An Overview of Advanced Antimicrobial Food Packaging: Emphasizing Antimicrobial Agents and Polymer-Based Films
Abstract
1. Introduction
2. Antimicrobial Food Packaging
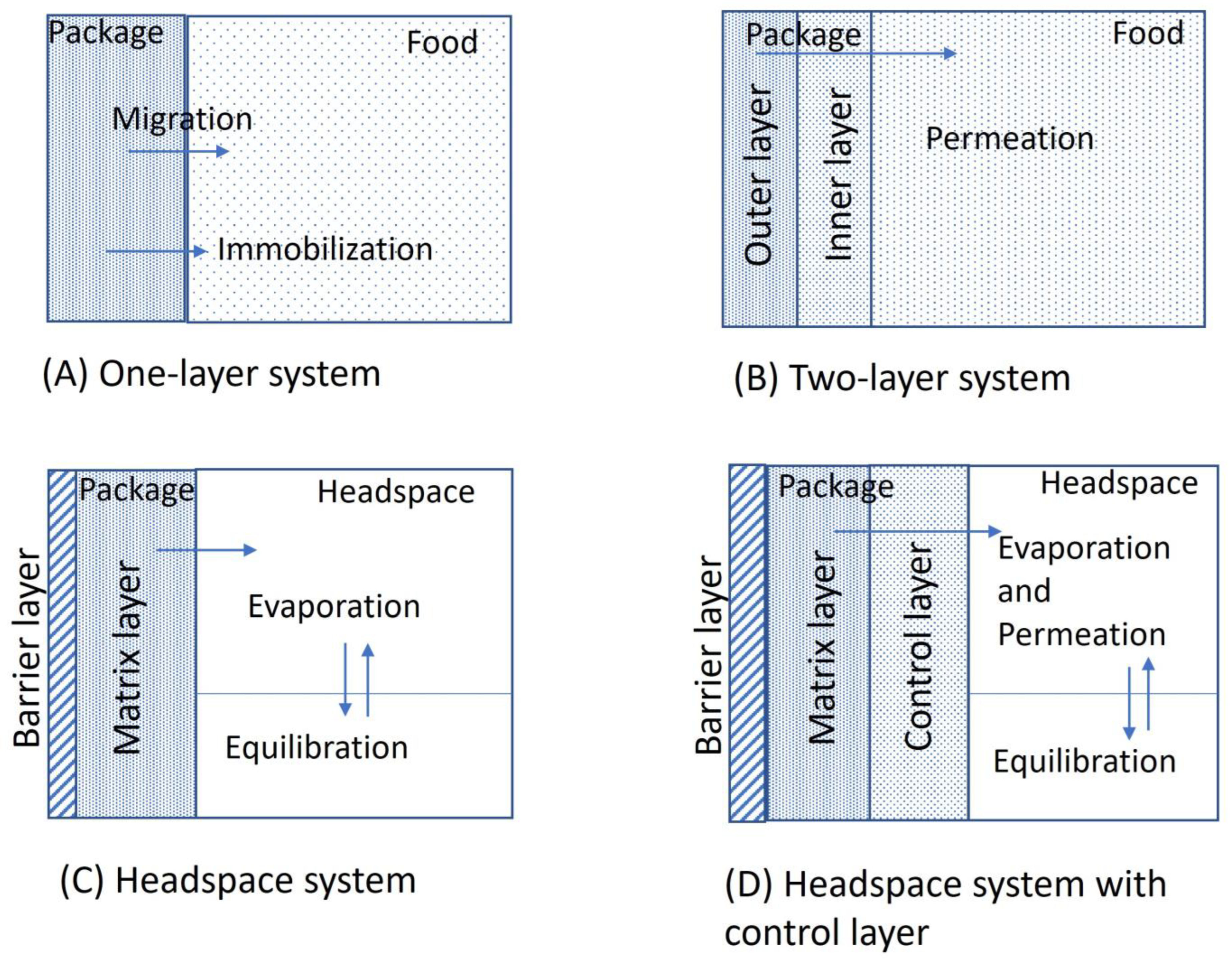
3. Antimicrobial Packaging Construction
Antimicrobial Mechanism
4. Composition of Antimicrobial Packaging Materials
4.1. Antimicrobial Packaging Material
4.1.1. Non-Biodegradable Packaging
4.1.2. Biodegradable Packaging
4.1.3. Antimicrobial Agents
4.1.4. Methods for Incorporating Antimicrobial Agents into Matrices
5. Types of Antimicrobial Packaging
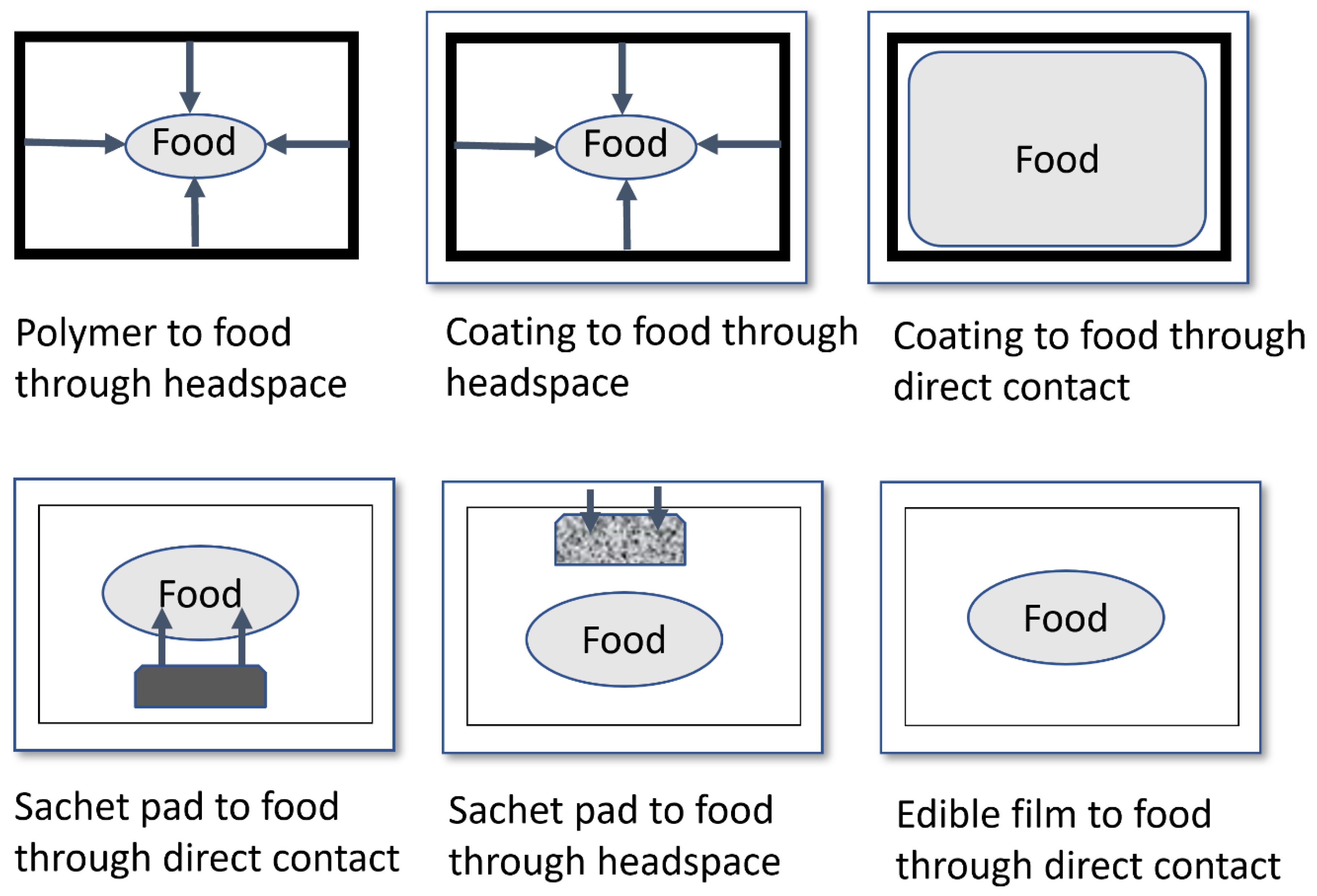
5.1. Sachets or Pads Containing Antimicrobial Agents Added into Packages
5.2. Antimicrobial Agents Directly Incorporated into Polymers
5.3. Coating or Adsorbing Antimicrobials to Polymer Surfaces
5.4. Antimicrobials Ionically or Covalently Immobilized to the Surface of the Polymer
5.5. Inherent Antimicrobial Polymers
6. Effectiveness of Antimicrobial Packaging
6.1. Antimicrobial Packaging of Plant Extracts and Essential oils
6.2. Bacteriocins
6.3. Enzyme-Lysozyme
6.4. Chitosan
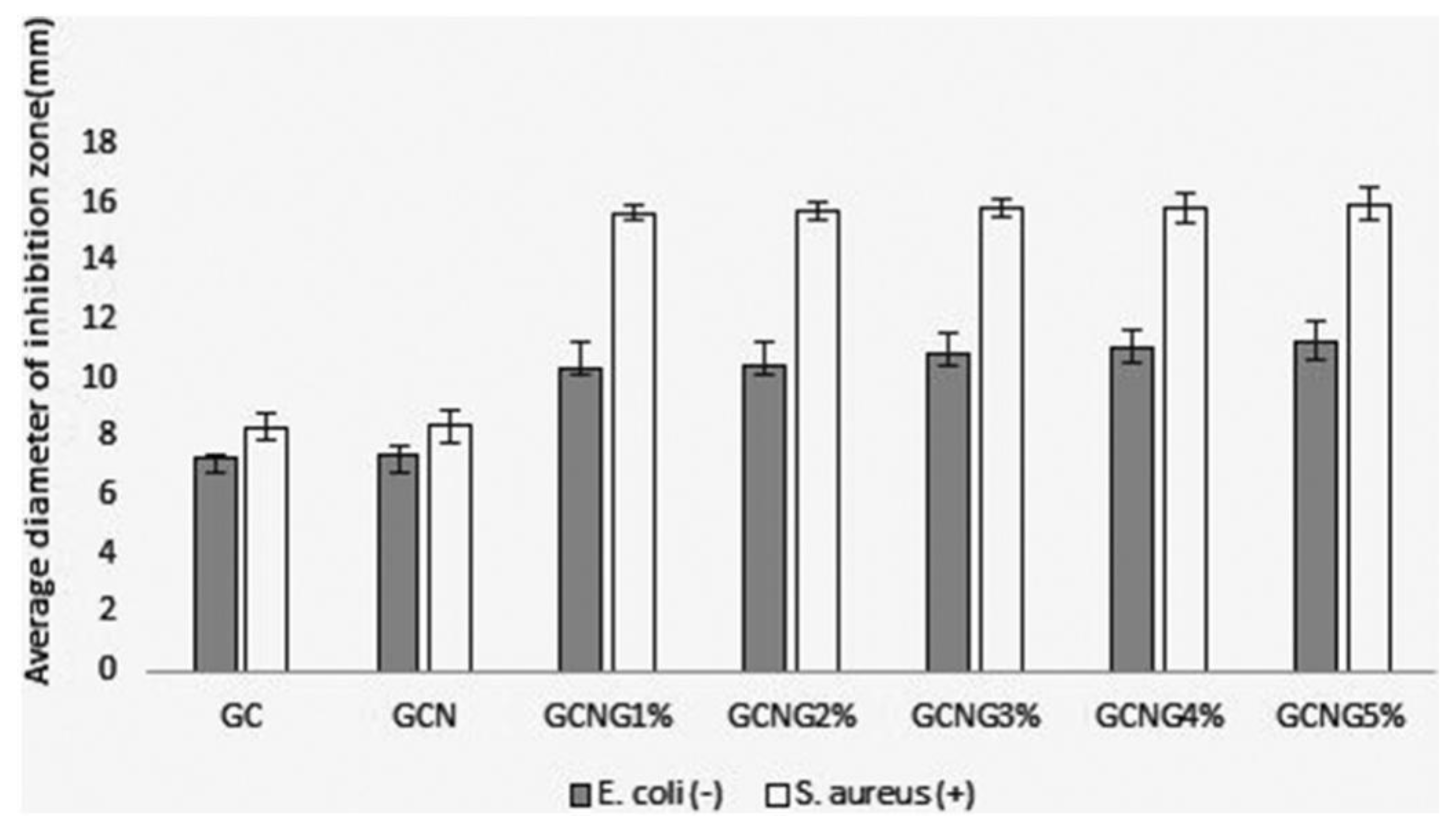
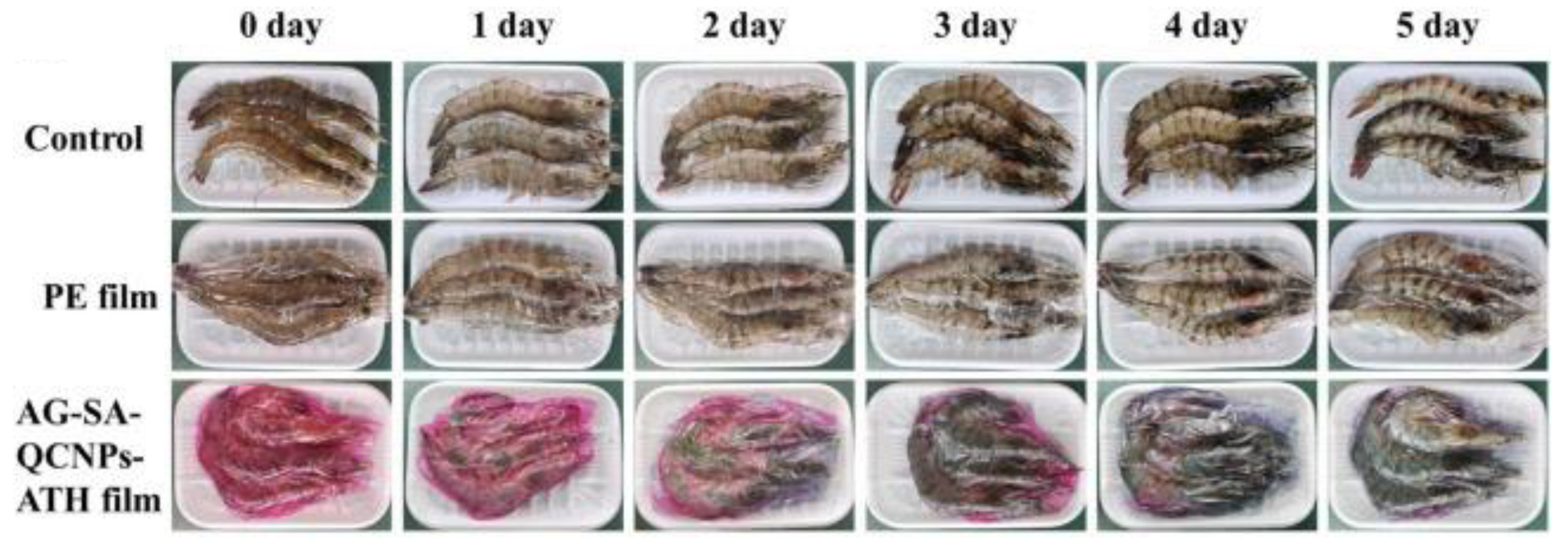
6.5. Chitosan Nanoparticles
7. Applications of Antimicrobial Packaging in Foods
8. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Omolayo, Y.; Feingold, B.J.; Neff, R.A.; Romeiko, X.X. Life cycle assessment of food loss and waste in the food supply chain. Resour. Conserv. Recycl. 2021, 164, 105119. [Google Scholar] [CrossRef]
- Zubair, M.; Ullah, A. Recent advances in protein derived bionanocomposites for food packaging applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 406–434. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Pradhan, R.A.; Arshad, M.; Ullah, A. Recent advances in lipid derived bio-based materials for food packaging applications. Macromol. Mater. Eng. 2021, 306, 2000799. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Bao, Y.; Tan, Y.; Lametsch, R.; Hong, H.; Luo, Y. Recent advances on characterization of protein oxidation in aquatic products: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2024, 64, 1572–1591. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H. Antimicrobial food packaging. Nov. Food Packag. Tech. 2003, 8, 50–70. [Google Scholar]
- Cruz, R.M.S.; Krauter, V.; Krauter, S.; Agriopoulou, S.; Weinrich, R.; Herbes, C.; Scholten, P.B.V.; Uysal-Unalan, I.; Sogut, E.; Kopacic, S.; et al. Bioplastics for Food Packaging: Environmental Impact, Trends and Regulatory Aspects. Foods 2022, 11, 3087. [Google Scholar] [CrossRef] [PubMed]
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, A.K. Linking microbial contamination to food spoilage and food waste: The role of smart packaging, spoilage risk assessments, and date labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, S.; Chen, X.; Sun, H.; Hu, M.; Bai, Z.; Zhuang, G.; Zhuang, X. Bacterial Communities Changes during Food Waste Spoilage. Sci. Rep. 2018, 8, 8220. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Brightwell, G. Effect of Hurdle Approaches Using Conventional and Moderate Thermal Processing Technologies for Microbial Inactivation in Fruit and Vegetable Products. Foods 2022, 11, 1811. [Google Scholar] [CrossRef]
- Safwa, S.M.; Ahmed, T.; Talukder, S.; Sarker, A.; Rana, M.R. Applications of non-thermal technologies in food processing Industries-A review. J. Agric. Food Res. 2023, 100917. [Google Scholar] [CrossRef]
- Goodman, S.; Vanderlee, L.; Acton, R.; Mahamad, S.; Hammond, D. The impact of front-of-package label design on consumer understanding of nutrient amounts. Nutrients 2018, 10, 1624. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.L.; Strupinsky, E.; Kline, L.R. Active Packaging for Food Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Zhang, W.; Roy, S.; Ezati, P.; Yang, D.-P.; Rhim, J.-W. Tannic acid: A green crosslinker for biopolymer-based food packaging films. Trends Food Sci. Technol. 2023, 136, 11–23. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Leistner, L. Further developments in the utilization of hurdle technology for food preservation. In Water in Foods; Elsevier: Amsterdam, The Netherlands, 1994; pp. 421–432. [Google Scholar]
