Synthesis and Physicochemical Properties of Thermally Sensitive Polymeric Derivatives of N-vinylcaprolactam
Abstract
1. Introduction
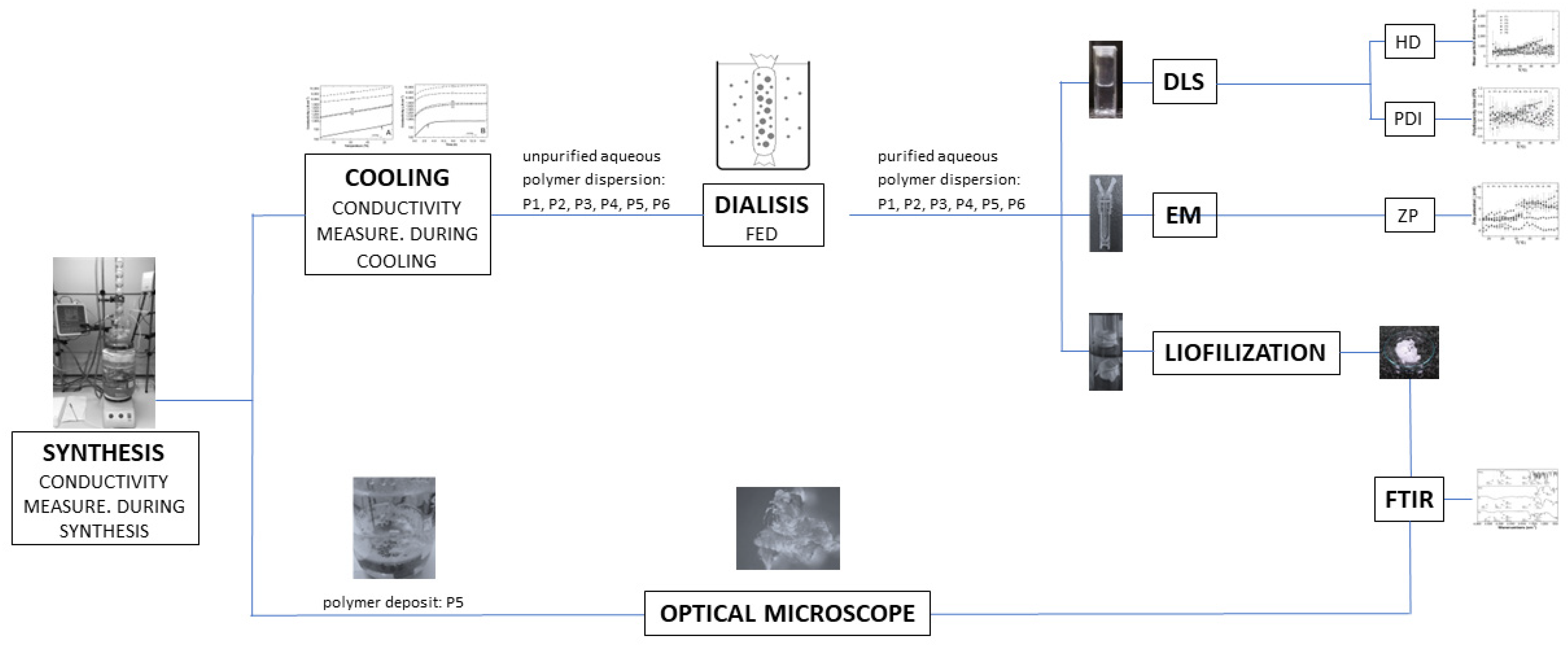
2. Materials and Methods
2.1. Materials
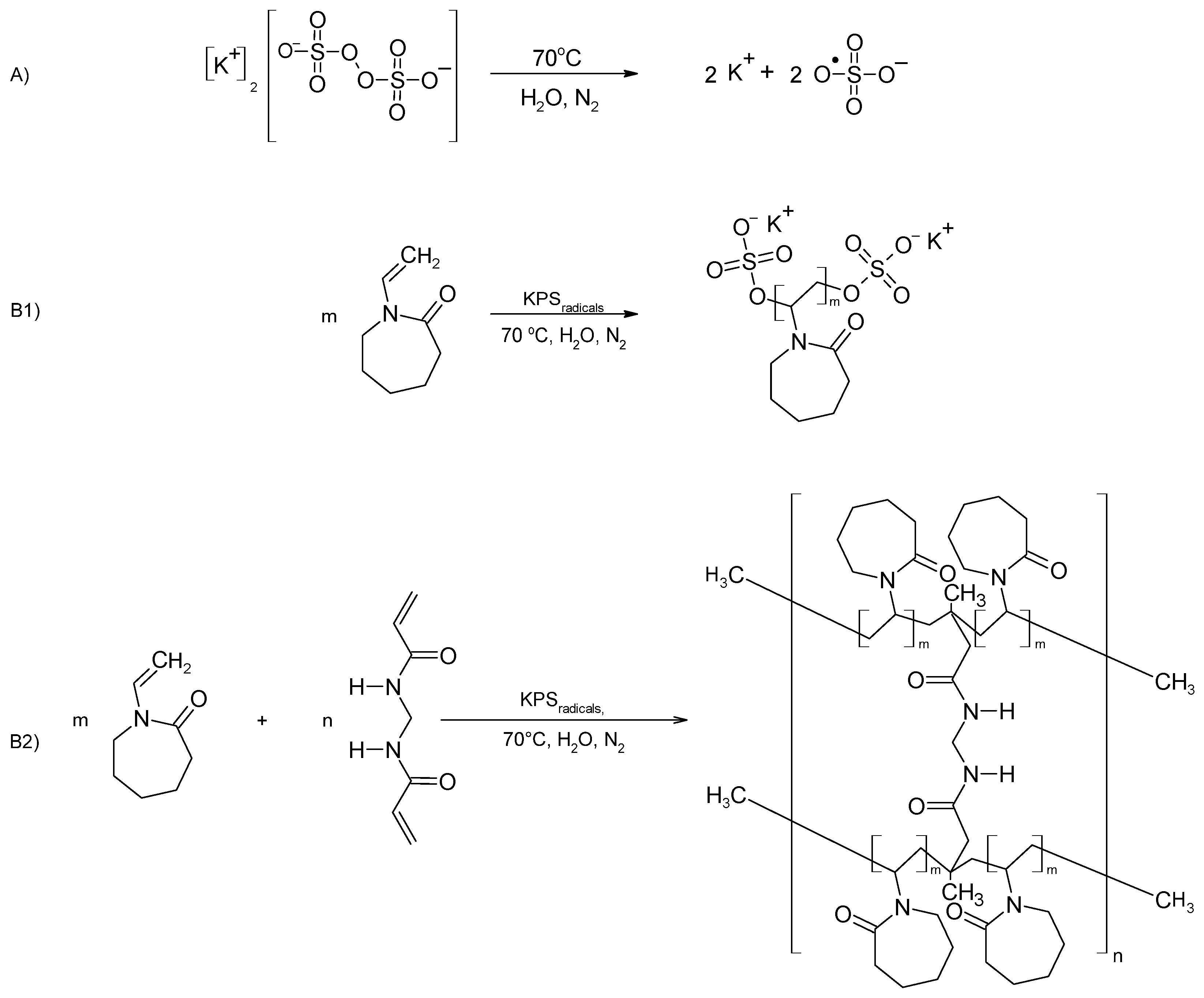
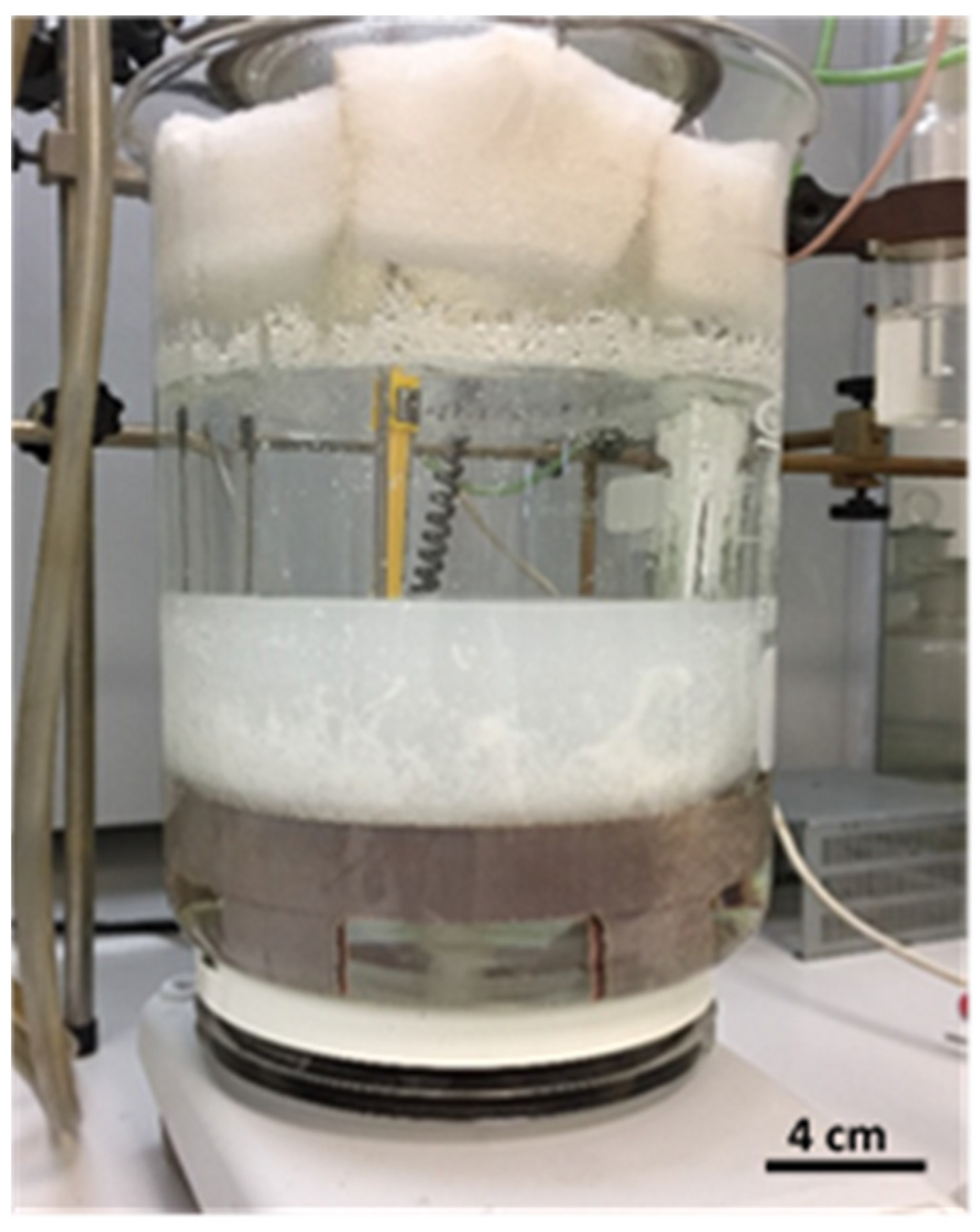
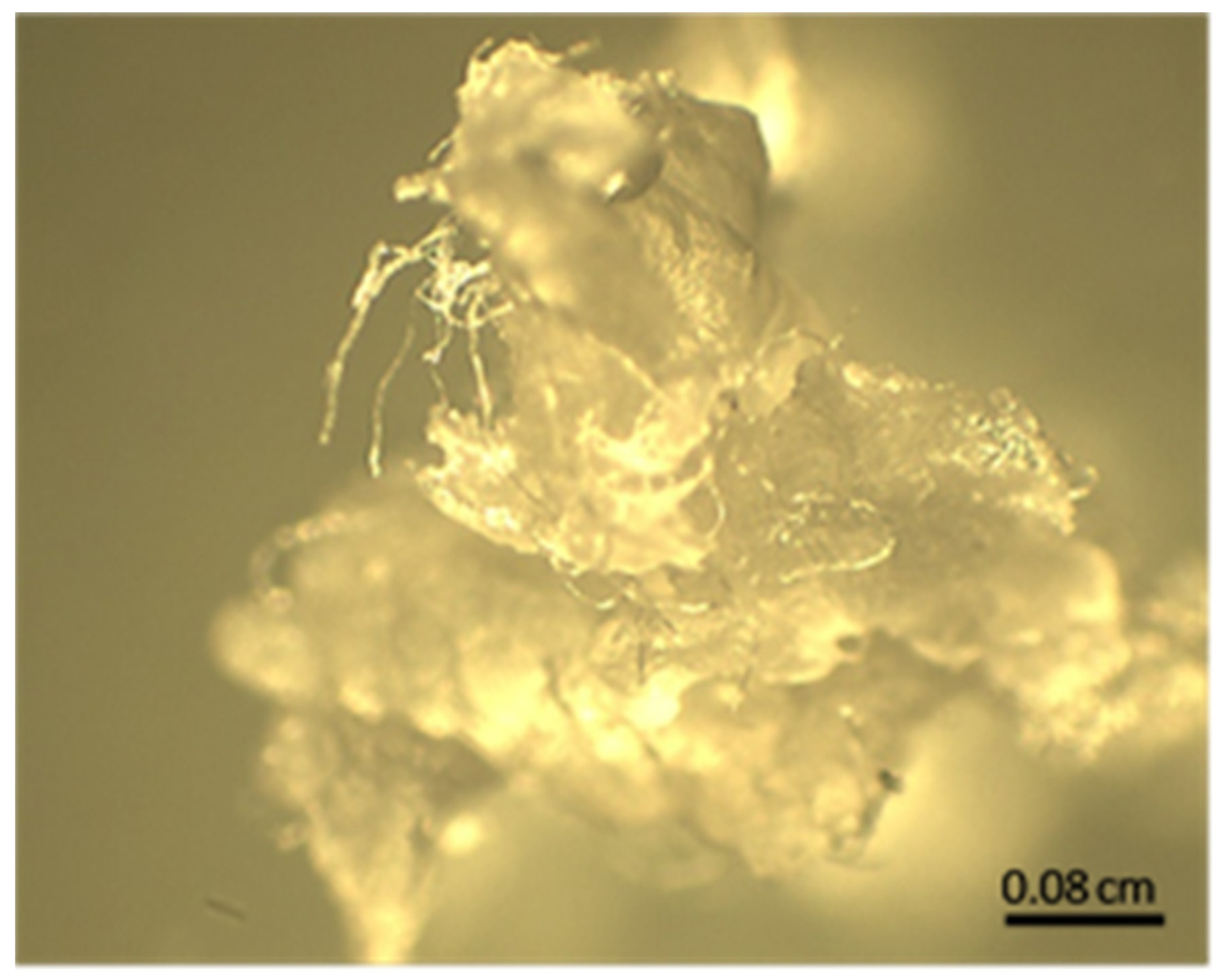
2.2. Synthesis
2.3. Conductivity Measurements
2.4. pH Measurements
2.5. Attenuated Total Reflection Fourier-Transformed Infrared Spectroscopy Measurements (ATR-FTIR)
2.6. Hydrodynamic Diameter (HD) and Polydispersity Index (PDI) Measurements
2.7. Zeta Potential (ZP) Measurements
3. Results
3.1. Synthesis
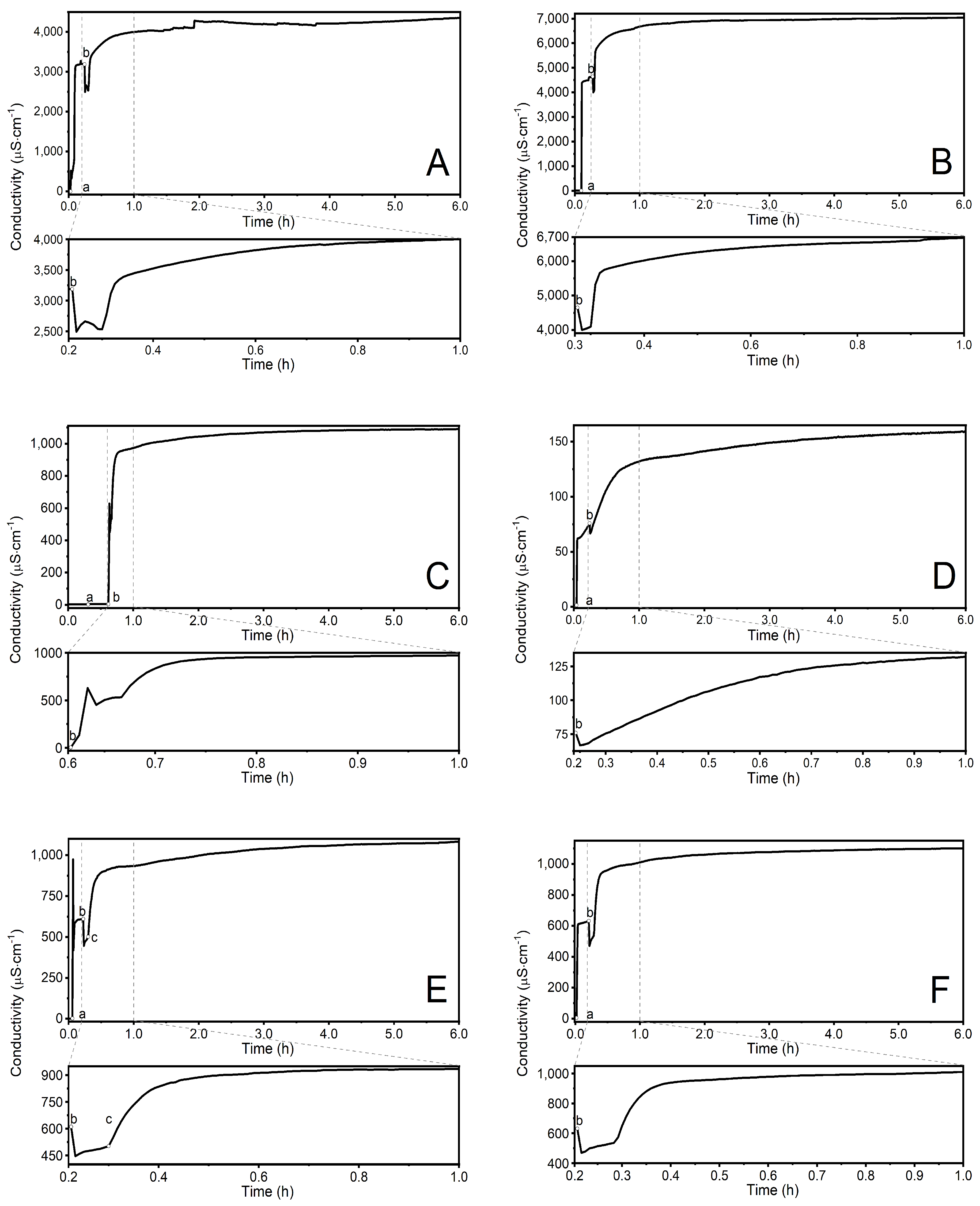
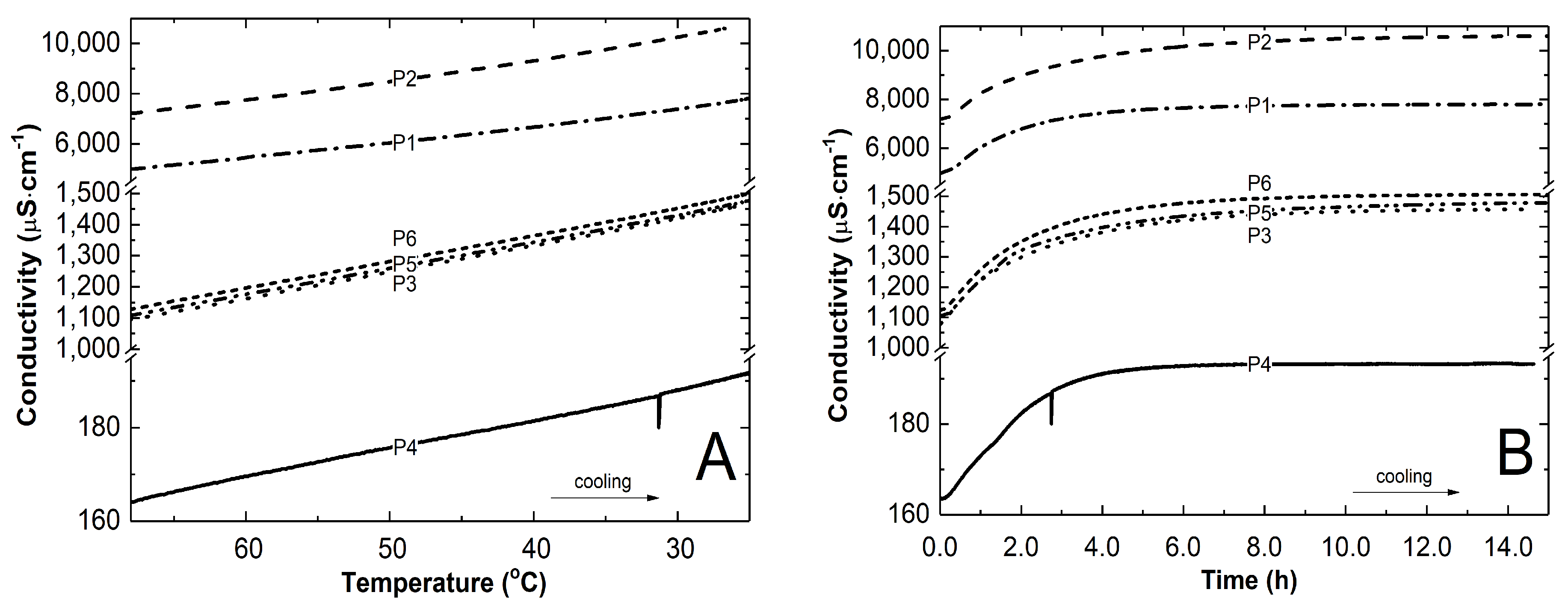
3.2. Conductivity Measurements
3.3. pH Measurements
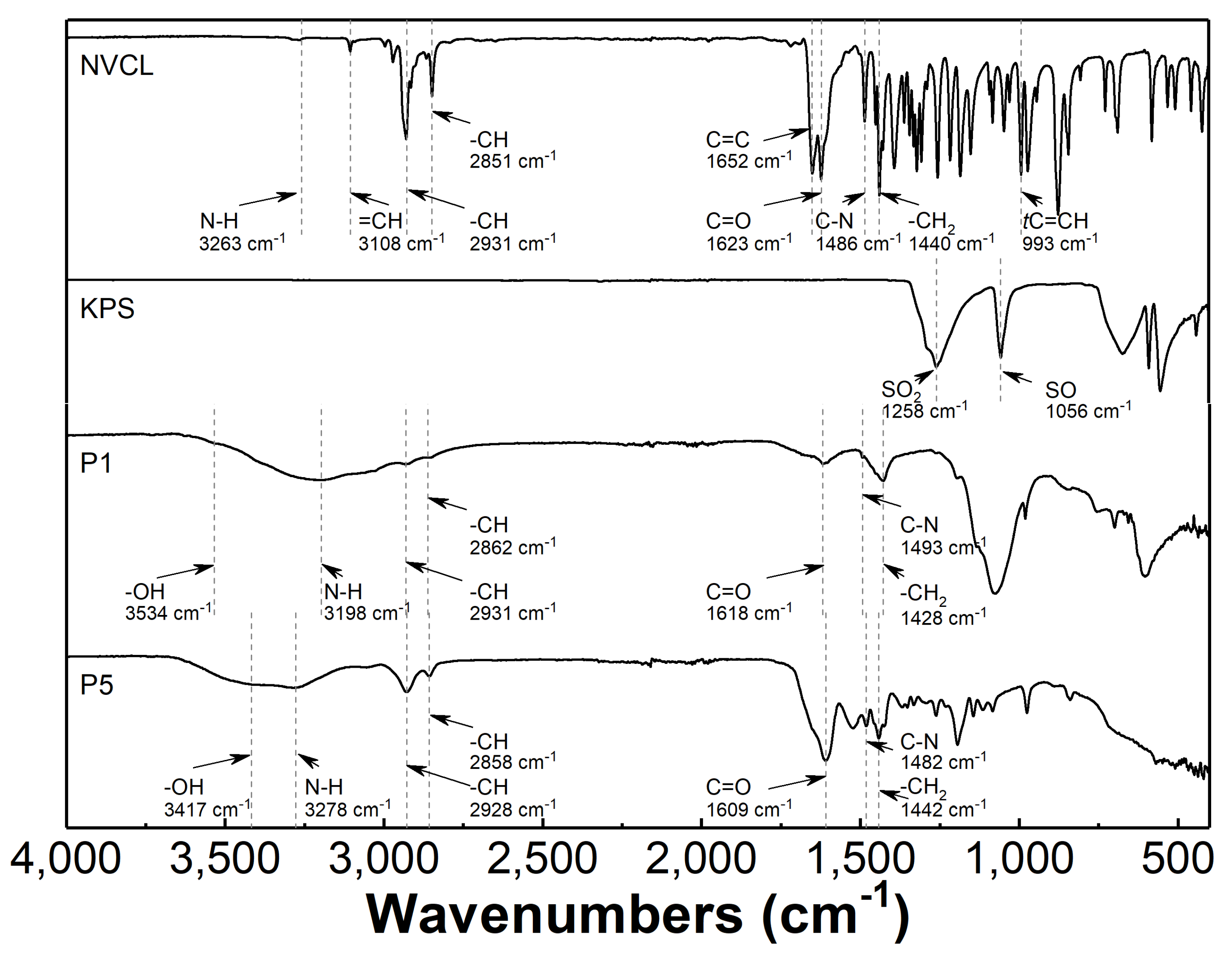
3.4. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy Analysis (ATR-FTIR)
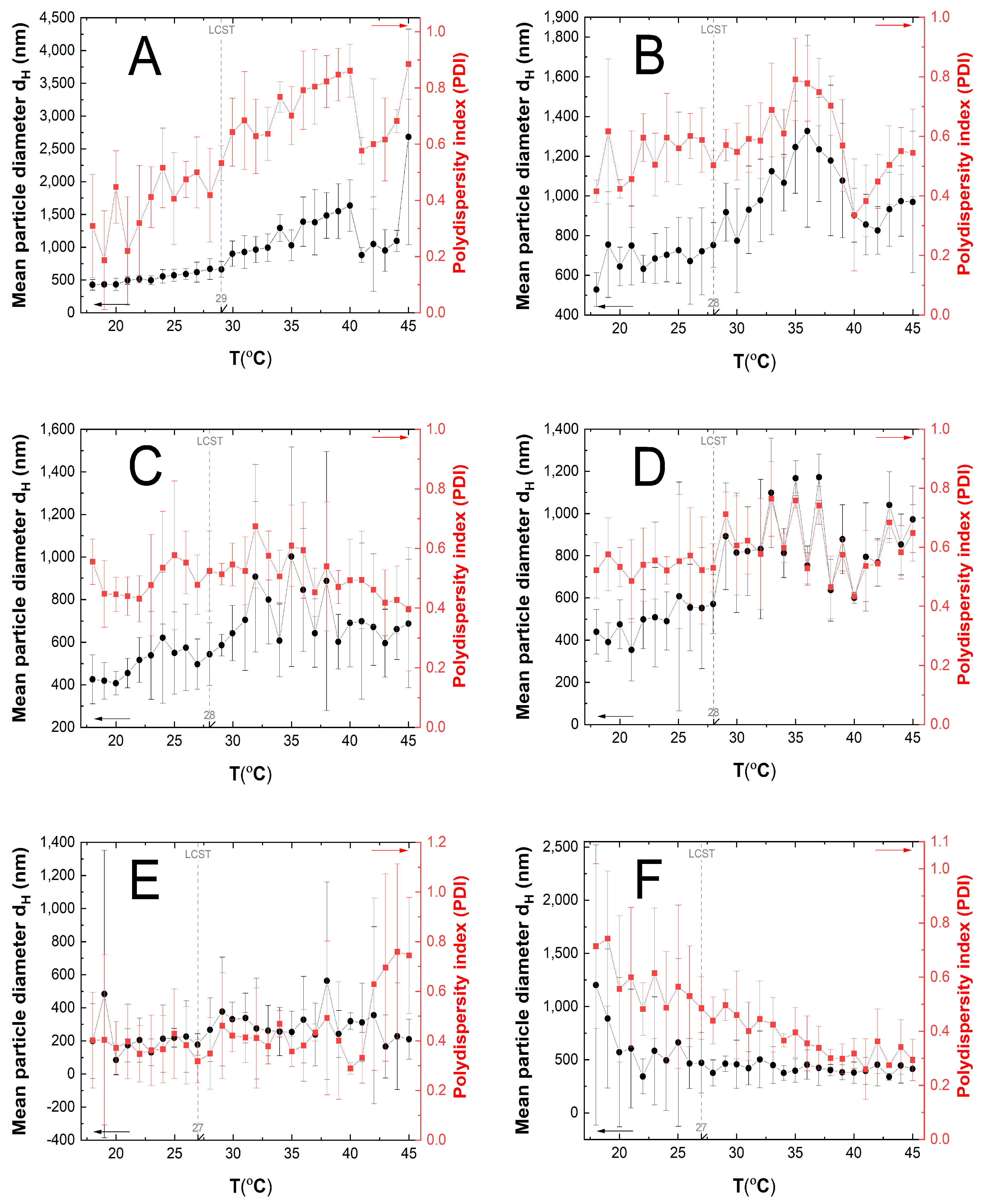
3.5. Hydrodynamic Diameter (HD) and Polydispersity Index (PDI) Measurements
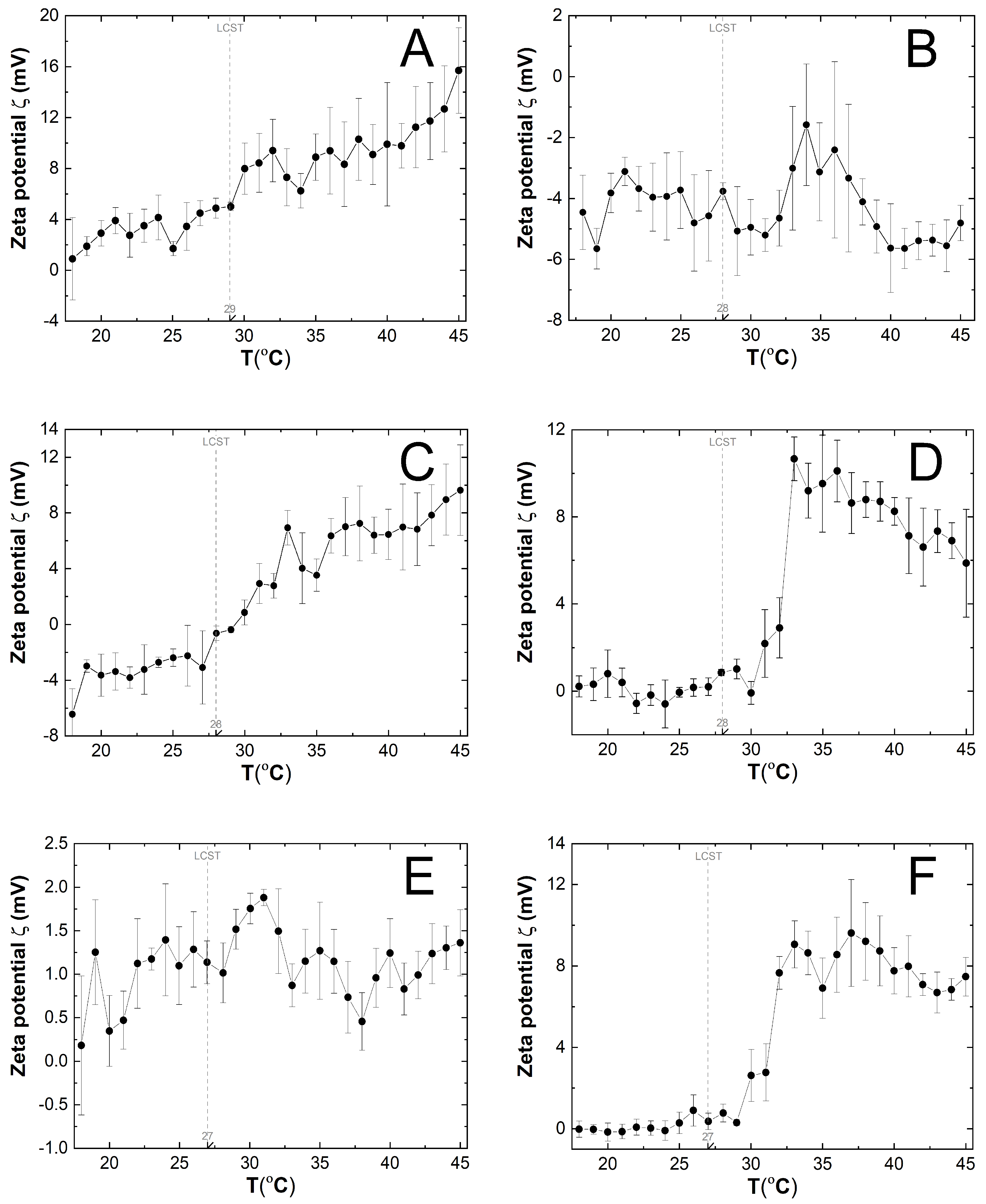
3.6. Zeta Potential (ZP) Measurements
4. Discussion
4.1. Synthesis
4.2. Conductivity
4.3. pH
4.4. ATR-FTIR
4.5. HD and PDI
4.6. ZP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abuwatfa, W.H.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive Polymers and Thermo-Responsive Liposomal Drug Delivery Systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef] [PubMed]
- Shakibania, S.; Ghazanfari, L.; Raeeszadeh-Sarmazdeh, M.; Khakbiz, M. Medical application of biomimetic 4D printing. Drug Dev. Ind. Pharm. 2021, 47, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Shirsath, N.; Singh, A.; Joshi, K.S.; Banerjee, R. Endogenous lung surfactant inspired pH responsive nanovesicle aerosols: Pulmonary compatible and site-specific drug delivery in lung metastases. Sci. Rep. 2014, 4, 7085. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Thakur, B.; Kumar, A.; Sharma, S.; Naushad, M.; Stadler, F.J. Atrazine removal using chitin-cl-poly(acrylamide-co-itaconic acid) nanohydrogel: Isotherms and pH responsive nature. Carbohydr. Polym. 2020, 241, 116258. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef] [PubMed]
