Cast Extruded Films Based on Polyhydroxyalkanoate/Poly(lactic acid) Blend with Herbal Extracts Hybridized with Zinc Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Additives
2.3. Film Preparation
2.4. Characterization of the Additives and the Biopolymer Films
3. Results
3.1. Results of Studies on the Active Additives
3.1.1. FTIR-ATR of the Additives
3.1.2. TGA Results
3.2. Results of Film Characterization
3.2.1. Mechanical Tests
3.2.2. SEM Results
3.2.3. Thermal Characterization
3.2.4. Barrier Properties
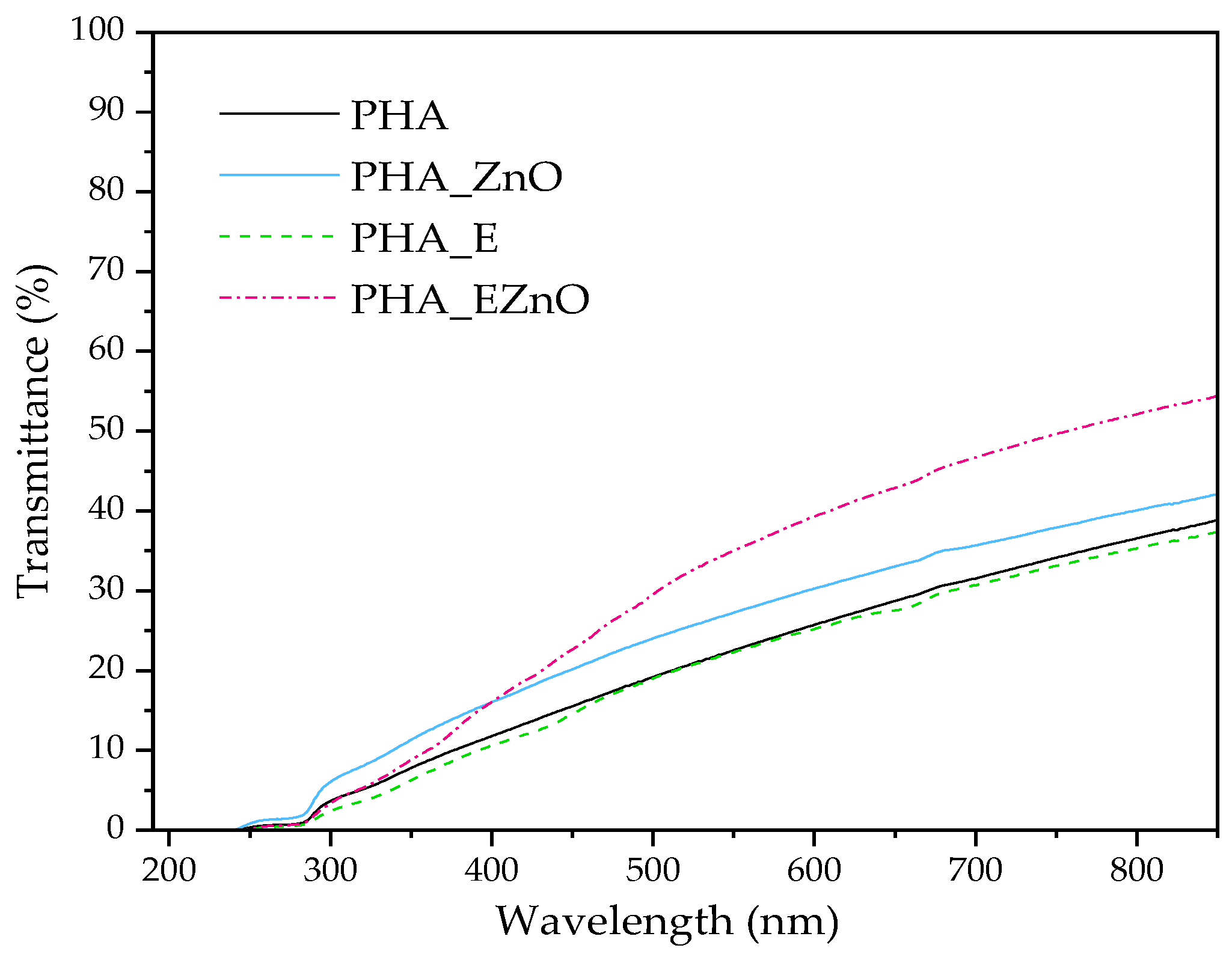
3.2.5. Optical Properties
3.3. Antimicrobial Activity of the PHA Films with Active Agents in the Biopolymer Matrix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://environment.ec.europa.eu/topics/plastics/single-use-plastics_en (accessed on 20 May 2024).