- Sung, S.-Y.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Rahmat, A.; Rahman, W.; Tan, A.-C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Han, J.H. Antimicrobial packaging systems. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2005; pp. 80–107. [Google Scholar]
- Ahvenainen, R. Novel Food Packaging Techniques; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Arshad, M.; Pradhan, R.A.; Ullah, A. Chitosan/chitin-based composites for food packaging applications. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 641–670. [Google Scholar]
- Chen, C.-S.; Liau, W.-Y.; Tsai, G.-J. Antibacterial effects of N-sulfonated and N-sulfobenzoyl chitosan and application to oyster preservation. J. Food Prot. 1998, 61, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Pusztahelyi, T. Chitin and chitin-related compounds in plant-fungal interactions. Mycology 2018, 9, 189–201. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety—Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef] [PubMed]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Singh, S.; Tambe, S.; Samui, A.; Raja, V.; Kumar, D. Maleic acid grafted low density polyethylene for thermally sprayable anticorrosive coatings. Prog. Org. Coat. 2006, 55, 20–26. [Google Scholar] [CrossRef]
- Sonia, A.K.; Dasan, K.P. Feasibility studies of cellulose microfiber (CMF) reinforced poly (ethylene-co-vinyl acetate)(EVA) composites for food packaging applications. Sci. Eng. Compos. Mater. 2016, 23, 489–494. [Google Scholar] [CrossRef]
- Sonia, A.; Dasan, K.P. Celluloses microfibers (CMF)/poly (ethylene-co-vinyl acetate)(EVA) composites for food packaging applications: A study based on barrier and biodegradation behavior. J. Food Eng. 2013, 118, 78–89. [Google Scholar] [CrossRef]
- Boonnattakorn, R.; Chonhenchob, V.; Siddiq, M.; Singh, S.P. Controlled release of mangiferin using ethylene vinyl acetate matrix for antioxidant packaging. Packag. Technol. Sci. 2015, 28, 241–252. [Google Scholar] [CrossRef]
- Kanavouras, A.; Hernandez-Munoz, P.; Coutelieris, F.A. Packaging of olive oil: Quality issues and shelf life predictions. Food Rev. Int. 2006, 22, 381–404. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Marino, A.; Nostro, A. Antimicrobial additives for poly (lactic acid) materials and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 7739–7756. [Google Scholar] [CrossRef]
- Sin, L.T. Polylactic Acid—PLA Biopolymer Technology and Applications; Elsevier: Exeter, UK, 2013. [Google Scholar]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. Oxygen scavenging films in food packaging. Environ. Chem. Lett. 2018, 16, 523–538. [Google Scholar] [CrossRef]
- Sinaga, M.Z.E.; Gea, S.; Panindia, N.; Sihombing, Y.A. The preparation of all-cellulose nanocomposite film from isolated cellulose of corncobs as food packaging. Orient. J. Chem. 2018, 34, 562. [Google Scholar] [CrossRef]
- Wang, G.-H. Inhibition and inactivation of five species of foodborne pathogens by chitosan. J. Food Prot. 1992, 55, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Reesha, K.; Panda, S.K.; Bindu, J.; Varghese, T. Development and characterization of an LDPE/chitosan composite antimicrobial film for chilled fish storage. Int. J. Biol. Macromol. 2015, 79, 934–942. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.; Dutta, P. Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef]
- Cooksey, K. Effectiveness of antimicrobial food packaging materials. Food Addit. Contam. 2005, 22, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Arcentales-Vera, B.; Estrella-Nuñez, J.; Yánez-Vega, H.; Bucio, E. Antimicrobial Activity of Composites-Based on Biopolymers. Macromol 2022, 2, 258–283. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and antimicrobial applications of biopolymers: A review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef] [PubMed]