- Torabiardekani, N.; Karami, F.; Khorram, M.; Zare, A.; Kamkar, M.; Zomorodian, K.; Zareshahrabadi, Z. Encapsulation of Zataria multiflora essential oil in polyvinyl alcohol/chitosan/gelatin thermo-responsive hydrogel: Synthesis, physico-chemical properties, and biological investigations. Int. J. Biol. Macromol. 2023, 243, 125073. [Google Scholar] [CrossRef]
- Yang, S.; Sarkar, S.; Xie, X.; Li, D.; Chen, J. Application of Optical Hydrogels in Environmental Sensing. Energy Environ. Mater. 2024, 7, e12646. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Kollarigowda, R.H.; Mathews, A.S.; Abraham, S. Super Mechanical Stimuli Responsive Hydrogel: Dynamic Cues for Cell Applications. ACS App. Bio Mater. 2019, 2, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Frachini, E.C.G.; Petri, D.F.S. Magneto-responsive hydrogels: Preparation, characterization, biotechnological and environmental applications. J. Braz. Chem. Soc. 2019, 30, 2010–2028. [Google Scholar] [CrossRef]
- Said, S.S.; Campbell, S.; Hoare, T. Externally Addressable Smart Drug Delivery Vehicles: Current Technologies and Future Directions. Chem. Mater. 2019, 31, 4971–4989. [Google Scholar] [CrossRef]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications—A Review. ACS Appl. Bio Mater. 2021, 4, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G. Stimuli-responsive polymeric nanocarriers for drug. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Liu, G.; Lovell, J.F.; Zhang, L.; Zhang, Y. Stimulus-responsive nanomedicines for disease diagnosis and treatment. Int. J. Mol. Sci. 2020, 21, 6380. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.A.; Lipson, J.E.G. LCST and UCST behavior in polymer solutions and blends. Polymer 2012, 53, 536–545. [Google Scholar] [CrossRef]
