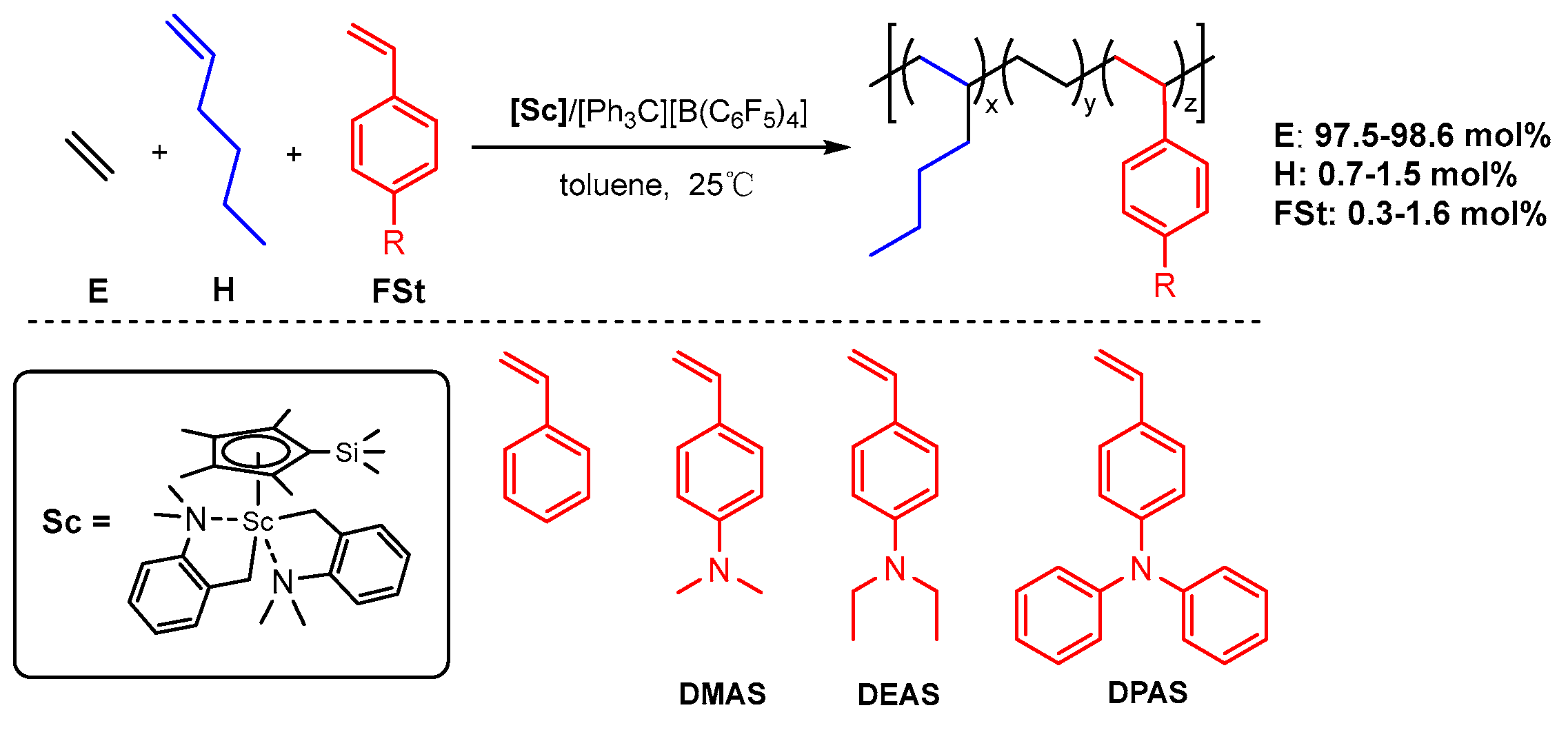
Terpolymerization of Ethylene with Hexene and Styrene Derivatives by Half-Sandwich Scandium Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods and Materials
2.2. Characterization
2.3. Typical Terpolymerization Procedure
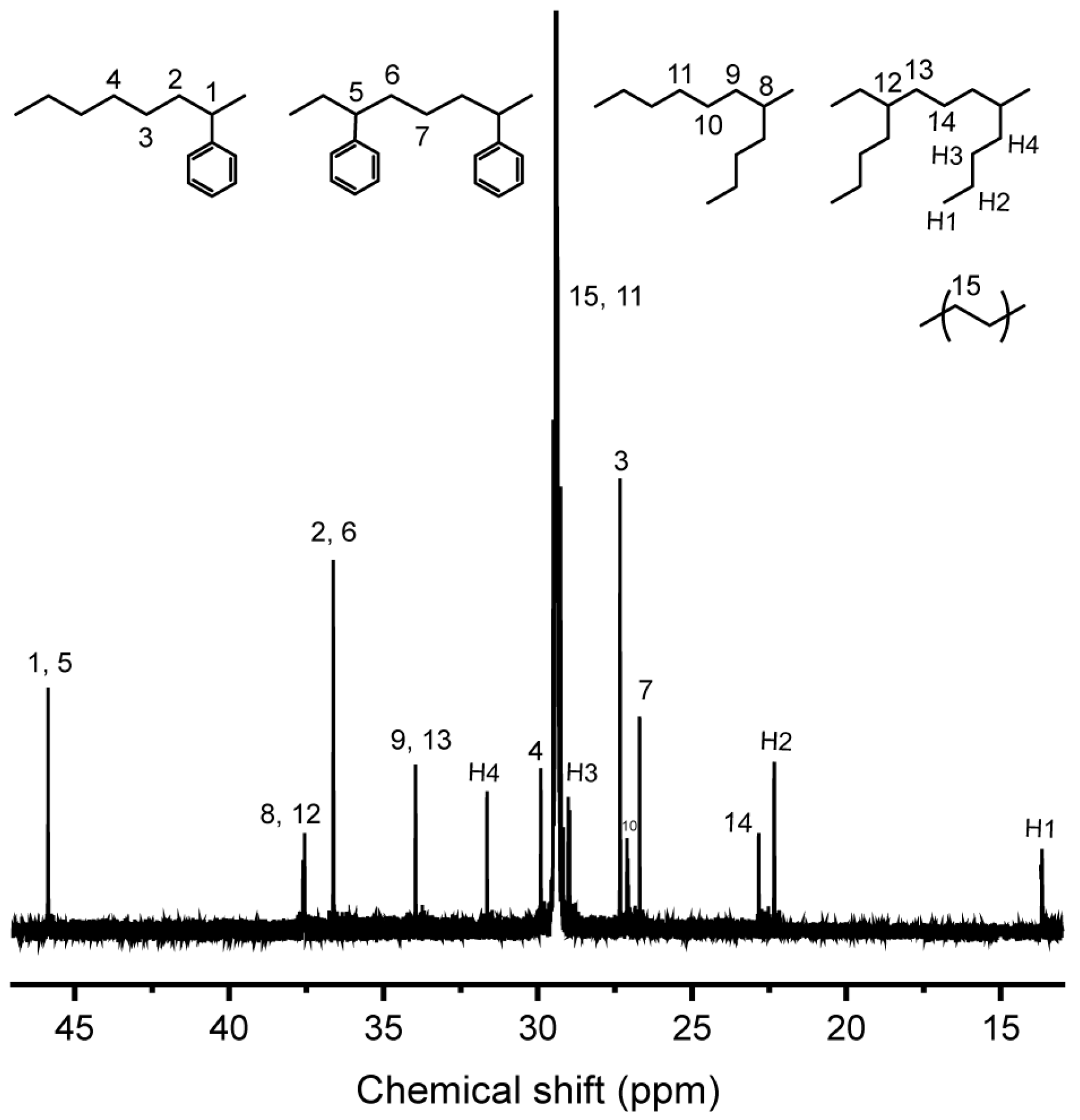
3. Results
3.1. Terpolymerization of Ethylene with Hexene and Styrene
3.2. Terpolymerization of Ethylene with Hexene and Styrene Derivatives
3.3. Mechanical Properties of Terpolymers
3.4. Surface Property of Terpolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Li, J.F.; Tao, W.J.; Sun, X.L.; Yang, X.H.; Tang, Y. Copolymerization of Ethylene with Functionalized Olefins by [ONX] Titanium Complexes. Macromolecules 2013, 46, 2870–2875. [Google Scholar] [CrossRef]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination-Insertion Copolymerization of Fundamental Polar Monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Hu, Y.L. Design and Synthesis of Structurally Well-defined Functional Polyolefins via Transition Metal-mediated Olefin Polymerization Chemistry. Coord. Chem. Rev. 2006, 250, 47–65. [Google Scholar] [CrossRef]
- Boffa, L.S.; Novak, B.M. Copolymerization of Polar Monomers with Olefins Using Transition-Metal Complexes. Chem. Rev. 2000, 100, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.X. Coordination Polymerization of Polar Vinyl Monomers by Single-Site Metal Catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef] [PubMed]
- Zinck, P.; Bonnet, F.; Mortreux, A.; Visseaux, M. Functionalization of Syndiotactic Polystyrene. Prog. Polym. Sci. 2009, 34, 369–392. [Google Scholar] [CrossRef]
- Franssen, N.M.G.; Reek, J.N.H.; Bruin, B. Synthesis of Functional ‘polyolefins’: State of the Art and Remaining Challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef] [PubMed]
- Boaen, N.K.; Hillmeyer, M.A. Post-polymerization Functionalization of Polyolefins. Chem. Soc. Rev. 2005, 34, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A. The Chemistry of Polyethylene. J. Macromol. Sci. Part C Polym. Rev. 2001, 41, 285–323. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wang, F.; Pan, L.; Wang, B.; Li, Y.S. Facile Synthesis of High-Molecular-Weight Vinyl Sulfone (Sulfoxide) Modified Polyethylenes via Coordination-Insertion Copolymerization. Macromolecules 2020, 53, 5177–5187. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Mu, H.L.; Pan, L.; Wang, X.L.; Li, Y.S. Robust Bulky [P, O] Neutral Nickel Catalysts for Copolymerization of Ethylene with Polar Vinyl Monomers. ACS Catal. 2018, 8, 5963–5976. [Google Scholar] [CrossRef]
- Mu, H.L.; Pan, L.; Song, D.P.; Li, Y.S. Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Zhang, Y.X.; Li, B.X.; Jian, Z.B. Fluorinated α-Diimine Nickel Mediated Ethylene (Co)Polymerization. Chem. Eur. J. 2021, 27, 11935–11942. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Han, Y.F.; Kou, S.Q.; Zhang, Y.X.; Jian, Z.B. Exploring Steric Effect of Electron-Donating Group in Palladium and Nickel Mediated Ethylene Polymerization and Copolymerization with Polar Monomers. Eur. Polym. J. 2021, 160, 110781. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, C.Q.; Mecking, S.; Jian, Z.B. Ultrahigh Branching of Main-Chain-Functionalized Polyethylenes by Inverted Insertion Selectivity. Angew. Chem. Int. Ed. 2020, 59, 14296–14302. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chen, C.L. Photoresponsive Palladium and Nickel Catalysts for Ethylene Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2021, 60, 22195–22200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, C.; Zhao, H.P.; Cai, Z.G.; Chen, C.L. Hydrogen-Bonding-Induced Heterogenization of Nickel and Palladium Catalysts for Copolymerization of Ethylene with Polar Monomers. Angew. Chem. Int. Ed. 2021, 60, 17446–17451. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, C.L. Direct and Tandem Routes for the Copolymerization of Ethylene with Polar Functionalized Internal Olefins. Angew. Chem. Int. Ed. 2020, 59, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Chen, C.L. Emerging Palladium and Nickel Catalysts for Copolymerization of Olefins with Polar Monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef]