- Cahan, R.; Goldstein, V.; Finkelstein, B.; Bormashenko, E. Development of the novel active packaging film preventing migration of antimicrobial component. Coll. Jud. Samaria Isr. 2003, 4, 89. [Google Scholar]
- Marın, S.; Guynot, M.; Neira, P.; Bernadó, M.; Sanchis, V.; Ramos, A. Risk assessment of the use of sub-optimal levels of weak-acid preservatives in the control of mould growth on bakery products. Int. J. Food Microbiol. 2002, 79, 203–211. [Google Scholar] [CrossRef]
- Pehlivan, H.; Balköse, D.; Ülkü, S.; Tihminlioglu, F. Characterization of pure and silver exchanged natural zeolite filled polypropylene composite films. Compos. Sci. Technol. 2005, 65, 2049–2058. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Zhang, L.; Xu, Q.; Che, Z.; Li, W.; Bai, Y.; Li, K. Effect of TiO2 nanoparticles on the antibacterial and physical properties of polyethylene-based film. Prog. Org. Coat. 2012, 73, 219–224. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Buonocore, G.; Sinigaglia, M.; Del Nobile, M.A. Development of immobilized lysozyme based active film. J. Food Eng. 2007, 78, 741–745. [Google Scholar] [CrossRef]
- Scannell, A.G.; Hill, C.; Ross, R.; Marx, S.; Hartmeier, W.; Arendt, E.K. Development of bioactive food packaging materials using immobilised bacteriocins Lacticin 3147 and Nisaplin®. Int. J. Food Microbiol. 2000, 60, 241–249. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Grapefruit seed extract incorporated antimicrobial LDPE and PLA films: Effect of type of polymer matrix. Lwt 2016, 74, 338–345. [Google Scholar] [CrossRef]
- Wang, K.; Lim, P.N.; Tong, S.Y.; Thian, E.S. Development of grapefruit seed extract-loaded poly(ε-caprolactone)/chitosan films for antimicrobial food packaging. Food Packag. Shelf Life 2019, 22, 100396. [Google Scholar] [CrossRef]
- Lv, Y.; Deng, Y.; Wang, M.; Li, C.; Xie, P.; Sun, B.; Yang, X.; Lang, Y. Effect of chitosan-gelatine edible coating containing nano-encapsulated clove ethanol extract on cold storage of chilled pork. Meat Sci. 2023, 204, 109288. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.; Ushimaru, P.I.; da Silva Probst, I.; Fernandes Jr, A. Essential oils against foodborne pathogens and spoilage bacteria in minced meat. Foodborne Pathog. Dis. 2009, 6, 725–728. [Google Scholar] [CrossRef]
- Yan, D.; Jin, C.; Xiao, X.-H.; Dong, X.-P. Antimicrobial properties of berberines alkaloids in Coptis chinensis Franch by microcalorimetry. J. Biochem. Biophys. Methods 2008, 70, 845–849. [Google Scholar] [CrossRef]
- Jung, D.-C.; Lee, S.-Y.; Yoon, J.-H.; Hong, K.-P.; Kang, Y.-S.; Park, S.-R.; Park, S.K.; Ha, S.-D.; Kim, G.-H.; Bae, D.-H. Inhibition of pork and fish oxidation by a novel plastic film coated with horseradish extract. LWT-Food Sci. Technol. 2009, 42, 856–861. [Google Scholar] [CrossRef]
- Sharma, H.; Ahuja, A.; Sharma, B.; Kulshreshtha, A.; Kadam, A.; Dutt, D. Vapor Phase Antimicrobial Active Packaging Application of Chitosan Capsules Containing Clove Essential Oil for the Preservation of Dry Cakes. Food Bioprocess Technol. 2024, 17, 780–790. [Google Scholar] [CrossRef]
- Ying, T.; Jiang, C.; Munir, S.; Liu, R.; Yin, T.; You, J.; Rong, J.; Xiong, S.; Hu, Y. Synthesis and application of gelatin-based controlled-release antibacterial films containing oregano essential oil/β-cyclodextrin microcapsules for chilling preservation of grass carp fillets. Food Chem. 2024, 451, 139465. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.S.; Ludueña, L.N.; Flores, S.K. Citric acid, chitosan and oregano essential oil impact on physical and antimicrobial properties of cassava starch films. Carbohydr. Polym. Technol. Appl. 2023, 5, 100307. [Google Scholar] [CrossRef]