- Xia, M.; Cheng, Y.; Meng, Z.; Jiang, X.; Chen, Z.; Theato, P.; Zhu, M. A novel nanocomposite hydrogel with precisely tunable UCST and LCST. Macromol. Rapid Commun. 2015, 36, 477–482. [Google Scholar] [CrossRef]
- Augé, A.; Zhao, Y. What determines the volume transition temperature of UCST acrylamide-acrylonitrile hydrogels? RSC Adv. 2016, 6, 70616–70623. [Google Scholar] [CrossRef]
- Audureau, N.; Coumes, F.; Rieger, J.; Stoffelbach, F. Poly(N -cyanoethylacrylamide), a new thermoresponsive homopolymer presenting both LCST and UCST behavior in water. Polym. Chem. 2022, 13, 1075–1083. [Google Scholar] [CrossRef]
- Li, L.; Guo, J.; Kang, C. LCST-UCST Transition Property of a Novel Retarding Swelling and Thermosensitive Particle Gel. Materials 2023, 16, 2761. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Stimuli-responsive water-soluble and amphiphilic (Co)polymers. ACS Symp. Ser. 2001, 780, 1–13. [Google Scholar] [CrossRef]
- Çaykara, T.; Turan, E. Effect of the amount and type of the crosslinker on the swelling behavior of temperature-sensitive poly(N-tert-butylacrylamide-co-acrylamide) hydrogels. Colloid. Polym. Sci. 2006, 284, 1038. [Google Scholar] [CrossRef]
- Thérien-Aubin, H.; Wu, Z.L.; Nie, Z.; Kumacheva, E. Multiple shape transformations of composite hydrogel sheets. J. Am. Chem. Soc. 2013, 135, 4834–4839. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.A.; Maikawa, C.L.; Lopez Hernandez, H.; Appel, E.A. Controlling properties of thermogels by tuning critical solution behaviour of ternary copolymers. Polym. Chem. 2021, 12, 1918–1923. [Google Scholar] [CrossRef]
- Ni, C.; Zhu, X.X. Synthesis and swelling behavior of thermosensitive hydrogels based on N-substituted acrylamides and sodium acrylate. Eur. Polym. J. 2004, 40, 1075–1080. [Google Scholar] [CrossRef]
