Impact of Silver Nanoparticle Treatment and Chitosan on Packaging Paper’s Barrier Effectiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation Procedures
2.2. Methods
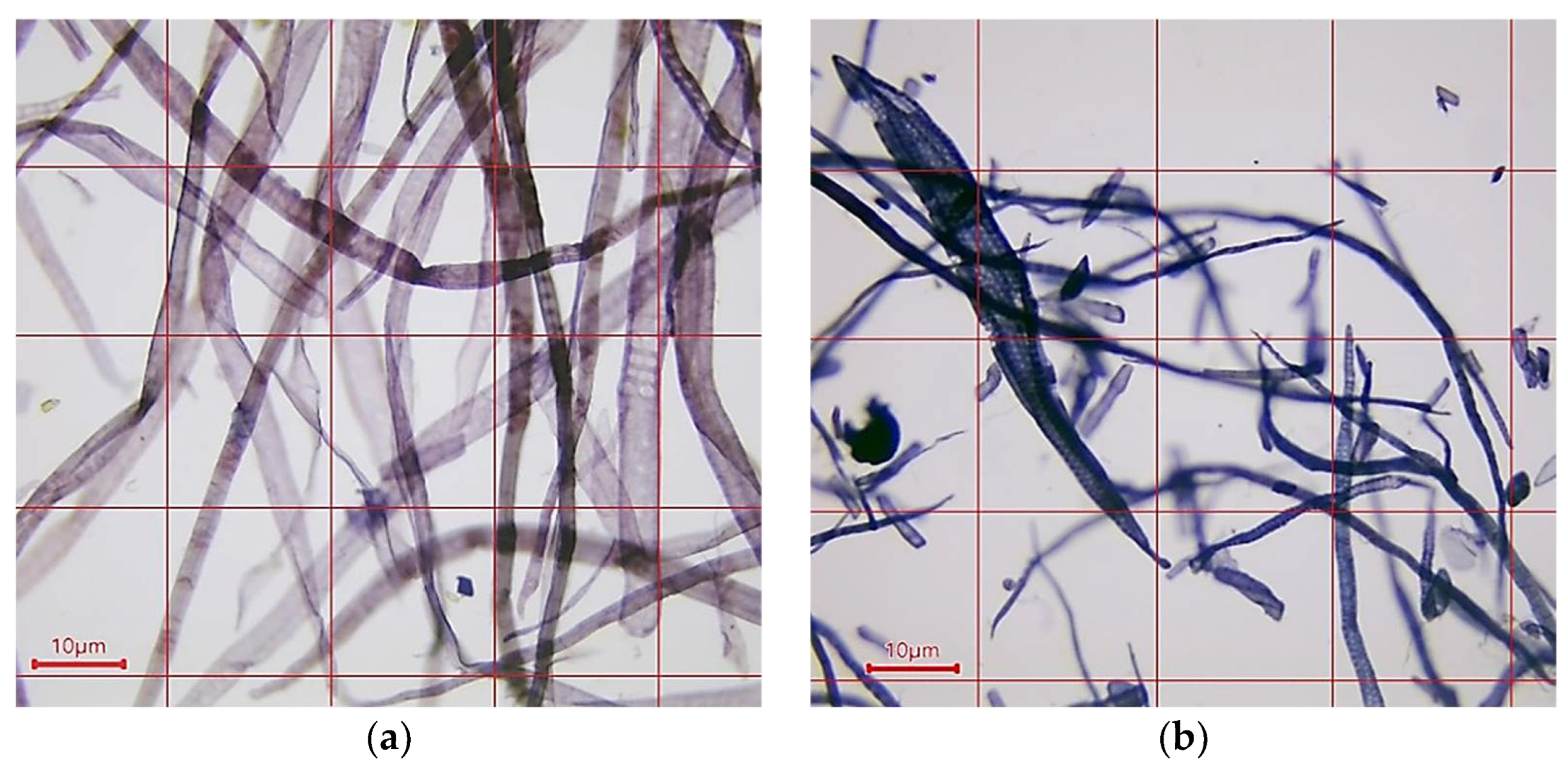
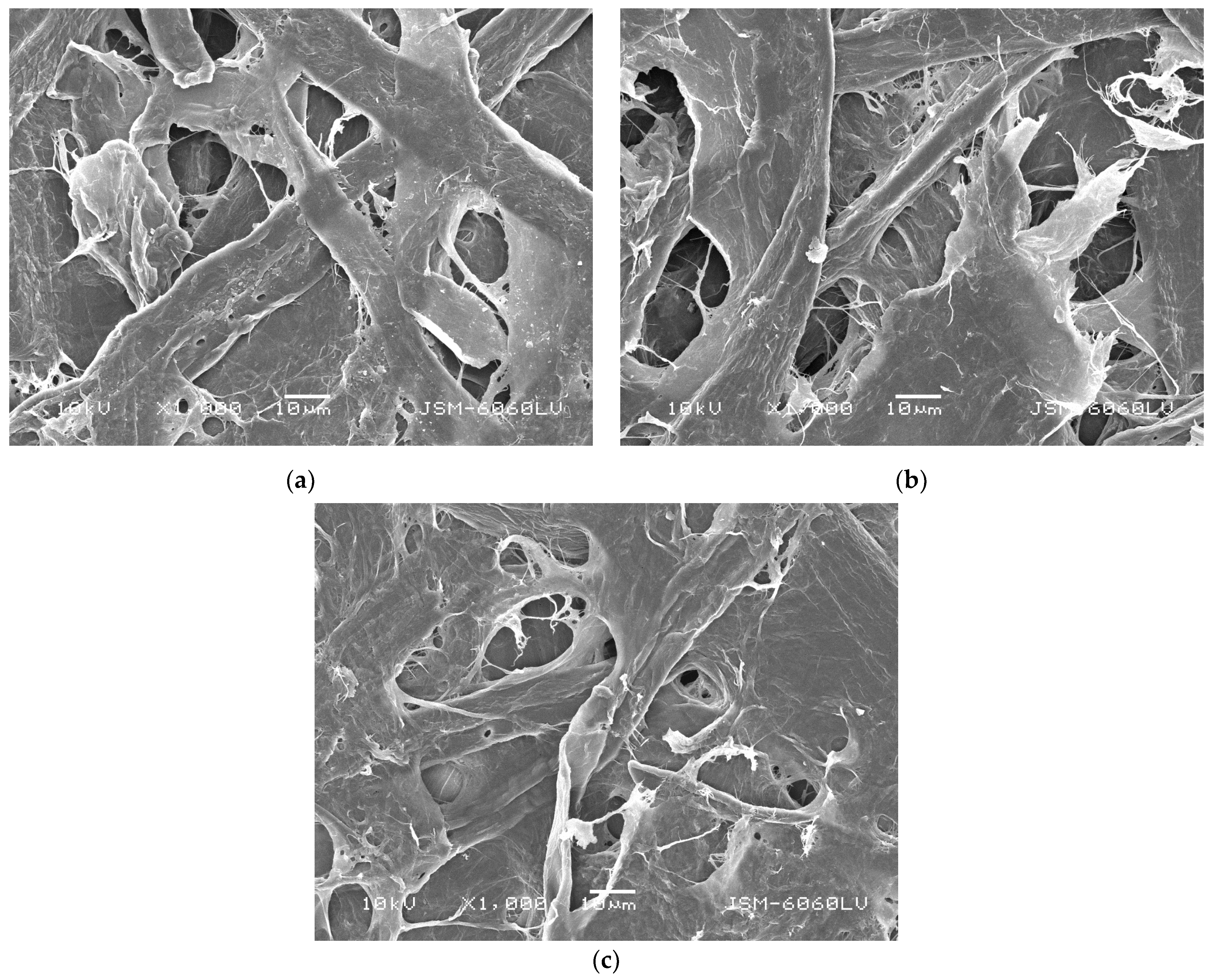
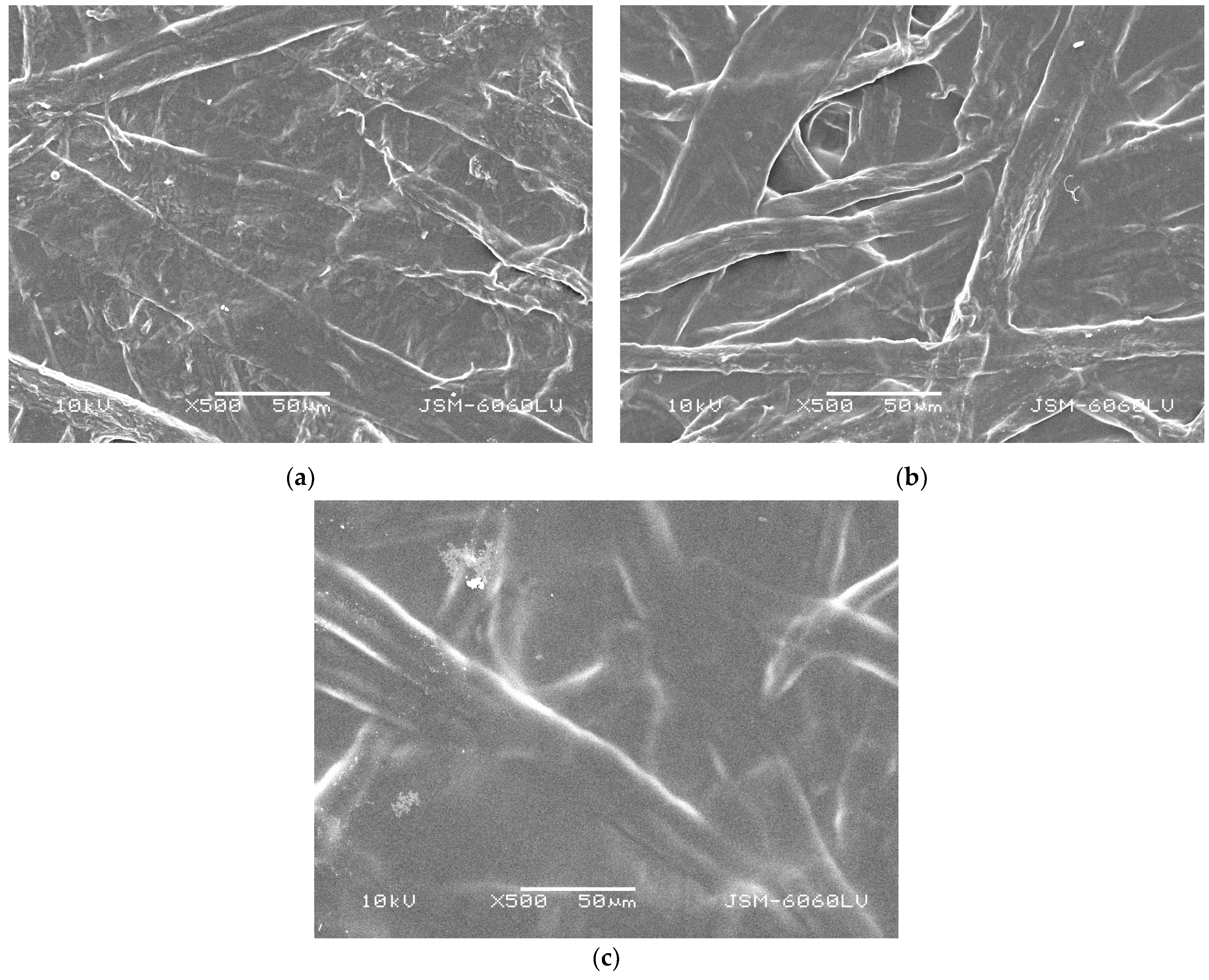
2.2.1. Morphology Characterization of Cellulose Fibers and Paper Samples
2.2.2. Structural Properties
2.2.3. Barrier Properties
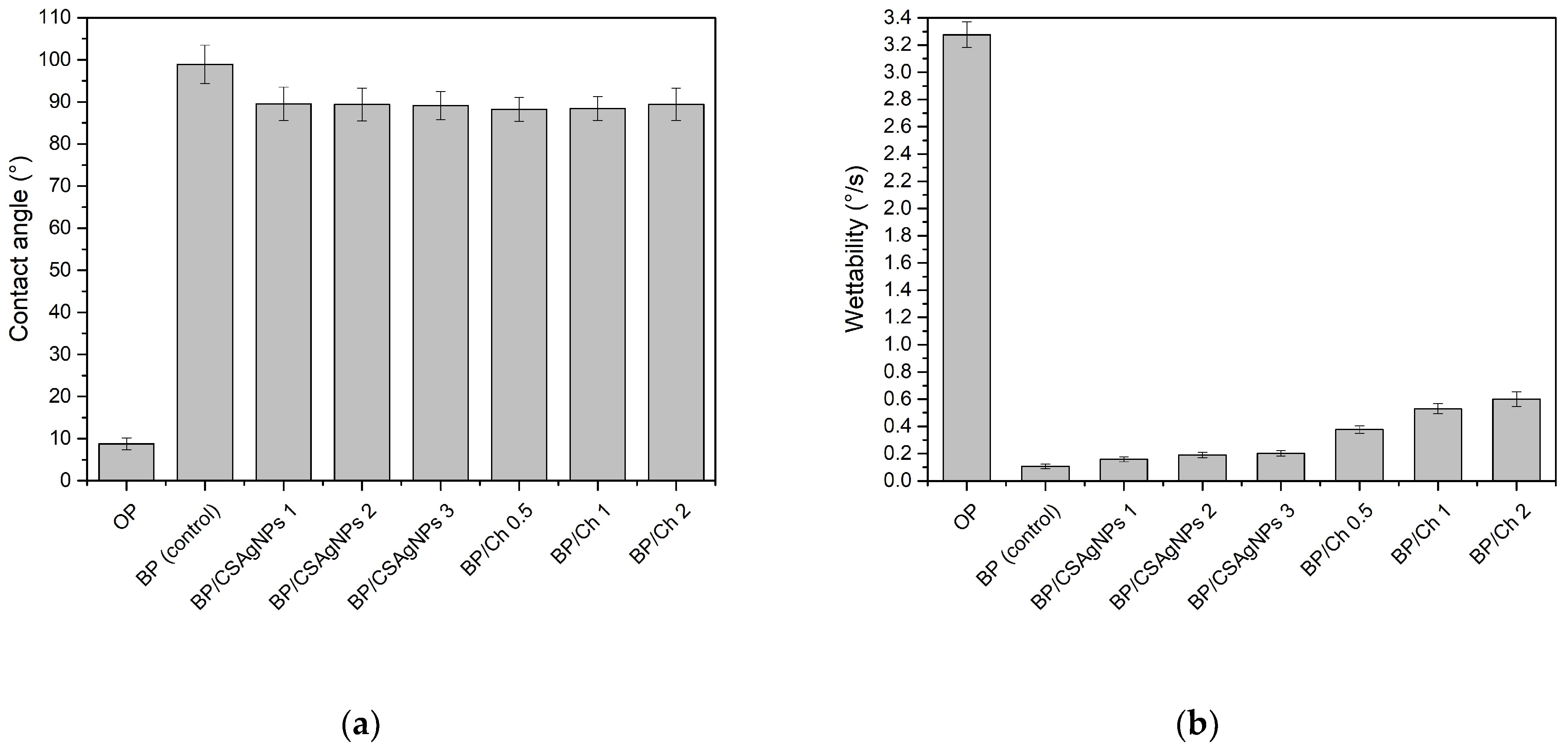
Contact Angle and Wettability
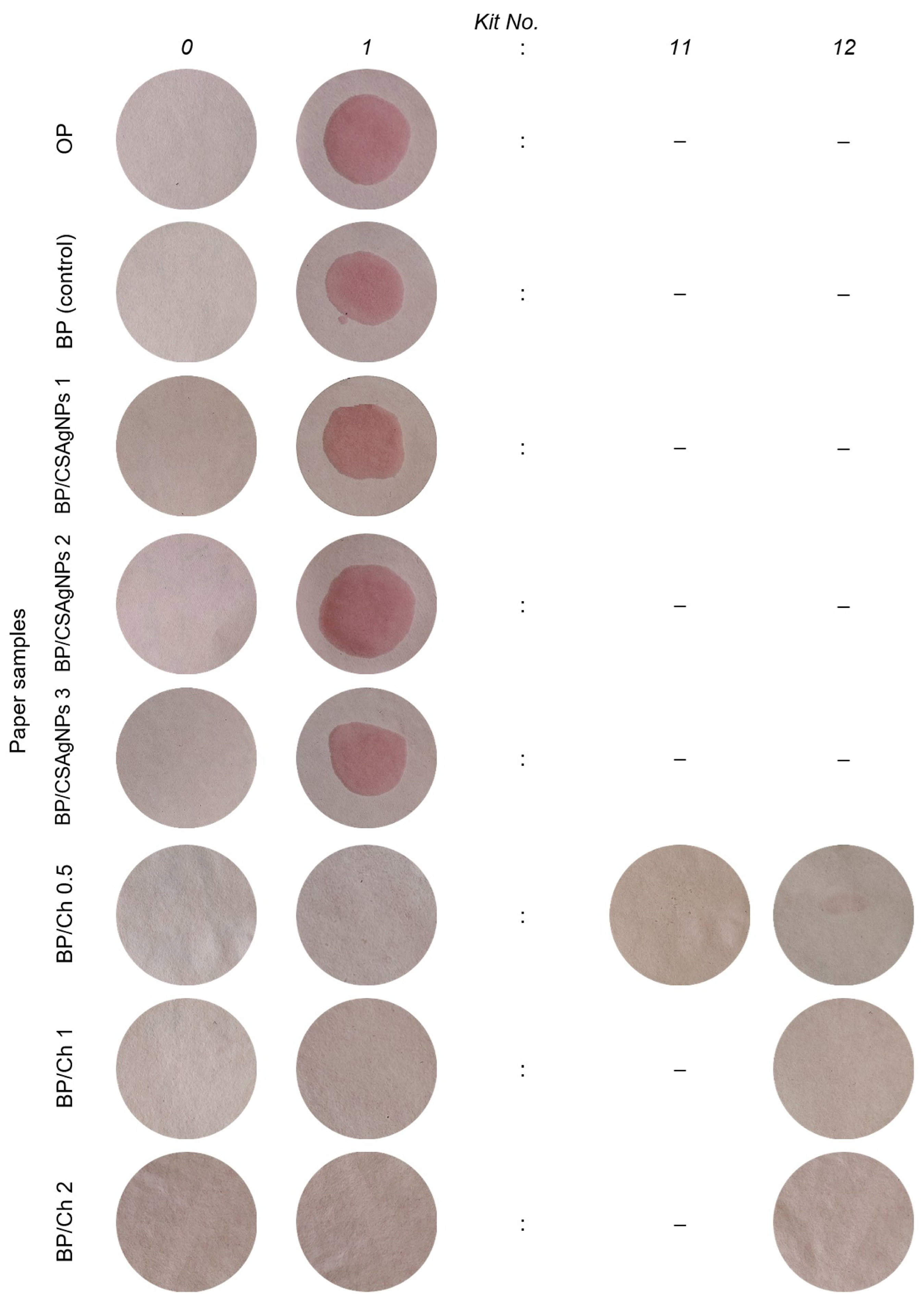
Grease Resistance Test (Kit Test)
2.2.4. Antimicrobial Testing
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Cellulose Fibers and Paper Samples
3.2. Barrier Properties
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Trinh, B.M.; Chang, B.P.; Mekonnen, T.H. The Barrier Properties of Sustainable Multiphase and Multicomponent Packaging Materials: A Review. Prog. Mater. Sci. 2023, 133, 101071. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Cascione, M.; Costa, D.; Martano, S.; Manno, D.; Cannavale, A.; Mazzotta, S.; Paladini, F.; Martino, M.; Rinaldi, R. Aloe Vera Silver Nanoparticles Addition in Chitosan Films: Improvement of Physicochemical Properties for Eco-Friendly Food Packaging Material. J. Mater. Res. Technol. 2023, 24, 1015–1033. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Sudhani, H.P.K.; Bajpai, V.K.; Chen, L.; Shukla, S.; Mukherjee, A. Plant Extract Mediated Silver Nanoparticles and Their Applications as Antimicrobials and in Sustainable Food Packaging: A State-of-the-Art Review. Trends Food Sci. Technol. 2021, 112, 651–666. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Kennedy, J.F. Chitosan-Based Nanocomposites as Coatings and Packaging Materials for the Postharvest Improvement of Agricultural Product: A Review. Carbohydr. Polym. 2023, 309, 120666. [Google Scholar] [CrossRef] [PubMed]