- Seydim, A.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Pan, Q.; Zhou, C.; Yang, Z.; Wang, C.; He, Z.; Liu, Y.; Song, S.; Chen, Y.; Xie, M.; Li, P. Preparation and characterization of functionalized chitosan/polyvinyl alcohol composite films incorporated with cinnamon essential oil as an active packaging material. Int. J. Biol. Macromol. 2023, 235, 123914. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Nerín, C.; Batlle, R. New cinnamon-based active paper packaging against Rhizopusstolonifer food spoilage. J. Agric. Food Chem. 2008, 56, 6364–6369. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ru, Y.; Ye, J.; Hong, Q.; Weng, H.; Zhang, Y.; Chen, J.; Xiao, A.; Xiao, Q. Active packaging coating based on agarose grafted with benzoic acid derivatives: Preparation, characterization and application in fish preservation. Food Hydrocoll. 2024, 153, 110002. [Google Scholar] [CrossRef]
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal packaging of sorbate and benzoate incorporated biodegradable films for fresh noodles. Food Control 2021, 123, 107763. [Google Scholar] [CrossRef]
- Chen, M.C.; Yeh, G.H.C.; Chiang, B.H. Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative. J. Food Process. Preserv. 1996, 20, 379–390. [Google Scholar] [CrossRef]
- Weng, Y.-M.; Chen, M.-J. Sorbic anhydride as antimycotic additive in polyethylene food packaging films. LWT-Food Sci. Technol. 1997, 30, 485–487. [Google Scholar] [CrossRef]
- Hoffman, K.; Han, I.; Dawson, P. Antimicrobial effects of corn zein films impregnated with nisin, lauric acid, and EDTA. J. Food Prot. 2001, 64, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, G.; Cutter, C.N.; Willett, J. Incorporation of bacteriocin in plastic retains activity and inhibits surface growth of bacteria on meat. Food Microbiol. 1999, 16, 229–235. [Google Scholar] [CrossRef]
- Natrajan, N.; Sheldon, B.W. Efficacy of nisin-coated polymer films to inactivate Salmonella typhimurium on fresh broiler skin. J. Food Prot. 2000, 63, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Cutter, C.N.; Siragusa, G.R. Population reductions of Gram-negative pathogens following treatments with nisin and chelators under various conditions. J. Food Prot. 1995, 58, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Serrano-León, J.S.; Bergamaschi, K.B.; Yoshida, C.M.P.; Saldaña, E.; Selani, M.M.; Rios-Mera, J.D.; Alencar, S.M.; Contreras-Castillo, C.J. Chitosan active films containing agro-industrial residue extracts for shelf life extension of chicken restructured product. Food Res. Int. 2018, 108, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.-H.; Kim, I.-H.; Choe, C.-H.; Seo, Y.-B.; Song, K.-B. Chitosan-coated packaging papers for storage of agricultural products. Appl. Biol. Chem. 1998, 41, 442–446. [Google Scholar]
- Hong, S.I.; Park, J.D.; Kim, D.M. Antimicrobial and physical properties of food rackaging films incorporated with some natural compounds. Food Sci. Biotechnol. 2000, 9, 38–42. [Google Scholar]
- Miller, W.; Spalding, D.; Risse, L.; Chew, V. The effects of an imazalil-impregnated film with chlorine and imazalil to control decay of bell peppers. Proc. Fla. State Hortic. Soc. 1984, 97, 108–111. [Google Scholar]
- Halek, G.; Garg, A. Fungal inhibition by a fungicide coupled to an ionomeric film. J. Food Saf. 1988, 9, 215–222. [Google Scholar] [CrossRef]
- Terrell, F.R.; Morris, J.; Johnson, M.; Gbur, E.; Makus, D. Yeast Inhibition in Grape. Juice Containing Sulfur Dioxide, Sorbic Acid, and Dimethyldicarbonate. J. Food Sci. 1993, 58, 1132–1134. [Google Scholar] [CrossRef]
- Vermeiren, L.; Devlieghere, F.; van Beest, M.; de Kruijf, N.; Debevere, J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Smith, J.; Ooraikul, B.; Koersen, W.; Van de Voort, F.; Jackson, E.; Lawrence, R. Shelf life extension of a bakery product using ethanol vapor. Food Microbiol. 1987, 4, 329–337. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Q.; Gao, Y.; Wan, S.; Meng, F.; Weng, W.; Zhang, Y. Characterization of silver nanoparticles loaded chitosan/polyvinyl alcohol antibacterial films for food packaging. Food Hydrocoll. 2023, 136, 108305. [Google Scholar] [CrossRef]