- Available online: https://eur-lex.europa.eu/eli/dir/2019/904/oj (accessed on 20 May 2024).
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.-Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From Production to Nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef]
- Kumar, V.; Rutika, S.; Reena, G. Blends and composites of polyhydroxyalkanoates (PHAs) and their applications. Eur. Polym. J. 2020, 161, 110824. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene Blends Intended for Biodegradable Food Packaging Applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Mangeon, C.; Michely, L.; Rios De Anda, A.; Thevenieau, F.; Renard, E.; Langlois, V. Natural Terpenes Used as Plasticizers for Poly(3-Hydroxybutyrate). ACS Sustain. Chem. Eng. 2018, 6, 16160–16168. [Google Scholar] [CrossRef]
- Dastan, S.D. Chemical and functional composition and biological activities of Anatolian Hypericum scabrum L. plant. J. Mol. Struct. 2023, 1275, 134561. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Rahnama, G.H.; Malekpoor, F.; Broujeni, H.R. Variation in antibacterial activity and phenolic content of Hypericum scabrum L. populations. J. Med. Plants Res. 2011, 5, 4119. [Google Scholar]
- Gilca, M.; Gaman, L.; Panait, E.; Stoian, I.; Atanasiu, V. Chelidonium majus—An integrative review: Traditional knowledge versus modern findings. Forsch. Komplementmed. 2010, 17, 241. [Google Scholar] [CrossRef]
- Terzic, M.; Fayez, S.; Fahmy, N.M.; Eldahshan, O.A.; Uba, A.I.; Ponniya, S.K.M.; Selvi, S.; Koyuncu, S.N.I.; Yüksekdağ, Ö.; Zengin, G. Chemical characterization of three different extracts obtained from Chelidonium majus L. (Greater celandine) with insights into their in vitro, in silico and network pharmacological properties. Fitoterapia 2024, 174, 105835. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Wang, H.; Nile, A.; Lin, X.; Dong, H.; Venkidasamy, B.; Sieniawska, E.; Enkhtaivan, G.; Kai, G. Comparative analysis of metabolic variations, antioxidant potential and cytotoxic effects in different parts of Chelidonium majus L. Food Chem. Toxicol. 2021, 156, 112483. [Google Scholar] [CrossRef] [PubMed]
- Kőszegi, K.; Végvári, G.Y.; Stefanovits-Bányai, É.; Békássy-Molnár, E.; Maráz, A. Influence of the harvesting seasons on the polyphenol composition and antimicrobial activity of stinging nettle (Urtica dioica L.) extracts. Acta Aliment. 2023, 52, 589–600. [Google Scholar] [CrossRef]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): A review. Heliyon 2022, 8, 09717. [Google Scholar] [CrossRef]
- Đurović, S.; Kojić, I.; Radić, D.; Smyatskaya, Y.A.; Bazarnova, J.G.; Filip, S.; Tosti, T. Chemical Constituents of Stinging Nettle (Urtica dioica L.): A Comprehensive Review on Phenolic and Polyphenolic Compounds and Their Bioactivity. Int. J. Mol. Sci. 2024, 25, 3430. [Google Scholar] [CrossRef]
- Mohammadian, M.; Biregani, Z.M.; Hassanloofard, Z.; Salami, M. Nettle (Urtica dioica L.) as a functional bioactive food ingredient: Applications in food products and edible films, characterization, and encapsulation systems. Trends Food Sci. Technol. 2024, 147, 104421. [Google Scholar] [CrossRef]
