Effect of Hydrochloric Acid Hydrolysis under Sonication and Hydrothermal Process to Produce Cellulose Nanocrystals from Oil Palm Empty Fruit Bunch (OPEFB)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
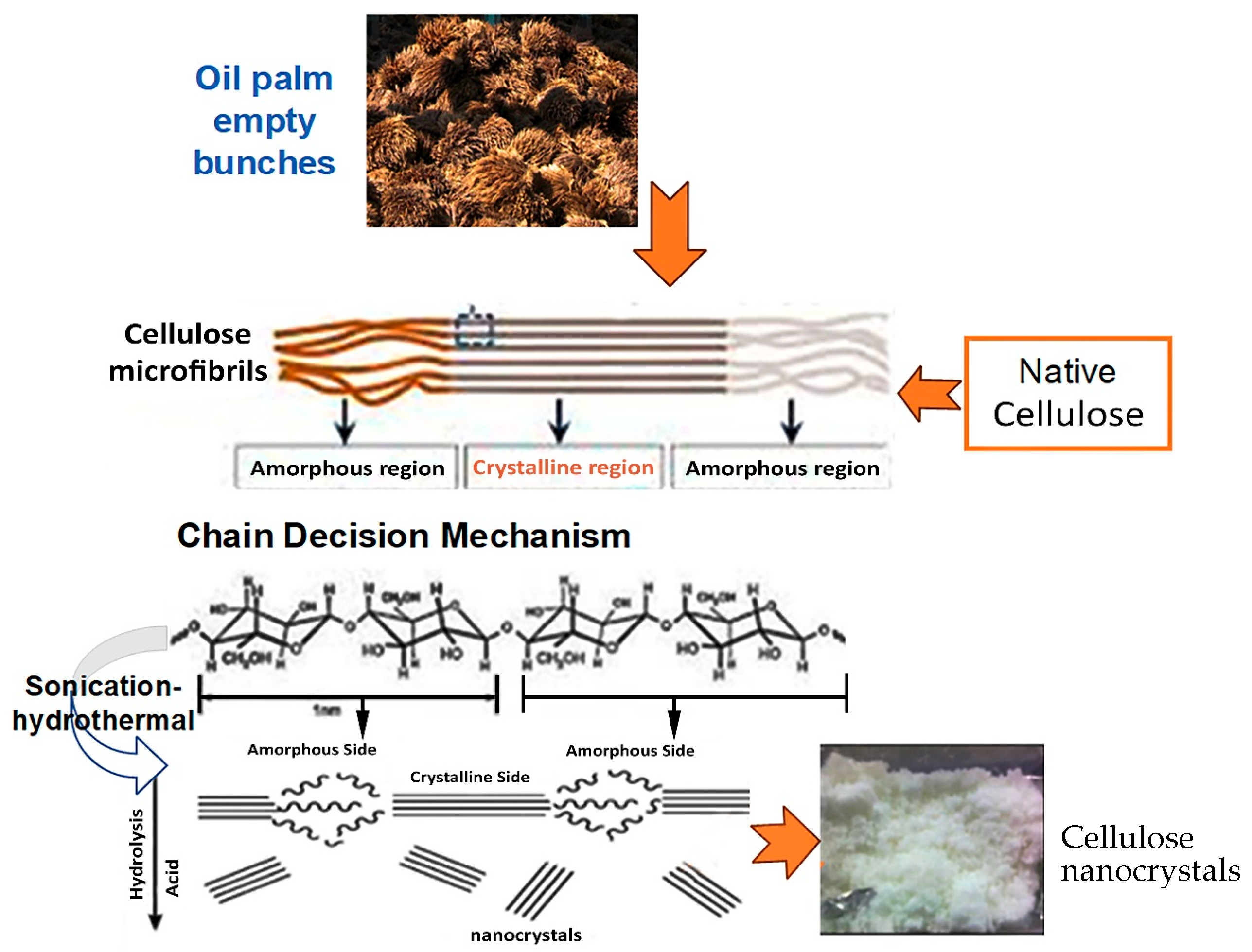
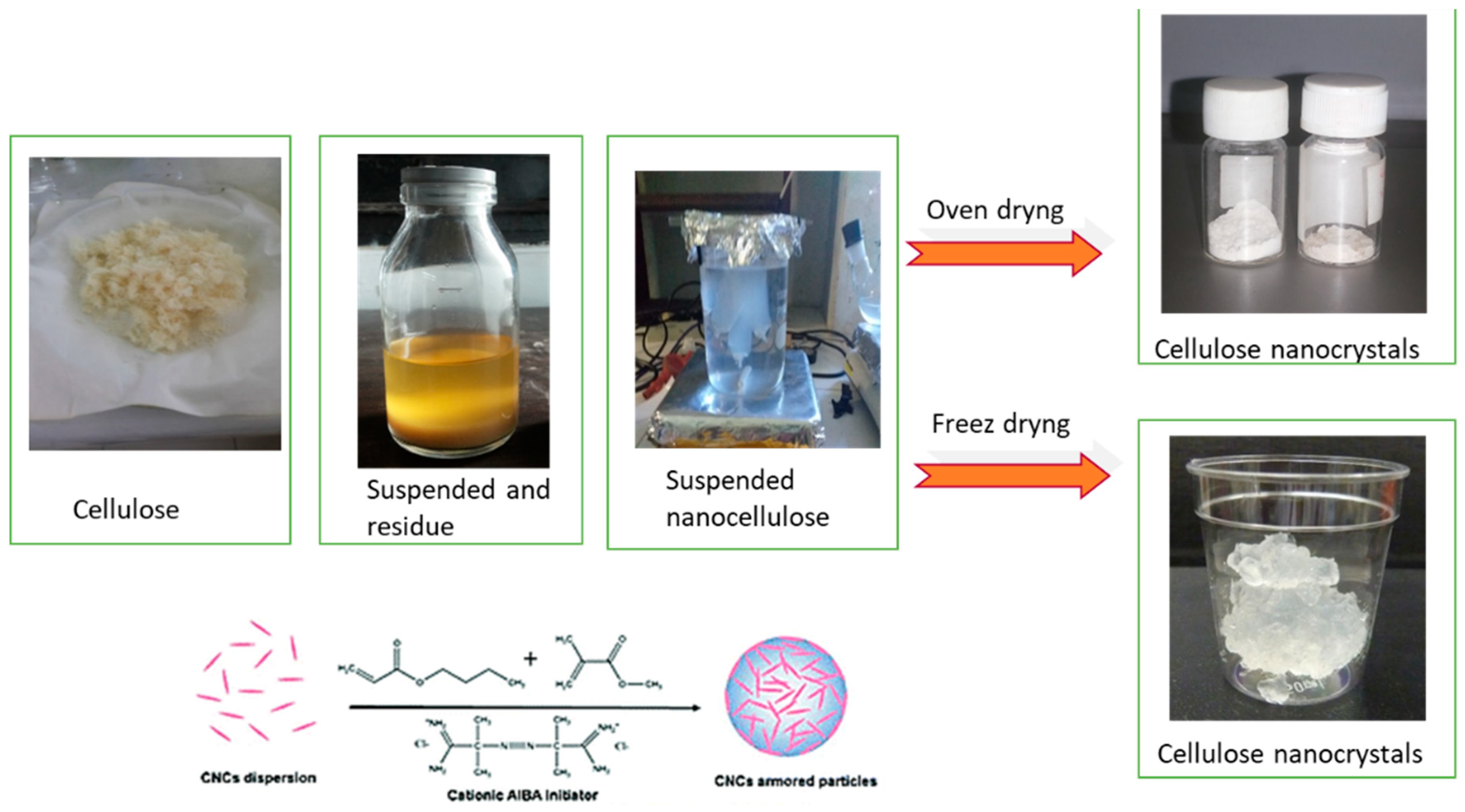
2.3. CNC Preparation
2.4. Characterization
2.4.1. Chemical Structure Analysis
2.4.2. Morphological Analysis
2.4.3. Crystal Structure Analysis (XRD)
2.4.4. Thermogravimetric Analysis
2.4.5. Particle Size Analysis by PSA
3. Results and Discussion
3.1. CNC Preparation Conditions
3.2. Scission Mechanism of Cellulose Chains into CNCs
3.3. FT-IR Analysis
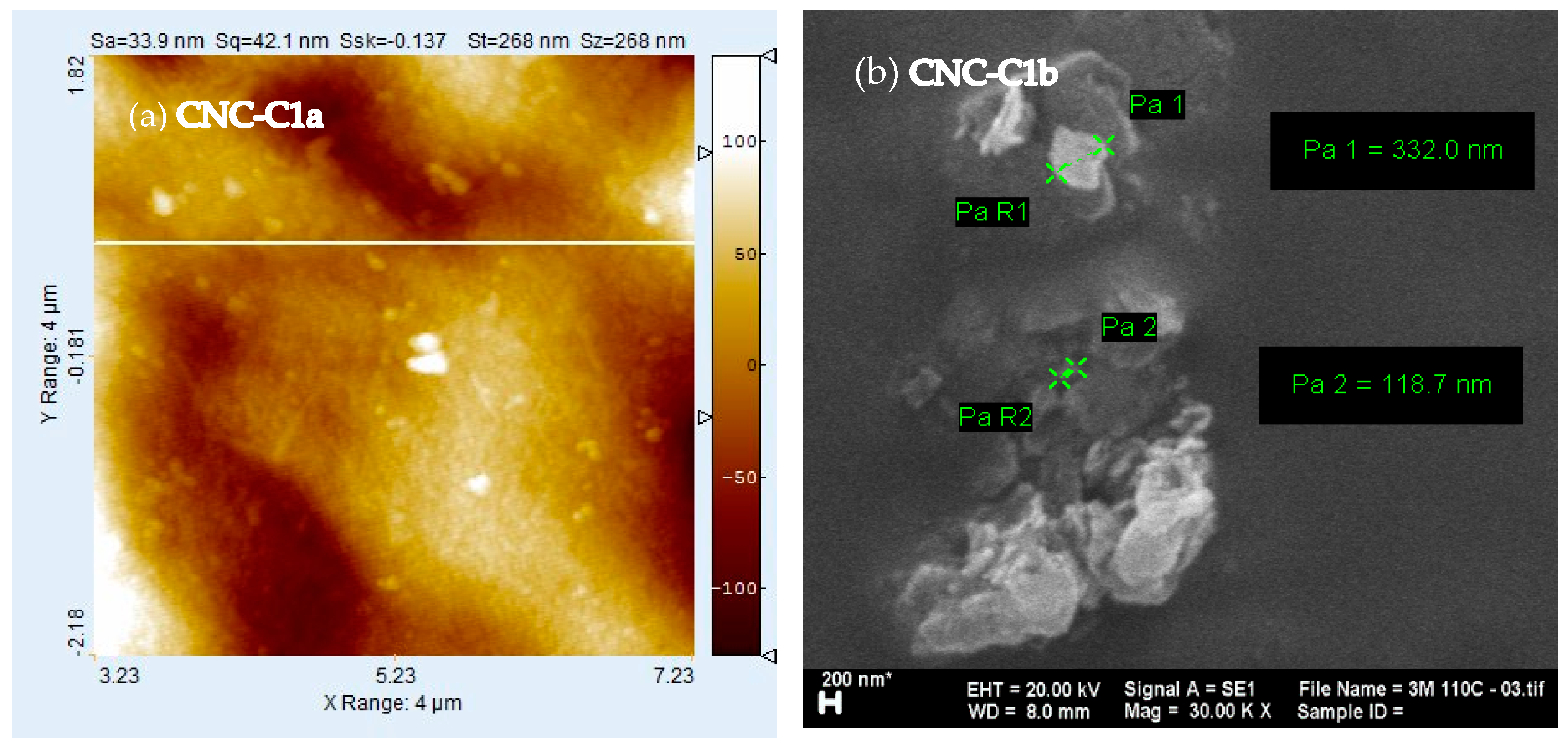
3.4. Morphological Analysis
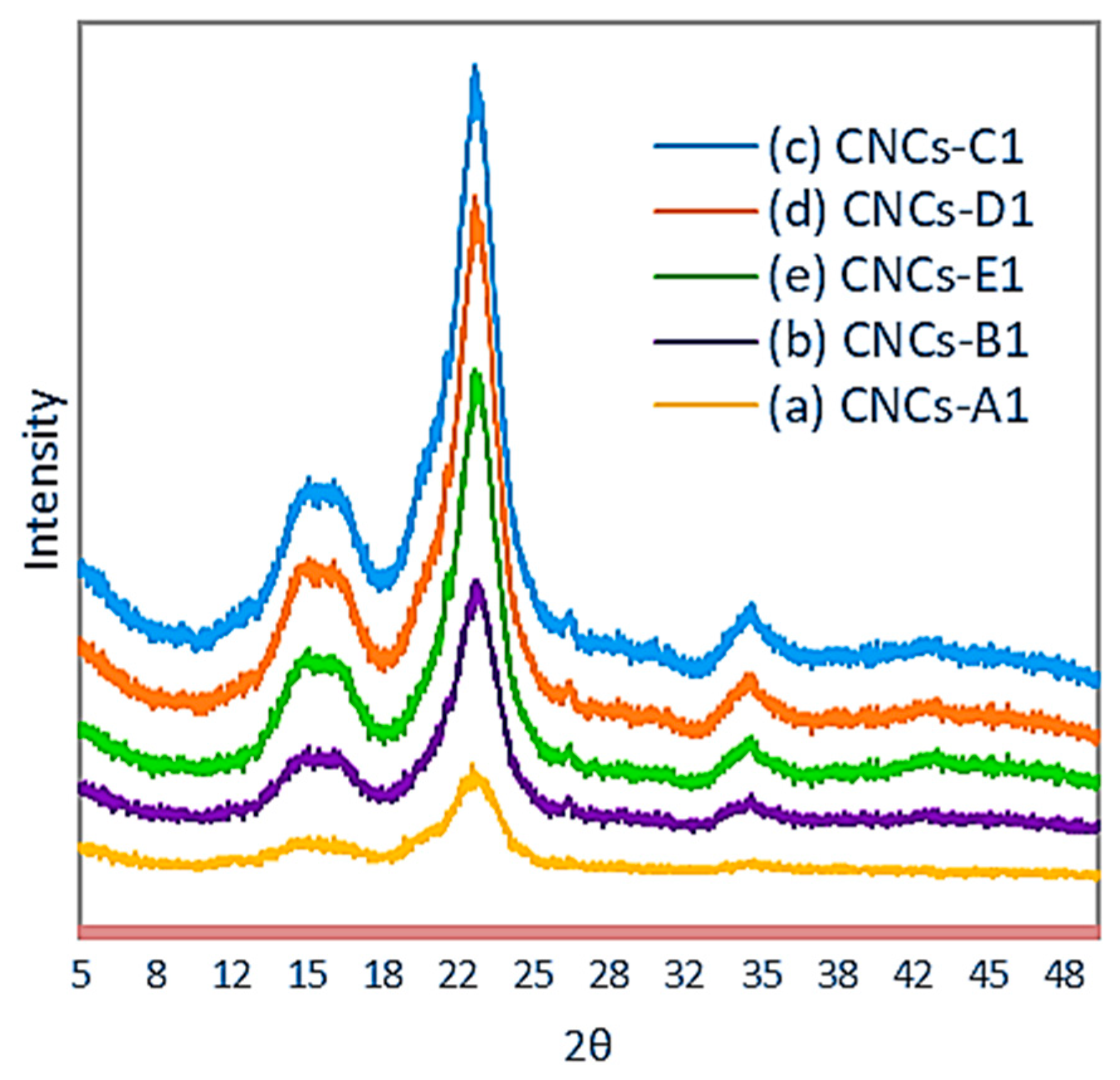
3.5. X-ray Diffraction Analysis
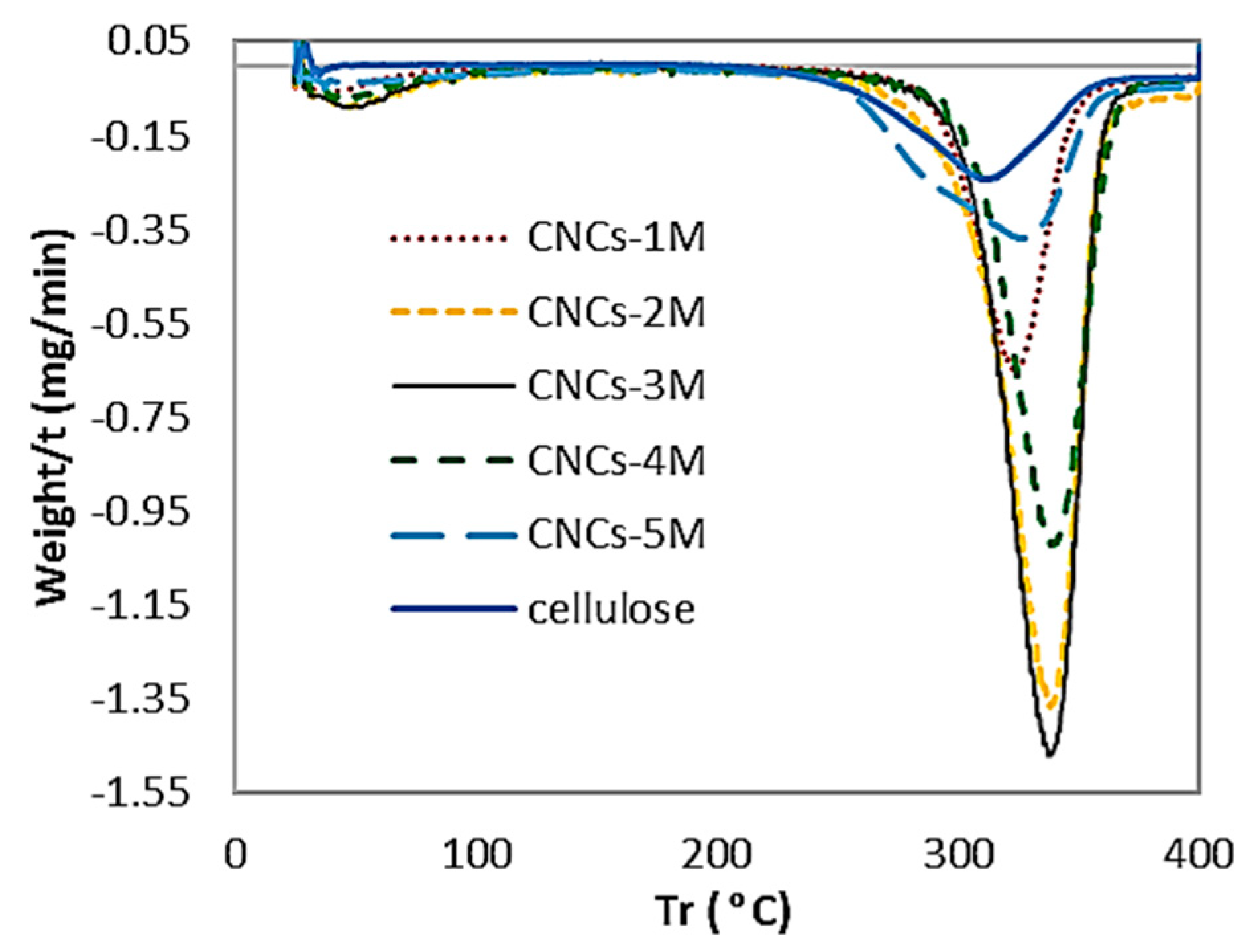
3.6. Thermogravimetric Analysis
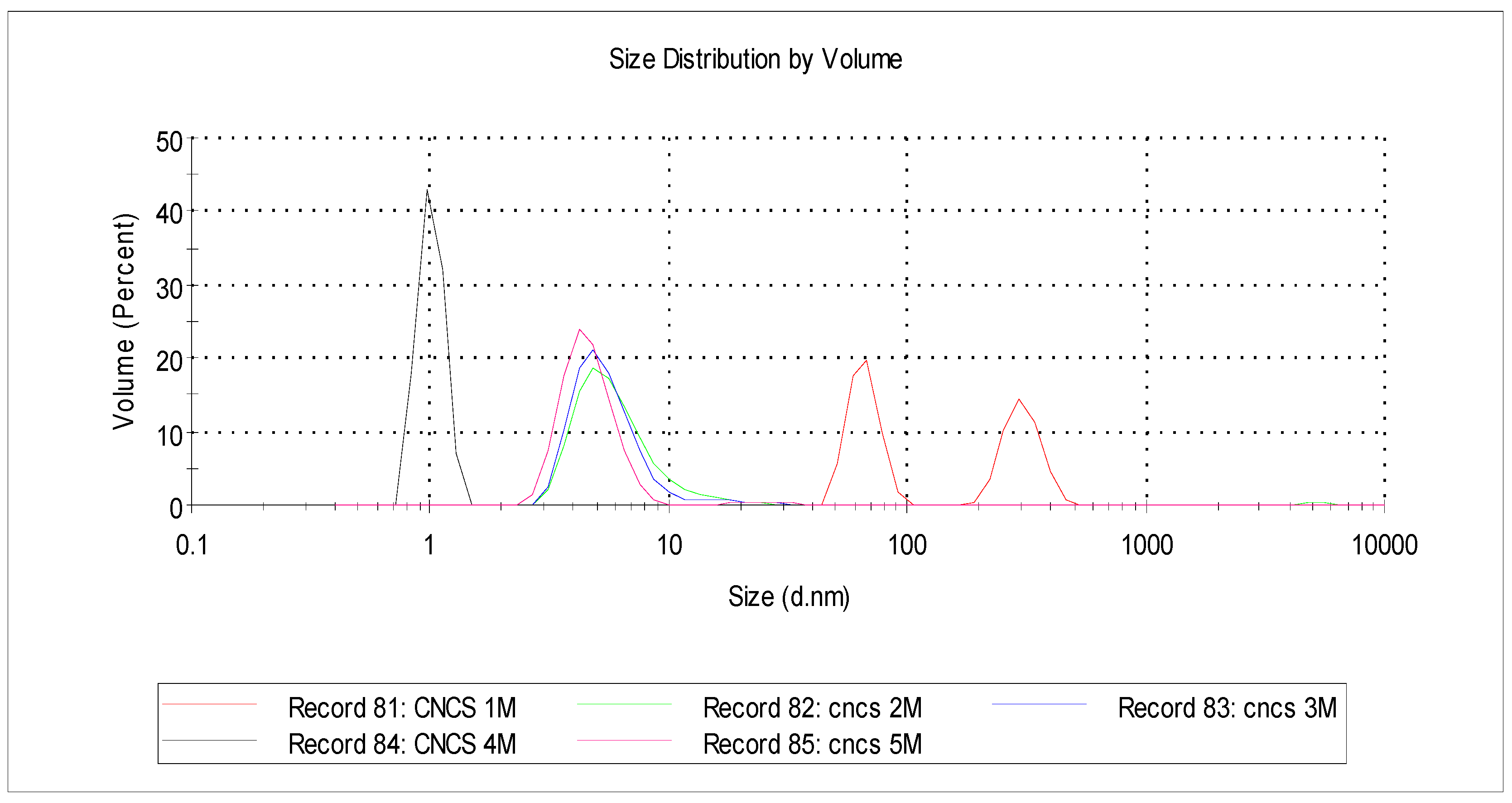
3.7. The CNC Size
3.8. CNC and Cellulose Comparisons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbasi Moud, A. Cellulose Nanocrystals Examined by Atomic Force Microscopy: Applications and Fundamentals. ACS Food Sci. Technol. 2022, 2, 1789–1818. [Google Scholar] [CrossRef]
- Chagas, J.S.; Almeida, J.N.S.; Pereira, A.C.L.; Silva, N.F.I.; Raimundo, R.A.; Medeiros, E.S.; Lima, B.A.S.G.; Galvão, L.S.; Santos, A.S.F.; Silva, F.I. Evaluation of the kinetics of low intensity ultrasound-assisted sulfuric acid hydrolysis to obtain cellulose nanocrystals (CNCs) from microcrystalline cellulose (MCC). Cellulose 2023, 30, 11455–11472. [Google Scholar] [CrossRef]
- Durairaj, A.; Maruthapandi, M.; Saravanan, A.; Luong JH, T.; Gedanken, A. Cellulose Nanocrystals (CNC)-Based Functional Materials for Supercapacitor Applications. Nanomaterials 2022, 12, 1828. [Google Scholar] [CrossRef] [PubMed]