- Lian, R.; Cao, J.; Jiang, X.; Rogachev, A.V. Physicochemical, antibacterial properties and cytocompatibility of starch/chitosan films incorporated with zinc oxide nanoparticles. Mater. Today Commun. 2021, 27, 102265. [Google Scholar] [CrossRef]
- Jebel, F.S.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef]
- Meshram, J.; Koli, V.; Phadatare, M.R.; Pawar, S. Anti-microbial surfaces: An approach for deposition of ZnO nanoparticles on PVA-Gelatin composite film by screen printing technique. Mater. Sci. Eng. C 2017, 73, 257–266. [Google Scholar] [CrossRef]
- Nguyen, S.V.; Lee, B.-K. Multifunctional nanocomposite based on polyvinyl alcohol, cellulose nanocrystals, titanium dioxide, and apple peel extract for food packaging. Int. J. Biol. Macromol. 2023, 227, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, J.; Skytta, E.; Ahvenainen-Rantala, R.; Enqvist, J. Antimicrobial and barrier properties of LDPE films containing Imazalil and EDTA. J. Plast. Film Sheeting 2003, 19, 249–261. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Cran, M.J.; Rupika, L.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Release of naturally derived antimicrobial agents from LDPE films. J. Food Sci. 2010, 75, E126–E133. [Google Scholar] [CrossRef] [PubMed]
- Torlak, E.; Nizamlioğlu, M. Antimicrobial effectiveness of chitosan-essential oil coated plastic films against foodborne pathogens. J. Plast. Film Sheeting 2011, 27, 235–248. [Google Scholar] [CrossRef]
- Ye, M.; Neetoo, H.; Chen, H. Control of Listeria monocytogenes on ham steaks by antimicrobials incorporated into chitosan-coated plastic films. Food Microbiol. 2008, 25, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Lara, M.; Gavara, R.; Hernández-Muñoz, P. Development of antimicrobial films for microbiological control of packaged salad. Int. J. Food Microbiol. 2012, 157, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Valderrama Solano, A.C.; de Rojas Gante, C. Two Different Processes to Obtain Antimicrobial Packaging Containing Natural Oils. Food Bioprocess Technol. 2012, 5, 2522–2528. [Google Scholar] [CrossRef]
- Rooney, M.L. Overview of active food packaging. In Active Food Packaging; Rooney, M.L., Ed.; Springer: Boston, MA, USA, 1995; pp. 1–37. [Google Scholar]
- Smith, J.; Hoshino, J.; Abe, Y. Interactive packaging involving sachet technology. In Active Food Packaging; Springer: Berlin/Heidelberg, Germany, 1995; pp. 143–173. [Google Scholar]
- Padgett, T.; Han, Y.; Dawson, P. Effect of lauric acid addition on the antimicrobial efficacy and water permeability of corn zein films containing nisin. J. Food Process. Preserv. 2000, 24, 423–432. [Google Scholar] [CrossRef]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K.K.; Dwelle, R.B. Disease and sprout control in individually film wrapped potatoes. Am. Potato J. 1990, 67, 705–718. [Google Scholar] [CrossRef]
- Labuza, T.P.; Breene, W. Applications of “active packaging” for improvement of shelf-life and nutritional quality of fresh and extended shelf-life foods 1. J. Food Process. Preserv. 1989, 13, 1–69. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Goldberg, S.; Doyle, R.; Rosenberg, M. Mechanism of enhancement of microbial cell hydrophobicity by cationic polymers. J. Bacteriol. 1990, 172, 5650–5654. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, B.; Simard, R.; Piette, G.; Begin, A.; Holley, R. Diffusion of acetic and propionic acids from chitosan-based antimicrobial packaging films. J. Food Sci. 2000, 65, 768–773. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Edible films and coatings as active layers. In Active Food Packaging; Rooney, M.L., Ed.; Springer: Boston, MA, USA, 1995; pp. 111–142. [Google Scholar]
- de Azeredo, H.M.; Otoni, C.G.; Assis, O.B.; Corrêa, D.S.; de Moura, M.R.; Mattoso, L.H.C. Nanoparticles and antimicrobial food packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands. [CrossRef]
