Terephthalate Copolyesters Based on 2,3-Butanediol and Ethylene Glycol and Their Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
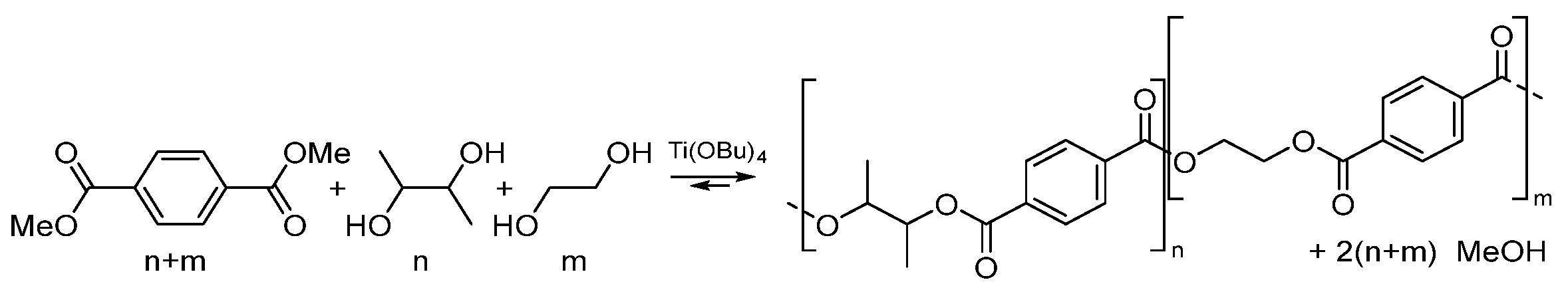
2.2. Synthesis of P23BET and P23BT
2.3. Characterization and Processing Techniques
3. Results and Discussion
3.1. Synthesis P23BET and P23BT
3.2. Reactivity and Molecular Weight
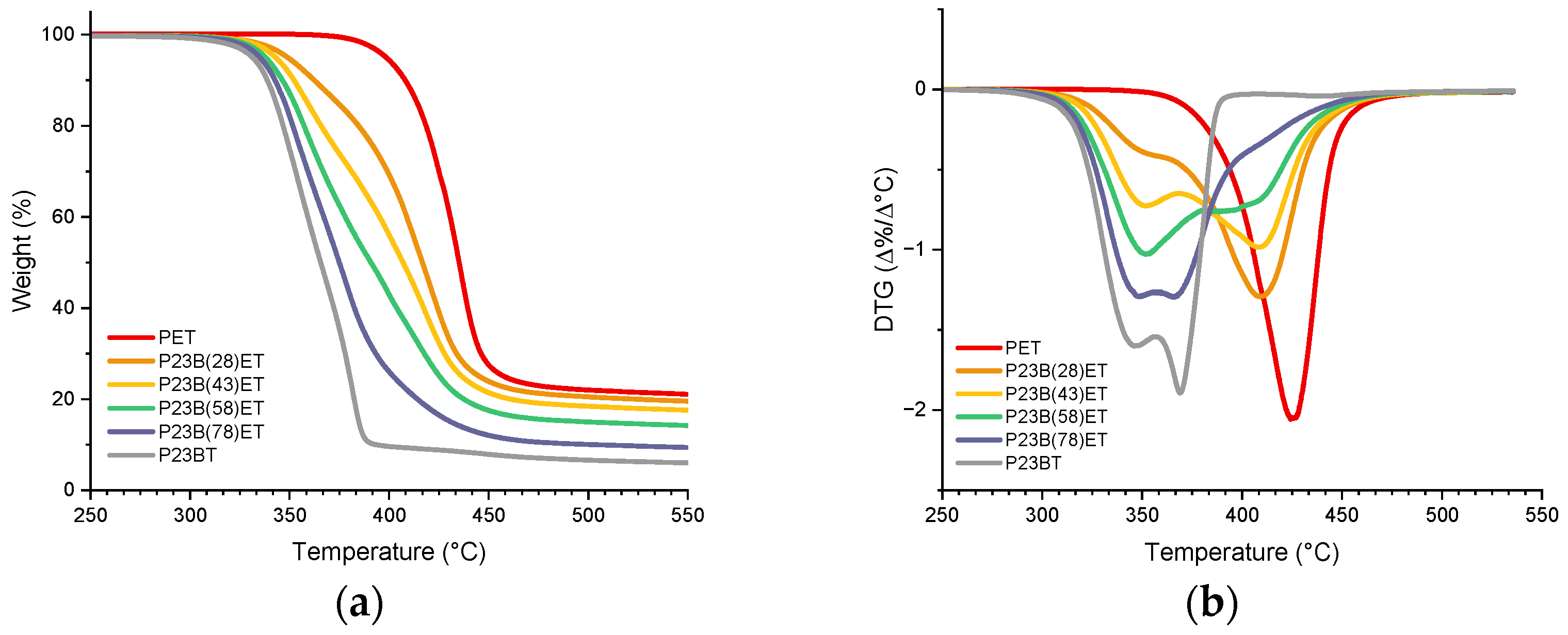
3.3. Thermostability
3.4. Side Reactions
3.5. Glass Transition Temperature and Crystallinity
3.6. Processing and Color
3.7. Water and Oxygen Permeability
3.8. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, S.; Kramer, T.S.; Santosa, D.S.; Heeres, A.; Heeres, H.J. Catalytic Conversion of Glycerol and Co-Feeds (Fatty Acids, Alcohols, and Alkanes) to Bio-Based Aromatics: Remarkable and Unprecedented Synergetic Effects on Catalyst Performance. Green Chem. 2022, 24, 941–949. [Google Scholar] [CrossRef]
- He, S.; Muizebelt, I.; Heeres, A.; Schenk, N.J.; Blees, R.; Heeres, H.J. Catalytic Pyrolysis of Crude Glycerol over Shaped ZSM-5/Bentonite Catalysts for Bio-BTX Synthesis. Appl. Catal. B Environ. 2018, 235, 45–55. [Google Scholar] [CrossRef]
- Cherubini, F.; Strømman, A.H. Chemicals from Lignocellulosic Biomass: Opportunities, Perspectives, and Potential of Biorefinery Systems. Biofuels Bioprod. Bioref. 2011, 5, 548–561. [Google Scholar] [CrossRef]
- Wong, M.K.; Lock, S.S.M.; Chan, Y.H.; Yeoh, S.J.; Tan, I.S. Towards Sustainable Production of Bio-Based Ethylene Glycol: Progress, Perspective and Challenges in Catalytic Conversion and Purification. Chem. Eng. J. 2023, 468, 143699. [Google Scholar] [CrossRef]
- Weinland, D.H.; van der Maas, K.; Wang, Y.; Bottega Pergher, B.; van Putten, R.-J.; Wang, B.; Gruter, G.-J.M. Overcoming the Low Reactivity of Biobased, Secondary Diols in Polyester Synthesis. Nat. Commun. 2022, 13, 7370. [Google Scholar] [CrossRef]
- Van Der Maas, K.; Wang, Y.; Weinland, D.H.; Van Putten, R.-J.; Wang, B.; Gruter, G.-J.M. PISOX Copolyesters—Bio- and CO2-Based Marine-Degradable High-Performance Polyesters. ACS Sustain. Chem. Eng. 2024, 12, 9822–9832. [Google Scholar] [CrossRef]
- Murcia Valderrama, M.A.; van Putten, R.-J.; Gruter, G.-J.M. PLGA Barrier Materials from CO2. The Influence of Lactide Co-Monomer on Glycolic Acid Polyesters. ACS Appl. Polym. Mater. 2020, 2, 2706–2718. [Google Scholar] [CrossRef]