- El-Lateef HM, A.; Gouda, M. Novel nanocomposites of nickel and copper oxide nanoparticles embedded in a melamine framework containing cellulose nanocrystals: Material features and corrosion protection applications. J. Mol. Liq. 2021, 342, 116960. [Google Scholar] [CrossRef]
- Jantachum, P.; Khumpaitool, B.; Utara, S. Effect of silane coupling agent and cellulose nanocrystals loading on the properties of acrylonitrile butadiene rubber/natural rubber nanocomposites. Ind. Crops Prod. 2023, 195, 116407. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; David, C.; Mountakis, N.; Papadakis, V.; Sfakiotakis, E.; Sagris, D.; Argyros, A. Optimization of cellulose nanocrystal (CNC) concentration in polycaprolactone bio-composites for bio-plotting: A robust interpretation of the reinforcement mechanisms. Cellulose 2024, 31, 3657–3680. [Google Scholar] [CrossRef]
- Uchida, T.; Nishioka, R.; Yanai, R. Preparation of cellulose nanocrystals coated with polymer crystals and their application in composite films. Polym. Adv. Technol. 2022, 33, 2511–2518. [Google Scholar] [CrossRef]
- Emenike, E.C.; Iwuozor, K.O.; Saliu, O.D.; Ramontja, J.; Adeniyi, A.G. Advances in the extraction, classification, modification, emerging and advanced applications of crystalline cellulose: A review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100337. [Google Scholar] [CrossRef]
- Jin, K.; Song, G.; Diao, H.; Zhang, X.; Ji, X.; Zhang, J.; Zhang, J. Hydrogen-bond assisted nonconventional photoluminescence of crystalline and amorphous cellulose. Cellulose 2023, 30, 8139–8150. [Google Scholar] [CrossRef]
