Changes in the Chemical Composition of Polyethylene Terephthalate under UV Radiation in Various Environmental Conditions
Abstract
1. Introduction
2. Method and Materials
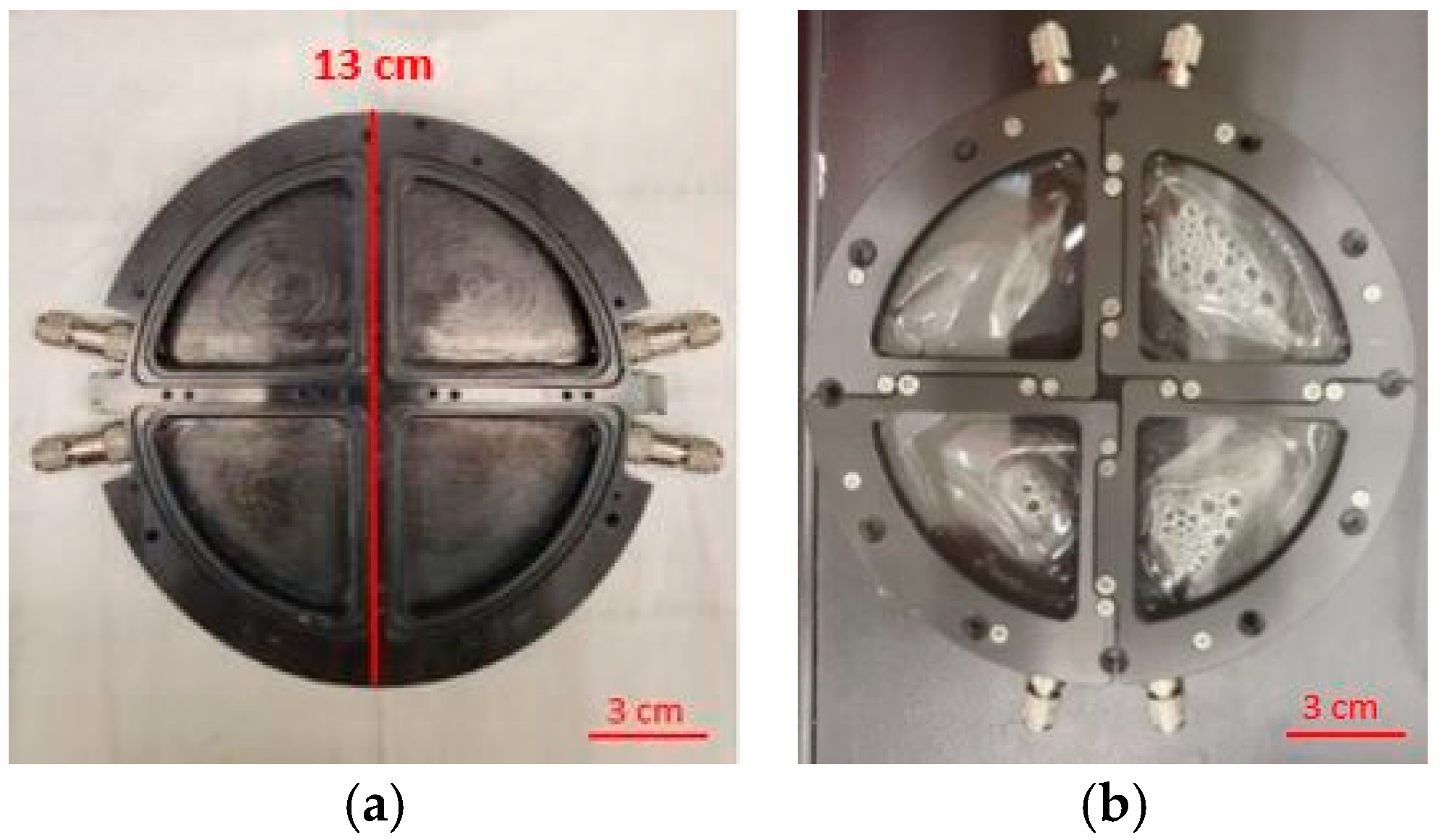
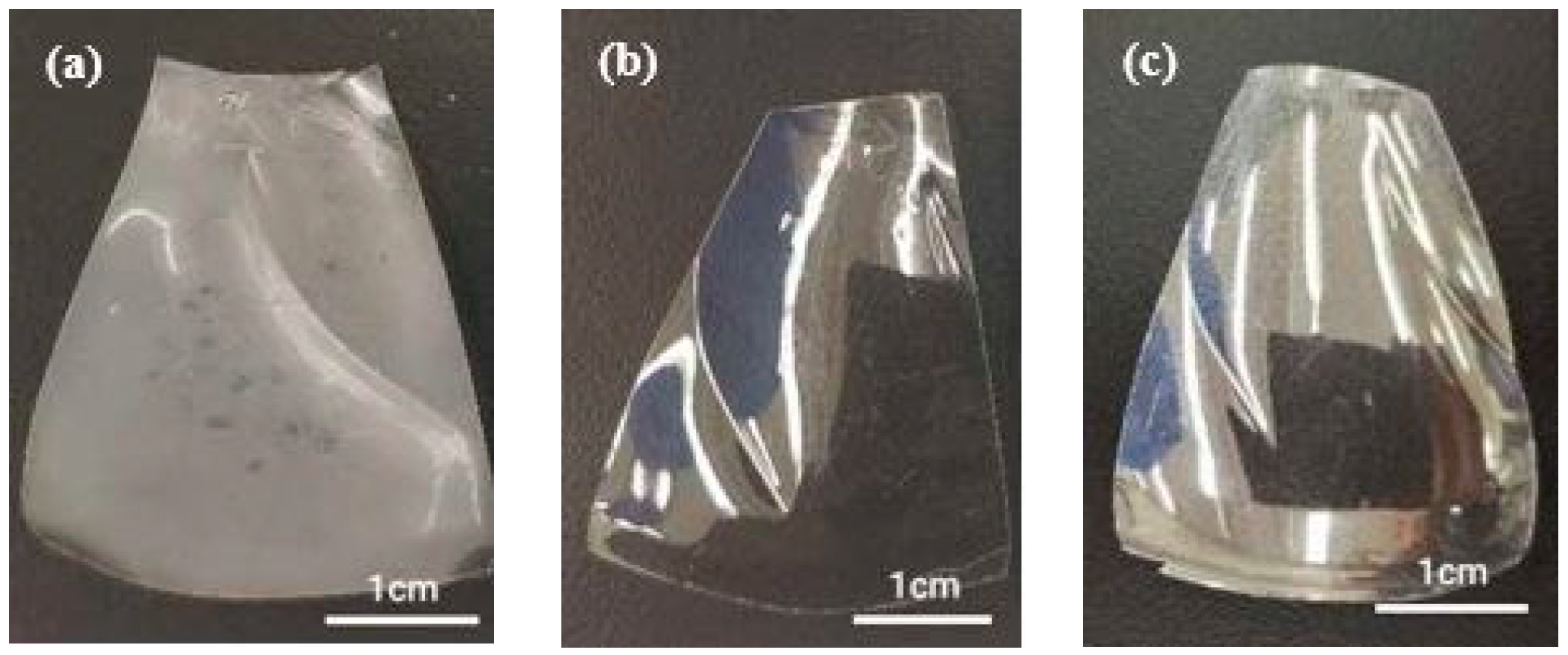
2.1. Sample Preparation
2.2. UV Exposures
2.3. Chemical Characterization by Fourier Transform Infrared (FTIR) Spectroscopy Measurements
3. Data Analysis
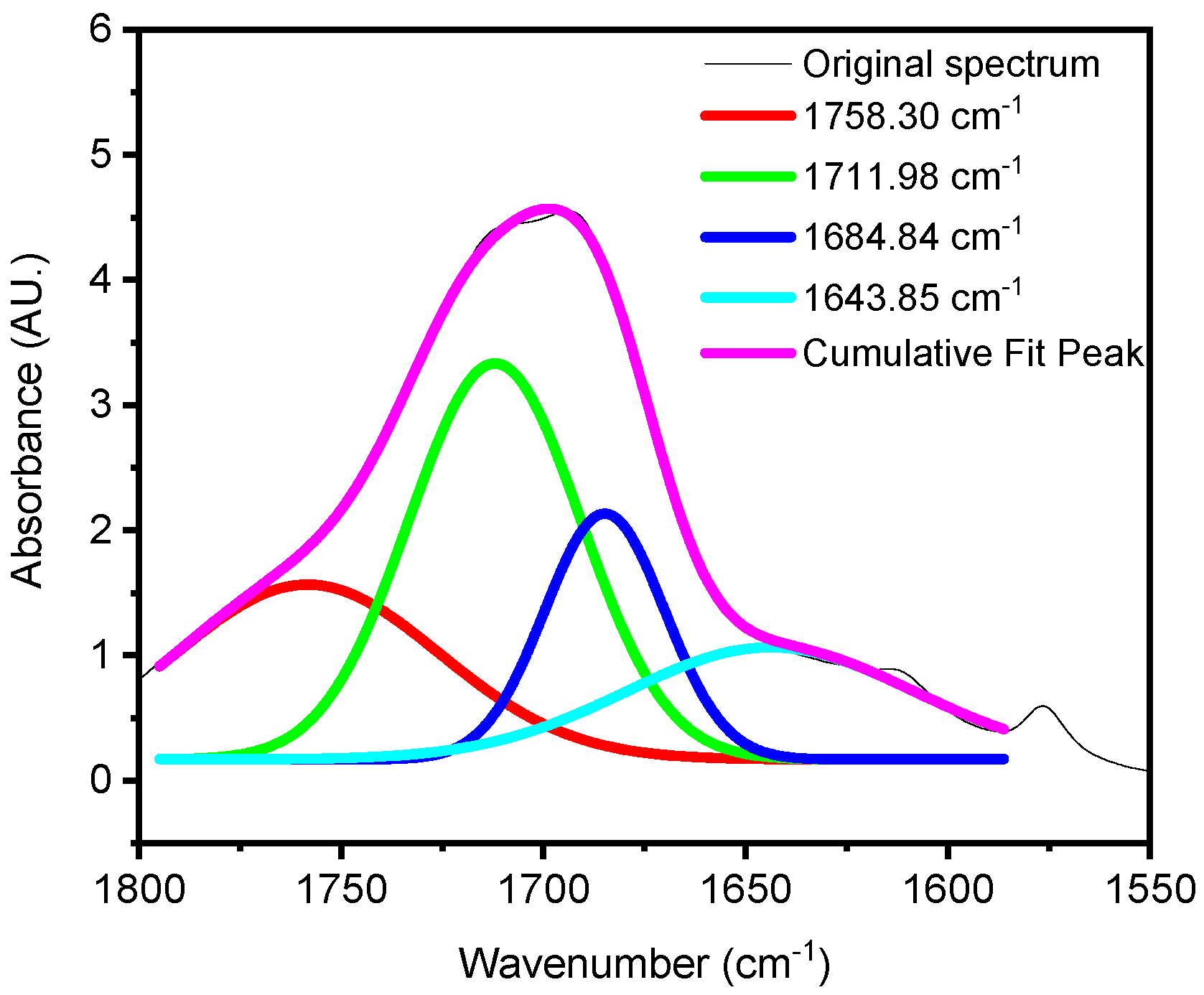
Deconvolution
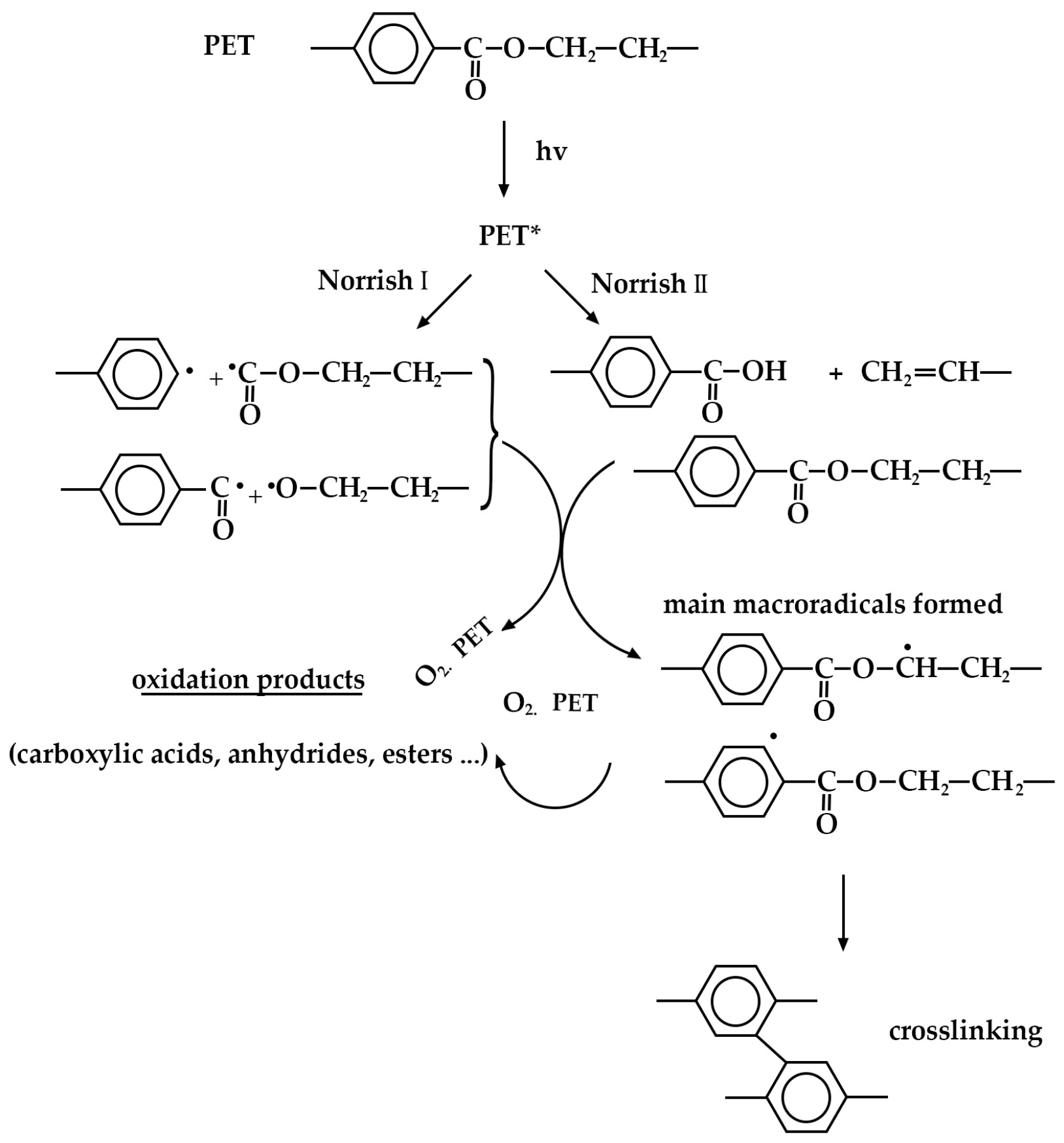
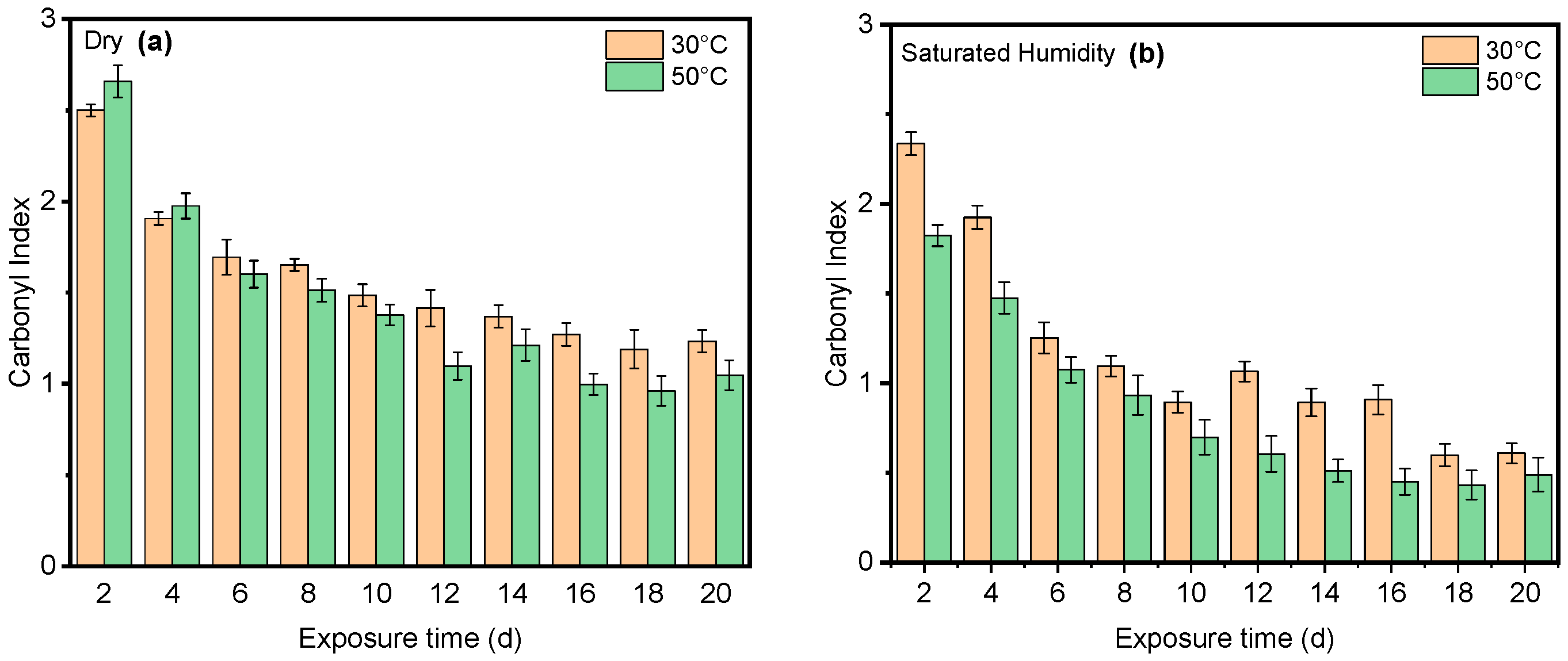
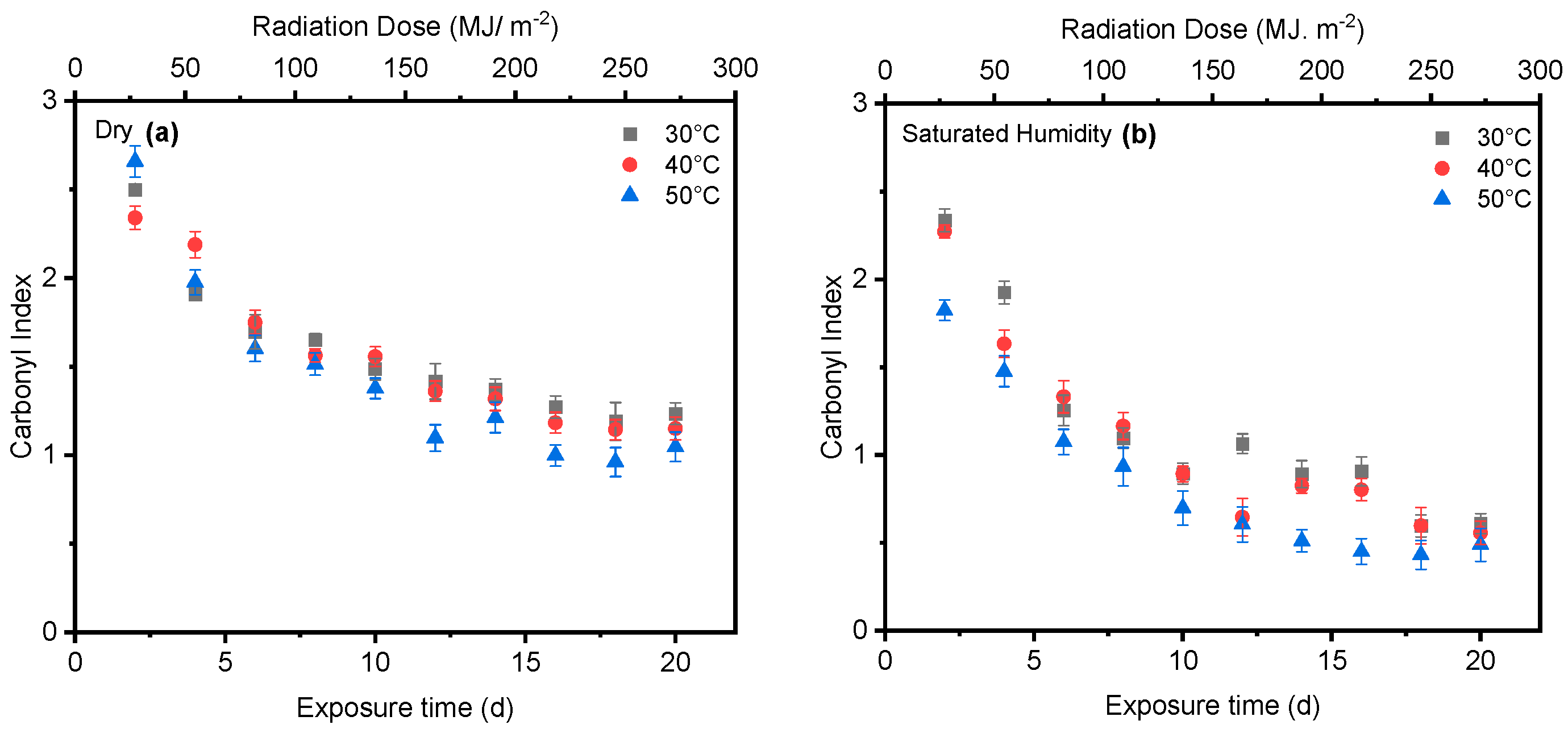
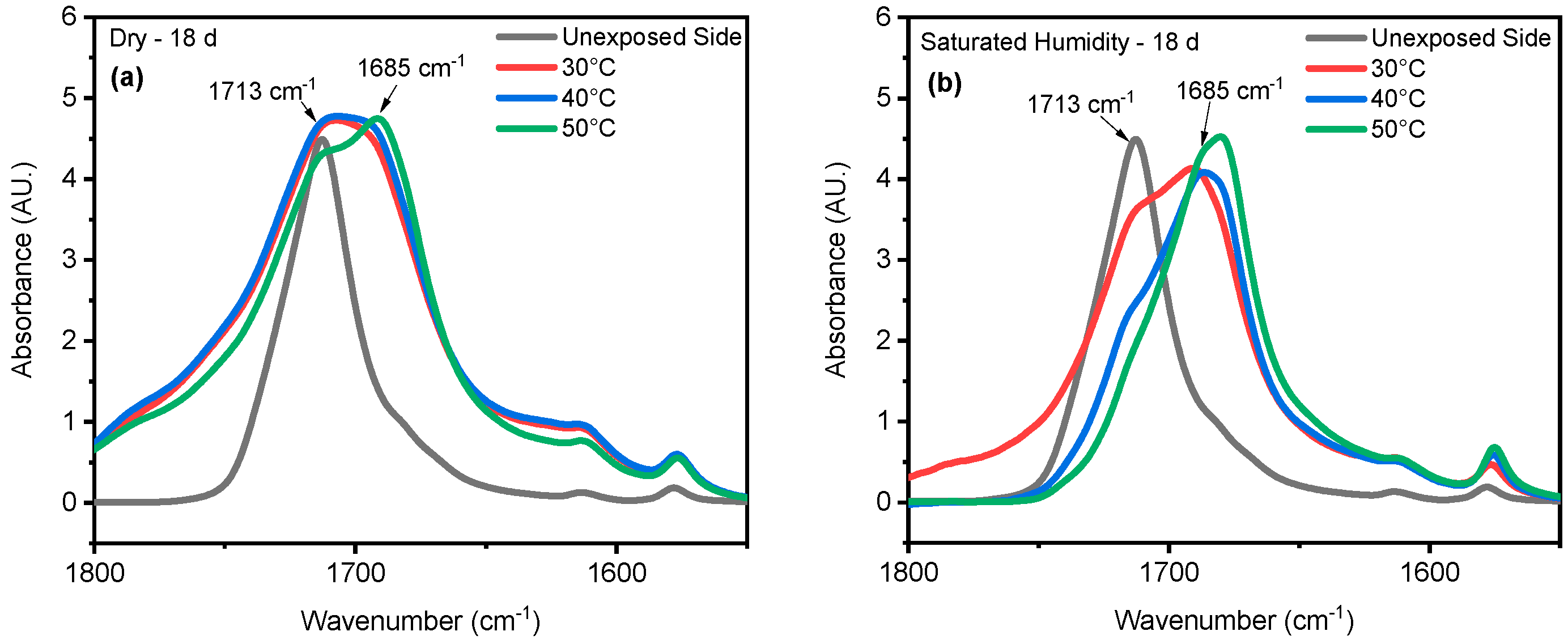
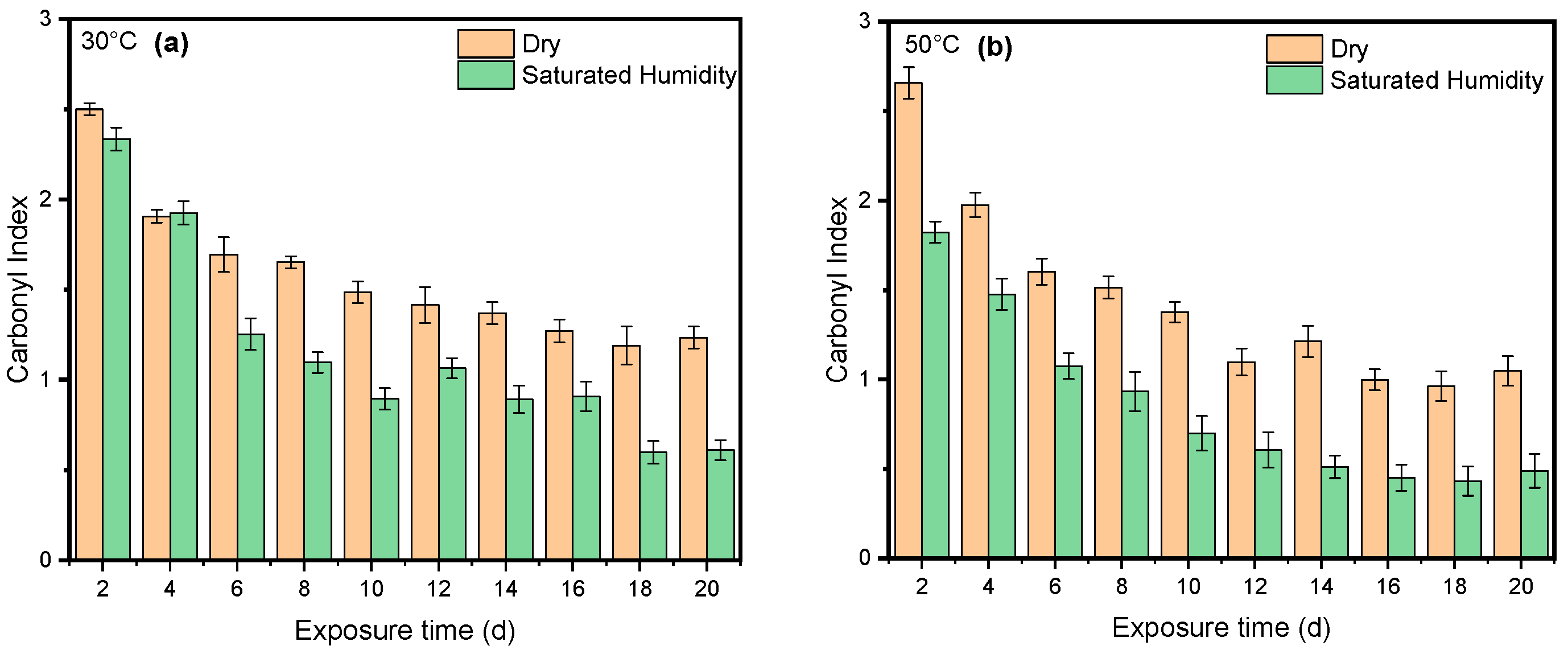
4. Results and Discussion
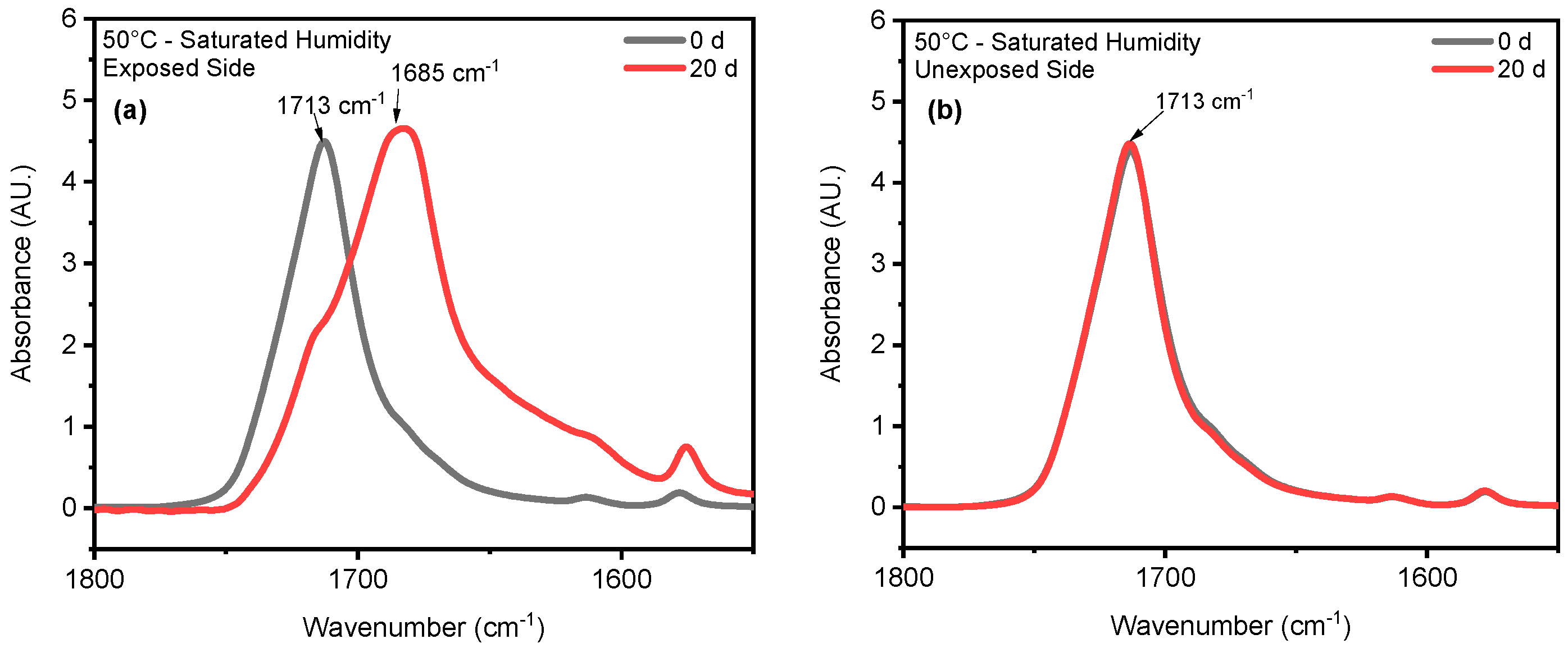
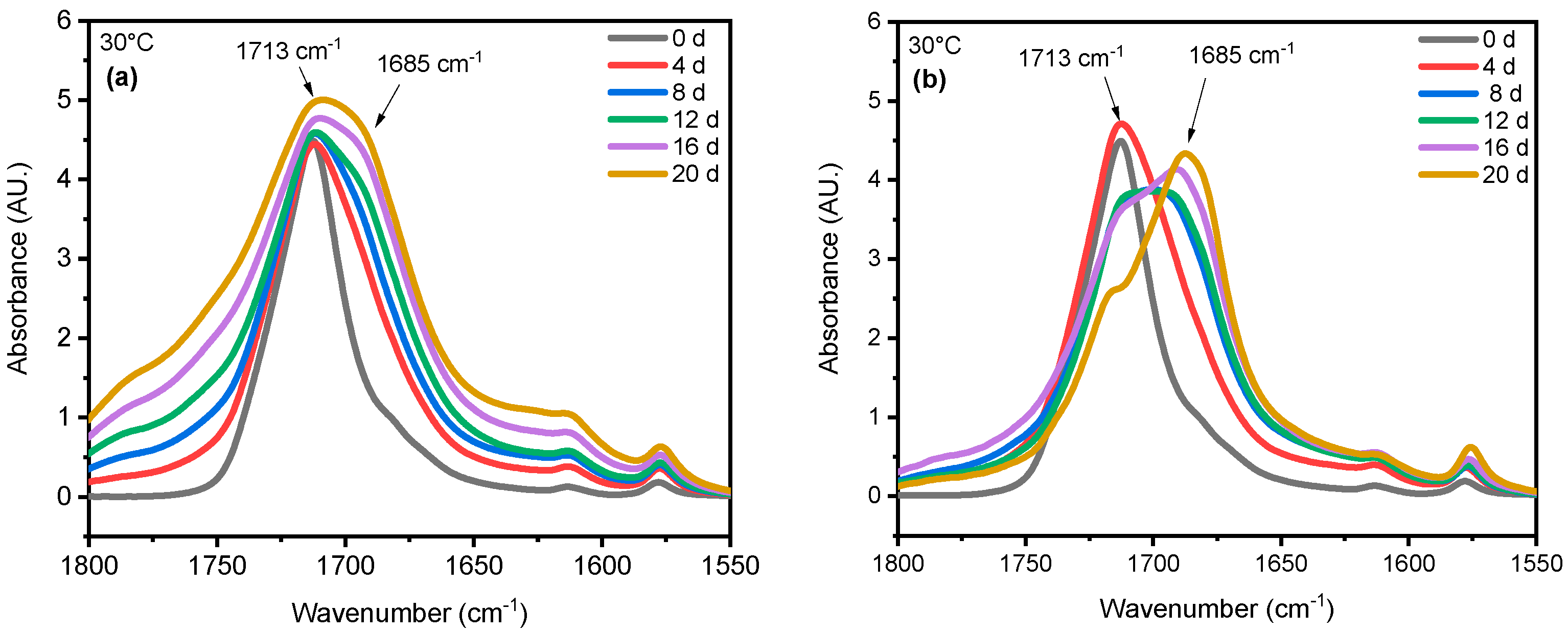
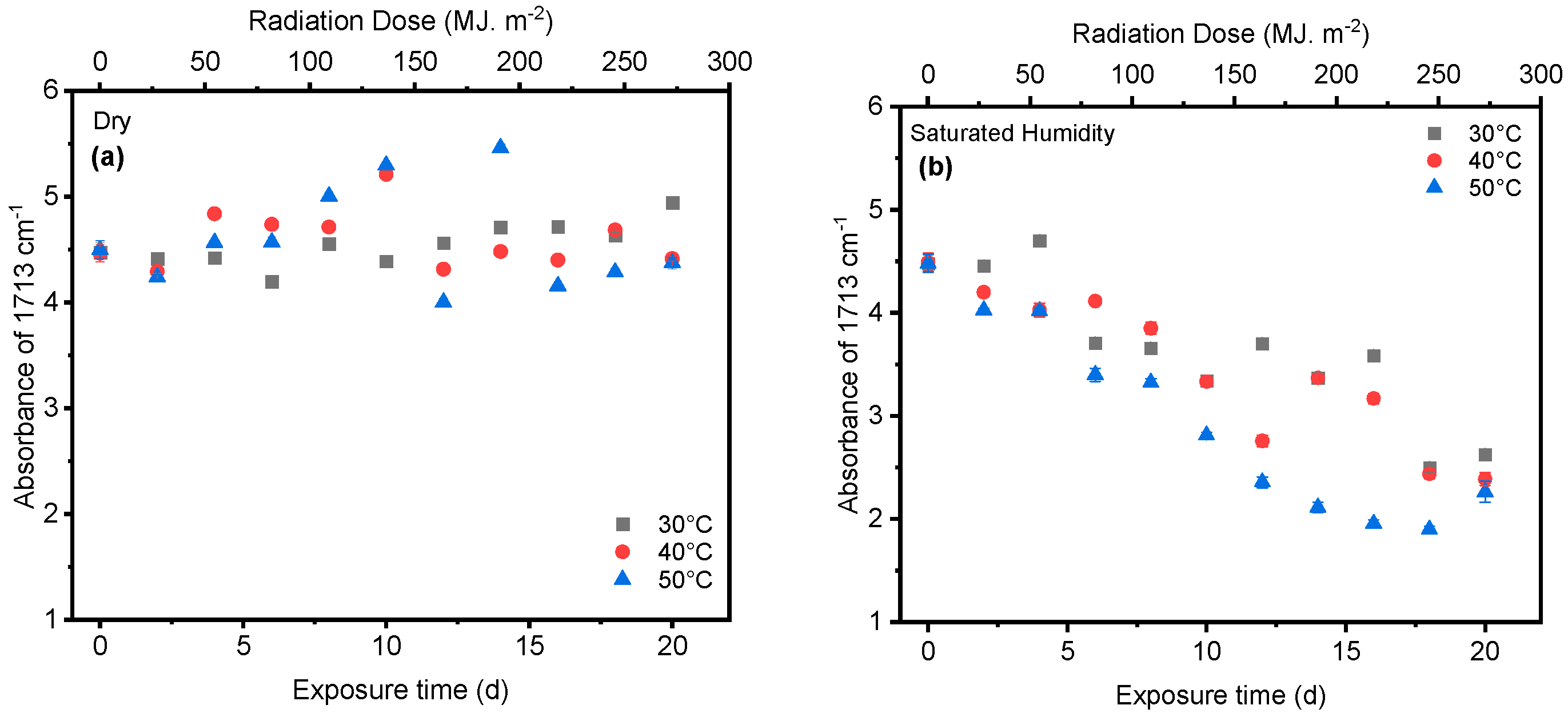
4.1. Effect of UV Radiation
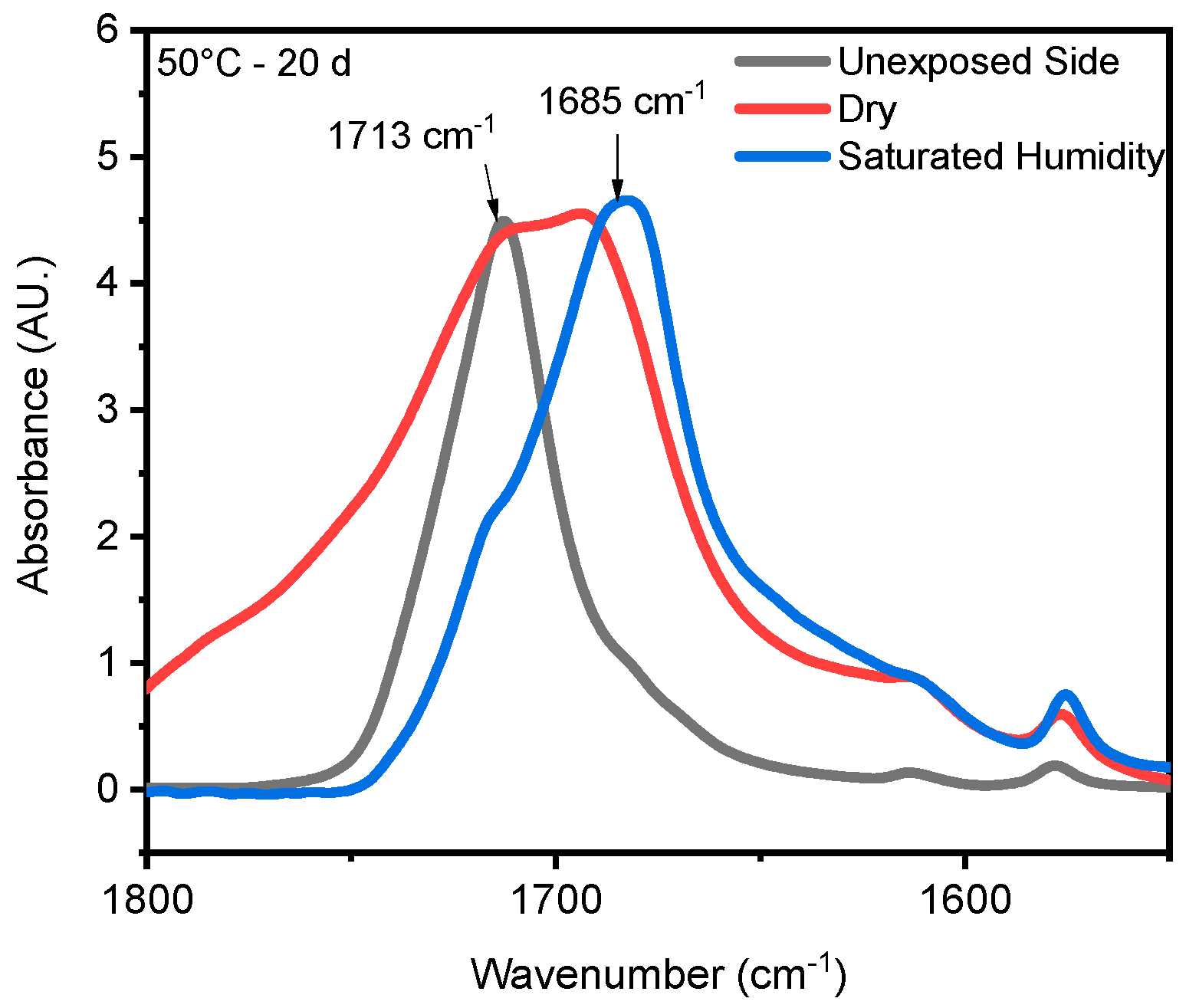
4.2. Effect of Saturated Humidity
4.3. Effect of Temperature
5. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
NIST Disclaimer
References
- Seeley, M.E.; Song, B.; Passie, R.; Hale, R.C. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Ivar Do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Singh, A.K.; Bedi, R.; Kaith, B.S. Composite materials based on recycled polyethylene terephthalate and their properties—A comprehensive review. Compos. Part B Eng. 2021, 219, 108928. [Google Scholar] [CrossRef]
- Nisticò, R. Polyethylene terephthalate (PET) in the packaging industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ahmed, J.; Ubaidullah, M.; Shaikh, S.F.; Ahamad, T.; Naushad, M.; Zheng, G. Utilization of waste polyethylene terephthalate bottles to develop metal-organic frameworks for energy applications: A clean and feasible approach. J. Clean. Prod. 2020, 248, 119251. [Google Scholar] [CrossRef]
- Llopis, S.F.; Verdejo, E.; Gil-Castell, O.; Ribes-Greus, A. Partial glycolytic depolymerisation of poly(ethylene terephthalate) (PET) in the solid state: Modelling the contribution of time and temperature. Polym. Degrad. Stab. 2024, 221, 110695. [Google Scholar] [CrossRef]