- Alimi, B.A.; Pathania, S.; Wilson, J.; Duffy, B.; Frias, J.M.C. Extraction, Quantification, Characterization, and Application in Food Packaging of Chitin and Chitosan from Mushrooms: A Review. Int. J. Biol. Macromol. 2023, 237, 124195. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Morais, L.D.O.; Macedo, E.V.; Granjeiro, J.M.; Delgado, I.F. Critical Evaluation of Migration Studies of Silver Nanoparticles Present in Food Packaging: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3083–3102. [Google Scholar] [CrossRef] [PubMed]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Z.; Alsammarraie, F.K.; Kong, F.; Lin, M.; Mustapha, A. Properties and Antimicrobial Activity of Polyvinyl Alcohol-Modified Bacterial Nanocellulose Packaging Films Incorporated with Silver Nanoparticles. Food Hydrocoll. 2020, 100, 105411. [Google Scholar] [CrossRef]
- Wang, L.; Periyasami, G.; Aldalbahi, A.; Fogliano, V. The Antimicrobial Activity of Silver Nanoparticles Biocomposite Films Depends on the Silver Ions Release Behaviour. Food Chem. 2021, 359, 129859. [Google Scholar] [CrossRef] [PubMed]
- Brito, S.d.C.; Bresolin, J.D.; Sivieri, K.; Ferreira, M.D. Low-density polyethylene films incorporated with silver nanoparticles to promote antimicrobial efficiency in food packaging. Food Sci. Technol. Int. 2020, 26, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Lazić, V.; Vivod, V.; Peršin, Z.; Stoiljković, M.; Ratnayake, I.S.; Ahrenkiel, P.S.; Nedeljković, J.M.; Kokol, V. Dextran-Coated Silver Nanoparticles for Improved Barrier and Controlled Antimicrobial Properties of Nanocellulose Films Used in Food Packaging. Food Packag. Shelf Life 2020, 26, 100575. [Google Scholar] [CrossRef]
- Istiqola, A.; Syafiuddin, A. A Review of Silver Nanoparticles in Food Packaging Technologies: Regulation, Methods, Properties, Migration, and Future Challenges. J. Chin. Chem. Soc. 2020, 67, 1942–1956. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Z.; Liu, Y.; Zheng, X.; Pei, Y.; Tang, K. Soluble Soybean Polysaccharide Films Containing In-Situ Generated Silver Nanoparticles for Antibacterial Food Packaging Applications. Food Packag. Shelf Life 2022, 31, 100800. [Google Scholar] [CrossRef]
