Durable, Photostable Omniphobic Synthetic Leather Surfaces with Anti-Biofouling Properties for Hygienic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Leather Samples and Chemicals
2.2. Preparation of FNPs Suspension
2.3. FNPs Deposition by the Dip-Coating Process
2.4. Surface Characterization
2.5. Bacteria Cultivation
2.6. Inoculation of Leather Surfaces and Bacterial Anti-Adhesion Assay
2.7. Self-Cleaning Efficiency of Leather Surfaces
2.8. Thermal Stability Test of Leather Surfaces
2.9. Mechanical Durability Test of Leather Surfaces
2.10. Photostability Test of Leather Surfaces
2.11. Statistical Analysis for Analyzing Bacterial Enumeration
3. Results and Discussion
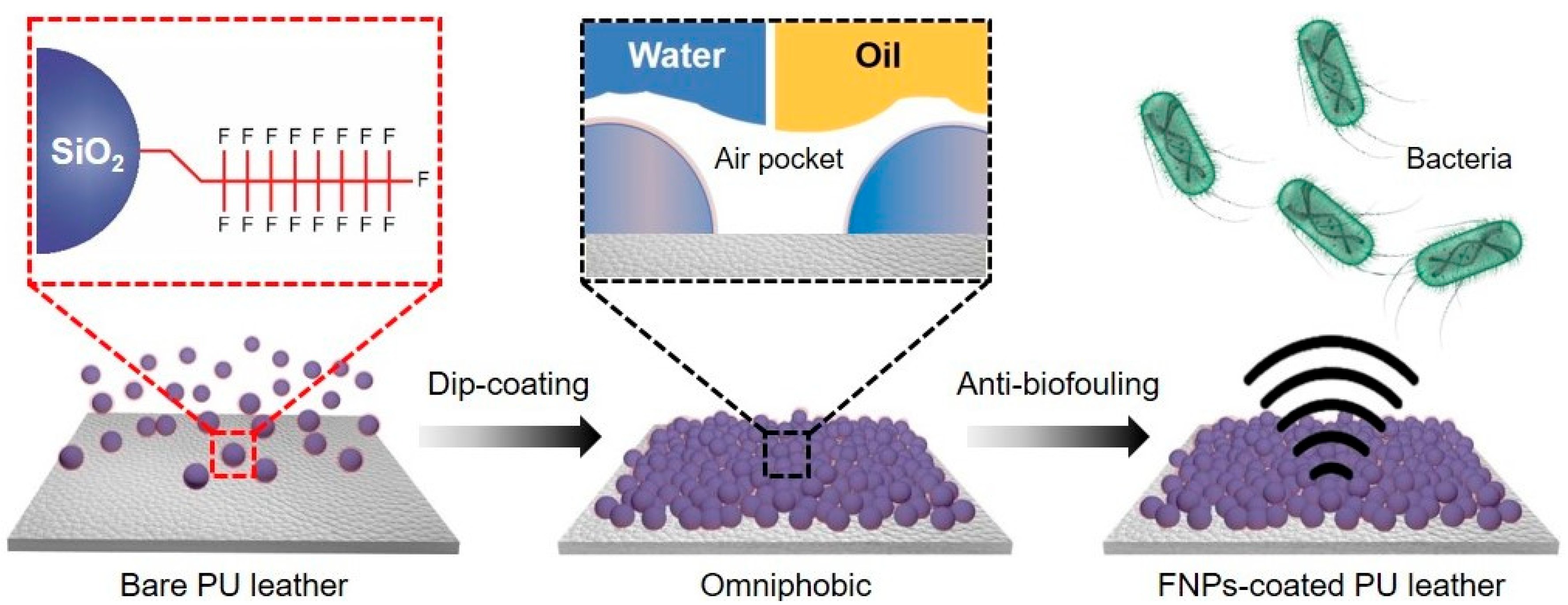
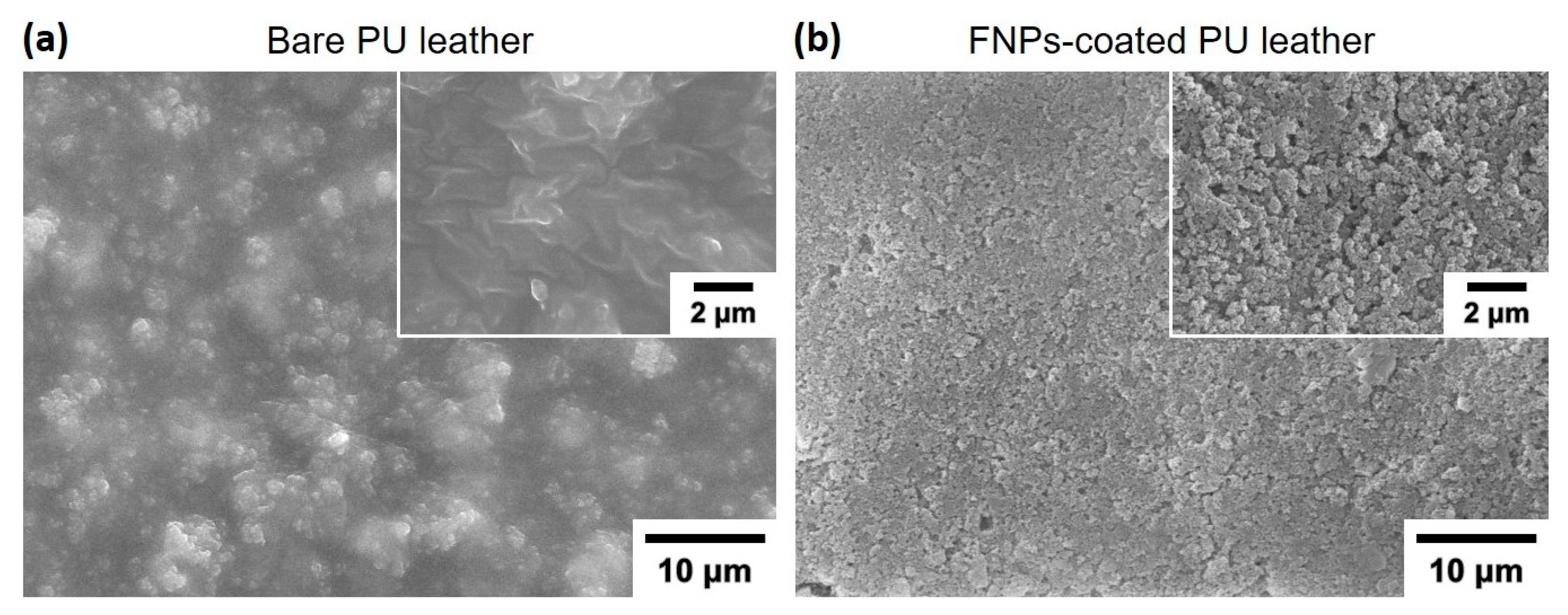
3.1. Characterization of FNPs-Coated Leather Surfaces
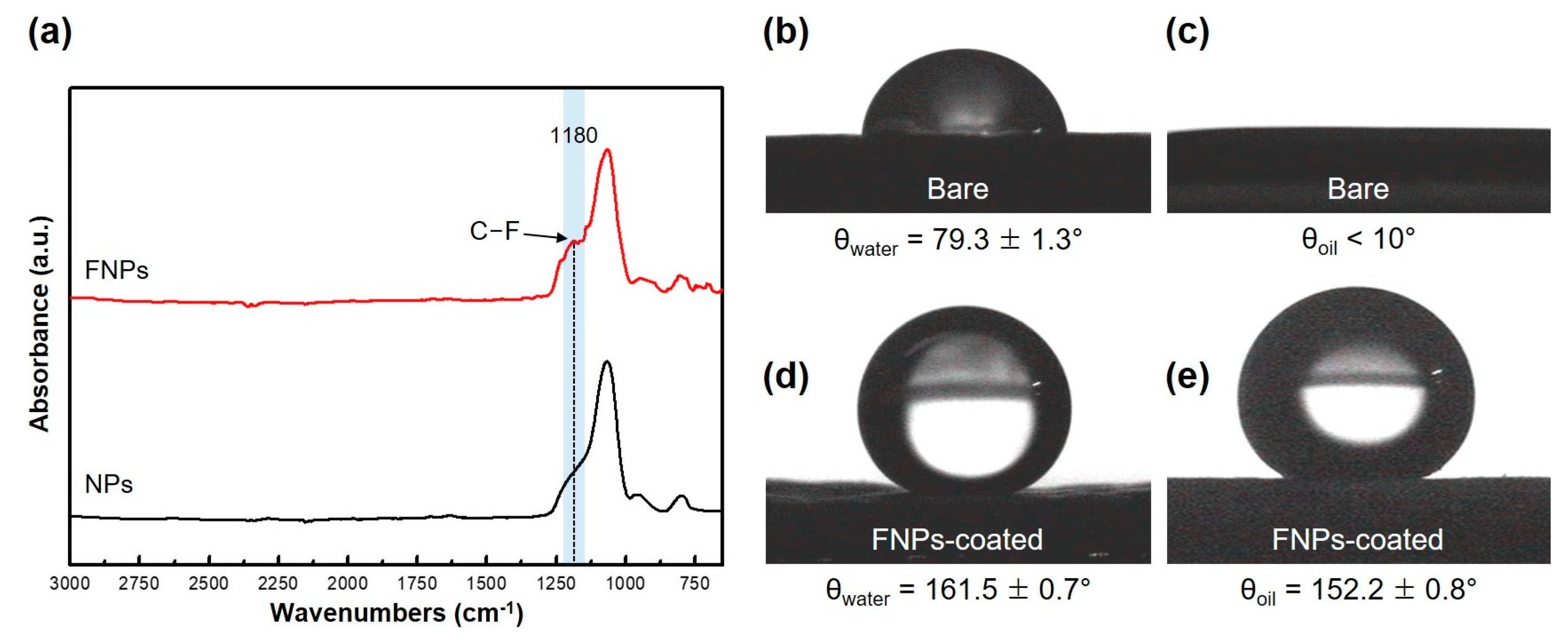
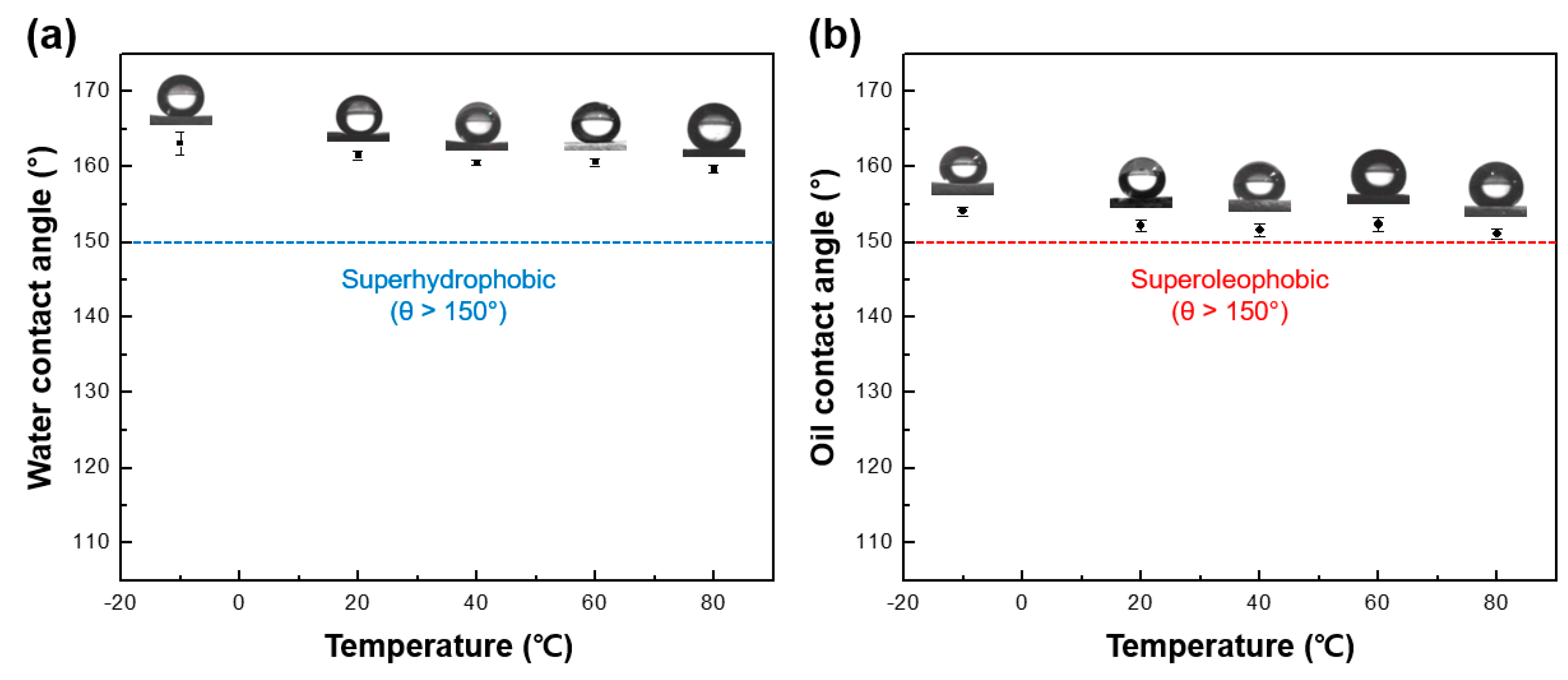
3.2. Wettability of Omniphobic FNPs-Coated Leather Surfaces
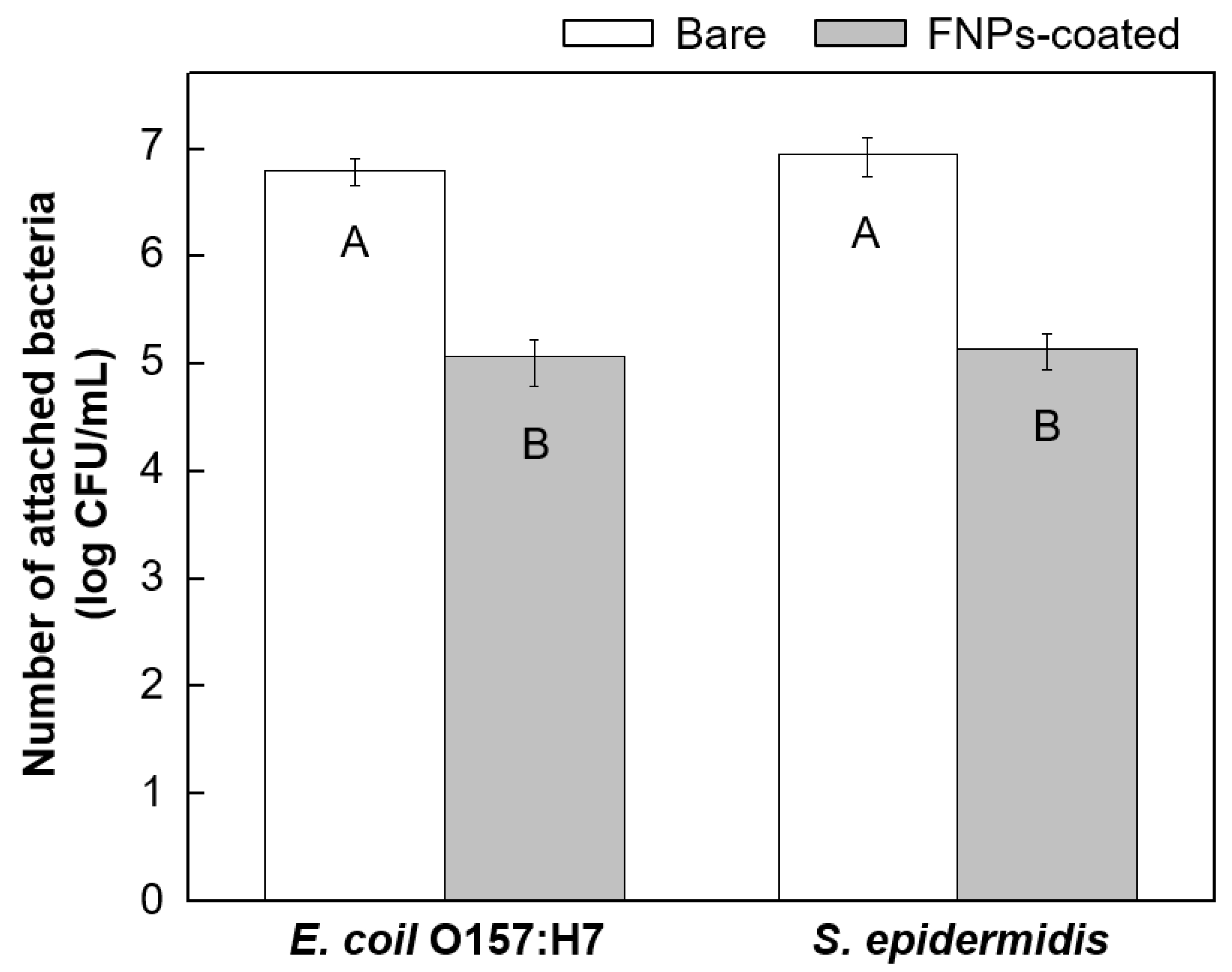
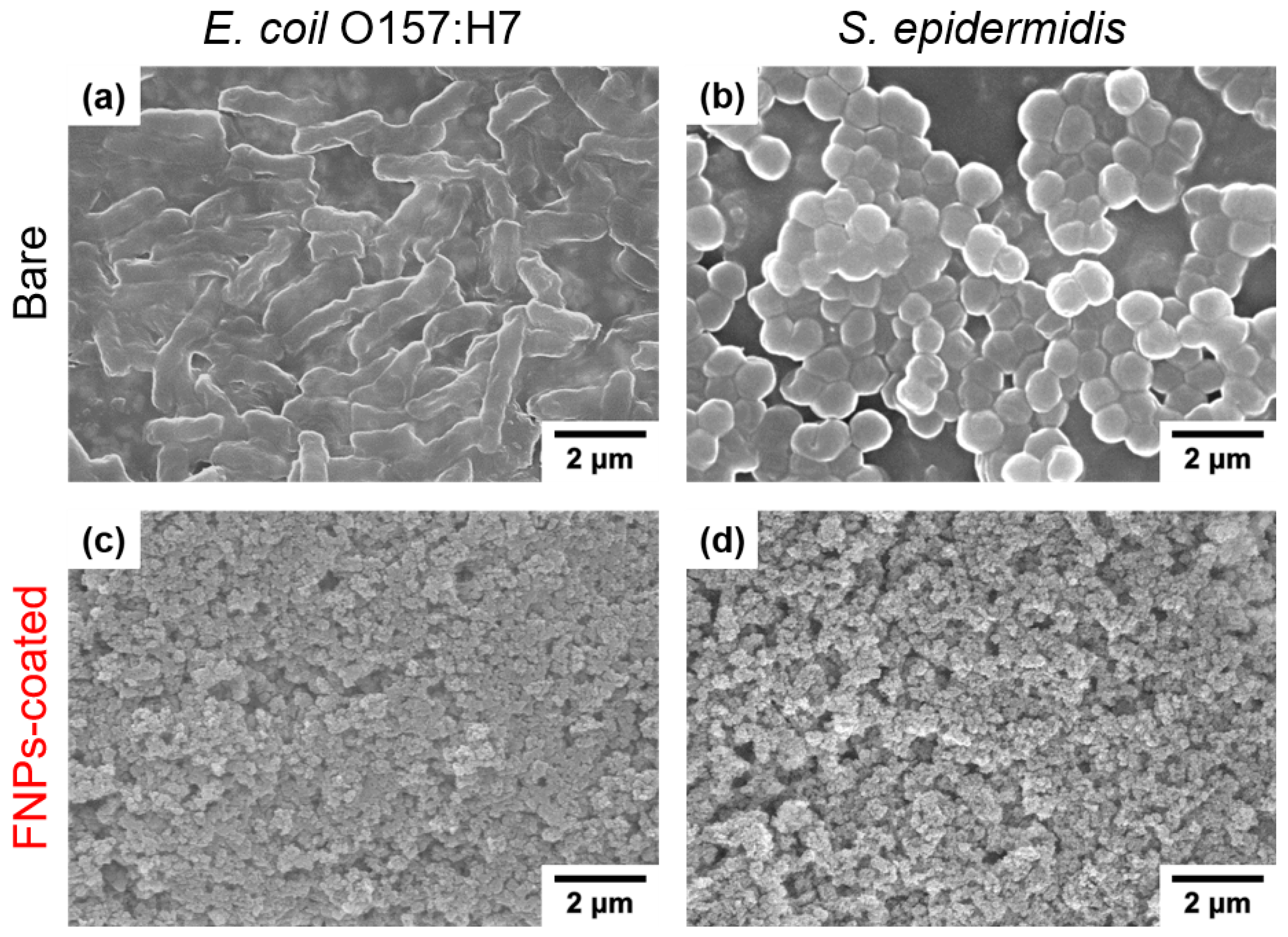
3.3. Anti-Biofouling Properties of FNPs-Coated Leather Surfaces
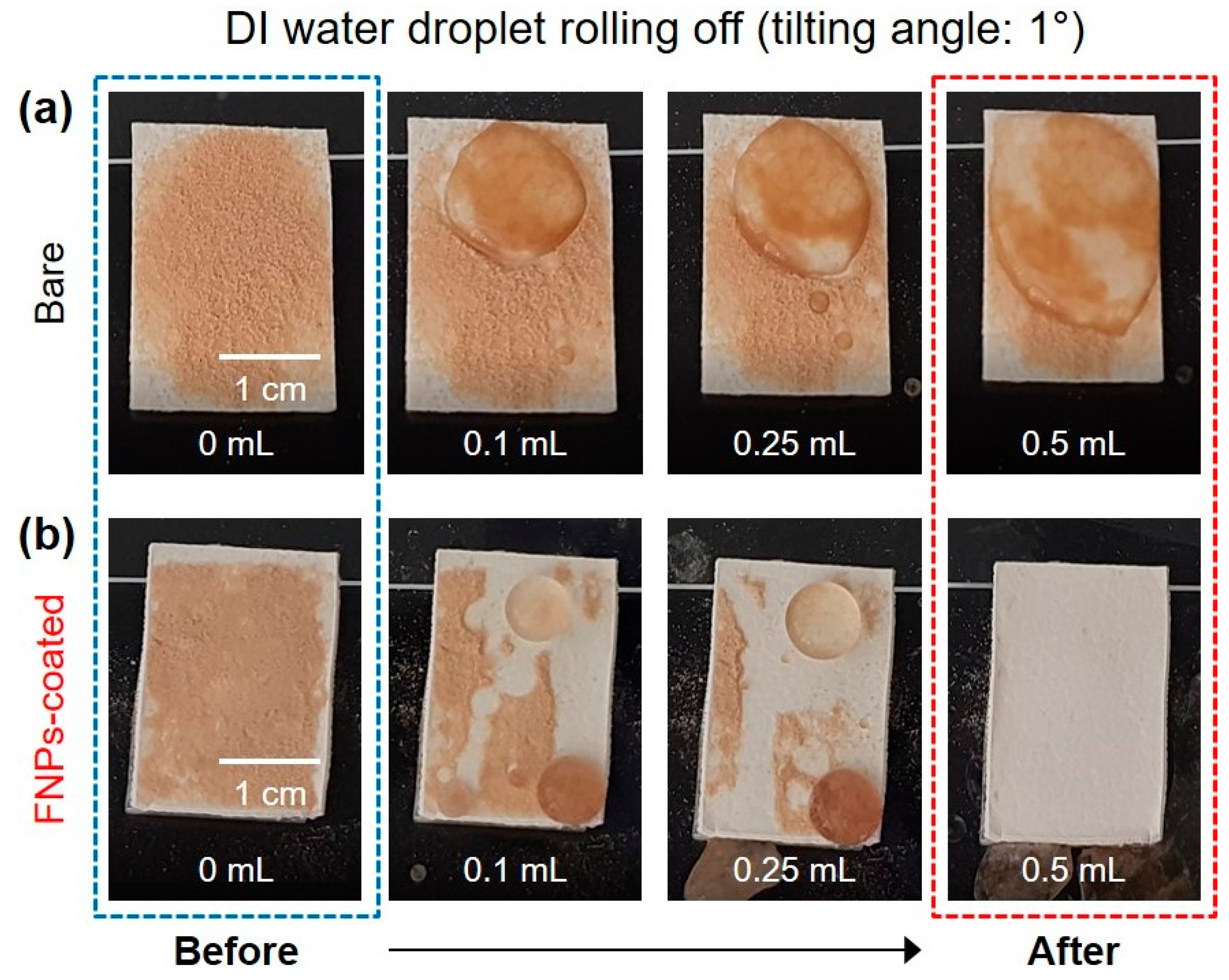
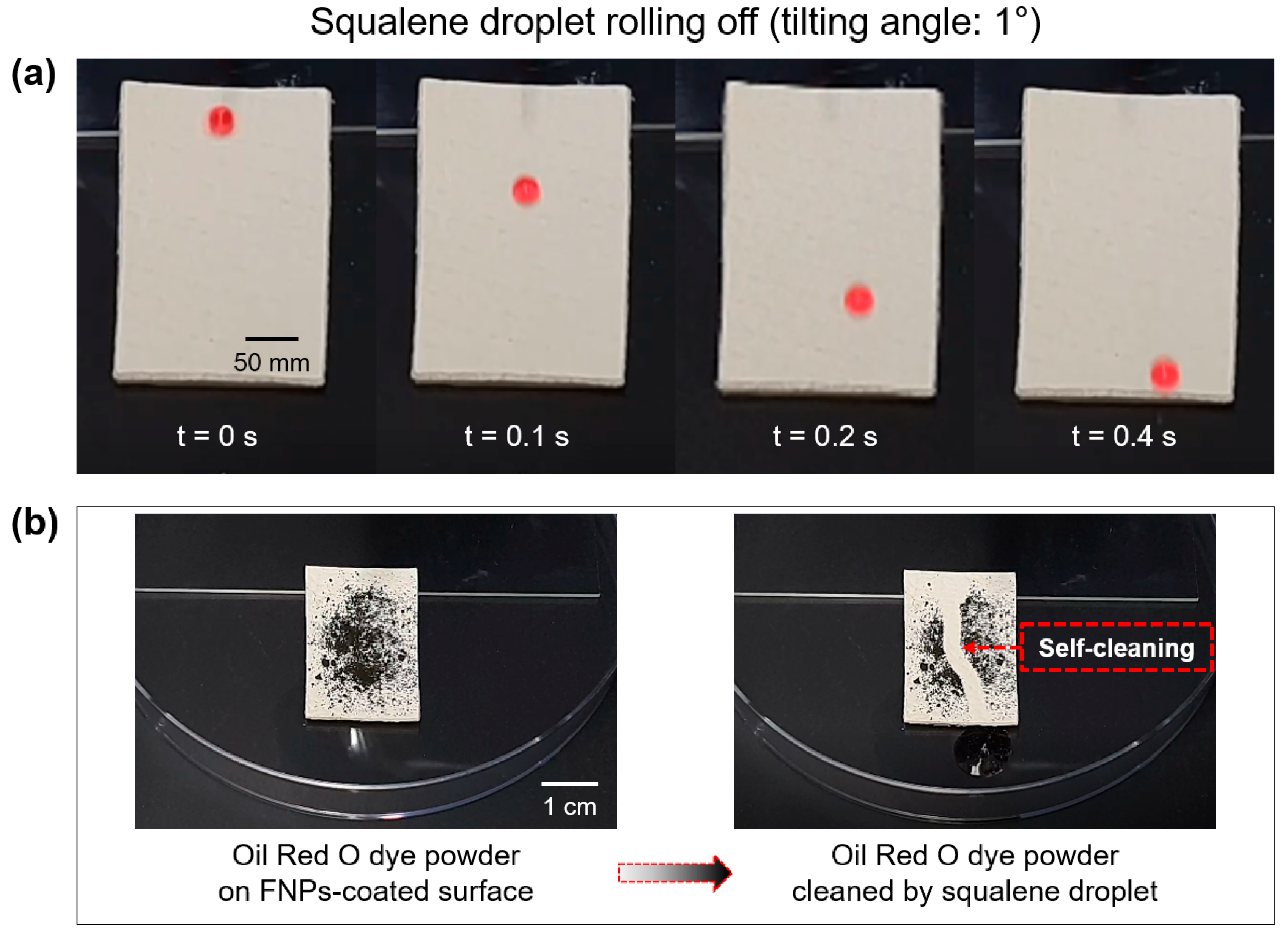
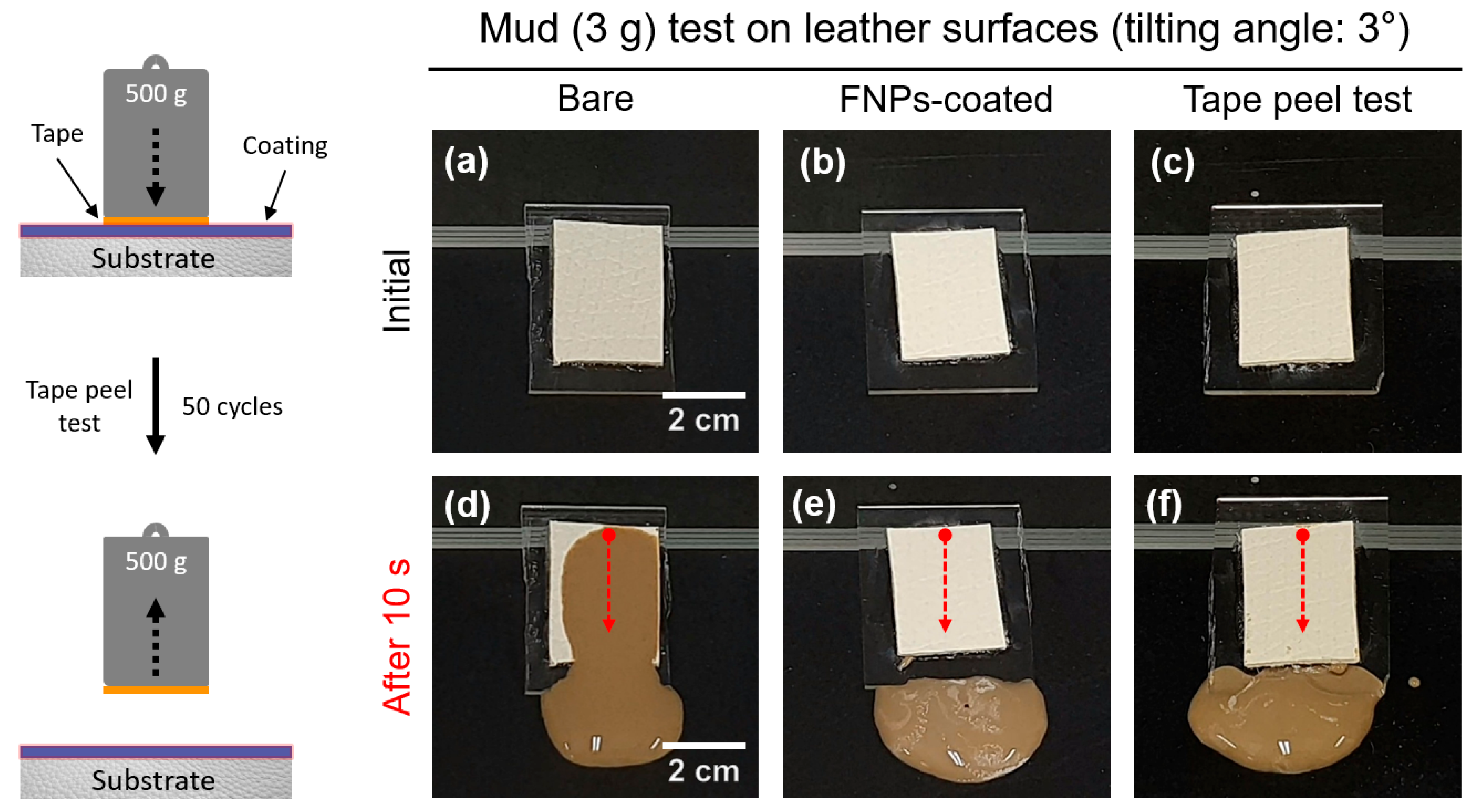
3.4. Self-Cleaning Properties of FNPs-Coated Leather Surfaces
3.5. Thermal Stability of Leather Surfaces
3.6. Mechanical Durability and Photostability of Leather Surfaces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurera, D.; Bhushan, B. Fabrication of Bioinspired Superliquiphobic Synthetic Leather with Self-Cleaning and Low Adhesion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 130–137. [Google Scholar] [CrossRef]
- Shi, Z.; Sheng, Y.; Wu, J.; Cui, J.; Lin, W.; Ngai, T. Porous Waterborne Polyurethane Films Templated from Pickering Foams for Fabrication of Synthetic Leather. Langmuir 2024, 40, 4751–4761. [Google Scholar] [CrossRef] [PubMed]
- Tian, S. Recent Advances in Functional Polyurethane and Its Application in Leather Manufacture: A Review. Polymers 2020, 12, 1996. [Google Scholar] [CrossRef] [PubMed]