- Dubelley, F.; Planes, E.; Bas, C.; Pons, E.; Yrieix, B.; Flandin, L. The hygrothermal degradation of PET in laminated multilayer. Eur. Polym. J. 2017, 87, 1–13. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Bian, X.; Xia, G.; Xin, J.H.; Jiang, S.; Ma, K. Applications of waste polyethylene terephthalate (PET) based nanostructured materials: A review. Chemosphere 2024, 350, 141076. [Google Scholar] [CrossRef] [PubMed]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An overview of plasticwaste generation and management in food packaging industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Mihai, F.C.; Gündogdu, S.; Markley, L.A.; Olivelli, A.; Khan, F.R.; Gwinnett, C.; Gutberlet, J.; Reyna-Bensusan, N.; Llanquileo-Melgarejo, P.; Meidiana, C.; et al. Plastic Pollution, Waste Management Issues, and Circular Economy Opportunities in Rural Communities. Sustainability 2022, 14, 20. [Google Scholar] [CrossRef]
- Andrady, A.L.; Barnes, P.W.; Bornman, J.F.; Gouin, T.; Madronich, S.; White, C.C.; Zepp, R.G.; Jansen, M.A.K. Oxidation and fragmentation of plastics in a changing environment; from UV-radiation to biological degradation. Sci. Total Environ. 2022, 851, 158022. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Chavda, P.; Kumar, R.; Pandit, R.; Joshi, M.; Kumar, M.; Joshi, C. Exploring genetic landscape of low-density polyethylene degradation for sustainable troubleshooting of plastic pollution at landfills. Sci. Total Environ. 2024, 912, 168882. [Google Scholar] [CrossRef] [PubMed]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Fan, C.; Liu, M.; Wolfe-Bryant, B. Effect of High-Density Polyethylene Microplastics on theSurvival and Development of Eastern Oyster (Crassostrea virginica) Larvae. Int. J. Environ. Res. Public Health 2023, 20, 6142. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- El-Dairi, M.; House, R.J. Microplastics in wastewater treatment plants: Detection, occurrence andremoval. Microplast. Seaf. Implic. Hum. Health 2019, 152, 285–287. [Google Scholar] [CrossRef]