- Adel, A.M.; Al-Shemy, M.T.; Diab, M.A.; El-Sakhawy, M.; Toro, R.G.; Montanari, R.; De Caro, T.; Caschera, D. Fabrication of Packaging Paper Sheets Decorated with Alginate/Oxidized Nanocellulose-silver Nanoparticles Bio-Nanocomposite. Int. J. Biol. Macromol. 2021, 181, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, Q.; Gao, Y.; Wan, S.; Meng, F.; Weng, W.; Zhang, Y. Characterization of Silver Nanoparticles Loaded Chitosan/Polyvinyl Alcohol Antibacterial Films for Food Packaging. Food Hydrocoll. 2023, 136, 108305. [Google Scholar] [CrossRef]
- Liao, M.; Pan, Y.; Fu, X.; Wu, S.; Gan, S.; Wu, Z.; Zhao, H.; Zheng, W.; Cao, Y.; Zhou, W.; et al. Electrospun Polylactic Acid Nanofiber Film Modified by Silver Oxide Deposited on Hemp Fibers for Antibacterial Fruit Packaging. Int. J. Biol. Macromol. 2023, 253, 126569. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of Inorganic Nanoparticles Reinforcement on Its Performance and Food Packaging Applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Samanvitha, S.K.; Shobana, N.; Renitta, E.R.; Senthilkumar, P.; Kumar, S.S.; Abirami, S.; Dhiva, S.; Bavanilatha, M.; Prakash, P.; et al. The Synthesis, Characterization and Applications of Polyhydroxyalkanoates (PHAs) and PHA-Based Nanoparticles. Polymers 2021, 13, 3302. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl Cellulose/Cellulose Nanocrystals Immobilized Silver Nanoparticles as an Effective Coating to Improve Barrier and Antibacterial Properties of Paper for Food Packaging Applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, C.; Wang, Y.; Xie, L.; Li, W.; Li, B.; Guo, R.; Yan, H. Magnesium Oxide/Silver Nanoparticles Reinforced Poly(Butylene Succinate-Co-Terephthalate) Biofilms for Food Packaging Applications. Food Packag. Shelf Life 2021, 30, 100748. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications—A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In Situ Reduction of Silver Nanoparticles by Sodium Alginate to Obtain Silver-Loaded Composite Wound Dressing with Enhanced Mechanical and Antimicrobial Property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hadidi, M.; Karaca, A.C.; Hedayati, S.; Tarahi, M.; Assadpour, E.; Jafari, S.M. Chitosan-Grafted Phenolic Acids as an Efficient Biopolymer for Food Packaging Films/Coatings. Carbohydr. Polym. 2023, 314, 120901. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.D.; Priyadarshi, R.; Bhaskar, R.; Han, S.S. Chitosan-Based Multifunctional Films Reinforced with Cerium Oxide Nanoparticles for Food Packaging Applications. Food Hydrocoll. 2023, 143, 108910. [Google Scholar] [CrossRef]
- Barik, M.; BhagyaRaj, G.V.S.; Dash, K.K.; Shams, R. A Thorough Evaluation of Chitosan-Based Packaging Film and Coating for Food Product Shelf-Life Extension. J. Agric. Food Res. 2024, 16, 101164. [Google Scholar] [CrossRef]
- Vrabič-Brodnjak, U.; Yavorov, N.; Lasheva, V.; Todorova, D. Chitosan-Coated Packaging Papers—Strength and Thermal Stability. Coatings 2023, 13, 828. [Google Scholar] [CrossRef]
- Harikrishnan, M.P.; Thampi, A.; Lal, A.M.N.; Warrier, A.S.; Basil, M.; Kothakota, A. Effect of Chitosan-Based Bio Coating on Mechanical, Structural and Physical Characteristics of Microfiber Based Paper Packaging: An Alternative to Wood Pulp/Plastic Packaging. Int. J. Biol. Macromol. 2023, 253, 126888. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Xiao, X.; Duan, Q.; Bai, H.; Cao, Y.; Zhang, Y.; Alee, M.; Yu, L. Improved Hydrophobicity, Antibacterial and Mechanical Properties of Polyvinyl Alcohol/Quaternary Chitosan Composite Films for Antibacterial Packaging. Carbohydr. Polym. 