- Khambhaty, Y.; Samidurai, S. An Insight into the Microbiome Associated with the Damage of Raw Animal Hide and Skin-Primarily Protein, during Leather Making. Int. J. Biol. Macromol. 2024, 264, 130640. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Oh, J.K.; Perez, K.; Zhou, W.; Wang, X.; Castillo, A.; Taylor, M.; Min, Y.; Cisneros-Zevallos, L.; Akbulut, M. Effect of Wax Chain Length on the Adhesion Dynamics and Interfacial Rigidity of Salmonella Typhimurium LT2. Surf. Interfaces 2024, 44, 103745. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and Superoleophobic Properties in Nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Guo, H.; Fuchs, P.; Casdorff, K.; Michen, B.; Chanana, M.; Hagendorfer, H.; Romanyuk, Y.E.; Burgert, I. Bio-inspired superhydrophobic and omniphobic wood surfaces. Adv. Mater. Interfaces 2017, 4, 1600289. [Google Scholar] [CrossRef]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and Self-Cleaning Properties of Artificial Superhydrophobic Surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Zhu, F.Y.; Han, M.D.; Sun, X.M.; Peng, X.H.; Zhang, H.X. Self-Cleaning Poly(Dimethylsiloxane) Film with Functional Micro/Nano Hierarchical Structures. Langmuir 2013, 29, 10769–10775. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, S.; DeFlorio, W.; Song, S.H.; Choi, H.; Cisneros-Zevallos, L.; Oh, J.K.; Akbulut, M.E.S. Nanostructured Antifouling Coatings for Galvanized Steel Food Storage and Container Surfaces to Enhance Hygiene and Corrosion Resistance against Bacterial, Fungal, and Mud Contamination. J. Food Eng. 2024, 363, 111784. [Google Scholar] [CrossRef]
- Kochkodan, V.; Johnson, D.J.; Hilal, N. Polymeric Membranes: Surface Modification for Minimizing (Bio)Colloidal Fouling. Adv. Colloid Interface Sci. 2014, 206, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, C.; Dong, Z.; Jiang, P.; Lü, Z.; Yu, S.; Gao, C. Improved Separation Performance and Durability of Polyamide Reverse Osmosis Membrane in Tertiary Treatment of Textile Effluent through Grafting Monomethoxy-Poly(Ethylene Glycol) Brushes. Sep. Purif. Technol. 