- Save, N.S.; Jassal, M.; Agrawal, A.K. Stimuli sensitive copolymer poly (N-tert-butylacrylamide-ran-acrylamide): Processing into thin films and their transitional behaviour. Polymer 2003, 44, 7979–7988. [Google Scholar] [CrossRef]
- Gosecki, M.; Ziemczonek, P.; Maczugowska, P.; Czaderna-Lekka, A.; Kozanecki, M.; Gosecka, M. The influence of 2-acrylamidephenylboronic acid on the phase behaviour of its copolymers with N -isopropylacrylamide in aqueous solution. Polym. Chem. 2021, 12, 3264–3275. [Google Scholar] [CrossRef]
- García-Peñas, A.; Biswas, C.S.; Liang, W.; Wang, Y.; Yang, P.; Stadler, F.J. Effect of Hydrophobic Interactions on Lower Critical Solution Temperature for. Polymers 2019, 11, 991. [Google Scholar] [CrossRef]
- Lee, B.; Jiao, A.; Yu, S.; You, J.B.; Kim, D.H.; Im, S.G. Initiated chemical vapor deposition of thermoresponsive poly (N-vinylcaprolactam) thin films for cell sheet engineering. Acta Biomater. 2013, 9, 7691–7698. [Google Scholar] [CrossRef]
- Roy, A.; Manna, K.; Pal, S. Recent advances in various stimuli-responsive hydrogels: From synthetic designs to emerging healthcare applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Anis, A.; Kim, D.; Pal, K. Environment sensitive hydrogels for drug delivery applications. Eur. Polym. J. 2019, 120, 109220. [Google Scholar] [CrossRef]
- Li, X.; Zhong, H.; Li, X.; Jia, F.; Cheng, Z.; Zhang, L.; Yin, J.; An, L.; Guo, L. Synthesis of attapulgite/N-isopropylacrylamide and its use in drug release. Mater. Sci. Eng. C. 2014, 45, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Higashi, K.; Azuma, T.; Okamoto, A. Supramolecular Polymeric Hydrogels for Ultrasound-Guided Protein Release. Biotechnol. J. 2019, 14, 1800530. [Google Scholar] [CrossRef] [PubMed]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.C.; Lai, J.Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kang, E.; Kang, S.W.; Huh, K.M. Thermo-irreversible glycol chitosan/hyaluronic acid blend hydrogel for injectable tissue engineering. Carbohydr. Polym. 2020, 244, 116432. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Imaz, A.; Forcada, J. Temperature-sensitive nanogels: Poly(N-vinylcaprolactam) versus poly(N-isopropylacrylamide). Polym. Chem. 2012, 3, 852–856. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2014, 53, 1–51. [Google Scholar] [CrossRef]
- Marsili, L.; Dal Bo, M.; Eisele, G.; Donati, I.; Berti, F.; Toffoli, G. Characterization of thermoresponsive poly-n-vinylcaprolactam polymers for biological applications. Polymers 2021, 13, 2639. [Google Scholar] [CrossRef]
- Siirilä, J.; Tenhu, H. Soft Poly(N-vinylcaprolactam) Based Aqueous Particles. Acta Chim. Slov. 2022, 69, 251–260. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Halligan, E.; Zhuo, S.; Colbert, D.M.; Alsaadi, M.; Tie, B.S.H.; Bezerra, G.S.N.; Keane, G.; Geever, L.M. Modulation of the Lower Critical Solution Temperature of Thermoresponsive Poly(N-vinylcaprolactam) Utilizing Hydrophilic and Hydrophobic Monomers. Polymers 2023, 15, 1595. [Google Scholar] [CrossRef] [PubMed]
- Vilos, C.; Velasquez, L.A. Therapeutic Strategies Based on Polymeric Microparticles. J. Biomed. Biotechnol. 2012, 2012, 672760. [Google Scholar] [CrossRef]
- Liu, J.; Debuigne, A.; Detrembleur, C.; Jérôme, C. Poly (N-vinylcaprolactam): A Thermoresponsive Macromolecule with Promising Future in Biomedical Field. Adv. Healthc. Mater. 2014, 3, 1941–1968. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Dal Bo, M.; Berti, F.; Toffoli, G. Thermoresponsive chitosan-grafted-poly(N-vinylcaprolactam) microgels via ionotropic gelation for oncological applications. Pharmaceutics 2021, 13, 1654. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering-A review. J. Mat. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Mohammed, M.N.; Bin Yusoh, K.; Shariffuddin, J.H.B.H. Poly(N-vinyl caprolactam) thermoresponsive polymer in novel drug delivery systems: A review. Mater. Express 2018, 8, 21–34. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Chen, J.-H.; Cao, Q.-T.; Duan, B.; Chen, H.-J.; Yu, X.-C.; Xiao, Y.-F. Operando monitoring transition dynamics of responsive polymer using optofluidic microcavities. Light: Sci. Appl. 2021, 10, 128. [Google Scholar] [CrossRef]
- Zheng, M.; Ji, Q.; Ullah, Z.; Zhang, Y.; Chen, M.; Li, W.; Li, Q.; Liu, L. High protection performance based on corrosion media-consumption and barrier properties of the supramolecular polymer reinforced graphene oxide composite coatings. J. Polym. Res. 2021, 28, 426. [Google Scholar] [CrossRef]
- Xu, X.; Bizmark, N.; Christie, K.S.S.; Datta, S.S.; Ren, Z.J.; Priestley, R.D. Thermoresponsive Polymers for Water Treatment and Collection. Macromolecules 2022, 55, 1894–1909. [Google Scholar] [CrossRef]
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D.H. Transformer Hydrogels: A Review. Adv. Mater. Technol. 2019, 4, 1900043. [Google Scholar] [CrossRef]
- Mushtaq, M.W.; Kanwal, F.; Batool, A.; Jamil, T.; Zia-ul-Haq, M.; Ijaz, B.; Huang, Q.; Ullah, Z. Polymer-coated CoFe2O4 nanoassemblies as biocompatible magnetic nanocarriers for anticancer drug delivery. J. Mater. Sci. 2017, 52, 9282–9293. [Google Scholar] [CrossRef]
- PN-EN ISO 3696:1999; Water for Analytical Laboratory Use. Specification and Test Methods. Polish Committee for Standardization: Warszawa, Poland, 1999.
- Pelton, R.H.; Chibante, P. Preparation of aqueous latices with N-isopropylacrylamide. Colloids Surf. 1986, 20, 247–256. [Google Scholar] [CrossRef]
- ISO 13321:1996; Methods for Determination of Particle Size Distribution; Photon Correlation Spectroscopy. International Organization for Standardization (ISO): Geneva, Switzerland, 1997; pp. 3406–3408.