- Long, Y.Y.; Wang, Y.X.; Li, B.X.; Li, Y.G.; Li, Y.S. Microstructure determination of ethylene-styrene-1-hexene terpolymers with fast 2D NMR by nonuniform sampling. Polymer 2019, 169, 185–194. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.L.; Xiang, B.; Liu, X.W.; Zhang, S.; Wu, Y.X. Imidazolidin-2-iminato vanadium complexes for the synthesis of ethylene/propylene/5-ethylidene-2-norbornene (ENB) terpolymers with high ENB incorporation and ultra-high molecular weight. Polym. Chem. 2024, 15, 2148–2156. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, P.; Wang, W.J.; Li, B.G. Dynamically Cross-Linked Polyolefin Elastomers with Highly Improved Mechanical and Thermal Performance. Macromolecules 2021, 54, 10381–10387. [Google Scholar] [CrossRef]
- Yang, F.; Ma, Z.; Lou, Y.; Li, Y.; Li, Y. Highly elastic, strong, and reprocessable cross-linked polyolefin elastomers enabled by boronic ester bonds. Polym. Chem. 2020, 11, 3285–3295. [Google Scholar] [CrossRef]
- Apisuk, W.; Suzuki, N.; Kim, H.J.; Kim, D.H.; Kitiyanan, B.; Nomura, K. Efficient terpolymerization of ethylene and styrene with α-olefins by aryloxo-modified half-titanocene-based catalysts and cocatalyst systems. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2565–2574. [Google Scholar] [CrossRef]
- Apisuk, W.; Nomura, K. Efficient Terpolymerization of Ethylene and Styrene with 1,7-Octadiene by Aryloxo Modified Half-Titanocenes–Cocatalyst Systems: Efficient Introduction of the Reactive Functionality. Mocromol. Chem. Phys. 2014, 215, 1785–1791. [Google Scholar] [CrossRef]
- Tritto, I.; Ravasio, A.; Boggioni, L.; Bertini, F.; Hitzbleck, J.; Okuda, J. Hydroxyl-Functionalized Norbornene Based Co- and Terpolymers by Scandium Half-Sandwich Catalyst. Macromol. Chem. Phys. 2010, 211, 897–904. [Google Scholar] [CrossRef]
- Wang, C.; Luo, G.; Nishiura, M.; Song, G.; Yamamoto, A.; Luo, Y.; Hou, Z. Heteroatom-assisted Olefin Polymerization by Rare-earth Metal Catalysts. Sci. Adv. 2017, 3, e1701011. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, Y.; Wang, B.; Lohr, T.L.; Marks, T.J. Scandium-Catalyzed Self-Assisted Polar Co-Monomer Enchainment in Ethylene Polymerization. Angew. Chem. Int. Ed. 2017, 56, 15964–15968. [Google Scholar] [CrossRef]