- Ordon, M.; Burdajewicz, W.; Sternal, J.; Okręglicki, M.; Mizielińska, M. The Antibacterial Effect of the Films Coated with the Layers Based on Uncaria tomentosa and Formitopsis betulina Extracts and ZnO Nanoparticles and Their Influence on the Secondary Shelf-Life of Sliced Cooked Ham. Appl. Sci. 2023, 13, 8853. [Google Scholar] [CrossRef]
- Mizielińska, M.; Zdanowicz, M.; Tarnowiecka-Kuca, A.; Bartkowiak, A. The Influence of Functional Composite Coatings on the Properties of Polyester Films before and after Accelerated UV Aging. Materials 2024, 17, 3048. [Google Scholar] [CrossRef]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Julianti, E.; Safitri, A.; Jaafar, M. Smart Packaging Based on Polylactic Acid: The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical–Mechanical Properties, Colony Reduction, Perishable Food Shelf Life, and Future Prospective. Polymers 2023, 15, 4103. [Google Scholar] [CrossRef]
- La Fuente Arias, C.I.; González-Martínez, C.; Chiralt, A. Active Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) Films Containing Phenolic Compounds with Different Molecular Structures. Polymers 2024, 16, 1574. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Biodegradable Polyester Blends Containing Multifunctional Substances of Plant Origin. Arch. Mater. Sci. Eng. 2023, 119, 5–11. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Ordon, M.; Nawrotek, P.; Stachurska, X.; Schmidt, A.; Mizielińska, M. Mixtures of Scutellaria baicalensis and Glycyrrhiza L. Extracts as Antibacterial and Antiviral Agents in Active Coatings. Coatings 2021, 11, 1438. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction—An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2007, 1, 1. [Google Scholar]
- Ordon, M.; Zdanowicz, M.; Nawrotek, P.; Stachurska, X.; Mizielińska, M. Polyethylene Films Containing Plant Extracts in the Polymer Matrix as Antibacterial and Antiviral Materials. Int. J. Mol. Sci. 2021, 22, 13438. [Google Scholar] [CrossRef]
- PN-EN ISO 11357-6:2018; Part 6: Determination of Oxidation Induction Time (Isothermal OIT) and Oxidation Induction Temperature (Dynamic OIT). Plastics—Differential Scanning Calorimetry (DSC). ISO: Geneva, Switzerland, 2018.
- ASTM D3985; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM F 1249-01; Standard Test Method for Water Vapor Transmission Rate through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM E 2180-01:2002; Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) in Polymeric or Hydrophobic Materials. ASTM: West Conshohocken, PA, USA, 2002.
- Bhetwal, A.; Maharjan, A.; Shakya, S.; Satyal, D.; Ghimire, S.; Khanal, P.R.; Parajuli, N.P. Isolation of Potential Phages against Multidrug-Resistant Bacterial Isolates: Promising Agents in the Rivers of Kathmandu, Nepal. BioMed Res. Int. 2017, 2017, 3723254. [Google Scholar] [CrossRef]
- Bonilla, N.; Rojas, M.J.; Cruz, G.N.F.; Hung, S.H.; Rohwer, F.; Barr, J.J. Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. Peer J. 2016, 4, 2261. [Google Scholar] [CrossRef]
- Skaradzińska, A.; Ochocka, M.; Śliwka, P.; Kuźmińska-Bajora, M.; Skaradziński, G.; Friese, A.; Roschanski, N.; Murugaiyan, J.; Roesler, U. Bacteriophage amplification–A comparison of selected methods. J. Virol. Methods 2020, 282, 113856. [Google Scholar] [CrossRef]
- ISO 22196-2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. Copy Purchased on 09.01.2021. ISO: Geneva, Switzerland, 2011.