- Sanchez, M.; Nobre, A.G.; Martinez, J.A.E.; Campanaro, J.F.; Vargas, V.M.L. Considerations about highly crystalline cellulose microfiber additive from Eucalyptus grandis for 3D-printing acrylonitrile butadiene styrene filament. Mater. Sci. Addit. Manuf. 2023, 2, 1000. [Google Scholar] [CrossRef]
- Serizawa, T.; Maeda, T.; Sawada, T. Neutralization-Induced Self-Assembly of Cellulose Oligomers into Antibiofouling Crystalline Nanoribbon Networks in Complex Mixtures. ACS Macro Lett. 2020, 9, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Listyanda, R.F.; Kusmono; Wildan, M.W.; Ilman, M.N. Extraction and characterization of nanocrystalline cellulose (NCC) from ramie fiber by sulphuric acid hydrolysis. AIP Conf. Proc. 2020, 2217, 030069. [Google Scholar] [CrossRef]
- Zahir, N.M.; Abdullah, M.K.; Jabit, N.A.; Ismail, S. Effects of Sulphuric Acid and Speed Rotation on Hydrolysis of Bamboo Sawdust. Key Eng. Mater. 2022, 908, 448–454. [Google Scholar] [CrossRef]
- Ndruru, S.T.C.L.; Asta, V.A.; Hidayat, R.A.R.; Mawarni, R.S.; Marlina, A.; Yulianti, E.; Handayani, A.S.; Hikmat; Siregar, R.A.; Heliawati, L.; et al. Isolation, Modification, and Characterization of Local Indonesia’s Sugarcane Bagasse Cellulose for Dye-Adsorbent Application. ChemistrySelect 2024, 9, e202302812. [Google Scholar] [CrossRef]
- Jiang, L.; Hinrichsen, G. Flax and cotton fiber reinforced biodegradable polyester amide composites, 2. Characterization of biodegradation. Angew. Makromol. Chem. 1999, 268, 18–21. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Bioresour. Technol. 2009, 100, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Medeiros, E.; Malmonge, J.; Gregorski, K.; Wood, D.; Mattoso, L.; Glenn, G.; Orts, W.; Imam, S. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Nazir, D.M.S.; Wahjoedi, B.; Yussof, A.; Abdullah, M. Eco-Friendly Extraction and Characterization of Cellulose from Oil Palm Empty Fruit Bunches. Bioresources 2013, 8, 2161–2172. [Google Scholar] [CrossRef]