2023, 312, 120755. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, B.; Mirza, B.; Ullah, A. Chitosan Based Bio-Nanocomposites Packaging Films with Unique Mechanical and Barrier Properties. Food Packag. Shelf Life 2023, 35, 101016. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kaur, P.; Bhardwaj, N.K.; Negi, Y.S. Surface Application of Different Concentrations of Chitosan on Recycled Paper and Its Impact on Packaging Properties. J. Coat. Technol. Res. 2023, 20, 1285–1298. [Google Scholar] [CrossRef]
- Nazarnezhad, N.; Avrand, M.; Resalati, H. The Effect of Chitosan Coating on the Strength and Barrier Properties of Liner Paper. Iran. J. Wood Pap. Sci. Res. 2023, 38, 37–47. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, J.; Su, M.; Xiao, N.; Zhong, L.; Zhang, X.; Chen, S.; Chen, Q.; Liang, W.; Liu, M. High-Barrier Oxidized Cellulose Nanofibril/Chitosan Coating for Functional Food Packaging Materials. ACS Appl. Polym. Mater. 2024, 6, 2877–2888. [Google Scholar] [CrossRef]
- Inthamat, P.; Karbowiak, T.; Tongdeesoontorn, W.; Siripatrawan, U. Biodegradable Active Coating from Chitosan/Astaxanthin Crosslinked with Genipin to Improve Water Resistance, Moisture and Oxygen Barrier and Mechanical Properties of Kraft Paper. Int. J. Biol. Macromol. 2024, 254, 127816. [Google Scholar] [CrossRef] [PubMed]
- Gürler, N. Development of Chitosan/Gelatin/Starch Composite Edible Films Incorporated with Pineapple Peel Extract and Aloe Vera Gel: Mechanical, Physical, Antibacterial, Antioxidant, and Sensorial Analysis. Polym. Eng. Sci. 2023, 63, 426–440. [Google Scholar] [CrossRef]
- Shan, P.; Wang, K.; Yu, F.; Yi, L.; Sun, L.; Li, H. Gelatin/Sodium Alginate Multilayer Composite Film Crosslinked with Green Tea Extract for Active Food Packaging Application. Colloids Surf. A 2023, 662, 131013. [Google Scholar] [CrossRef]