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Efenberger-Szmechtyk, M.; Strzelec, K. Antioxidant and Anti–Aging Activity of Freeze–Dried Alcohol–Water Extracts from Common Nettle (Urtica Dioica L.) and Peppermint (Mentha Piperita L.) in Elastomer Vulcanizates. Polymers 2022, 14, 1460. [Google Scholar] [CrossRef]
- Elsayed, N.; Hasanin, M.S.; Abdelraof, M. Utilization of Olive Leaves Extract Coating Incorporated with Zinc/Selenium Oxide Nanocomposite to Improve the Postharvest Quality of Green Beans Pods. Bioact. Carbohydr. Dietary Fibre 2022, 28, 100333. [Google Scholar] [CrossRef]
- Mascia, L.; Kouparitsas, Y.; Nocita, D.; Bao, X. Antiplasticization of Polymer Materials: Structural Aspects and Effects on Mechanical and Diffusion-Controlled Properties. Polymers 2020, 12, 769. [Google Scholar] [CrossRef]
- Berrabah, I.; Dehouche, N.; Kaci, M.; Bruzaud, S.; Deguines, C.H.; Delaite, C. Effect of ZnO Nanoparticles on Tensile and Viscoelastic Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Bionanocomposites. Macromol. Symp. 2022, 405, 2100273. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Bas Gil, N.J.; González-Martínez, C.; Chiralt, A. Antioxidant Poly (Lactic Acid) Films with Rice Straw Extract for Food Packaging Applications. Food Pack. Shelf Life 2022, 34, 101003. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Schrader, J.A.; Gowrishankar, S.; Kunwei, L.; McCabe, K.G.; Grewell, D.; Graves, W.R.; Kessler, M.R. Biodegradation behavior of bacterial-based polyhydroxyalkanoate (PHA) and DDGS composites. Green. Chem. 2014, 16, 1911–1920. [Google Scholar] [CrossRef]
- Shamala, T.R.; Divyashree, M.S.; Davis, R.; Kumari, K.S.L.; Vijayendra, S.V.N.; Raj, B. Production and characterization of bacterial polyhydroxyalkanoate copolymers and evaluation of their blends by fourier transform infrared spectroscopy and scanning electron microscopy. Indian J. Microbiol. 2019, 49, 251–258. [Google Scholar] [CrossRef]