- Shao, B.; Han, Z.; Pang, R.; Wu, D.; Xie, B.; Su, Y. The crystalline structure transition and hydrogen bonds shift determining enhanced enzymatic digestibility of cellulose treated by ultrasonication. Sci. Total Environ. 2023, 876, 162631. [Google Scholar] [CrossRef]
- Baksi, S.; Saha, S.; Birgen, C.; Sarkar, U.; Preisig, H.A.; Markussen, S.; Wittgens, B.; Wentzel, A. Valorization of Lignocellulosic Waste (Crotalaria juncea) Using Alkaline Peroxide Pretreatment under Different Process Conditions: An Optimization Study on Separation of Lignin, Cellulose, and Hemicellulose. J. Nat. Fibers 2019, 16, 662–676. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Tang, L.; Huang, B.; Lu, Q.; Wang, S.; Ou, W.; Lin, W.; Chen, X. Ultrasonication-assisted manufacture of cellulose nanocrystals esterified with acetic acid. Bioresour. Technol. 2013, 127, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Pawcenis, D.; Leśniak, M.; Szumera, M.; Sitarz, M.; Profic-Paczkowska, J. Effect of hydrolysis time, pH and surfactant type on stability of hydrochloric acid hydrolyzed nanocellulose. Int. J. Biol. Macromol. 2022, 222, 1996–2005. [Google Scholar] [CrossRef]
- Rubleva, N.V.; Voronova, M.I.; Surov, O.V.; Zakharov, A.G.; Lebedeva, E.O.; Fineevskii, A.V. Production of cellulose nanocrystals by hydrolysis in mixture of hydrochloric and nitric acids. Izv. Vyss. Uchebnykh Zaved. Khimiya Khimicheskaya Tekhnologiya 2019, 62, 85–93. [Google Scholar] [CrossRef]