2019, 209, 443–451. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Pan, G.; Shi, H.; Yan, H.; Xu, J.; Guo, M.; Wang, Z.; Liu, Y. Surface Modification of Polyamide Reverse Osmosis Membrane with Sulfonated Polyvinyl Alcohol for Antifouling. Appl. Surf. Sci. 2017, 419, 177–187. [Google Scholar] [CrossRef]
- Junter, G.A.; Thébault, P.; Lebrun, L. Polysaccharide-Based Antibiofilm Surfaces. Acta Biomater. 2016, 30, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, K.; Zhang, M.; Shen, L.; Zhou, G.; Ding, Y.; Xin, Q.; Luo, J.; Xie, J.; Li, J. Self-Adhesive, Strong Antifouling, and Mechanically Reinforced Methacrylate Hyaluronic Acid Cross-Linked Carboxybetaine Zwitterionic Hydrogels. Biomacromolecules 2024, 25, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Liu, S.; DeFlorio, W.; Hao, L.; Wang, X.; Salazar, K.S.; Taylor, M.; Castillo, A.; Cisneros-Zevallos, L.; Oh, J.K.; et al. Influence of Surface Roughness, Nanostructure, and Wetting on Bacterial Adhesion. Langmuir 2023, 39, 5426–5439. [Google Scholar] [CrossRef]
- Koch, K.; Bohn, H.F.; Barthlott, W. Hierarchically Sculptured Plant Surfaces and Superhydrophobicity. Langmuir 2009, 25, 14116–14120. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Wetting Behavior of Water and Oil Droplets in Three-Phase Interfaces for Hydrophobicity/Philicity and Oleophobicity/Philicity. Langmuir 2009, 25, 14165–14173. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Oh, J.K. Silica Nanoparticle-Infused Omniphobic Polyurethane Foam with Bacterial Anti-Adhesion and Antifouling Properties for Hygiene Purposes. Nanomaterials 2023, 13, 2035. [Google Scholar] [CrossRef] [PubMed]
- Fenero, M.; Knez, M.; Saric, I.; Petravic, M.; Grande, H.; Palenzuela, J. Omniphobic Etched Aluminum Surfaces with Anti-Icing Ability. Langmuir 2020, 36, 10916–10922. [Google Scholar] [CrossRef]
- Villegas, M.; Cetinic, Z.; Shakeri, A.; Didar, T.F. Fabricating Smooth PDMS Microfluidic Channels from Low-Resolution 3D Printed Molds Using an Omniphobic Lubricant-Infused Coating. Anal. Chim. Acta 2018, 1000, 248–255. [Google Scholar] [CrossRef]