- Mania, S.; Cieślik, M.; Konzorski, M.; Święcikowski, P.; Nelson, A.; Banach, A.; Tylingo, R. The Synergistic Microbiological Effects of Industrial Produced Packaging Polyethylene Films Incorporated with Zinc Nanoparticles. Polymers 2020, 12, 1198. [Google Scholar] [CrossRef]
- Dai, L.; Li, R.; Liang, Y.; Liu, Y.; Zhang, W.; Shi, S. Development of Pomegranate Peel Extract and Nano ZnO Co-Reinforced Polylactic Acid Film for Active Food Packaging. Membranes 2022, 12, 1108. [Google Scholar] [CrossRef]
- Zembouai, I.; Kaci, M.; Bruzaud, S.; Benhamida, A.; Corre, Y.-M.; Grohens, Y. A Study of Morphological, Thermal, Rheological and Barrier Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Polylactide Blends Prepared by Melt Mixing. Polym. Test. 2013, 32, 842–851. [Google Scholar] [CrossRef]
- D’anna, A.; Arrigo, R.; Frache, A. PLA/PHB Blends: Biocompatibilizer Effects. Polymers 2019, 11, 1416. [Google Scholar] [CrossRef]
- Genovesi, A.; Aversa, C.; Barletta, M. Polyhydroxyalkanoates-Based Cast Film as Bio-Based Packaging for Fresh Fruit and Vegetables: Manufacturing and Characterization. J. Polym. Environ. 2023, 31, 4522–4532. [Google Scholar] [CrossRef]
- Baran, A.; Vrábel, P.; Olčák, D.; Chodák, I. Solid State 13C-NMR Study of a Plasticized PLA/PHB Polymer Blend. J. Appl. Pol. Sci. 2018, 135, 46296. [Google Scholar] [CrossRef]
- Pérez Amaro, L.; Cicogna, F.; Passaglia, E.; Morici, E.; Oberhauser, W.; Al-Malaika, S.; Dintcheva, N.T.; Coiai, S. Thermo-Oxidative Stabilization of Poly(Lactic Acid) with Antioxidant Intercalated Layered Double Hydroxides. Polym. Degrad. Stab. 2016, 133, 92–100. [Google Scholar] [CrossRef]
- Yao, J.; Luo, F.; Mao, J.; Li, Y.; Sun, X.; Ma, D.; Luo, C.; Li, L. Effects of Crystal Planes of ZnO Nanocrystal on Crystalline, Thermal and Thermal-Oxidation Stability of iPP. J. Polym. Res. 2021, 28, 172. [Google Scholar] [CrossRef]
- Plota-Pietrzak, A.; Masek, A. Functionalized Metal Oxide Particles with Antioxidant as New Carriers Providing Higher Stability of Polyolefin Products. Sustain. Mater. Technol. 2024, 40, e00885. [Google Scholar] [CrossRef]