- Rohadi, T.N.T.; Ridzuan, M.J.M.; Majid, M.S.A.; Sulaiman, M.H. Biodegradability of Bioplastic Film Using Different Regions of Pennisetum Purpureum Incorporated with Gelatine and Chitosan. Int. J. Environ. Sci. Technol. 2023, 20, 10313–10324. [Google Scholar] [CrossRef]
- Wawrzyńczak, A.; Chudzińska, J.; Feliczak-Guzik, A. Metal and Metal Oxides Nanoparticles as Nanofillers for Biodegradable Polymers. ChemPhysChem 2024, 25, e202300823. [Google Scholar] [CrossRef]
- Saleem, S.; Khan, S.T. Engineered Nanomaterials for Food Preservation and Packaging: Focus on Antimicrobial and Antibiofilm Activities. In Quality Control in Fruit and Vegetable Processing; Apple Academic Press: New York, NY, USA, 2023; pp. 229–259. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Baurai, M.; Loria, N.; Dubey, N.; Tokala, V.Y. Implications of Nanotechnology in Food Packaging. In Nanotechnology Horizons in Food Process Engineering; Apple Academic Press: New York, NY, USA, 2023; pp. 161–205. [Google Scholar] [CrossRef]
- Advaita; Malik, K.; Bhagabati, V.P.; Sharma, K. Nanostructured Film and Coating Materials. In Biopolymer-Based Films and Coatings; CRC Press: Boca Raton, FL, USA, 2023; pp. 361–384. [Google Scholar] [CrossRef]
- Cherian, R.M.; Antony, T.; Zakuwan, S.Z.; Jose, C.; Kargarzadeh, H.; Thomas, S. Developments in Chitosan-Based Nanocomposites for Food Packaging Applications. In Nanomaterials from Renewable Resources for Emerging Applications; CRC Press: Boca Raton, FL, USA, 2022; pp. 55–92. [Google Scholar] [CrossRef]
- Sharma, H.; Ahuja, A.; Sharma, B.; Kulshreshtha, A.; Kadam, A.; Dutt, D. Vapor Phase Antimicrobial Active Packaging Application of Chitosan Capsules Containing Clove Essential Oil for the Preservation of Dry Cakes. Food Bioprocess Technol. 2024, 17, 780–790. [Google Scholar] [CrossRef]
- ISO 5264-1:1979; Pulps—Laboratory Beating, Part 1: Valley Beater Method. ISO-International Standards Organization: Geneva, Switzerland, 1979.