- Murcia Valderrama, M.A.; van Putten, R.-J.; Gruter, G.-J.M. The Potential of Oxalic—And Glycolic Acid Based Polyesters (Review). Towards CO2 as a Feedstock (Carbon Capture and Utilization—CCU). Eur. Polym. J. 2019, 119, 445–468. [Google Scholar] [CrossRef]
- De Jong, E.; Visser, H.A.; Dias, A.S.; Harvey, C.; Gruter, G.-J.M. The Road to Bring FDCA and PEF to the Market. Polymers 2022, 14, 943. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased Polyesters and Other Polymers from 2,5-Furandicarboxylic Acid: A Tribute to Furan Excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Hakizimana, O.; Matabaro, E.; Lee, B.H. The Current Strategies and Parameters for the Enhanced Microbial Production of 2,3-Butanediol. Biotechnol. Rep. 2020, 25, e00397. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Yao, M.; Yang, W.; Han, Y.; Liu, L.; Zhang, J.; Liu, J. Mechanism of Microbial Production of Acetoin and 2,3-Butanediol Optical Isomers and Substrate Specificity of Butanediol Dehydrogenase. Microb. Cell. Fact. 2023, 22, 165. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.E.; Castro, A.M.; Freire, D.M.G.; Mesquita, R.D. Enzymes and Pathways in Microbial Production of 2,3-Butanediol and 3-Acetoin Isomers. Crit. Rev. Biotechnol. 2023, 43, 67–81. [Google Scholar] [CrossRef]
- Segovia-Hernández, J.G.; Sanchez-Ramirez, E.; Alcocer-Garcia, H.; Romero-Garcia, A.G.; Quiroz-Ramirez, J.J. 2,3-Butanediol. In Sustainable Production of Biofuels Using Intensified Processes; Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2022; pp. 91–110. ISBN 978-3-031-13215-5. [Google Scholar]
- Tinôco, D.; Pateraki, C.; Koutinas, A.A.; Freire, D.M.G. Bioprocess Development for 2,3-Butanediol Production by Paenibacillus Strains. ChemBioEng Rev. 2021, 8, 44–62. [Google Scholar] [CrossRef]
- Xie, S.; Li, Z.; Zhu, G.; Song, W.; Yi, C. Cleaner Production and Downstream Processing of Bio-Based 2,3-Butanediol: A Review. J. Clean. Prod. 2022, 343, 131033. [Google Scholar] [CrossRef]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial Production of 2,3-Butanediol for Industrial Applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef]
- GSCALTEX. Available online: https://www.gscaltex.com/downloadfile/GreenDiol(en).pdf (accessed on 25 July 2024).