- Dikareva, N.; Simon, K.S. Microplastic pollution in streams spanning an urbanisation gradient. Environ. Pollut. 2019, 250, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; He, H.; Zeng, E.Y. Plastic Pollution in Waterways and in the Oceans; INC: New York, NY, USA, 2022; Volume 2004, ISBN 9780323998758. [Google Scholar]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in human food chains: Food becoming a threat to health safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef]
- Muncke, J. Exposure to endocrine disrupting compounds via the food chain: Is packaging a relevant source? Sci. Total Environ. 2009, 407, 4549–4559. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, T.B.; Hunt, C.J.; Meyer, A.S. Influence of substrate crystallinity and glass transition temperature on enzymatic degradation of polyethylene terephthalate (PET). New Biotechnol. 2022, 69, 28–35. [Google Scholar] [CrossRef]
- O’Brine, T.; Thompson, R.C. Degradation of plastic carrier bags in the marine environment. Mar. Pollut. Bull. 2010, 60, 2279–2283. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef]
- Andrady, A.L.; Heikkilä, A.M.; Pandey, K.K.; Bruckman, L.S.; White, C.C.; Zhu, M.; Zhu, L. Effects of UV radiation on natural and synthetic materials. Photochem. Photobiol. Sci. 2023, 22, 1177–1202. [Google Scholar] [CrossRef]
- Sørensen, L.; Groven, A.S.; Hovsbakken, I.A.; Del Puerto, O.; Krause, D.F.; Sarno, A.; Booth, A.M. UV degradation of natural and synthetic microfibers causes fragmentation and release of polymer degradation products and chemical additives. Sci. Total Environ. 2021, 755, 143170. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Biodegradation of Plastics: Monitoring what Happens; Springer: Berlin/Heidelberg, Germany, 1998; pp. 32–40. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Surface properties of beached plastics. Environ. Sci. Pollut. Res. 2015, 22, 11022–11032. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. Handb. Environ. Chem. 2019, 78, 71–92. [Google Scholar] [CrossRef]