- ISO 22412; Particle Size Analysis–Dynamic Light Scattering. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2008.
- Worldwide, M.I. Dynamic Light Scattering, Common Terms Defined; Inform White Paper; Malvern Instruments Limited: Malvern, UK, 2011; pp. 1–6. [Google Scholar]
- Kozanoǧlu, S.; Özdemir, T.; Usanmaz, A. Polymerization of N-Vinylcaprolactam and Characterization of Poly (N-Vinylcaprolactam). J. Macromol. Sci. Part. A Pure Appl. Chem. 2011, 4, 467. [Google Scholar] [CrossRef]
- Gola, A.; Niżniowska, A.; Musiał, W. The influence of initiator concentration on selected properties on poly-N-vinylcaprolactam nanoparticles. Nanomaterials 2019, 9, 1577. [Google Scholar] [CrossRef]
- Finnegan, M.; Duffy, E.; Morrin, A. The determination of skin surface pH via the skin volatile emission using wearable colorimetric sensors. Sens. Bio-Sens. Res. 2022, 35, 100473. [Google Scholar] [CrossRef]
- Prakash, C.; Bhargava, P.; Tiwari, S.; Majumdar, B.; Bhargava, R.K. Skin Surface pH in Acne Vulgaris: Insights from an Observational Study and Review of the Literature. J. Clin. Aesthet. Dermatol. 2017, 10, 33–39. [Google Scholar] [PubMed] [PubMed Central]
- Luebberding, S.; Krueger, N.; Kerscher, M. Skin physiology in men and women: In vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int. J. Cosmet. Sci. 2013, 35, 477–483. [Google Scholar] [CrossRef]
- Thankachan, D.; Anbazhagan, R.; Tsai, H.C.; Thuy, D.V.T.; Gebrie, H.T.; Chen, K.J.; Chen, W.L.; Chen, J.K. pH dependent biocompatible room temperature covalent organic polymers for selective chemotherapeutic drug delivery. Micropor. Mesopor. Mat. 2024, 365, 112903. [Google Scholar] [CrossRef]
- Richard, A. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra, 1st ed.; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- SDBSWeb. National Institute of Advanced Industrial Science and Technology. Available online: http://sdbs.db.aist.go.jp (accessed on 25 March 2024).