- Anžlovar, A.; Kržan, A.; Žagar, E. Degradation of PLA/ZnO and PHBV/ZnO Composites Prepared by Melt Processing. Arab. J. Chem. 2018, 11, 343–352. [Google Scholar] [CrossRef]
- Yaptenco, K.F.; Kim, J.G.; Lim, B.S. Gas transmission rates of commercially available polyethylene and polypropylene films for modified atmosphere packaging. Philipp. Agricult. Sci. 2007, 90, 22. [Google Scholar]
- Paszkiewicz, S.; Kwiatkowska, M.; Rosłaniec, Z.; Szymczyk, A.; Jotko, M.; Lisiecki, S. The Influence of Different Shaped Nanofillers (1D, 2D) on Barrier and Mechanical Properties of Polymer Hybrid Nanocomposites Based on PET Prepared by in Situ Polymerization. Polym. Comp. 2016, 37, 1949–1959. [Google Scholar] [CrossRef]
- Wróbei, D.; Zandvoort, M.A.M.J.V.; Lettinga, P.; Ginkel, G.V.; Levine, Y.K. Chlorophylls in polymers. II. pheophytin α in polymers and the influence of stretching on the state of chlorophylls in anhydrous polymer films. Photochem. Photobiol. 1995, 62, 290–298. [Google Scholar] [CrossRef]
- Kang, Y.-R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, Characterization, and Functional Properties of Chlorophylls, Pheophytins, and Zn-Pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Mizielińska, M.; Kowalska, U.; Jarosz, M.; Sumińska, P.; Landercy, N.; Duquesne, E. The Effect of UV Aging on Antimicrobial and Mechanical Properties of PLA Films with Incorporated Zinc Oxide Nanoparticles. Int. J. Environ. Res. Public Health 2018, 15, 794. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; Thamer, B.M.; Abdulhameed, M.M.; El-Newehy, M.H. Fabrication of biodegradable and antibacterial films of chitosan/polyvinylpyrrolidone containing Eucalyptus citriodora extracts. Int. J. Biol. Macromol. 2024, 266, 131001. [Google Scholar] [CrossRef]