- Rykaczewski, K.; Paxson, A.T.; Staymates, M.; Walker, M.L.; Sun, X.; Anand, S.; Srinivasan, S.; McKinley, G.H.; Chinn, J.; Scott, J.H.J.; et al. Dropwise Condensation of Low Surface Tension Fluids on Omniphobic Surfaces. Sci. Rep. 2014, 4, 4158. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wei, P.; Li, F.; Liu, L.; Zhao, X. Liquid-Assisted Strategy for Dual-Purpose Oil-Water Separation with Super-Omniphobic Mesh. Chem. Eng. J. 2023, 475, 146094. [Google Scholar] [CrossRef]
- Brown, P.S.; Bhushan, B. Bioinspired, Roughness-Induced, Water and Oil Super-Philic and Super-Phobic Coatings Prepared by Adaptable Layer-by-Layer Technique. Sci. Rep. 2015, 5, 14030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Park, D.S.; Choi, J.; Park, S.; Soper, S.A.; Murphy, M.C. Flexible-Templated Imprinting for Fluorine-Free, Omniphobic Plastics with Re-Entrant Structures. J. Colloid Interface Sci. 2021, 585, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Wang, Z.; Zhao, J.; Liang, Y.; Li, X.; Ren, L. Improved Dynamic Stability of Superomniphobic Surfaces and Droplet Transport on Slippery Surfaces by Dual-Scale Re-Entrant Structures. Chem. Eng. J. 2020, 394, 124871. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Huo, J.; Hou, X. Superoleophobic Surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef]
- Kim, H.; Han, H.; Lee, S.; Woo, J.; Seo, J.; Lee, T. Nonfluorinated Superomniphobic Surfaces through Shape-Tunable Mushroom-like Polymeric Micropillar Arrays. ACS Appl. Mater. Interfaces 2019, 11, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.N.H.; Hwang, H.S.; Lee, J.; Park, I. Synthesis of New Semi-Fluorinated Polysilazanes and Their Amphiphobic Coating Applications. Prog. Org. Coat. 2020, 148, 105853. [Google Scholar] [CrossRef]
- Liang, J.; Liu, K.; Wang, D.; Li, H.; Li, P.; Li, S.; Su, S.; Xu, S.; Luo, Y. Facile Fabrication of Superhydrophilic/Superhydrophobic Surface on Titanium Substrate by Single-Step Anodization and Fluorination. Appl. Surf. Sci. 2015, 338, 126–136. [Google Scholar] [CrossRef]