- Gulmine, J.; Janissek, P.; Heise, H.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Mendiburu-Valor, E.; Mondragon, G.; González, N.; Kortaberria, G.; Martin, L.; Eceiza, A.; Peña-Rodriguez, C. Valorization of urban and marine PET waste by optimized chemical recycling. Resour. Conserv. Recycl. 2022, 184, 106413. [Google Scholar] [CrossRef]
- Le, D.K.; Leung, R.I.H.; Er, A.S.R.; Zhang, X.; Tay, X.J.; Thai, Q.B.; Phan-Thien, N.; Duong, H.M. Applications of functionalized polyethylene terephthalate aerogels from plastic bottle waste. Waste Manag. 2019, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, W.; Du, R.; Zhou, X.; Liu, X.; Xu, S.; Wang, Y.Z. Cosolvent-promoted selective non-aqueous hydrolysis of PET wastes and facile product separation. Green Chem. 2022, 24, 3284–3292. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Degradation profile of polyethylene after artificial accelerated weathering. Polym. Degrad. Stab. 2003, 79, 385–397. [Google Scholar] [CrossRef]
- Ding, L.; Yu, X.; Guo, X.; Zhang, Y.; Ouyang, Z.; Liu, P.; Zhang, C.; Wang, T.; Jia, H.; Zhu, L. The photodegradation processes and mechanisms of polyvinyl chloride and polyethylene terephthalate microplastic in aquatic environments: Important role of clay minerals. Water Res. 2022, 208, 117879. [Google Scholar] [CrossRef]
- Du, B.; Yang, R.; Xie, X. Investigation of Hydrolysis in Poly (ethylene terephthalate) by FTIR-ATR. Chin. J. Polym. Sci. 2014, 32, 230–235. [Google Scholar] [CrossRef]
- Persin, Z.; Mohan, T.; Stana-kleinschek, K. Chemical modification and characterization of poly (ethylene terephthalate) surfaces for collagen immobilization. Cent. Eur. J. Chem. 2013, 11, 1786–1798. [Google Scholar] [CrossRef]