- ISO 5267-1:1999/Cor 1:2001; Pulps—Determination of Drainability—Part 1: Schopper-Riegler methodTechnical Corrigendum 1. ISO-International Standards Organization: Geneva, Switzerland, 2001.
- ISO 5269-2:2004; Pulps—Preparation of Laboratory Sheets for Physical Testing—Part 2: Rapid-Köthen Method. ISO-International Standards Organization: Geneva, Switzerland, 2004.
- ISO 9184-3:1990; Paper, Board and Pulps—Fibre Furnish Analysis. ISO-International Standards Organization: Geneva, Switzerland, 2021.
- ISO 536:2019; Paper and Board—Determination of Grammage. ISO-International Standards Organization: Geneva, Switzerland, 2019.
- ISO 534:2011; Paper and Board—Determination of Thickness, Density and Specific Volume. ISO-International Standards Organization: Geneva, Switzerland, 2022.
- ISO 5627:1995/Cor 1:2002; Paper and Board—Determination of Smoothness (Bekk Method)—Technical Corrigendum 1. ISO-International Standards Organization: Geneva, Switzerland, 2002.
- ASTM D724-99:2003; Standard Test Method for Surface Wettability of Paper (Angle-of-Contact Method). ASTM International: West Conshohocken, PA, USA, 2009.
- ISO 535:2023; Paper and Board—Determination of Water Absorptiveness—Cobb Method. ISO-International Standards Organization: Geneva, Switzerland, 2023.
- Salmén, L.; Larsson, P.A. On the Origin of Sorption Hysteresis in Cellulosic Materials. Carbohydr. Polym. 2018, 182, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kjellgren, H.; Gällstedt, M.; Engström, G.; Järnström, L. Barrier and Surface Properties of Chitosan-Coated Greaseproof Paper. Carbohydr. Polym. 2006, 65, 453–460. [Google Scholar] [CrossRef]
- Gal, M.R.; Rahmaninia, M.; Hubbe, M.A. A Comprehensive Review of Chitosan Applications in Paper Science and Technologies. Carbohydr. Polym. 2023, 309, 120665. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Choi, I.; Lee, J.-S.; Chang, Y.; Yoon, C.S.; Han, J. Whey Protein Isolate Coating Material for High Oxygen Barrier Properties: A Scale-up Study from Laboratory to Industrial Scale and Its Application to Food Packaging. Food Packag. Shelf Life 2022, 31, 100765. [Google Scholar] [CrossRef]
- Zakaria, S.; Chin, H.C.; Wan Ahmad, W.H.; Kaco, H.; Soon, W.C.; Chi, H.C. Mechanical and Antibacterial Properties of Paper Coated with Chitosan. Sains Malays. 2015, 44, 905–911. [Google Scholar] [CrossRef]
- Bordenave, N.; Grelier, S.; Coma, V. Hydrophobization and Antimicrobial Activity of Chitosan and Paper-Based Packaging Material. Biomacromolecules 2010, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C. New Ways to Enhance the Functionality of Paperboard by Surface Treatment—A Review. Packag. Technol. Sci. 2008, 21, 339–373. [Google Scholar] [CrossRef]

| Paper Samples | Description | CSAgNP Treatment (mL/20 cm2) | Chitosan Coating (g/m2) |
|---|---|---|---|
| OP | Mixture of only pulp | - | - |
| BP (control) | Base paper | - | - |
| BP/CSAgNPs 1 | Base paper treated with CSAgNPs | 1 | - |
| BP/CSAgNPs 2 | 2 | - | |
| BP/CSAgNPs 3 | 3 | - | |
| BP/Ch 0.5 | Base paper coated with chitosan | - | 0.5 |
| BP/Ch 1 | - | 1 | |
| BP/Ch 2 | - | 2 |
| Paper Samples | Grammage (g/m2) | Thickness (mm) | Smoothness (Bekk, Top Side) (s) |
|---|---|---|---|