- Cortez-Lemus, N.A.; Hermosillo-Ochoa, E.; Licea-Claverie, Á. Effective end-group modification of star-shaped PNVCL from xanthate to trithiocarbonate avoiding chemical crosslinking. Polymers 2021, 13, 3677. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Salah, I.; Shamat, M.A.; Cook, M.T. Soluplus solutions as thermothickening materials for topical drug delivery. J. Appl. Polym. Sci. 2019, 136, 46915. [Google Scholar] [CrossRef]
- Delgado, A.V.; Gonz’alez-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid. Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef]
- Gasztych, M.; Malamis, A.; Musiał, W. The Influence of Initiators, Particle Size and Composition on the Electrokinetic Potential of N-(Isopropyl)acrylamide Derivatives. Polymers 2024, 16, 907. [Google Scholar] [CrossRef] [PubMed]
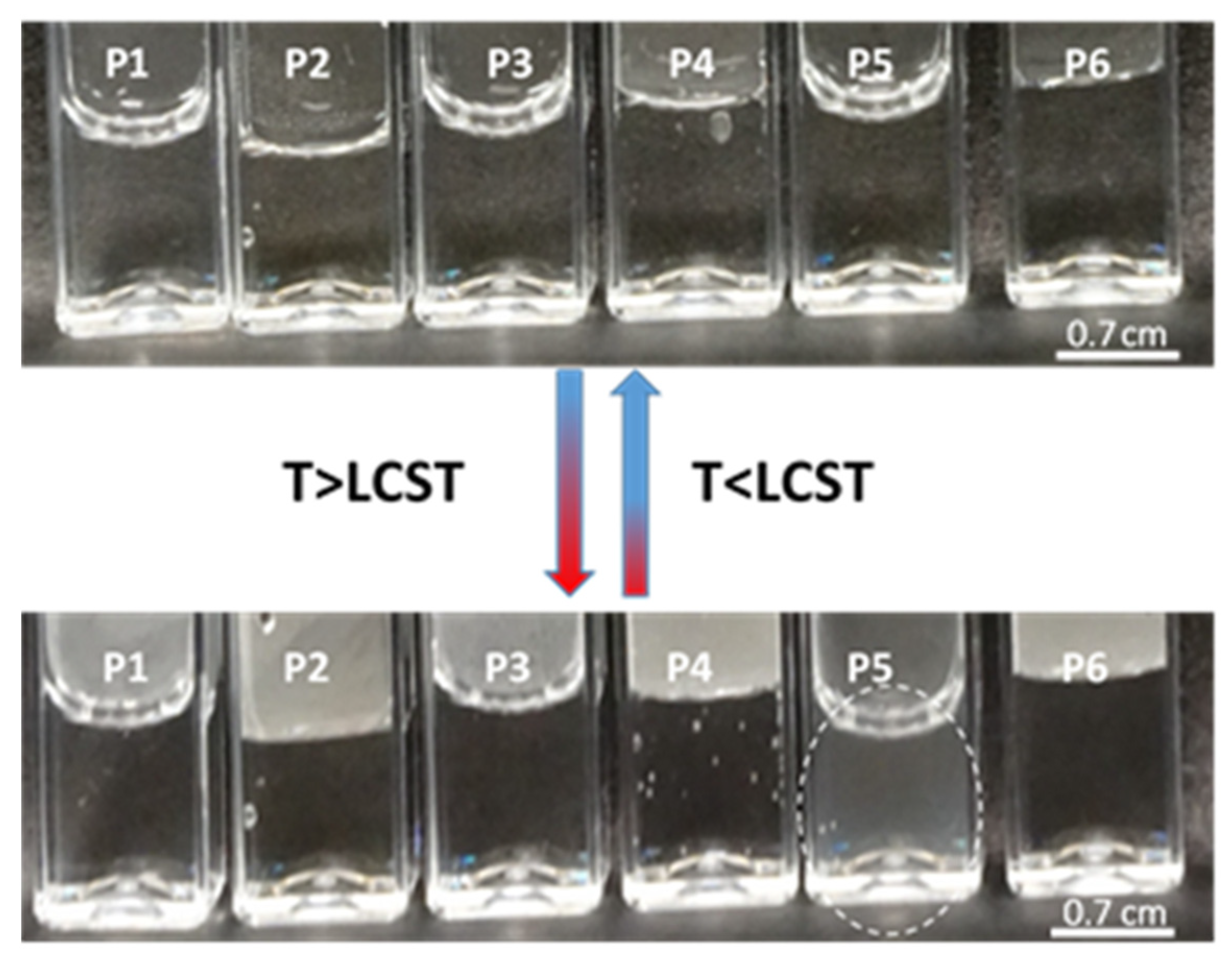
| Components | Polymer Code | ||||||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | ||
| Monomer (g) | NVCL | 3.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Anionic initiator (g) | KPS | 2.91 | 4.85 | 0.49 | 0.049 | 0.49 | 0.49 |
| Cross-linker (g) | MBA | - | - | - | 0.55 | 0.0055 | |
| Polymer Code | Polymer Dyspersion | |
|---|---|---|
| Unpurified before FED | Purified after FED | |
| P1 | 1.90 | 5.29 |
| P2 | 1.67 | 6.08 |
| P3 | 2.45 | 5.00 |
| P4 | 3.50 | 5.10 |
| P5 | 2.50 | 4.80 |
| P6 | 2.50 | 4.85 |
| Assignation | Wavenumbers /cm−1 | |||
|---|---|---|---|---|
| Reference [60] | Observed | |||
| NVCL/ pNVCL | NVCL | P1 | P5 | |
| N-H | 3274 | 3263 | 3198 | 3278 |
| C-H | 2926 2856 | 2931 2851 | 2931 2862 | 2928 2858 |
| C=O | 1631 | 1623 | 1618 | 1609 |
| C-N | 1479 | 1486 | 1493 | 1482 |
| -CH2- | 1441 | 1440 | 1428 | 1442 |
| C=C | 1659 | 1652 | - | - |
| =CH2 | 994 | 993 | - | - |
| O-H | 3507 | - | 3534 | 3417 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gola, A.; Pietrańczyk, R.; Musiał, W. Synthesis and Physicochemical Properties of Thermally Sensitive Polymeric Derivatives of N-vinylcaprolactam. Polymers 2024, 16, 1917. https://doi.org/10.3390/polym16131917
Gola A, Pietrańczyk R, Musiał W. Synthesis and Physicochemical Properties of Thermally Sensitive Polymeric Derivatives of N-vinylcaprolactam. Polymers. 2024; 16(13):1917. https://doi.org/10.3390/polym16131917
Chicago/Turabian StyleGola, Agnieszka, Rafał Pietrańczyk, and Witold Musiał. 2024. "Synthesis and Physicochemical Properties of Thermally Sensitive Polymeric Derivatives of N-vinylcaprolactam" Polymers 16, no. 13: 1917. https://doi.org/10.3390/polym16131917
APA StyleGola, A., Pietrańczyk, R., & Musiał, W. (2024). Synthesis and Physicochemical Properties of Thermally Sensitive Polymeric Derivatives of N-vinylcaprolactam. Polymers, 16(13), 1917. https://doi.org/10.3390/polym16131917