- Prasad, S.G.; De, A.; De, U. Structural and Optical Investigations of Radiation Damage in Transparent PET Polymer Films. Int. J. Spectrosc. 2011, 2011, 810936. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Nunes, A.R.; Santos, P.S.M.; Girão, A.V.; Duarte, A.C.; Rocha-Santos, T. Degradation of polyethylene microplastics in seawater: Insights into the environmental degradation of polymers. J. Environ. Sci. Health Part A 2018, 53, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, P.; Gräsing, D.; Bielytskyi, P.; Zimmermann, W.; Matysik, J.; Wei, R.; Song, C. UV Pretreatment Impairs the Enzymatic Degradation of Polyethylene Terephthalate. Front. Microbiol. 2020, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Pegram, J.E.; Andrady, A.L. Outdoor weathering of selected polymeric materials under marine exposure conditions. Polym. Degrad. Stab. 1989, 26, 333–345. [Google Scholar] [CrossRef]
- Pickett, J.E.; Kuvshinnikova, O.; Sung, L.P.; Ermi, B.D. Accelerated weathering parameters for some aromatic engineering thermoplastics. Polym. Degrad. Stab. 2019, 166, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A.; Hsueh, H.; Kim, J.H.; Jacobs, D.; Perry, L.; Goodwin, D.; White, C.; Watson, S.; Sung, L. Temperature and light intensity effects on photodegradation of high-density polyethylene. Polym. Degrad. Stab. 2019, 165, 153–160. [Google Scholar] [CrossRef]
- Subba Reddy, M.; Srinivasulu Reddy, P.; Venkata, G.; And, S.; Venkata Subbaiah, H. Effect of Plastic Pollution on Environment. J. Chem. Pharm. Sci. 2014, 14, 28–29. [Google Scholar]
- Wu, B.; Wu, H.; Xu, S.M.; Wang, Y.Z. Comparative study of the aging degradation behaviors of PET under artificially accelerated and typical marine environment. Polym. Degrad. Stab. 2023, 217, 110515. [Google Scholar] [CrossRef]
- Ioakeimidis, C.; Fotopoulou, K.N.; Karapanagioti, H.K.; Geraga, M.; Zeri, C. The degradation potential of PET bottles in the marine environment: An ATR-FTIR based approach. Sci. Rep. 2016, 6, 23501. [Google Scholar] [CrossRef]