- Köpke, M.; Mihalcea, C.; Liew, F.; Tizard, J.H.; Ali, M.S.; Conolly, J.J.; Al-Sinawi, B.; Simpson, S.D. 2,3-Butanediol Production by Acetogenic Bacteria, an Alternative Route to Chemical Synthesis, Using Industrial Waste Gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef]
- Ghadermazi, P.; Re, A.; Ricci, L.; Chan, S.H.J. Metabolic Engineering Interventions for Sustainable 2,3-Butanediol Production in Gas-Fermenting Clostridium autoethanogenum. Msystems 2022, 7, e01111-21. [Google Scholar] [CrossRef]
- Wei, H.; Wang, W.; Chou, Y.-C.; Himmel, M.E.; Chen, X.; Bomble, Y.J.; Zhang, M. Prospects for Engineering Ralstonia Eutropha and Zymomonas Mobilis for the Autotrophic Production of 2,3-Butanediol from CO2 and H2. Eng. Microbiol. 2023, 3, 100074. [Google Scholar] [CrossRef]
- Debuissy, T.; Pollet, E.; Avérous, L. Synthesis of Potentially Biobased Copolyesters Based on Adipic Acid and Butanediols: Kinetic Study between 1,4- and 2,3-Butanediol and Their Influence on Crystallization and Thermal Properties. Polymer 2016, 99, 204–213. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-R.; Ahn, C.-H. Novel Biobased Copolyesters Based on 1,2-Propanediol or 2,3-Butanediol with the Same Ethylene Skeletal Structure as PETG. Polymer 2018, 135, 314–326. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Hu, X.; Wu, W.; Wang, Z.; Wang, R.; Zhang, L. Preparation and Properties of Novel Thermoplastic Vulcanizate Based on Bio-Based Polyester/Polylactic Acid, and Its Application in 3D Printing. Polymers 2017, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.; Gao, Y.; Wang, R.; Wang, Z.; Kang, H.; Zhang, L. Renewable and Super-Toughened Poly (Butylene Succinate) with Bio-Based Elastomers: Preparation, Compatibility and Performances. Eur. Polym. J. 2019, 116, 438–444. [Google Scholar] [CrossRef]
- Hu, X.; Shen, X.; Huang, M.; Liu, C.; Geng, Y.; Wang, R.; Xu, R.; Qiao, H.; Zhang, L. Biodegradable Unsaturated Polyesters Containing2,3-Butanediol for Engineering Applications: Synthesis, Characterization and Performances. Polymer 2016, 84, 343–354. [Google Scholar] [CrossRef]
- Debuissy, T.; Pollet, E.; Avérous, L. Enzymatic Synthesis of Biobased Poly(1,4-Butylene Succinate-Ran-2,3-Butylene Succinate) Copolyesters and Characterization. Influence of 1,4- and 2,3-Butanediol Contents. Eur. Polym. J. 2017, 93, 103–115. [Google Scholar] [CrossRef]
- Kirchberg, A.; Wegelin, S.; Grutke, L.; Meier, M.A.R. Unexpected Performance of Iron(III) Chloride in the Polymerization of Renewable 2,3-Butanediol and the Depolymerization of Poly(Ethylene Terephthalate). RSC Sustain. 2024, 2, 435–444. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Vogelzang, W.; Knoop, R.J.I.; Frissen, A.E.; van Haveren, J.; van Es, D.S. Biobased Furandicarboxylic Acids (FDCAs): Effects of Isomeric Substitution on Polyester Synthesis and Properties. Green Chem. 2014, 16, 1957–1966. [Google Scholar] [CrossRef]
- Gubbels, E.; Jasinska-Walc, L.; Koning, C.E. Synthesis and Characterization of Novel Renewable Polyesters Based on 2,5-furandicarboxylic Acid and 2,3-butanediol. J. Polym. Sci. A Polym. Chem. 2013, 51, 890–898. [Google Scholar] [CrossRef]