- Liu, B.; Qiao, K.N.; Fang, J.; Wang, T.T.; Wang, Z.C.; Liu, D.T.; Xie, Z.G.; Maron, L.; Cui, D.M. Mechanism and Effect of Polar Styrenes on Scandium-Catalyzed Copolymerization with Ethylene. Angew. Chem. Int. Ed. 2018, 57, 14896–14901. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Liu, D.T.; Wang, Z.C.; Cui, D.M. Development of Group 3 Catalysts for Alternating Copolymerization of Ethylene and Styrene Derivatives. ACS Catal. 2018, 8, 6086–6093. [Google Scholar] [CrossRef]
- Zhang, K.; Dou, Y.L.; Zhang, Z.; Jiang, Y.; Li, S.H.; Cui, D.M. Copolymerization of Ethylene and Halogenated Styrenes Using Scandium Catalysts. Polymer 2020, 209, 123057. [Google Scholar] [CrossRef]
- Wang, T.T.; Wu, C.J.; Wang, B.L.; Cui, D.M. Isospecific Alternating Copolymerization of Unprotected Polar Styrenes and Ethylene by the Cs Symmetric Scandium Precursor via Synergistic Effects of Two Substituent Groups. Giant 2021, 7, 100061. [Google Scholar] [CrossRef]
- Wang, T.T.; Wu, C.J.; Ji, X.L.; Cui, D.M. Cui; Direct Synthesis of Functional Thermoplastic Elastomer with Excellent Mechanical Properties by Scandium-Catalyzed Copolymerization of Ethylene and Fluorostyrenes. Angew. Chem. Int. Ed. 2021, 60, 25735–25740. [Google Scholar] [CrossRef] [PubMed]
- Morishima, Y.; Hashimoto, T.; Itoh, Y.; Kamachi, M.; Nozakura, S.I. Synthesis of amphiphilic block copolymers. Block copolymers of methacrylic acid and p-N,N-dimethylaminostyrene. J. Polym. Sci. Part A Polym. Chem. 1982, 20, 299–310. [Google Scholar] [CrossRef]
- Li, X.; Nishiura, M.; Mori, K.; Mashiko, T.; Hou, Z. Cationic scandium aminobenzyl complexes. synthesis, structure and unprecedented catalysis of copolymerization of 1-hexene and dicyclopentadiene. Chem. Commun. 2007, 40, 4137–4139. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.C.W.; Tsai, W.M.; Rausch, M.D. Isospecific polymerization of propylene catalyzed by rac-ethylenebis(indenyl)methylzirconium cation. J. Am. Chem. Soc. 1991, 113, 8570–8571. [Google Scholar] [CrossRef]
- Wunderlich, B.; Czornyj, G. A Study of Equilibrium Melting of Polyethylene. Macromolecules 1977, 10, 906–913. [Google Scholar] [CrossRef]
- Luo, Y.J.; Hou, Z.M. Polymerization of 1-Hexene and Copolymerization of Ethylene with 1-Hexene Catalyzed by Cationic Half-Sandwich Scandium Alkyls. Stud. Surf. Sci. Catal. 2006, 161, 95–104. [Google Scholar]