- Fan, C.; Huang, Y.Z.; Lin, J.N.; Li, J. Microplastic constituent identification from admixtures by Fourier-transform infrared (FTIR) spectroscopy: The use of polyethylene terephthalate (PET), polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC) and nylon (NY) as the model constituent. Environ. Technol. Innov. 2021, 23, 101798. [Google Scholar] [CrossRef]
- Rathore, C.; Saha, M.; Gupta, P.; Kumar, M.; Naik, A.; de Boer, J. Standardization of micro-FTIR methods and applicability for the detection and identification of microplastics in environmental matrices. Sci. Total Environ. 2023, 888, 164157. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, M.E.; Lemos, V.A.S.; López, G.; Soares, S.A.; Feitosa, J.P.M.; Araújo, B.S.; Ayala, A.P.; de Azevedo, M.M.F.; Santos, F.E.P.; Cavalcante, R.M. Morphology, Chemical Characterization and Sources of Microplastics in a Coastal City in the Equatorial Zone with Diverse Anthropogenic Activities (Fortaleza city, Brazil). J. Polym. Environ. 2022, 30, 2862–2874. [Google Scholar] [CrossRef]
- Lee, H.L.; Chiu, C.W.; Lee, T. Engineering terephthalic acid product from recycling of PET bottles waste for downstream operations. Chem. Eng. J. Adv. 2021, 5, 100079. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Wang, Q. Automated Classification of Undegraded and Aged Polyethylene Terephthalate Microplastics from ATR-FTIR Spectroscopy using Machine Learning Algorithms. J. Polym. Environ. 2024, 1–16. [Google Scholar] [CrossRef]
- Celina, M.C.; Linde, E.; Martinez, E. Carbonyl Identification and Quantification Uncertainties for Oxidative Polymer Degradation. Polym. Degrad. Stab. 2021, 188, 109550. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly (ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]
- Endo, T.; Reddy, L.; Nishikawa, H.; Kaneko, S.; Nakamura, Y.; Endo, K. Composite Engineering—Direct Bonding of plastic PET Films by Plasma Irradiation. Procedia Eng. 2017, 171, 88–103. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Wang, W.; Gonzalez-Gil, G.; Vrouwenvelder, J.S.; Li, Z. Effects of nano- and microplastics on kidney: Physicochemical properties, bioaccumulation, oxidative stress and immunoreaction. Chemosphere 2022, 288, 132631. [Google Scholar] [CrossRef]
