Cellulose Acetate and Polycaprolactone Fibre Coatings on Medical-Grade Metal Substrates for Controlled Drug Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. SS Substrate Preparation
2.2.2. Membrane Preparation
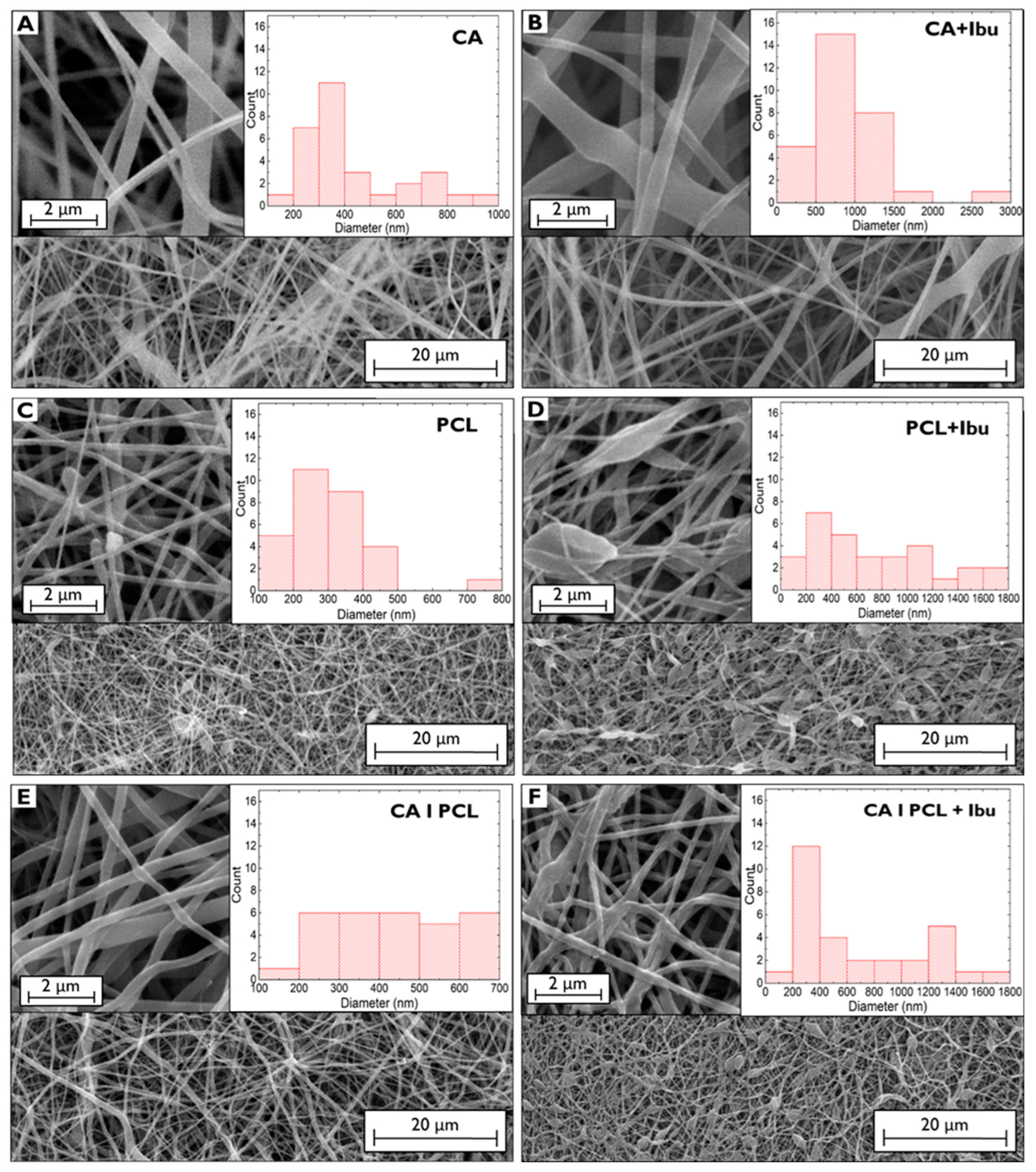
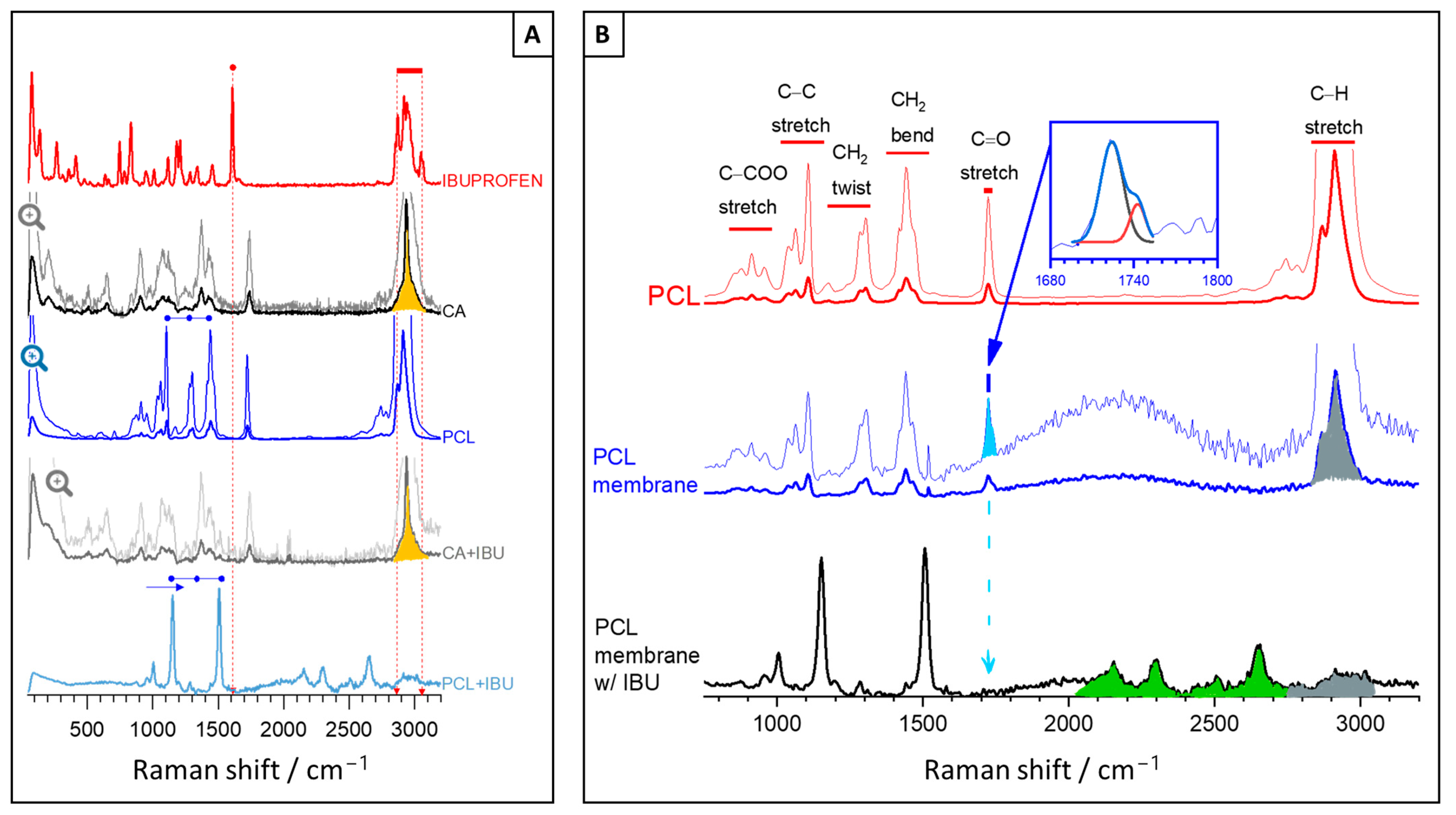
2.2.3. Morphological and Chemical Characterisation
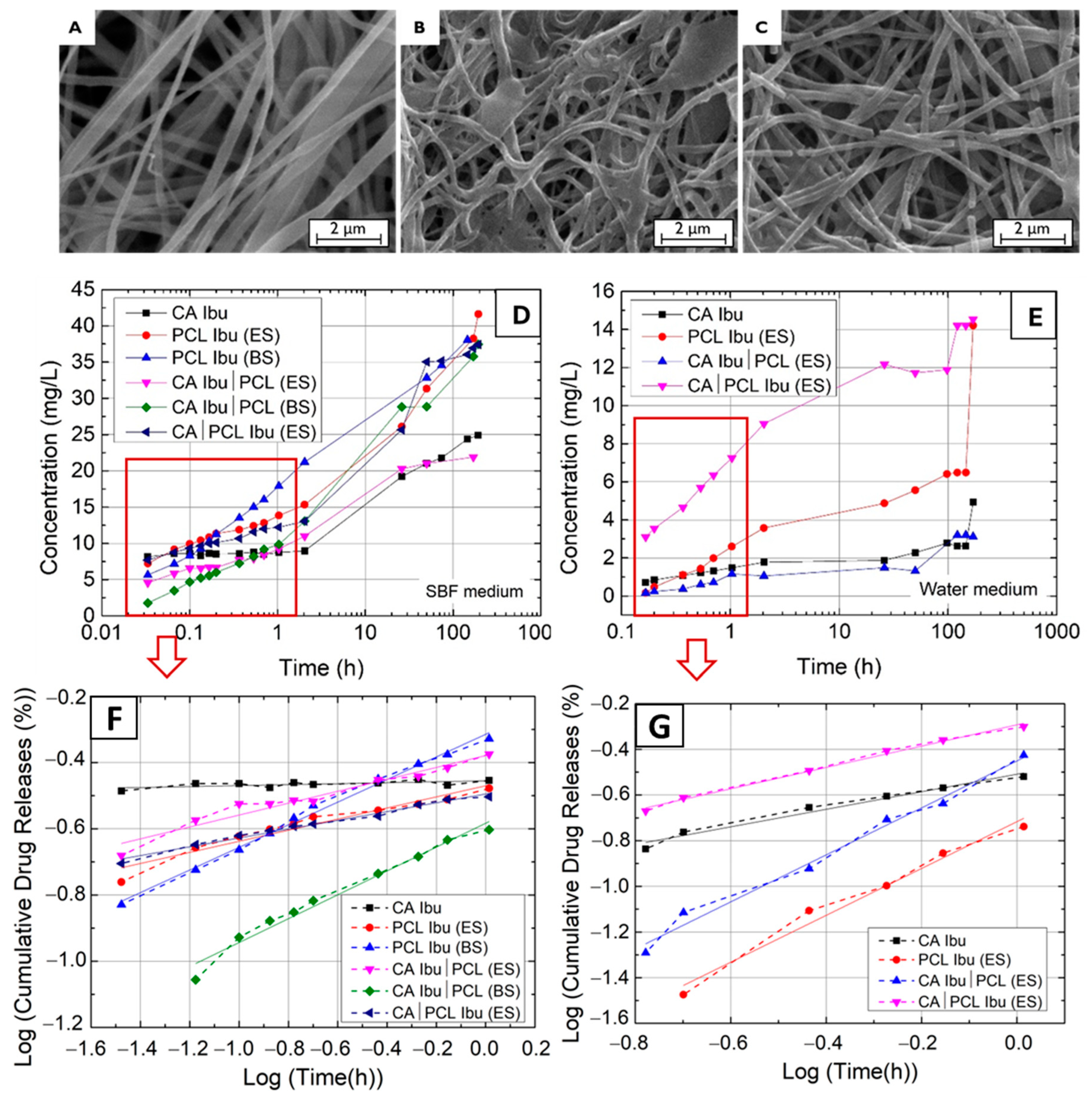
2.2.4. Drug Release Methodology
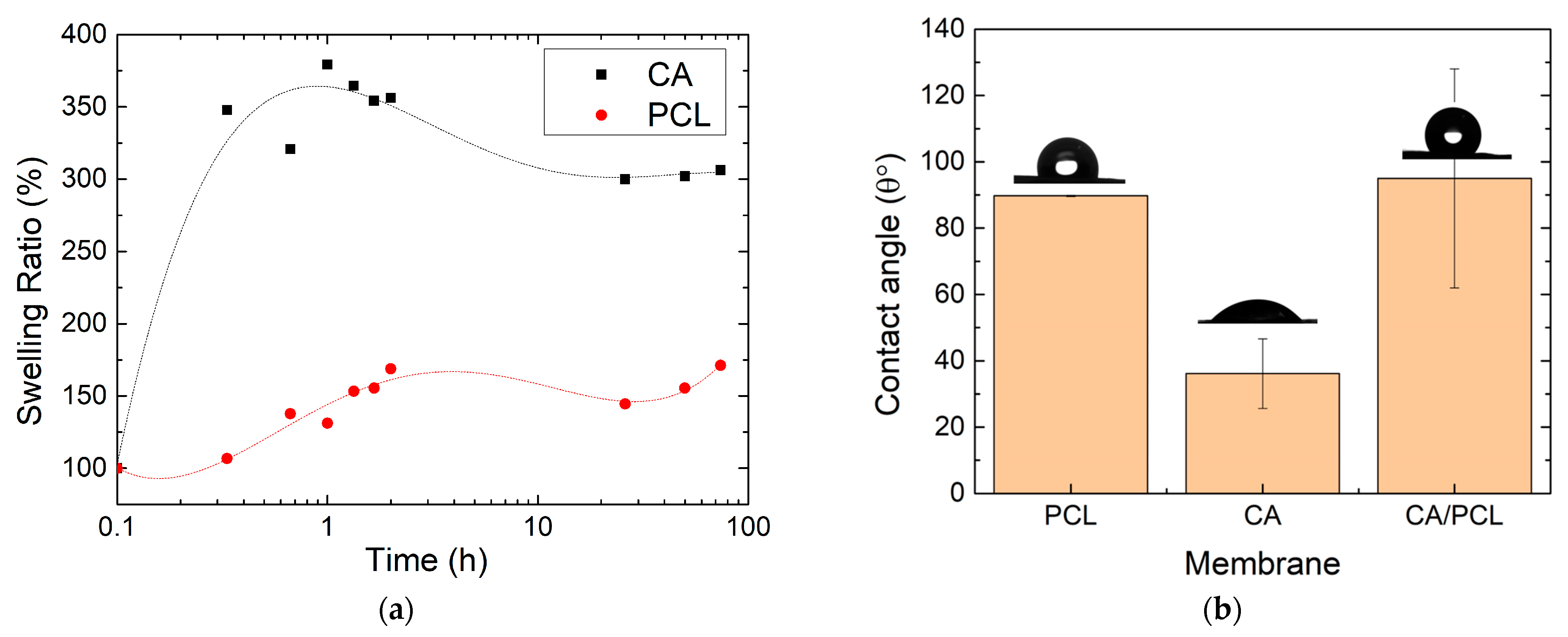
2.2.5. Mechanical Tests
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hashemi, P.M.; Borhani, E.; Nourbakhsh, M.S. A Review on Nanostructured Stainless Steel Implants for Biomedical Application. Nanomed. J. 2016, 3, 202–216. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial Adherence and Biofilm Formation on Medical Implants: A Review. Proc. Inst. Mech. Eng. Part H 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Lee, J.; Nam, J. Effectiveness of Orthopedic Implant Removal Surgery in Patients with No Implant-Related Symptoms after Fracture Union of Isolated Lower Extremity Shaft Fractures: Patient-Centered Evaluation. Arch. Orthop. Trauma. Surg. 2023, 143, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose Acetate in Fabrication of Polymeric Membranes: A Review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose Acetate Electrospun Nanofibers for Drug Delivery Systems: Applications and Recent Advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.S.N.K.; Yadav, K.S. Fabrication and Characterization of Polycaprolactone-Based Green Materials for Drug Delivery. In Applications of Advanced