- Ordon, M.; Nawrotek, P.; Stachurska, X.; Mizielińska, M. Polyethylene Films Coated with Antibacterial and Antiviral Layers Based on CO2 Extracts of Raspberry Seeds, of Pomegranate Seeds and of Rosemary. Coatings 2021, 11, 1179. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Hosseini, M. A new active nanocomposite film based on PLA/ZnO nanoparticle/essential oils for the preservation of refrigerated Otolithes ruber fillets. Food Packag. Shelf Life 2019, 19, 94–103. [Google Scholar] [CrossRef]
- Pan, M.; Carol, L.; Lednicky, J.A.; Eiguren-Fernandez, A.; Hering, S.; Fan, Z.H.; Wu, C.Y. Determination of the distribution of infectious viruses in aerosol particles using water-based condensational growth technology and a bacteriophage MS2 model. Aerosol Sci. Technol. 2019, 53, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, N.; Toulouse, M.J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of Five Bacteriophages as Models for Viral Aerosol Studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef]
- Prussin, A.J., II; Schwake, D.O.; Lin, K.; Gallagher, D.L.; Buttling, L.; Marr, L.C. Survival of the Enveloped Virus Phi6 in Droplets as a Function of Relative Humidity, Absolute Humidity and Temperature. Appl. Environ. Microbiol. 2018, 84, e00551-18. [Google Scholar] [CrossRef]
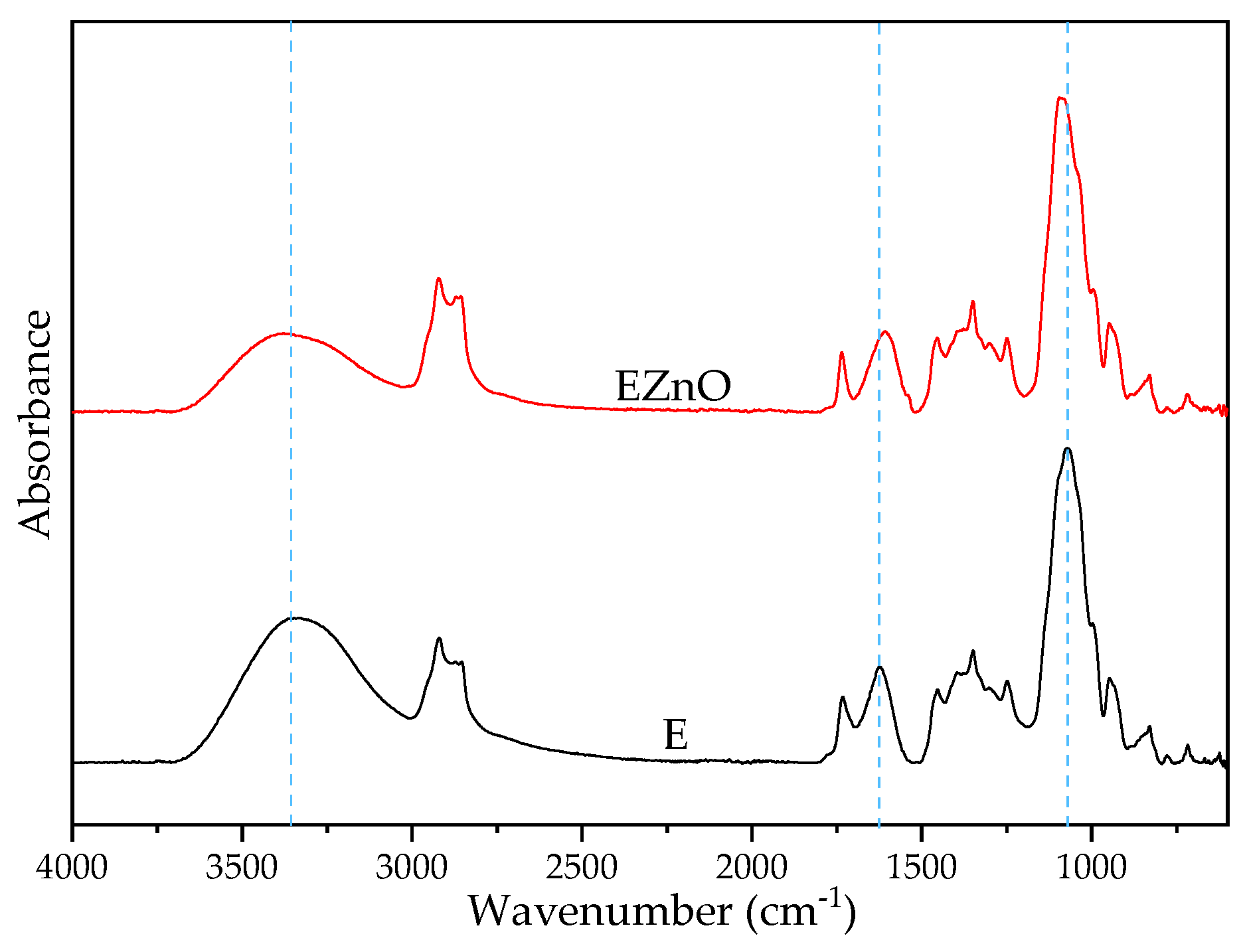
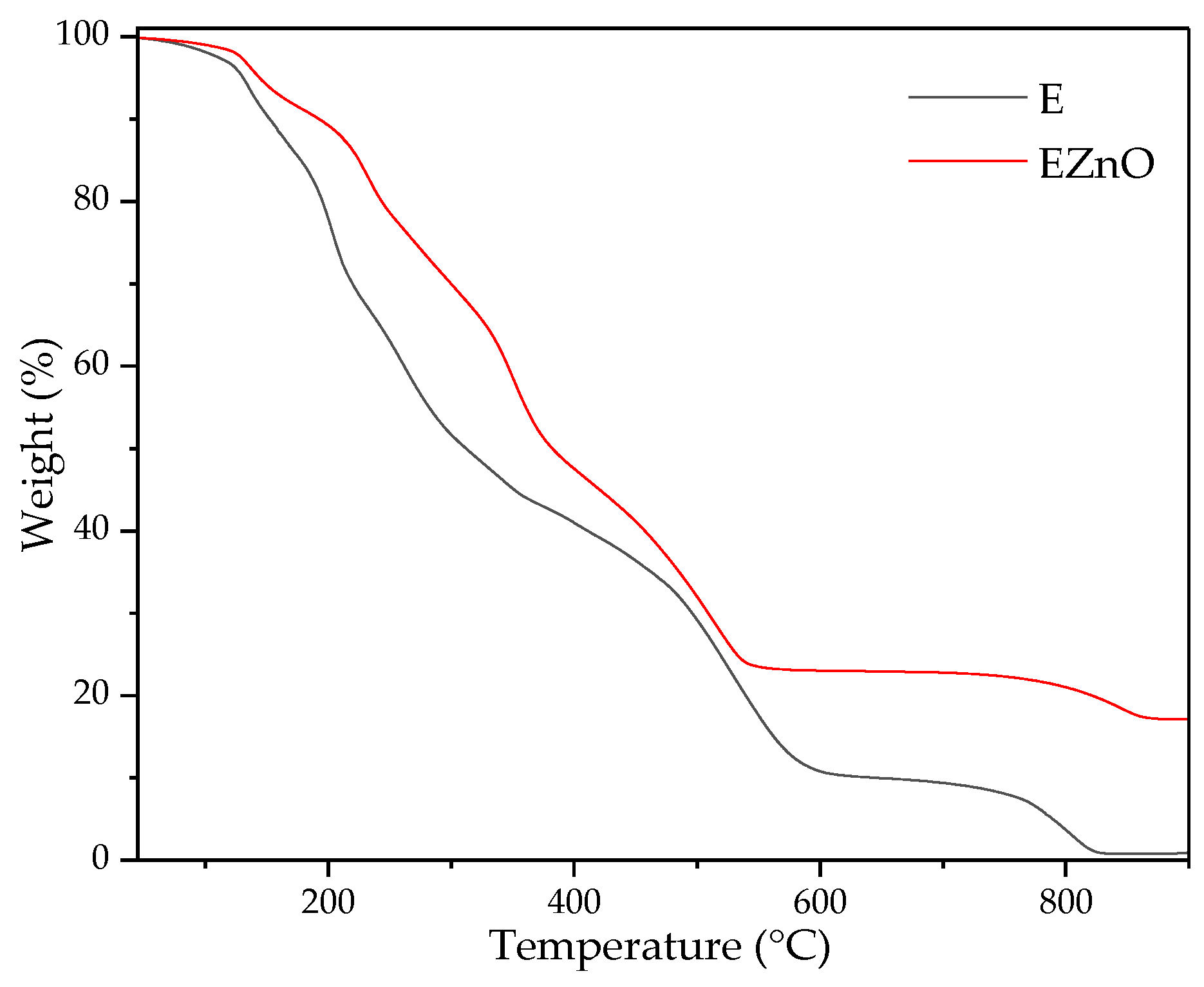





| Components | ZnO | E | EZnO |
|---|---|---|---|
| Atmer 110 | 1.33 | 1.33 | 1.33 |
| Extract mixture * | - | 1 | 1 |
| ZnO | 0.04 | - | 0.04 |
| Sample Acronym | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Thickness (µm) |
|---|---|---|---|---|
| PHA | 846 (±79.7) a | 20.6 (±1.39) b | 4.2 (±0.28) a | 157 |
| PHA-ZnO | 965 (±98.1) a | 20.6 (±2.18) b | 5.2 (±1.18) a | 130 |
| PHA-E | 939 (±52.9) a | 20.9 (±1.23) b | 4.6 (±0.57) a | 156 |
| PHA-EZnO | 982 (±74.1) a | 24.3 (±2.08) a | 4.7 (±0.16) a | 150 |
| Sample | Time to Oxidation Peak (min) | End Set (min) |
|---|---|---|
| PHA | 2.62 | 13.4 |
| PHA-ZnO | 3.36 | 15.7 |
| PHA-E | 2.53 | 12.9 |
| PHA-EZnO | 6.5 | 14.8 |