| OP | 50.89 ± 0.3 | 0.081 ± 0.02 | 10.98 ± 1.13 |
| BP (control) | 50.96 ± 0.2 | 0.081 ± 0.01 | 10.96 ± 1.11 |
| BP/CSAgNPs 1 | 50.48 ± 0.5 | 0.080 ± 0.03 | 14.89 ± 1.29 |
| BP/CSAgNPs 2 | 49.68 ± 0.5 | 0.071 ± 0.03 | 14.92 ± 1.25 |
| BP/CSAgNPs 3 | 49.95 ± 0.7 | 0.070 ± 0.03 | 14.03 ± 1.18 |
| BP/Ch 0.5 | 50.48 ± 0.5 | 0.080 ± 0.02 | 12.27 ± 1.10 |
| BP/Ch 1 | 49.68 ± 0.4 | 0.079 ± 0.02 | 14.56 ± 1.26 |
| BP/Ch 2 | 49.95 ± 0.4 | 0.083 ± 0.03 | 16.42 ± 1.32 |
| Cobb60 (g/m2) | OP | BP | BP/CSAgNPs 1 | BP/CSAgNPs 2 | BP/CSAgNPs 3 |
| 68.81 ± 2.2 | 20.92 ± 1.2 | 18.54 ± 0.7 | 18.63 ± 0.5 | 18.74 ± 0.5 |
| Cobb60 (g/m2) | OP | BP | BP/Ch 0.5 | BP/Ch 1 | BP/Ch 2 |
| 68.81 ± 2.2 | 20.92 ± 1.2 | 21.65 ± 1.1 | 22.16 ± 1.2 | 21.66 ± 1.1 |
| Sample | Tested Microorganisms | Inhibition Efficiency of CSAgNPs Treated (%) | |||
|---|---|---|---|---|---|
| BP/CSAgNPs 1 | BP/CSAgNPs 2 | BP/CSAgNPs 3 | |||
| A | Gram-positive bacteria | Staphylococcus aureus ATCC 6538 | 47.5 | 67.3 | 81.6 |
| B | Bacillus cereus ATCC 10876 | 11.7 | 31.5 | 40.4 | |
| C | Gram-negative bacteria | Escherichia coli ATCC 8739 | 46.8 | 66.0 | 75.8 |
| D | Pseudomonas aeruginosa ATCC 9027 | 16.3 | 20.4 | 38.8 | |
| E | Salmonella abony NTCC 6017 | 27.7 | 35.7 | 45.6 | |
| F | Yeast | Candida albicans ATCC 10231 | 18.6 | 34.4 | 37.1 |
| G | Saccharomyces cerevisiae ATCC 2601 | 33.4 | 41.1 | 51.2 | |
| H | Fungal strain | Aspergillus brasiliensis ATCC 16404 | 15.1 | 28.3 | 35.5 |
| I | Fusarium moniliforme | 27.1 | 33.8 | 36.9 | |
| Sample | Tested Microorganisms | Inhibition Efficiency of Chitosan Coating (%) | |||
|---|---|---|---|---|---|
| BP/Ch 0.5 | BP/Ch 1 | BP/Ch 2 | |||
| A | Gram-positive bacteria | Staphylococcus aureus ATCC 6538 | 76.4 | 78.5 | 86.5 |
| B | Bacillus cereus АТСС 10876 | 31.2 | 79.5 | 88.2 | |
| C | Gram-negative bacteria | Escherichia coli ATCC 8739 | 51.8 | 73.7 | 89.5 |
| D | Pseudomonasaeruginosa ATCC 9027 | 32.3 | 54.8 | 56.3 | |
| E | Salmonella abony NTCC 6017 | 20.2 | 47.4 | 48.6 | |
| F | Yeast | Candida albicans ATCC 10231 | 62.9 | 81.7 | 82.6 |
| G | Saccharomyces cerevisiae ATCC 2601 | 76.6 | 82.8 | 89.9 | |
| H | Fungal strain | Aspergillus brasiliensis ATCC 16404 | 65.9 | 70.1 | 82.4 |
| I | Fusarium moniliforme | 74.7 | 82.9 | 87.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, D.; Yavorov, N.; Vrabič-Brodnjak, U. Impact of Silver Nanoparticle Treatment and Chitosan on Packaging Paper’s Barrier Effectiveness. Polymers 2024, 16, 2127. https://doi.org/10.3390/polym16152127
Todorova D, Yavorov N, Vrabič-Brodnjak U. Impact of Silver Nanoparticle Treatment and Chitosan on Packaging Paper’s Barrier Effectiveness. Polymers. 2024; 16(15):2127. https://doi.org/10.3390/polym16152127
Chicago/Turabian StyleTodorova, Dimitrina, Nikolay Yavorov, and Urška Vrabič-Brodnjak. 2024. "Impact of Silver Nanoparticle Treatment and Chitosan on Packaging Paper’s Barrier Effectiveness" Polymers 16, no. 15: 2127. https://doi.org/10.3390/polym16152127
APA StyleTodorova, D., Yavorov, N., & Vrabič-Brodnjak, U. (2024). Impact of Silver Nanoparticle Treatment and Chitosan on Packaging Paper’s Barrier Effectiveness. Polymers, 16(15), 2127. https://doi.org/10.3390/polym16152127