- Tian, R.; Li, K.; Lin, Y.; Lu, C.; Duan, X. Characterization Techniques of Polymer Aging: From Beginning to End. Chem. Rev. 2022, 123, 3007–3088. [Google Scholar] [CrossRef]
- Gok, A.; Fagerholm, C.L.; French, R.H.; Bruckman, L.S. Temporal evolution and pathway models of poly(ethylene-terephthalate) degradation under multi-factor accelerated weathering exposures. PLoS ONE 2019, 14, e0212258. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, V.; Singh, S.; Anil, A.G.; Sunil Kumar Naik, T.S.; Garg, S.; Samuel, J.; Kumar, M.; Ramamurthy, P.C.; Singh, J. Occurrence, toxicity and remediation of polyethylene terephthalate plastics. A review. Environ. Chem. Lett. 2022, 20, 1777–1800. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Steck, J.; Yang, J.; Wang, Y.; Suo, Z. Degradable Plastics Are Vulnerable to Cracks. Engineering 2021, 7, 624–629. [Google Scholar] [CrossRef]
- Min, K.; Cuiffi, J.D.; Mathers, R.T. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Kadoma, A.; Jiao, Q.; Vlassak, J.J.; Suo, Z. Hydrolytic crack growth and embrittlement in poly(ethylene terephthalate). J. Mech. Phys. Solids 2023, 176, 105303. [Google Scholar] [CrossRef]
- Blanco-Gutiérrez, V.; Li, P.; Berzal-Cabetas, R.; Dos santos-García, A.J. Exploring the photocatalytic activity of nanometric magnetite for PET materials degradation under UV light. J. Solid State Chem. 2022, 316, 123509. [Google Scholar] [CrossRef]
- Sang, T.; Wallis, C.J.; Hill, G.; Britovsek, G.J.P. Polyethylene terephthalate degradation under natural and accelerated weathering conditions. Eur. Polym. J. 2020, 136, 109873. [Google Scholar] [CrossRef]
- Al-azzawi, F. Degradation Studies on Recycled Polyethylene Terephthalate. Doctoral Dissertation, London Metropolitan University, London, UK, 2015. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. Indonesian Journal of Science & Technology How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar]