- Tang, L.-R.; Huang, B.; Ou, W.; Chen, X.-R.; Chen, Y.-D. Manufacture of cellulose nanocrystals by cation exchange resin-catalyzed hydrolysis of cellulose. Bioresour. Technol. 2011, 102, 10973–10977. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Mathematisch-Physikalische Klasse 1918, 1918, 98–100. [Google Scholar]
- Zulnazri, Z.; Anjana, F.; Roesyadi, A. Temperature Effect of Crystalinity in Cellulose Nanocrystal from Oil Palm Empty Fruit Bunch (OPEFB) using Sonication-Hydrothermal Methods. J. Pure Appl. Chem. Res. 2017, 6, 14–21. [Google Scholar] [CrossRef]
- Hastuti, N.; Kanomata, K.; Kitaoka, T. Hydrochloric Acid Hydrolysis of Pulps from Oil Palm Empty Fruit Bunches to Produce Cellulose Nanocrystals. J. Polym. Environ. 2018, 26, 3698–3709. [Google Scholar] [CrossRef]
- Mohamad Haafiz, M.K.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Saeed, A.; He, Z.; Ni, Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 2011, 18, 451–459. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Nacos, M.; Katapodis, P.; Pappas, C.; Daferera, D.; Tarantilis, P.; Christakopoulos, P.; Polissiou, M. Kenaf xylan—A source of biologically active acidic oligosaccharides. Carbohydr. Polym. 2006, 66, 126–134. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Corrêa, A.; Teixeira, E.; Pessan, L.; Mattoso, L. Cellulose nanofibers from curaua fibers. Cellulose 2010, 17, 1183–1192. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Abdullah, I. Isolation and Characterization of Cellulose Nanocrystals from Agave angustifolia Fibre. BioResources 2013, 8, 2161–2172. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Isolation, preparation, and characterization of nanofibers from oil palm empty-fruit-bunch (OPEFB). Cellulose 2010, 17, 977–985. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.; Pereira, A.G.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly(acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef]
