---|
| O-phenylenediamine | −796.34267 ± 0.63241 | −20,356.87821 ± 16.37264 | ||
| Cortisol | −2193.66542 ± 1.38562 | −61,573.38429 ± 23.58513 | ||
| Complex | −2915.22996 ± 1.94675 | −79,166.75309 ± 26.79324 | 74.77813 ± 0.073281 | 2013.50941 ± 1.079312 |
| Electrode | Sa (µm) | Ssk (µm) |
|---|---|---|
| GCE/MOF/NIP | 10.31 ± 0.24 | −2.871 ± 0.031 |
| GCE/MOF/MIP before elution | 10.37 ± 0.24 | −4.116 ± 0.039 |
| GCE/MOF/MIP after elution | 11.76 ± 0.26 | −5.378 ± 0.045 |
| Sample | Spiked (nM) | Found (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 1 | 0.947 | 94.7 | 4.15 |
| 2 | 10 | 10.12 | 101.2 | 2.86 |
| 3 | 100 | 103.6 | 103.6 | 4.03 |
| 4 | 500 | 493.7 | 98.7 | 4.17 |
| 5 | 1000 | 1013.4 | 101.3 | 3.95 |
| Electrode | Sensing Unit | Sample | Detection Range (nM) | LOD (nM) | Ref |
|---|---|---|---|---|---|
| ITO | Ferrocene tagged antibodies | Saliva | 0.028–137.9 | 0.028 | [52] |
| Reduced graphene oxide | Anti-cortisol antibodies | Saliva/sweat | 0.2758–551.7 | 0.2758 | [53] |
| Carbon | Carbon nanotubes/ metalloporphyrins | Saliva | 0.00005–100 | 0.00005 | [54] |
| Graphene + AuNPs-triamcinolone | Aptamer | Serum | 0.0827–27,500 | 0.275 | [55] |
| GCE/MOF/MIP | MIP | Sweat | 0.0027–1000 | 0.0027 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.; He, F. A Wearable Electrochemical Sensor Based on a Molecularly Imprinted Polymer Integrated with a Copper Benzene-1,3,5-Tricarboxylate Metal-Organic Framework for the On-Body Monitoring of Cortisol in Sweat. Polymers 2024, 16, 2289. https://doi.org/10.3390/polym16162289
Tang P, He F. A Wearable Electrochemical Sensor Based on a Molecularly Imprinted Polymer Integrated with a Copper Benzene-1,3,5-Tricarboxylate Metal-Organic Framework for the On-Body Monitoring of Cortisol in Sweat. Polymers. 2024; 16(16):2289. https://doi.org/10.3390/polym16162289
Chicago/Turabian StyleTang, Pingping, and Feiyu He. 2024. "A Wearable Electrochemical Sensor Based on a Molecularly Imprinted Polymer Integrated with a Copper Benzene-1,3,5-Tricarboxylate Metal-Organic Framework for the On-Body Monitoring of Cortisol in Sweat" Polymers 16, no. 16: 2289. https://doi.org/10.3390/polym16162289
APA StyleTang, P., & He, F. (2024). A Wearable Electrochemical Sensor Based on a Molecularly Imprinted Polymer Integrated with a Copper Benzene-1,3,5-Tricarboxylate Metal-Organic Framework for the On-Body Monitoring of Cortisol in Sweat. Polymers, 16(16), 2289. https://doi.org/10.3390/polym16162289