Green Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 395–423. ISBN 9780128204849. [Google Scholar]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral. Biol. Craniofac Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Sionkowska, A. Current Research on the Blends of Natural and Synthetic Polymers as New Biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Demina, T.S.; Bolbasov, E.N.; Peshkova, M.A.; Efremov, Y.M.; Bikmulina, P.Y.; Birdibekova, A.V.; Popyrina, T.N.; Kosheleva, N.V.; Tverdokhlebov, S.I.; Timashev, P.S.; et al. Electrospinning vs. Electro-Assisted Solution Blow Spinning for Fabrication of Fibrous Scaffolds for Tissue. Polymers 2022, 14, 5254. [Google Scholar] [CrossRef]
- Czarnecka, K.; Wojasiński, M.; Ciach, T.; Sajkiewicz, P. Solution Blow Spinning of Polycaprolactone-Rheological Determination of Spinnability and the Effect of Processing Conditions on Fiber Diameter and Alignment. Materials 2021, 14, 1463. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, Z.; Zhao, H.; Liu, T.; Dong, A.; Zhang, J. Electrospinning of Ibuprofen-Loaded Composite Nanofibers for Improving the Performances of Transdermal Patches. J. Nanosci. Nanotechnol. 2013, 13, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, W.; Ngai, T. Polymer Coatings on Magnesium-Based Implants for Orthopedic Applications. J. Polym. Sci. 2022, 60, 32–51. [Google Scholar] [CrossRef]
- Faria, J.; Dionísio, B.; Soares, Í.; Baptista, A.C.; Marques, A.; Gonçalves, L.; Bettencourt, A.; Baleizão, C.; Ferreira, I. Cellulose Acetate Fibres Loaded with Daptomycin for Metal Implant Coatings. Carbohydr. Polym. 2022, 276, 118733. [Google Scholar] [CrossRef] [PubMed]