- Chu, D.; Singh, S.C.; Yong, J.; Zhan, Z.; Sun, X.; Duan, J.A.; Guo, C. Superamphiphobic Surfaces with Controllable Adhesion Fabricated by Femtosecond Laser Bessel Beam on PTFE. Adv. Mater. Interfaces 2019, 6, 1900550. [Google Scholar] [CrossRef]
- Meng, L.; Wang, L.; Wang, Z. Membrane Distillation with Electrospun Omniphobic Membrane for Treatment of Hypersaline Chemical Industry Wastewater. Desalination 2023, 564, 116782. [Google Scholar] [CrossRef]
- Koh, E.; Lee, Y.T. Preparation of an Omniphobic Nanofiber Membrane by the Self-Assembly of Hydrophobic Nanoparticles for Membrane Distillation. Sep. Purif. Technol. 2021, 259, 118134. [Google Scholar] [CrossRef]
- Kwon, J.; Jung, H.; Jung, H.; Lee, J. Micro/Nanostructured Coating for Cotton Textiles That Repel Oil, Water, and Chemical Warfare Agents. Polymers 2020, 12, 1826. [Google Scholar] [CrossRef]
- Abu Jarad, N.; Rachwalski, K.; Bayat, F.; Khan, S.; Shakeri, A.; MacLachlan, R.; Villegas, M.; Brown, E.D.; Soleymani, L.; Didar, T.F. An Omniphobic Spray Coating Created from Hierarchical Structures Prevents the Contamination of High-Touch Surfaces with Pathogens. Small 2023, 19, 2205761. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wu, Y.; Xu, R.; Li, Z.; Liu, Y.; Liu, J.; Cai, M.; Bu, W.; Zhou, F. Robust Hybrid Omniphobic Surface for Stain Resistance. ACS Appl. Mater. Interfaces 2021, 13, 14562–14568. [Google Scholar] [CrossRef]
- Wang, Y.; McCarthy, T.J. Dip-Coating Deposition on Chemically Patterned Surfaces: A Mechanistic Analysis and Comparison with Topographically Patterned Surfaces. Langmuir 2014, 30, 2419–2428. [Google Scholar] [CrossRef]
- Wu, S.; Altenried, S.; Zogg, A.; Zuber, F.; Maniura-Weber, K.; Ren, Q. Role of the Surface Nanoscale Roughness of Stainless Steel on Bacterial Adhesion and Microcolony Formation. ACS Omega 2018, 3, 6456–6464. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.S.; Sadasivuni, K.K.; Liu, S.; Xing, R. Recent Developments in Air-Trapped Superhydrophobic and Liquid-Infused Slippery Surfaces for Anti-Icing Application. Prog. Org. Coat. 2019, 137, 105373. [Google Scholar] [CrossRef]