- Guo, F.; Nishiura, M.; Koshino, H.; Hou, Z. Scandium-Catalyzed Cyclocopolymerization of 1,5-Hexadiene with Styrene and Ethylene: Efficient Synthesis of Cyclopolyolefins Containing Syndiotactic Styrene Styrene Sequences and Methylene-1,3-cyclopentane Units. Macromolecules 2011, 44, 6335–6344. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, F.; Li, Y.; Hou, Z. Synthesis of amino-containing syndiotactic polystyrene as efficient polymer support for palladium nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 5–9. [Google Scholar] [CrossRef]
- Park, J.H.; Yun, C.; Park, M.H.; Do, Y.; Yoo, S.; Lee, M.H. Vinyl-Type Polynorbornenes with Triarylamine Side Groups: A New Class of Soluble Hole-Transporting Materials for OLEDs. Macromolecules 2009, 42, 6840–6843. [Google Scholar] [CrossRef]
- Fang, Y.K.; Liu, C.L.; Yang, G.Y.; Chen, P.C.; Chen, W.C. New Donor-Acceptor Random Copolymers with Pendent Triphenylamine and 1,3,4-Oxadiazole for High-Performance Memory Device Applications. Macromolecules 2011, 44, 2604–2612. [Google Scholar] [CrossRef]
- Shang, R.; Gao, H.; Luo, F.; Li, Y.; Wang, B.; Ma, Z.; Pan, L.; Li, Y. Functional Isotactic Polypropylenes via Efficient Direct Copolymerizations of Propylene with Various Amino-Functionalized α-Olefins. Macromolecules 2019, 52, 9280–9290. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, D.; Chen, F.; Zhu, J.; Guo, W.; Zhang, Y.; Guo, J.; Huang, Q. Functionalized amphiphilic polyethylene via direct copolymerizations of ethylene with α-olefin containing amino functionalization. J. Macromol. Sci. Part A 2022, 59, 202–210. [Google Scholar] [CrossRef]



| Run (a) | FSt | Hex (mmoL) | FSt (mmol) | Act. (b) | fE (c) (mol%) | fHex (c) (mol%) | fFSt (c) (mol%) | Mn (d) [×104 Da] | Mw/Mn (d) | Tm (e) (oC) | Xc (e) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | St | 20 | 10 | 3684 | 98.2 | 1.2 | 0.6 | 12.9 | 1.8 | 127.7 | 51.4 |
| 2 | St | 20 | 20 | 3810 | 97.8 | 1.1 | 1.1 | 11.3 | 2.1 | 123.6 | 49.1 |
| 3 | St | 20 | 30 | 4166 | 97.5 | 1.0 | 1.5 | 10.7 | 1.7 | 123.0 | 46.8 |
| 4 | St | 30 | 20 | 4550 | 97.7 | 1.2 | 1.1 | 10.1 | 2.4 | 123.4 | 48.6 |
| 5 | St | 40 | 10 | 4780 | 97.9 | 1.5 | 0.6 | 9.7 | 1.7 | 126.9 | 51.1 |