- Soares, Í.; Faria, J.; Marques, A.; Ribeiro, I.A.C.; Baleizão, C.; Bettencourt, A.; Ferreira, I.M.M.; Baptista, A.C. Drug Delivery from PCL/Chitosan Multilayer Coatings for Metallic Implants. ACS Omega 2022, 7, 23096–23106. [Google Scholar] [CrossRef]
- Rafique, A.; Sequeira, I.; Bento, A.S.; Moniz, M.P.; Carmo, J.; Oliveira, E.; Oliveira, J.P.; Marques, A.; Ferreira, I.; Baptista, A.C. A Facile Blow Spinning Technique for Green Cellulose Acetate/Polystyrene Composite Separator for Flexible Energy Storage Devices. Chem. Eng. J. 2023, 464, 142515. [Google Scholar] [CrossRef]
- Baptista, A.C.; Brito, M.; Marques, A.; Ferreira, I. Electronic Control of Drug Release from Gauze or Cellulose Acetate Fibres for Dermal Applications. J. Mater. Chem. B 2021, 9, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Cuk Neyt Tas, A. Synthesis of Biomimetic Ca-Hydroxyapatite Powders at 37 °C in Synthetic Body Fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The Effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Puyathorn, N.; Senarat, S.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical and Bioactivity Characteristics of Doxycycline Hyclate-Loaded Solvent Removal-Induced Ibuprofen-Based In Situ Forming Gel. Gels 2023, 9, 128. [Google Scholar] [CrossRef]
- Azari, A.; Golchin, A.; Maymand, M.M.; Mansouri, F.; Ardeshirylajimi, A. Electrospun Polycaprolactone Nanofibers: Current Research and Applications in Biomedical Application. Adv. Pharm. Bull. 2022, 12, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Korczyc, A.; Warowicka, A.; Jasiurkowska-Delaporte, M.; Grześkowiak, B.; Jarek, M.; Maciejewska, B.M.; Jurga-Stopa, J.; Jurga, S. Antimicrobial Electrospun Poly(ε-Caprolactone) Scaffolds for Gingival Fibroblast Growth. RSC Adv. 2016, 6, 19647–19656. [Google Scholar] [CrossRef]
- Kamath, S.M.; Sridhar, K.; Jaison, D.; Gopinath, V.; Ibrahim, B.K.M.; Gupta, N.; Sundaram, A.; Sivaperumal, P.; Padmapriya, S.; Patil, S.S. Fabrication of Tri-Layered Electrospun Polycaprolactone Mats with Improved Sustained Drug Release Profile. Sci. Rep. 2020, 10, 18179. [Google Scholar] [CrossRef]
- Ahmad Wsoo, M.; Izwan Abd Razak, S.; Shahir, S.; Ahmed Abdullah Al-Moalemi, H.; Rafiq Abdul Kadir, M.; Hasraf Mat Nayan, N. Development of Prolonged Drug Delivery System Using Electrospun Cellulose Acetate/Polycaprolactone Nanofibers: Future Subcutaneous Implantation. Polym. Adv. Technol. 2021, 32, 3664–3678. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- del Ángel-Sánchez, K.; Borbolla-Torres, C.I.; Palacios-Pineda, L.M.; Ulloa-Castillo, N.A.; Elías-Zúñiga, A. Development, Fabrication, and Characterization of Composite Polycaprolactone Membranes Reinforced with TiO2 Nanoparticles. Polymers 2019, 11, 1955. [Google Scholar] [CrossRef] [PubMed]
- Kalita, N.K.; Hakkarainen, M. Triggering Degradation of Cellulose Acetate by Embedded Enzymes: Accelerated Enzymatic Degradation and Biodegradation under Simulated Composting Conditions. Biomacromolecules 2023, 24, 3290–3303. [Google Scholar] [CrossRef]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.H.; Kim, C.S. Electrospun Bioactive Poly (ɛ-Caprolactone)-Cellulose Acetate-Dextran Antibacterial Composite Mats for Wound Dressing Applications. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Ramos Avilez, H.V.; Castilla Casadiego, D.A.; Vega Avila, A.L.; Perales Perez, O.J.; Almodovar, J. Production of Chitosan Coatings on Metal and Ceramic Biomaterials. In Chitosan Based Biomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 1, pp. 255–293. ISBN 9780081002575. [Google Scholar]
| In SBF | In Water | |||||
|---|---|---|---|---|---|---|
| Membranes | Ibu Released (mg) | n | log(M) = n × log(t) + log(K) | Ibu Released (mg) | n | log(M) = n × log(t) + log(K) |
| CA Ibu | 0.498 | 0.014 | y = 0.014x − 0.455 | 0.098 | 0.384 | y = 0.384x − 0.509 |
| PCL Ibu (ES) | 0.832 | 0.168 | y = 0.168x − 0.470 | 0.284 | 1.026 | y = 1.026x − 0.717 |
| PCL Ibu (BS) | 0.762 | 0.341 | y = 0.341x − 0.315 | - | - | - |
| CA Ibu|PCL (ES) | 0.438 | 0.180 | y = 0.180x − 0.379 | 0.062 | 1.029 | y = 1.029x − 0.451 |
| CA Ibu|PCL (BS) | 0.789 | 0.360 | y = 0.3601x − 0.583 | - | - | - |
| CA|PCL Ibu (ES) | 0.779 | 0.133 | y = 0.133x − 0.495 | 0.290 | 0.468 | y = 0.468x − 0.292 |
| Membrane | Thickness (μm) | Yield Strength (kPa) | Ultimate Strength (kPa) |
|---|---|---|---|
| CA | 39 ± 15 | - | 0.86 ± 0.08 |
| CA Ibu | 53 ± 4 | - | 5.42 ± 0.74 |
| PCL (ES) | 27 ± 7 | - | 2.40 ± 0.11 |
| PCL Ibu (ES) | 67 ± 10 | - | 3.86 ± 0.18 |
| PCL (BS) | 89 ± 21 | 21.94 ± 0.64 | 89 ± 21 |
| PCL Ibu (BS) | 113 ± 23 | 13.83 ± 1.28 | 113 ± 23 |
| CA Ibu|PCL (ES) | 19 ± 2 | - | 1.84 ± 0.42 |
| CA Ibu|PCL (BS) | 45 ± 6 | 5.21 ± 0.45 | 45 ± 6 |