- Zulnazri; Roesyadi, A.; Sumarno, S. Effects of hydrolysis conditions on the crystallinity, chemical structure, morphology, and thermal stability of cellulose nanocrystals extracted from oil palm biomass residue. Int. J. ChemTech Res. 2016, 9, 456–464. [Google Scholar]
- Zulnazri, Z.; Dewi, R.; Sulhatun, S.; Nasrun, N. Kinetics study the decomposition of the cellulose into cellulose nanocrystals by hydrothermal with hydrochloric acid catalyst. Int. J. Plant Biol. 2019, 10, 7440. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Satyamurthy, P.; Jain, P.; Balasubramanya, R.H.; Vigneshwaran, N. Preparation and characterization of cellulose nanowhiskers from cotton fibres by controlled microbial hydrolysis. Carbohydr. Polym. 2011, 83, 122–129. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline celluloseby acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
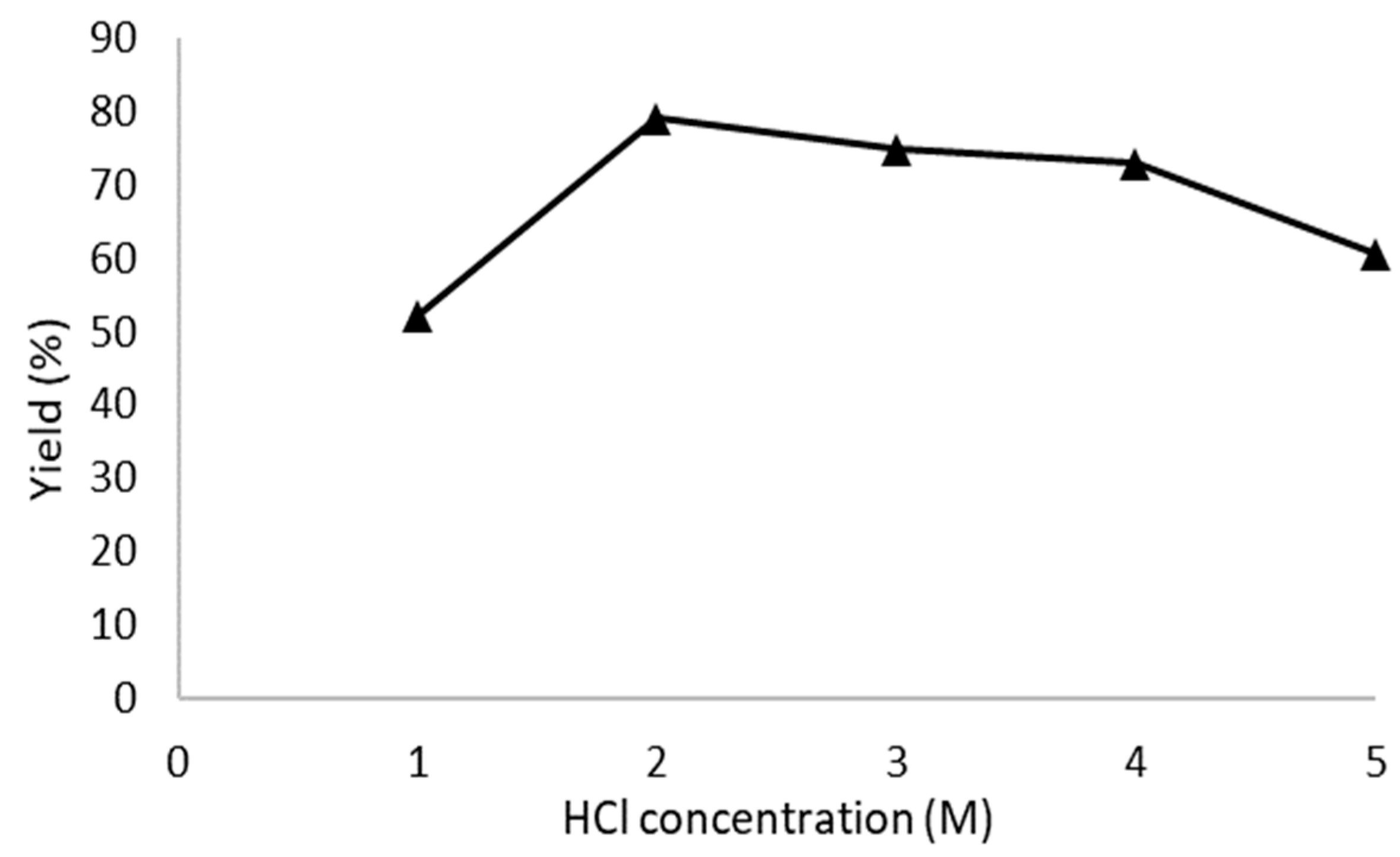




| Sample | Hydrolysis HCl (M) | Reaction Time (h) | Yield (%) | Crystallinity (%) | Dhkl (002) (nm) | PSA (nm) | (TGA) T0(°C) | (DTG) Tm(°C) | Temp. Process (°C) |
|---|---|---|---|---|---|---|---|---|---|
| CNC-A0 | 1 | 0 | 35.20 | 65.32 | 15.64 | - | - | - | |
| CNC-A1 | 1 | 1 | 52.20 | 70.63 | 15.64 | 65.93 | 294.81 | 326.72 | |
| CNC-B0 | 2 | 0 | 66.80 | 72.82 | 4.69 | - | - | - | |
| CNC-B1 | 2 | 1 | 79.09 | 75.87 | 4.69 | 6.25 | 305.20 | 340.15 | |
| CNC-C0-110 | 3 | 0 | 48.90 | 73.40 | 11.74 | - | - | - | 110 |
| CNC-C1-110 | 3 | 1 | 74.82 | 78.59 | 7.83 | 5.83 | 305.66 | 339.82 | |