| Sample Acronym | OTR RH 0% (cm3/m2/24 h) | WVTR RH 100% (g/m2/24 h) | WVTR RH 90% * (g/m2/24 h) |
|---|---|---|---|
| PHA | 70.7 (±7.97) | 55.9 (±0.84) | 50.3 (±0.78) |
| PHA-ZnO | 60.7 (±3.81) | 55.9 (±0.85) | 51.3 (±2.53) |
| PHA-E | 72.7 (±9.15) | 53.6 (±1.23) | 48.2 (±0.13) |
| PHA-EZnO | 57.4 (±0.69) | 49.4 (±4.60) | 44.4 (±4.10) |
| Sample Acronym | L* | a* | b* | C* | YI | ∆E | T (700 nm) |
|---|---|---|---|---|---|---|---|
| PHA | 98.2 (±0.06) | 0.17 (±0.02) | 1.92 (±0.20) | 1.95 | 2.82 | - | 32% |
| PHA-ZnO | 97.8 (±0.15) | 0.10 (±0.04) | 1.50 (±0.26) | 1.50 | 2.19 | 0.44 | 36% |
| PHA-E | 94.6 (±0.28) | −5.70 (±0.35) | 16.32 (±0.93) | 17.29 | 24.64 | 15.86 | 31% |
| PHA-E stored | 94.8 (±0.23) | −2.87 (±0.30) | 13.33 (±1.28) | 13.65 | 20.09 | 12.16 | - |
| PHA-EZnO | 92.1 (±0.03) | −7.40 (±0.38) | 21.43 (±1.13) | 22.60 | 33.24 | 21.67 | 47% |
| PHA-EZnO stored | 92.1 (±0.15) | −1.92 (±0.30) | 14.72 (±1.09) | 14.84 | 22.83 | 14.15 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdanowicz, M.; Mizielińska, M.; Kowalczyk, A. Cast Extruded Films Based on Polyhydroxyalkanoate/Poly(lactic acid) Blend with Herbal Extracts Hybridized with Zinc Oxide. Polymers 2024, 16, 1954. https://doi.org/10.3390/polym16141954
Zdanowicz M, Mizielińska M, Kowalczyk A. Cast Extruded Films Based on Polyhydroxyalkanoate/Poly(lactic acid) Blend with Herbal Extracts Hybridized with Zinc Oxide. Polymers. 2024; 16(14):1954. https://doi.org/10.3390/polym16141954
Chicago/Turabian StyleZdanowicz, Magdalena, Małgorzata Mizielińska, and Agnieszka Kowalczyk. 2024. "Cast Extruded Films Based on Polyhydroxyalkanoate/Poly(lactic acid) Blend with Herbal Extracts Hybridized with Zinc Oxide" Polymers 16, no. 14: 1954. https://doi.org/10.3390/polym16141954
APA StyleZdanowicz, M., Mizielińska, M., & Kowalczyk, A. (2024). Cast Extruded Films Based on Polyhydroxyalkanoate/Poly(lactic acid) Blend with Herbal Extracts Hybridized with Zinc Oxide. Polymers, 16(14), 1954. https://doi.org/10.3390/polym16141954