- Brown, P.S.; Bhushan, B. Durable, Superoleophobic Polymer–Nanoparticle Composite Surfaces with Re-Entrant Geometry via Solvent-Induced Phase Transformation. Sci. Rep. 2016, 6, 21048. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, Q.; Liu, J.; Liu, X.; Zhou, F. Low Surface Energy Surfaces from Self-Assembly of Perfluoropolymer with Sticky Functional Groups. J. Colloid Interface Sci. 2010, 351, 261–266. [Google Scholar] [CrossRef]
- Tsibouklis, J.; Nevell, T.G. Ultra-Low Surface Energy Polymers: The Molecular Design Requirements. Adv. Mater. 2003, 15, 647–650. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The Influence of Surface Chemistry on the Kinetics and Thermodynamics of Bacterial Adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef]
- Demeester, K.E.; Liang, H.; Jensen, M.R.; Jones, Z.S.; D’Ambrosio, E.A.; Scinto, S.L.; Zhou, J.; Grimes, C.L. Synthesis of Functionalized N-Acetyl Muramic Acids to Probe Bacterial Cell Wall Recycling and Biosynthesis. J. Am. Chem. Soc. 2018, 140, 9458–9465. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Tan, L.P.; Pisharath, S.; Hng, H.H. Nanothermite Composites with a Novel Cast Curable Fluoropolymer. Chem. Eng. J. 2021, 414, 128786. [Google Scholar] [CrossRef]
- Lee, S.G.; Ham, D.S.; Lee, D.Y.; Bong, H.; Cho, K. Transparent superhydrophobic/translucent superamphiphobic coatings based on silica–fluoropolymer hybrid nanoparticles. Langmuir 2013, 29, 15051–15057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ao, J.; Zhang, J.; Gao, J.; Hao, L.; Jiang, R.; Zhang, Z.; Liu, Z.; Zhao, J.; Ren, L. Bioinspired Superhydrophobic Surfaces, Inhibiting or Promoting Microbial Contamination? Mater. Today 2023, 67, 468–494. [Google Scholar] [CrossRef]
- Giacomello, A.; Meloni, S.; Chinappi, M.; Casciola, C.M. Cassie-Baxter and Wenzel States on a Nanostructured Surface: Phase Diagram, Metastabilities, and Transition Mechanism by Atomistic Free Energy Calculations. Langmuir 2012, 28, 10764–10772. [Google Scholar] [CrossRef]