| Run (a) | FSt | Hex (mmoL) | FSt (mmol) | Act. (b) | fE (c) mol% | fHex (c) mol% | fFSt (c) mol% | Mn (d) [×104 Da] | Mw/Mn (d) | Tm(e) | Xc (e) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DMAS | 20 | 10 | 1720 | 98.0 | 1.3 | 0.7 | 12.4 | 2.5 | 134.2 | 43.8 |
| 2 | DMAS | 20 | 20 | 2080 | 97.9 | 1.0 | 1.1 | 11.6 | 2.1 | 132.4 | 38.4 |
| 3 | DMAS | 20 | 30 | 2130 | 97.5 | 1.0 | 1.5 | 10.2 | 2.3 | 128.5 | 35.1 |
| 4 | DMAS | 30 | 20 | 2360 | 97.6 | 1.3 | 1.1 | 10.1 | 1.9 | 130.5 | 35.7 |
| 5 | DMAS | 40 | 10 | 2550 | 97.7 | 1.5 | 0.8 | 8.5 | 2.7 | 130.6 | 35.9 |
| 6 | DEAS | 20 | 10 | 1404 | 98.0 | 1.1 | 0.9 | 11.9 | 1.9 | 135.5 | 36.5 |
| 7 | DEAS | 20 | 20 | 1609 | 97.8 | 0.9 | 1.3 | 11.2 | 2.6 | 133.9 | 35.7 |
| 8 | DEAS | 20 | 30 | 1845 | 97.7 | 0.8 | 1.5 | 10.6 | 2.1 | 131.6 | 35.5 |
| 9 | DEAS | 30 | 20 | 2057 | 97.8 | 1.1 | 1.1 | 9.8 | 2.6 | 134.9 | 36.0 |
| 10 | DEAS | 40 | 10 | 2280 | 98.0 | 1.2 | 0.8 | 8.2 | 2.4 | 137.0 | 36.7 |
| 11 | DPAS | 20 | 10 | 5020 | 98.6 | 1.0 | 0.4 | 12.7 | 1.8 | 129.6 | 48.6 |
| 12 | DPAS | 20 | 20 | 5150 | 98.5 | 0.8 | 0.7 | 12.2 | 2.3 | 129.2 | 46.3 |
| 13 | DPAS | 20 | 30 | 5380 | 98.3 | 0.7 | 0.9 | 10.3 | 2.1 | 127.2 | 43.1 |
| 14 | DPAS | 30 | 20 | 5510 | 98.4 | 1.0 | 0.6 | 8.9 | 1.8 | 129.4 | 44.2 |
| 15 | DPAS | 40 | 10 | 5730 | 98.4 | 1.3 | 0.3 | 7.4 | 1.9 | 130.0 | 44.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, X.; Leng, X.; Liu, C.; Yao, Q.; Li, Y. Terpolymerization of Ethylene with Hexene and Styrene Derivatives by Half-Sandwich Scandium Catalyst. Polymers 2024, 16, 2290. https://doi.org/10.3390/polym16162290
Mu X, Leng X, Liu C, Yao Q, Li Y. Terpolymerization of Ethylene with Hexene and Styrene Derivatives by Half-Sandwich Scandium Catalyst. Polymers. 2024; 16(16):2290. https://doi.org/10.3390/polym16162290
Chicago/Turabian StyleMu, Xiaochun, Xuefei Leng, Chuanchuan Liu, Qiang Yao, and Yang Li. 2024. "Terpolymerization of Ethylene with Hexene and Styrene Derivatives by Half-Sandwich Scandium Catalyst" Polymers 16, no. 16: 2290. https://doi.org/10.3390/polym16162290
APA StyleMu, X., Leng, X., Liu, C., Yao, Q., & Li, Y. (2024). Terpolymerization of Ethylene with Hexene and Styrene Derivatives by Half-Sandwich Scandium Catalyst. Polymers, 16(16), 2290. https://doi.org/10.3390/polym16162290