- Arnaud, S.P.; Wu, L.; Wong Chang, M.-A.; Comerford, J.W.; Farmer, T.J.; Schmid, M.; Chang, F.; Li, Z.; Mascal, M. New Bio-Based Monomers: Tuneable Polyester Properties Using Branched Diols from Biomass. Faraday Discuss. 2017, 202, 61–77. [Google Scholar] [CrossRef]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Biosurfaces: A Materials Science and Engineering Perspective, 1st ed.; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-1-118-29997-5. [Google Scholar]
- Little, A.; Pellis, A.; Comerford, J.W.; Naranjo-Valles, E.; Hafezi, N.; Mascal, M.; Farmer, T.J. Effects of Methyl Branching on the Properties and Performance of Furandioate-Adipate Copolyesters of Bio-Based Secondary Diols. ACS Sustain. Chem. Eng. 2020, 8, 14471–14483. [Google Scholar] [CrossRef]
- Wu, F.; Yang, G. Synthesis and Properties of Poly(Trimethylene Terephthalate-Co-2-Methyl-Ethylene Terephthalate) Random Copolyesters. J. Appl. Polym. Sci. 2010, 116, 3419–3426. [Google Scholar] [CrossRef]
- Van der Vegt, A.K.; Govaert, L.E. Polymeren van Keten tot Kunststof, 5th ed.; VSSD: Kanpur, India, 2005; ISBN 10-90-71301-86-9. [Google Scholar]
- Groenewoud, W.M. Characterisation of Polymers by Thermal Analysis, 1st ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 2001; ISBN 978-0-444-50604-7. [Google Scholar]
- Birkle, M.; Mehringer, H.S.; Nelson, T.F.; Mecking, S. Aliphatic Polyester Materials from Renewable 2,3-Butanediol. ACS Sustain. Chem. Eng. 2024, 12, 4156–4163. [Google Scholar] [CrossRef]
- Abedsoltan, H. A Focused Review on Recycling and Hydrolysis Techniques of Polyethylene Terephthalate. Polym. Eng. Sci. 2023, 63, 2651–2674. [Google Scholar] [CrossRef]
- Nisticò, R. Polyethylene Terephthalate (PET) in the Packaging Industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Lee, S.W.; Ree, M.; Park, C.E.; Jung, Y.K.; Park, C.-S.; Jin, Y.S.; Bae, D.C. Synthesis and Non-Isothermal Crystallization Behaviors of Poly(Ethylene Isophthalate-Co-Terephthalate)s. Polymer 1999, 40, 7137–7146. [Google Scholar] [CrossRef]
- Haile, W.A.; Dean, L.R.; Mcconnell, R.L. Polyesters Containing Neopentyl Glycol and Fibers Formed Therefrom. U.S. Patent No. 6,139,954, 2005. [Google Scholar]
- Tsai, Y.; Fan, C.-H.; Hung, C.-Y.; Tsai, F.-J. Amorphous Copolyesters Based on 1,3/1,4-Cyclohexanedimethanol: Synthesis, Characterization and Properties. J. Appl. Polym. Sci. 2008, 109, 2598–2604. [Google Scholar] [CrossRef]
- Amza, C.G.; Zapciu, A.; Constantin, G.; Baciu, F.; Vasile, M.I. Enhancing Mechanical Properties of Polymer 3D Printed Parts. Polymers 2021, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- ASTM F1927-14; Standard Test Method for Determination of Oxygen Gas Transmission Rate, Permeability and Permeance at Controlled Relative Humidity Through Barrier Materials Using a Coulometric Detector 1. ASTM: West Conshohocken, PA, USA, 2014.
- ASTM E96/E96M-15; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2015.
- ISO 179-1:2010; lastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/44852.html (accessed on 23 July 2024).