| CNC-D0 | 4 | 0 | 58.50 | 67.75 | 2.61 | - | - | - | |
| CNC-D1 | 4 | 1 | 72.90 | 77.19 | 3.35 | 1.013 | 307.09 | 340.56 | |
| CNC-E0 | 5 | 0 | 45.82 | 68.77 | 4.69 | - | - | - | |
| CNC-E1 | 5 | 1 | 60.54 | 76.36 | 2.93 | 4.64 | 269.53 | 337.87 | - |
| CNC-C0-120 | 3 | 0 | 45.64 | 57.70 | 3.91 | - | - | - | |
| CNC-C1-120 | 3 | 1 | 63.70 | 61.22 | 2.35 | - | - | - | |
| CNC-C2 | 3 | 2 | 54.05 | 64.05 | 3.88 | - | - | - | 120 |
| CNC-C3 | 3 | 3 | 40.50 | 58.91 | 17.46 | - | - | - | |
| CNC-C4 | 3 | 4 | 31.02 | 57.89 | 17.46 | - | - | - | |
| CNC-C5 | 3 | 5 | 25.07 | 57.17 | 3.79 | - | - | - | |
| CNC-C0-100 | 3 | 0 | 48.10 | 64.16 | 3.88 | - | - | - | |
| CNC-C1-100 | 3 | 1 | 75.20 | 68.24 | 4.69 | - | - | - | |
| CNC-C2 | 3 | 2 | 56.50 | 73.07 | 3.91 | - | - | - | |
| CNC-C3 | 3 | 3 | 44.70 | 68.04 | 3.96 | - | - | - | 100 |
| CNC-C4 | 3 | 4 | 48.00 | 66.02 | 3.35 | - | - | - | |
| CNC-C5 | 3 | 5 | 24.32 | 64.75 | 3.35 | - | - | - | |
| Cellulose | NaOH extracted | 2 | 50.04 | 63.02 | 22.9 | - | 233.43 | 314.17 |
| Peak Frequency (cm−1) for OPEFB Pulp, Cellulose, and CNCs | Peak Assignment |
|---|---|
| 3250–3500 | O-H bending |
| 2917, 2914, 2897 | CH2 groups |
| 1600–1650 | O-H stretching |
| 1300–1450 | CH2 aromatics |
| 1238 | C-O-C aryl–alkyl |
| 1104, 1158, 1160 | C-O-C stretching |
| Sample | Thermograv. Analysis (TGA) T0 (°C) | Derivative Thermograv (DTG) Tmax (°C) |
|---|---|---|
| Cellulose | 233.43 | 314.17 |
| CNC-A1 | 294.81 | 326.72 |
| CNC-B1 | 305.20 | 340.15 |
| CNC-C1 | 305.66 | 339.82 |
| CNC-D1 | 307.09 | 340.56 |
| CNC-E1 | 269.53 | 337.87 |
| CNC-A1 | CNC-B1 | CNC-C1 | CNC-D1 | CNC-E1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| % Volume | Size (d.nm) | % Volume | Size (d.nm) | % Volume | % Volume | Size (d.nm) | % Volume | Size (d.nm) | % Volume |