- Talebi, F.; Misaghi, A.; Khanjari, A.; Kamkar, A.; Gandomi, H.; Rezaeigolestani, M. Incorporation of spice essential oils into poly-lactic acid film matrix with the aim of extending microbiological and sensorial shelf life of ground beef. LWT 2018, 96, 482–490. [Google Scholar] [CrossRef]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Impact of sweet potato starch-based nanocomposite films activated with thyme essential oil on the shelf-life of baby spinach leaves. Foods 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Limjaroen, P.; Ryser, E.; Lockhart, H.; Harte, B. Development of a food packaging coating material with antimicrobial properties. J. Plast. Film Sheeting 2003, 19, 95–109. [Google Scholar] [CrossRef]
- Soto, K.M.; Hernández-Iturriaga, M.; Loarca-Piña, G.; Luna-Bárcenas, G.; Gómez-Aldapa, C.A.; Mendoza, S. Stable nisin food-grade electrospun fibers. J. Food Sci. Technol. 2016, 53, 3787–3794. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Bharti, A. Evaluating the antimicrobial activity of Nisin, Lysozyme and Ethylenediaminetetraacetate incorporated in starch based active food packaging film. J. Food Sci. Technol. 2015, 52, 3504–3512. [Google Scholar] [CrossRef]
- Divsalar, E.; Tajik, H.; Moradi, M.; Forough, M.; Lotfi, M.; Kuswandi, B. Characterization of cellulosic paper coated with chitosan-zinc oxide nanocomposite containing nisin and its application in packaging of UF cheese. Int. J. Biol. Macromol. 2018, 109, 1311–1318. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Talbert, J.N.; Hernandez-Munoz, P.; Gavara, R.; Goddard, J. Covalent immobilization of lysozyme on ethylene vinyl alcohol films for nonmigrating antimicrobial packaging applications. J. Agric. Food Chem. 2013, 61, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Irkin, R.; Esmer, O.K. Novel food packaging systems with natural antimicrobial agents. J. Food Sci. Technol. 2015, 52, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.S.; Chinnan, M.S. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Corradini, C.; Alfieri, I.; Cavazza, A.; Lantano, C.; Lorenzi, A.; Zucchetto, N.; Montenero, A. Antimicrobial films containing lysozyme for active packaging obtained by sol–gel technique. J. Food Eng. 2013, 119, 580–587. [Google Scholar] [CrossRef]
- Lago, M.; Sendón, R.; de Quirós, A.R.-B.; Sanches-Silva, A.; Costa, H.; Sánchez-Machado, D.; Valdez, H.S.; Angulo, I.; Aurrekoetxea, G.; Torrieri, E. Preparation and characterization of antimicrobial films based on chitosan for active food packaging applications. Food Bioprocess Technol. 2014, 7, 2932–2941. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Murphy, T.; Morris, M.; Cummins, E.; Kerry, J. Antimicrobial activity of chitosan, organic acids and nano-sized solubilisates for potential use in smart antimicrobially-active packaging for potential food applications. Food Control 2013, 34, 393–397. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable antimicrobial food packaging: Trends and perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.; Chawla, S.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
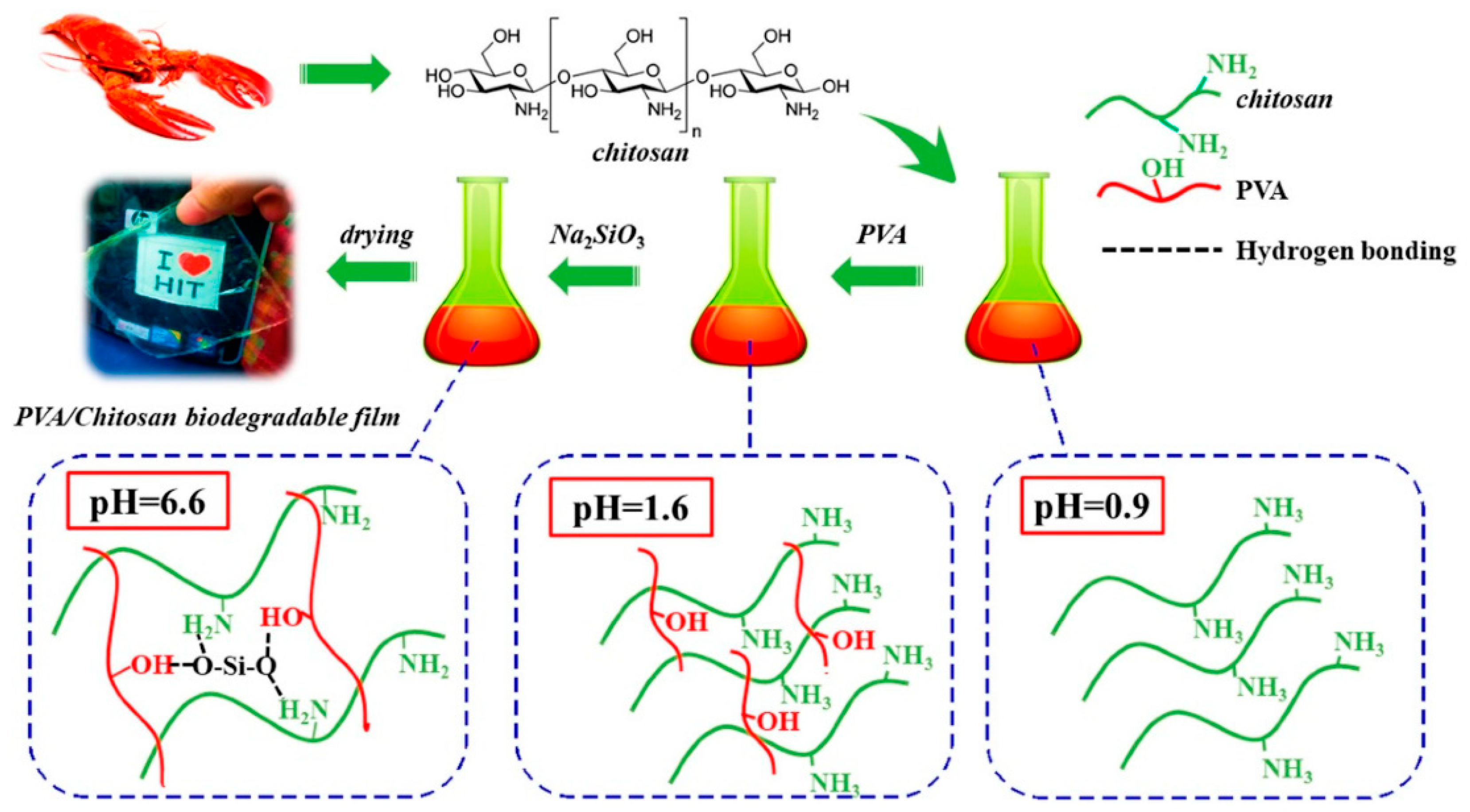
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Biswas, D.; Rhim, J.-W. Antimicrobial nanoparticles in active food packaging applications. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2024; pp. 21–32. [Google Scholar]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 1383–1416. [Google Scholar] [CrossRef]
- Piryaei, M.; Azimi, S. Preparation and evaluation of smart food packaging films with anthocyanin Sardasht black grape based on Astragalus gummifer and chitosan nanoparticles. Int. J. Biol. Macromol. 2024, 254, 127974. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; Castillo, R.; Jiménez, A.J.L.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. Polysaccharide film containing cinnamaldehyde-chitosan nanoparticles, a new eco-packaging material effective in meat preservation. Food Chem. 2024, 437, 137710. [Google Scholar] [CrossRef] [PubMed]