- Montaudo, G.; Puglisi, C.; Samperi, F. Primary Thermal Degradation Mechanisms of PET and PBT. Polym. Degrad. Stab. 1993, 42, 13–28. [Google Scholar] [CrossRef]
- Duan, H.; Sun, D.; Yamada, Y.; Sato, S. Dehydration of 2,3-Butanediol into 3-Buten-2-Ol Catalyzed by ZrO2. Catal. Commun. 2014, 48, 1–4. [Google Scholar] [CrossRef]
- Duan, H.; Yamada, Y.; Kubo, S.; Sato, S. Vapor-Phase Catalytic Dehydration of 2,3-Butanediol to 3-Buten-2-Ol over ZrO2 Modified with Alkaline Earth Metal Oxides. Appl. Catal. A Gen. 2017, 530, 66–74. [Google Scholar] [CrossRef]
- Geurts, K.; Fletcher, S.P.; Feringa, B.L. Copper Catalyzed Asymmetric Synthesis of Chiral Allylic Esters. J. Am. Chem. Soc. 2006, 128, 15572–15573. [Google Scholar] [CrossRef] [PubMed]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.; Pascault, J.P.; Fuertes, P.; Saint-Loup, R. Synthesis and Properties of Poly(Butylene Succinate): Efficiency of Different Transesterification Catalysts. J. Polym. Sci. A Polym. Chem. 2011, 49, 5301–5312. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Tsanaktsis, V.; Nikolaidis, N.; Kostoglou, M.; Papageorgiou, G.Z.; Lambropoulou, D.A.; Bikiaris, D.N. Effect of Catalyst Type on Molecular Weight Increase and Coloration of Poly(Ethylene Furanoate) Biobased Polyester during Melt Polycondensation. Polym. Chem. 2017, 8, 6895–6908. [Google Scholar] [CrossRef]
- Dong, L.; Xu, T.; Dai, J.; Chen, W.; Lu, W. Tracking the Conjugated Yellowing Derivatives during the Production of Poly(Ethylene Furanoate) Using High-Definition Mass Spectrometry. ACS Sustain. Chem. Eng. 2024, 12, 6412–6423. [Google Scholar] [CrossRef]





| Polymer | DMT (mol) | EG:23BDO:DMT (mol Feed Ratio) | PC Time (h) | Mn (kDa) | Mw (kDa) | PDI (Mw/Mn) | Tg (°C) |
|---|---|---|---|---|---|---|---|
| P23B(28)ET | 0.090 | 1.30:1.20:1 | 4 | 31.5 | 75.1 | 2.4 | 88 |
| P23B(43)ET | 0.180 | 0.82:1.40:1 | 4 | 24.1 | 50.5 | 2.1 | 92 |
| P23B(46)ET | 0.075 | 0.80:1.40:1 | 4 | 26.6 | 59.9 | 2.3 | 94 |
| P23B(58)ET | 0.090 | 0.57:1.65:1 | 6 | 24.6 | 56.4 | 2.2 | 99 |
| P23B(78)ET | 0.090 | 0.27:1.95:1 | 7 | 18.1 | 38.7 | 2.1 | 104 |
| P23BT | 0.090 | 0.00:2.10:1 | 7 | 9.8 | 18.5 | 1.9 | 104 |
| Polymer | acN (kJ/m2) | St. Dev. |
|---|---|---|
| PET | 3.07 | 0.64 |
| PETG | 3.86 | 0.11 |
| P23B(43)ET | 2.13 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blom, M.; van Putten, R.-J.; van der Maas, K.; Wang, B.; Klink, G.P.M.v.; Gruter, G.-J.M. Terephthalate Copolyesters Based on 2,3-Butanediol and Ethylene Glycol and Their Properties. Polymers 2024, 16, 2177. https://doi.org/10.3390/polym16152177
Blom M, van Putten R-J, van der Maas K, Wang B, Klink GPMv, Gruter G-JM. Terephthalate Copolyesters Based on 2,3-Butanediol and Ethylene Glycol and Their Properties. Polymers. 2024; 16(15):2177. https://doi.org/10.3390/polym16152177
Chicago/Turabian StyleBlom, Marian, Robert-Jan van Putten, Kevin van der Maas, Bing Wang, Gerard P. M. van Klink, and Gert-Jan M. Gruter. 2024. "Terephthalate Copolyesters Based on 2,3-Butanediol and Ethylene Glycol and Their Properties" Polymers 16, no. 15: 2177. https://doi.org/10.3390/polym16152177
APA StyleBlom, M., van Putten, R.-J., van der Maas, K., Wang, B., Klink, G. P. M. v., & Gruter, G.-J. M. (2024). Terephthalate Copolyesters Based on 2,3-Butanediol and Ethylene Glycol and Their Properties. Polymers, 16(15), 2177. https://doi.org/10.3390/polym16152177