| Membranes on SS Substrates | Average Force | SS Substrates after Adhesion Test | |
|---|---|---|---|
| Without CHI | With CHI | ||
| CA | 0.15 ± 0.02 | 0.21 ± 0.03 | 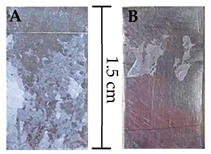 |
| CA Ibu | 0.20 ± 0.03 | 0.22 ± 0.03 | |
| PCL | 0.20 ± 0.03 | 0.30 ± 0.02 | |
| PCL Ibu | 0.22 ± 0.02 | 0.27 ± 0.03 | |
| CA|PCL | 0.21 ± 0.04 | 0.46 ± 0.06 | |
| CA Ibu|PCL Ibu | 0.22 ± 0.05 | 0.74 ± 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cidade do Carmo, C.; Brito, M.; Oliveira, J.P.; Marques, A.; Ferreira, I.; Baptista, A.C. Cellulose Acetate and Polycaprolactone Fibre Coatings on Medical-Grade Metal Substrates for Controlled Drug Release. Polymers 2024, 16, 2006. https://doi.org/10.3390/polym16142006
Cidade do Carmo C, Brito M, Oliveira JP, Marques A, Ferreira I, Baptista AC. Cellulose Acetate and Polycaprolactone Fibre Coatings on Medical-Grade Metal Substrates for Controlled Drug Release. Polymers. 2024; 16(14):2006. https://doi.org/10.3390/polym16142006
Chicago/Turabian StyleCidade do Carmo, Catarina, Miguel Brito, J. P. Oliveira, Ana Marques, Isabel Ferreira, and Ana Catarina Baptista. 2024. "Cellulose Acetate and Polycaprolactone Fibre Coatings on Medical-Grade Metal Substrates for Controlled Drug Release" Polymers 16, no. 14: 2006. https://doi.org/10.3390/polym16142006
APA StyleCidade do Carmo, C., Brito, M., Oliveira, J. P., Marques, A., Ferreira, I., & Baptista, A. C. (2024). Cellulose Acetate and Polycaprolactone Fibre Coatings on Medical-Grade Metal Substrates for Controlled Drug Release. Polymers, 16(14), 2006. https://doi.org/10.3390/polym16142006