- Amaregouda, Y.; Kamanna, K. Carboxymethyl cellulose/starch-based films incorporating chitosan nanoparticles for multifunctional food packaging. Cellulose 2024, 31, 2413–2427. [Google Scholar] [CrossRef]
- Santhosh, R.; Sarkar, P. Fabrication of jamun seed starch/tamarind kernel xyloglucan bio-nanocomposite films incorporated with chitosan nanoparticles and their application on sapota (Manilkara zapota) fruits. Int. J. Biol. Macromol. 2024, 260, 129625. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, Y.; Lu, D.; Gao, W.; Zhao, Q.; Shi, X. Multifunctional intelligent film integrated with purple sweet potato anthocyanin and quercetin-loaded chitosan nanoparticles for monitoring and maintaining freshness of shrimp. Food Packag. Shelf Life 2023, 35, 101022. [Google Scholar] [CrossRef]
- Fang, M.; Wang, J.; Fang, S.; Zuo, X. Fabrication of carboxymethyl chitosan films for cheese packaging containing gliadin-carboxymethyl chitosan nanoparticles co-encapsulating natamycin and theaflavins. Int. J. Biol. Macromol. 2023, 246, 125685. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, V.S.; Lorevice, M.V.; Baccarin, G.S.; da Costa, F.M.; da Silva Fernandes, R.; Aouada, F.A.; de Moura, M.R. Combining chitosan nanoparticles and garlic essential oil as additive fillers to produce pectin-based nanocomposite edible films. Polymers 2023, 15, 2244. [Google Scholar] [CrossRef]
- Hotchkiss, J.H. Food-packaging interactions influencing quality and safety. Food Addit. Contam. 1997, 14, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Cutter, C.; Willett, J.; Siragusa, G. Improved antimicrobial activity of nisin-incorporated polymer films by formulation change and addition of food grade chelator. Lett. Appl. Microbiol. 2001, 33, 325–328. [Google Scholar] [CrossRef]
- An, D.-S.; Hwang, Y.-I.; Cho, S.-H.; Lee, D.-S. Packaging of fresh curled lettuce and cucumber by using low density polyethylene films impregnated with antimicrobial agents. J.-Korean Soc. Food Sci. Nutr. 1998, 27, 675–681. [Google Scholar]
- Han, J.H.; Floros, J.D. Simulating diffusion model and determining diffusivity of potassium sorbate through plastics to develop antimicrobial packaging films. J. Food Process. Preserv. 1998, 22, 107–122. [Google Scholar] [CrossRef]
- Nayik, G.A.; Muzaffar, K. Developments in packaging of fresh fruits-shelf life perspective: A review. Am. J. Food Sci. Nutr. Res. 2014, 1, 34–39. [Google Scholar]


| Classification | Antimicrobial Agents and Biopolymers |
|---|---|
| Organic acid/acid salts | Acetic, citric, sorbic, malic, lactic, and succinic acid, sodium benzoate, and potassium sorbate |
| Para benzoic acids | Ethanol |
| Bacteriocins/enzymes | Nisin, pediocin, subtilin, lacticin Lysozyme, lactoperoxidase, glucose oxidase |
| Fatty acids/esters | Laurie acid, palmitoleic acid, Gycerol mono laurate |
| Chelating agents/metals | EDTA and lactoferrin Copper, silver, and zirconium |
| Polysaccharide | Chitosan and Starch |
| Phenolics | Catechin, cresol, and hydroquinone |
| Plant/spice extracts/plant volatiles | Grape seed extract, grapefruit seed extract, rosemary oil, oregano oil, basil oil, and other herbs/spice oils Cinnamaldehyde, thymol, terpineol, Allyl isothiocyanate, eugenol, and pinene |
| Antimicrobial Agents | Packaging Materials | Food | Microorganism | References |
|---|---|---|---|---|
| Natural Extracts | ||||
| LDPE, PLA/TPS PCL, Chitosan | Minced fish paste Salmon | E-coli and Listeria monocytogenes E-coli, Psedomonas aeruginosa | [51] [52] |
| Chitosan-gelatine LDPE | Chilled pork Pork, culture media | E.Coli, Psedomonas aeruginosa E. coli, S. Cervisiae | [53] [54] |
| LDPE, paper | Ground beef | E. coli | [55] |
| Chitosan, paper | Pork, fish fillet | E. coli | [56] |
| Essential Oils | ||||
| Chitosan Gelatin-chitosan, cypurs, cintronella | Dry cakes Cold fish fillets | E. coli and S. aureus Aspergillus niger, Bacillus coagulans | [57] [54] |
| Gelatin Starch Whey and milk protein | Grass carp fillets Fresh beef, chicken breast | Pseudomonas, Aeromonas Zygosaccharomyces bailii Pseudomonas spp. | [58] [59] [60] |