- Hongru, A.; Xiangqin, L.; Shuyan, S.; Ying, Z.; Tianqing, L. Measurement of Wenzel Roughness Factor by Laser Scanning Confocal Microscopy. RSC Adv. 2017, 7, 7052–7059. [Google Scholar] [CrossRef]
- Lloyd, B.P.; Bartlett, P.N.; Wood, R.J.K. Wetting of Surfaces Made of Hydrophobic Cavities. Langmuir 2015, 31, 9325–9330. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.M.L.; Amirfazli, A.; Elliott, J.A.W. Modeling and Measurement of Contact Angle Hysteresis on Textured High-Contact-Angle Surfaces. J. Phys. Chem. C 2014, 118, 18554–18563. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [PubMed]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Lu, X.; Min, Y.; Cisneros-Zevallos, L.; Akbulut, M. Bacterially Antiadhesive, Optically Transparent Surfaces Inspired from Rice Leaves. ACS Appl. Mater. Interfaces 2015, 7, 19274–19281. [Google Scholar] [CrossRef] [PubMed]
- van Loosdrecht, M.C.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A.J. The Role of Bacterial Cell Wall Hydrophobicity in Adhesion. Appl. Environ. Microbiol. 1987, 53, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Higaki, Y.; Otsuka, H.; Takahara, A. Perfluoropolyether-Infused Nano-Texture: A Versatile Approach to Omniphobic Coatings with Low Hysteresis and High Transparency. Chem. Commun. 2013, 49, 597–599. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic Acid: A Crosslinker Leading to Versatile Functional Polymeric Networks: A Review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhao, X.; Wang, W.; Gong, X. Durable Self-Cleaning Surfaces with Superhydrophobic and Highly Oleophobic Properties. Langmuir 2019, 35, 8404–8412. [Google Scholar] [CrossRef]
- Mountfort, K.A.; Bronstein, H.; Archer, N.; Jickells, S.M. Identification of Oxidation Products of Squalene in Solution and in Latent Fingerprints by ESI-MS and LC/APCI-MS. Anal. Chem. 2007, 79, 2650–2657. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Y.; Chen, W.L.; Hong, R.J. Preparation, Durability and Thermostability of Hydrophobic Antireflective Coatings for Solar Glass Covers. Sol. Energy 2015, 118, 222–231. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-Icing Performance of Superhydrophobic Surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Kim, A.; Lee, C.; Kim, J. Durable, Scalable, and Tunable Omniphobicity on Stainless Steel Mesh for Separation of Low Surface Tension Liquid Mixtures. Surf. Coatings Technol. 2018, 344, 394–401. [Google Scholar] [CrossRef]
- Mondal, P.; Arunachalam, S. Finite Element Modelling of Car Seat with Hyperelastic and Viscoelastic Foam Material Properties to Assess Vertical Vibration in Terms of Acceleration. Engineering 2020, 12, 177–193. [Google Scholar] [CrossRef]
- Dall Agnol, L.; Dias, F.T.G.; Ornaghi, H.L.; Sangermano, M.; Bianchi, O. UV-Curable Waterborne Polyurethane Coatings: A State-of-the-Art and Recent Advances Review. Prog. Org. Coatings 2021, 154, 106156. [Google Scholar] [CrossRef]
| Sample | Liquid | UV Exposure Time | CAwater (θ, before UV) | CAoil (θ, after UV) |
|---|---|---|---|---|
| FNPs-coated leather | Water | 1 h | 160.2 ± 0.3° | 158.3 ± 0.6° |
| FNPs-coated leather | Oil | 1 h | 152.1 ± 0.5° | 151.7 ± 0.7° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Oh, J.K. Durable, Photostable Omniphobic Synthetic Leather Surfaces with Anti-Biofouling Properties for Hygienic Applications. Polymers 2024, 16, 1983. https://doi.org/10.3390/polym16141983
Lee H, Oh JK. Durable, Photostable Omniphobic Synthetic Leather Surfaces with Anti-Biofouling Properties for Hygienic Applications. Polymers. 2024; 16(14):1983. https://doi.org/10.3390/polym16141983
Chicago/Turabian StyleLee, Hanna, and Jun Kyun Oh. 2024. "Durable, Photostable Omniphobic Synthetic Leather Surfaces with Anti-Biofouling Properties for Hygienic Applications" Polymers 16, no. 14: 1983. https://doi.org/10.3390/polym16141983
APA StyleLee, H., & Oh, J. K. (2024). Durable, Photostable Omniphobic Synthetic Leather Surfaces with Anti-Biofouling Properties for Hygienic Applications. Polymers, 16(14), 1983. https://doi.org/10.3390/polym16141983