| 54.7 | 6.25 | 99.3 | 5.83 | 99.5 | 54.7 | 6.25 | 99.3 | 5.83 | 99.5 |
| 45.3 | 357.90 | 0.1 | 631.8 | 0.1 | 45.3 | 357.90 | 0.1 | 631.8 | 0.1 |
| 5014 | 0.6 | 4810 | 0.4 | 5014 | 0.6 | 4810 | 0.4 | ||
| 100 | 100 | 100 | 100 | 100 | 100 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulnazri, Z.; Dewi, R.; Muarif, A.; Fikri, A.; Fithra, H.; Roesyadi, A.; Sangian, H.F.; Alva, S. Effect of Hydrochloric Acid Hydrolysis under Sonication and Hydrothermal Process to Produce Cellulose Nanocrystals from Oil Palm Empty Fruit Bunch (OPEFB). Polymers 2024, 16, 1866. https://doi.org/10.3390/polym16131866
Zulnazri Z, Dewi R, Muarif A, Fikri A, Fithra H, Roesyadi A, Sangian HF, Alva S. Effect of Hydrochloric Acid Hydrolysis under Sonication and Hydrothermal Process to Produce Cellulose Nanocrystals from Oil Palm Empty Fruit Bunch (OPEFB). Polymers. 2024; 16(13):1866. https://doi.org/10.3390/polym16131866
Chicago/Turabian StyleZulnazri, Zulnazri, Rozanna Dewi, Agam Muarif, Ahmad Fikri, Herman Fithra, Achmad Roesyadi, Hanny F. Sangian, and Sagir Alva. 2024. "Effect of Hydrochloric Acid Hydrolysis under Sonication and Hydrothermal Process to Produce Cellulose Nanocrystals from Oil Palm Empty Fruit Bunch (OPEFB)" Polymers 16, no. 13: 1866. https://doi.org/10.3390/polym16131866
APA StyleZulnazri, Z., Dewi, R., Muarif, A., Fikri, A., Fithra, H., Roesyadi, A., Sangian, H. F., & Alva, S. (2024). Effect of Hydrochloric Acid Hydrolysis under Sonication and Hydrothermal Process to Produce Cellulose Nanocrystals from Oil Palm Empty Fruit Bunch (OPEFB). Polymers, 16(13), 1866. https://doi.org/10.3390/polym16131866