| Chitosan/PVA Chitosan film, active paper | Mangoes Rainbow trout, sliced bread | E. coli and Staphylococcus aureus and C. lagenarium Oncorhynchus mykiss, Rhizopusstolonifer | [61] [62] |
| Organic Acids/Anhydride | ||||
| Agarose | Fish | E. coli and Staphylococcus aureus | [63] |
| PBAT/TPS LDPE, MC/chitosan | Fresh noodles Cheese, chicken breast | Aspergillus niger and Rhizopus sp. Yeast, mold | [64] [65] |
| PE | Culture media | Mold, Saccharomyce S.cerevisiae | [66] |
| Enzymes | ||||
| SPI, Zein | Culture media | E. coli, Lactobacillus plantarum | [67] |
| PVOH, nylon, cellulose acetate | Culture media | Lysozyme activity test | [49] |
| Bacteriocins | ||||
| PE | Beef culture media | Total aerobes | [68] |
| PVC, nylon, LLDPE | Chicken | Staphylococcus aureus Sal. Typhimurium | [69] [70] |
| Zein | Simulants | Migration test | [67] |
| Biopolymers | ||||
| Chitosan/peanut skin/pink peper | Chicken Strawberry | psychrotrophic E. coli | [71] [72] |
| Chitosan/paper LDPE | Culture media | E. coli, S.cerevisiae, Fusarium oxysporum | [73] |
| Fungicides | ||||
| LDPE, PE | Bell paper, cheese | Molds | [74] |
| Ionomer | Culture media | Molds | [75] |
| Grape juice | Yeast | [76] | |
| Others | ||||
| Sachet | Bread | Molds | [77] |
| Silica oxide sachet | Bakery | Molds | [78] |
| Metal/Metal Oxide Nanoparticles | ||||
| Chitosan/PVA | Strawberry | E. coli | [79] |
| TPS | Meat fresh products | E. coli and S. aureus | |
| Starch/Chitosan Bacterial cellulose LDPE PVA-gelatin | Food packaging Culture media Culture media | Staphylococcus aureus Bacillus subtilis and Enterobacter aerogenes Bacillus subtilis, E. coli | [80] [81] [82] |
| PVA/CNC/Apple peel extract Chitosan | Cherry tomatoes Culture media | Fungi, Mold and bacteria E. coli, Staphylococcus aureus, Candida albicans | [83] [84] |
| Antimicrobial Compound | Tradename | Company Name | Packaging Forms |
|---|---|---|---|
| Silver substituted zeolite | AgIonTM Novaron® | AgIon Technologies Inc. (Wakefield, MA, USA) Toagosei, Co. Ltd. (Tokyo, Japan) | Bulk food storage containers, paperboard cartons, plastic or paper food wraps, and milk containers |
| Triclosan | Microban® | Microban Product (Toronto, ON, Canada) | Deli wrap, reheatable food containers |
| Allyl isothiocyanate | WasaOuro | Lintec Corporation (Tokyo, Japan) Dry Company Ltd. (Tokyo, Japan) | Pressure-sensitive labels, sheets Sachets |
| Chlorine dioxide | MicrosphereTM | Bernard Technologies Inc. (Aalen, Germany) | Produce storage bags, paperboard coating, rigid containers, pressure-sensitive labels |
| Carbon dioxide | FreshpaxTM Verifrais | Multisorb Technologies (Buffalo, NY, USA) SARL Codimer (Paris, France) | Sachets Sachets |
| Ethanol vapor | Ethicap® Negamold® Fretek® OitechTM | Freund (Tokyo, Japan) Nippon Kayaku (Chiyoda City, Tokyo) | Sachets Sachets Sachets |
| Glucose oxidase | Bioka | Bioka Ltd. (Kantvik, Finland) | Sachets |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, P.; Zubair, M.; Roopesh, M.S.; Ullah, A. An Overview of Advanced Antimicrobial Food Packaging: Emphasizing Antimicrobial Agents and Polymer-Based Films. Polymers 2024, 16, 2007. https://doi.org/10.3390/polym16142007
Upadhyay P, Zubair M, Roopesh MS, Ullah A. An Overview of Advanced Antimicrobial Food Packaging: Emphasizing Antimicrobial Agents and Polymer-Based Films. Polymers. 2024; 16(14):2007. https://doi.org/10.3390/polym16142007
Chicago/Turabian StyleUpadhyay, Punita, Muhammad Zubair, M. S. Roopesh, and Aman Ullah. 2024. "An Overview of Advanced Antimicrobial Food Packaging: Emphasizing Antimicrobial Agents and Polymer-Based Films" Polymers 16, no. 14: 2007. https://doi.org/10.3390/polym16142007
APA StyleUpadhyay, P., Zubair, M., Roopesh, M. S., & Ullah, A. (2024). An Overview of Advanced Antimicrobial Food Packaging: Emphasizing Antimicrobial Agents and Polymer-Based Films. Polymers, 16(14), 2007. https://doi.org/10.3390/polym16142007