- Fechine, G.J.M.; Rabello, M.S.; Maior, R.M.S.; Catalani, L.H. Surface characterization of photodegraded poly (ethylene terephthalate). The effect of ultraviolet absorbers. Polymer 2004, 45, 2303–2308. [Google Scholar] [CrossRef]
- Marchetti, B.; Karsili, T.N.V.; Ashfold, M.N.R. Exploring Norrish type i and type II reactions: An: Ab initio mechanistic study highlighting singlet-state mediated chemistry. Phys. Chem. Chem. Phys. 2019, 21, 14418–14428. [Google Scholar] [CrossRef]
- Gardette, J.L.; Colin, A.; Trivis, S.; German, S.; Therias, S. Impact of photooxidative degradation on the oxygen permeability of poly(ethyleneterephthalate). Polym. Degrad. Stab. 2014, 103, 35–41. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Kinetic studies related to polymer degradation and stability. In Plastics to Energy; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128131404. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostampour, S.; Cook, R.; Jhang, S.-S.; Li, Y.; Fan, C.; Sung, L.-P. Changes in the Chemical Composition of Polyethylene Terephthalate under UV Radiation in Various Environmental Conditions. Polymers 2024, 16, 2249. https://doi.org/10.3390/polym16162249
Rostampour S, Cook R, Jhang S-S, Li Y, Fan C, Sung L-P. Changes in the Chemical Composition of Polyethylene Terephthalate under UV Radiation in Various Environmental Conditions. Polymers. 2024; 16(16):2249. https://doi.org/10.3390/polym16162249
Chicago/Turabian StyleRostampour, Sara, Rachel Cook, Song-Syun Jhang, Yuejin Li, Chunlei Fan, and Li-Piin Sung. 2024. "Changes in the Chemical Composition of Polyethylene Terephthalate under UV Radiation in Various Environmental Conditions" Polymers 16, no. 16: 2249. https://doi.org/10.3390/polym16162249
APA StyleRostampour, S., Cook, R., Jhang, S.-S., Li, Y., Fan, C., & Sung, L.-P. (2024). Changes in the Chemical Composition of Polyethylene Terephthalate under UV Radiation in Various Environmental Conditions. Polymers, 16(16), 2249. https://doi.org/10.3390/polym16162249






