Nanostructured Carbon Fibres (NCF): Fabrication and Application in Supercapacitor Electrode
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Carbon Nanofibre
2.3. Materials Characterisation
2.4. Electrochemical Characterisation
3. Results and Discussion
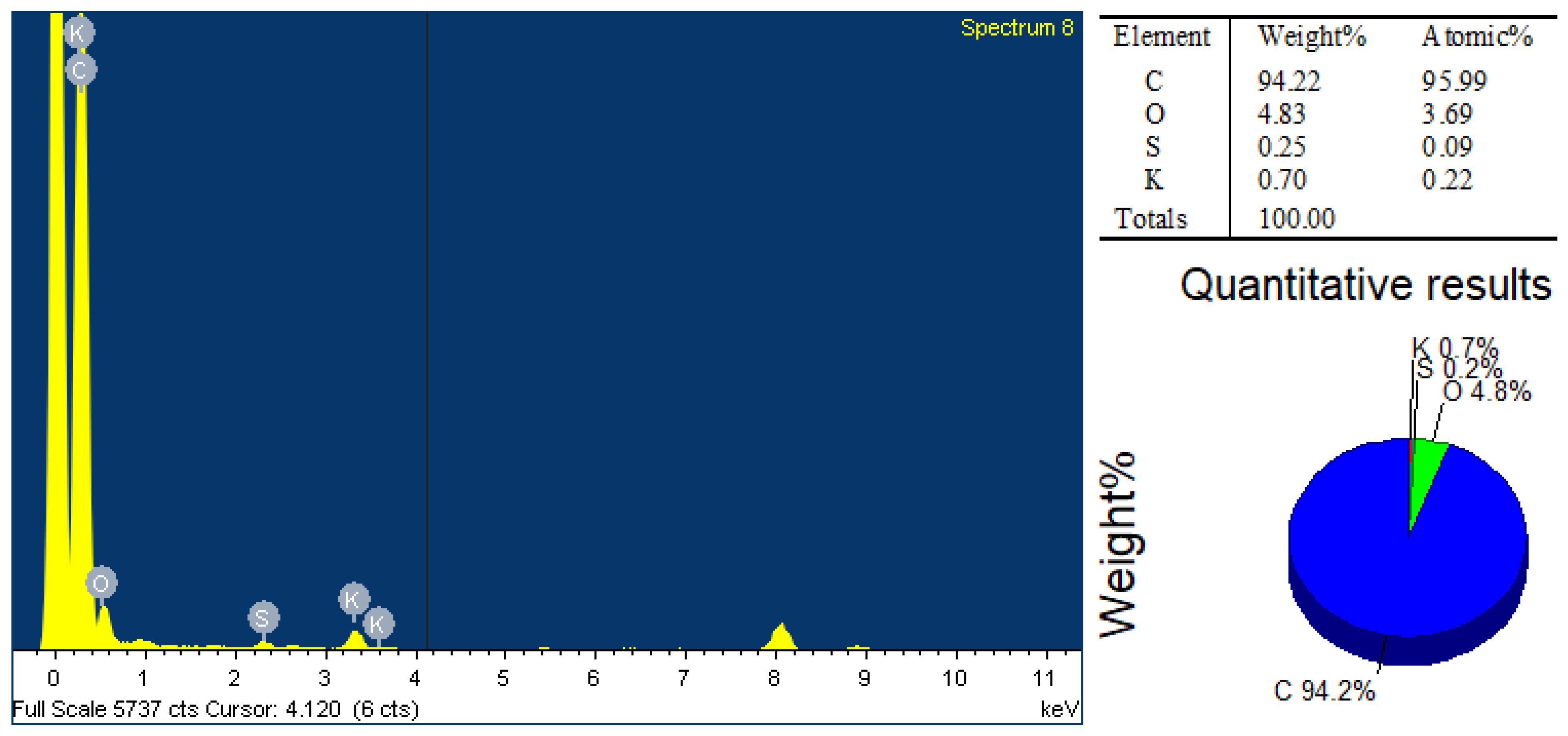
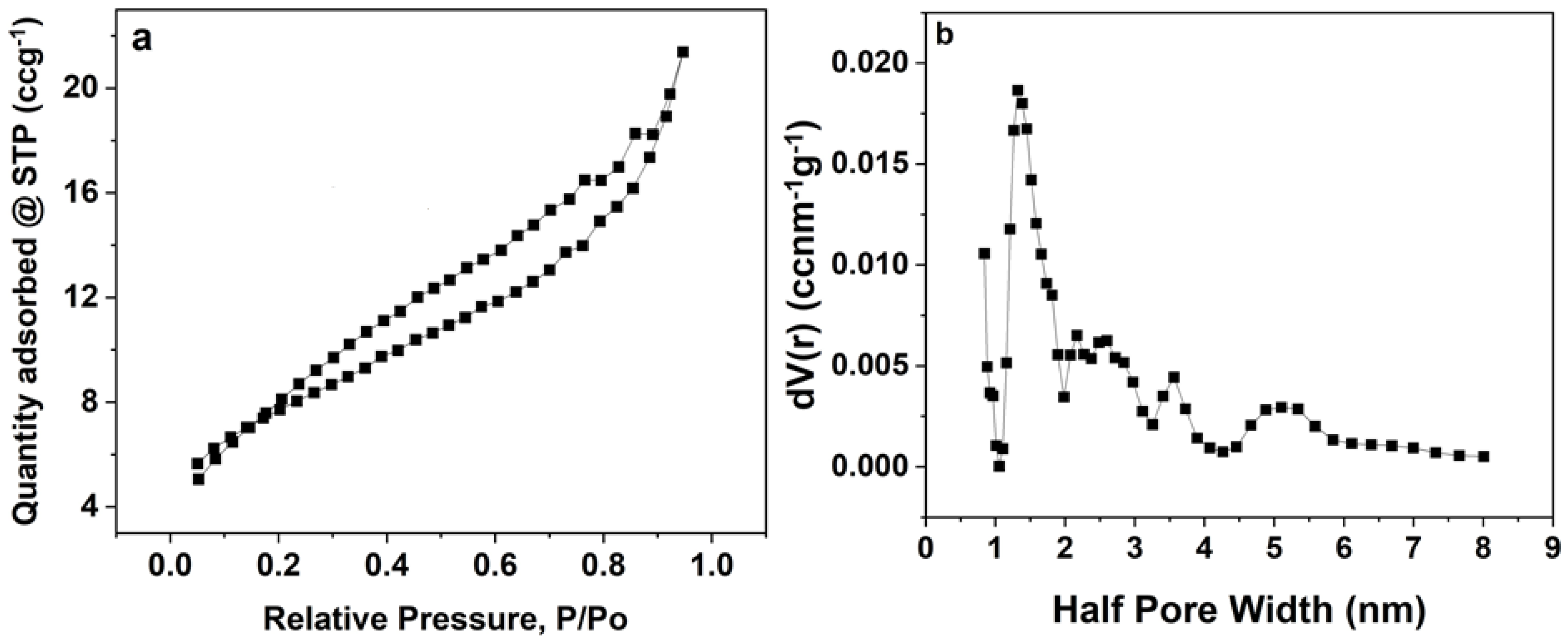
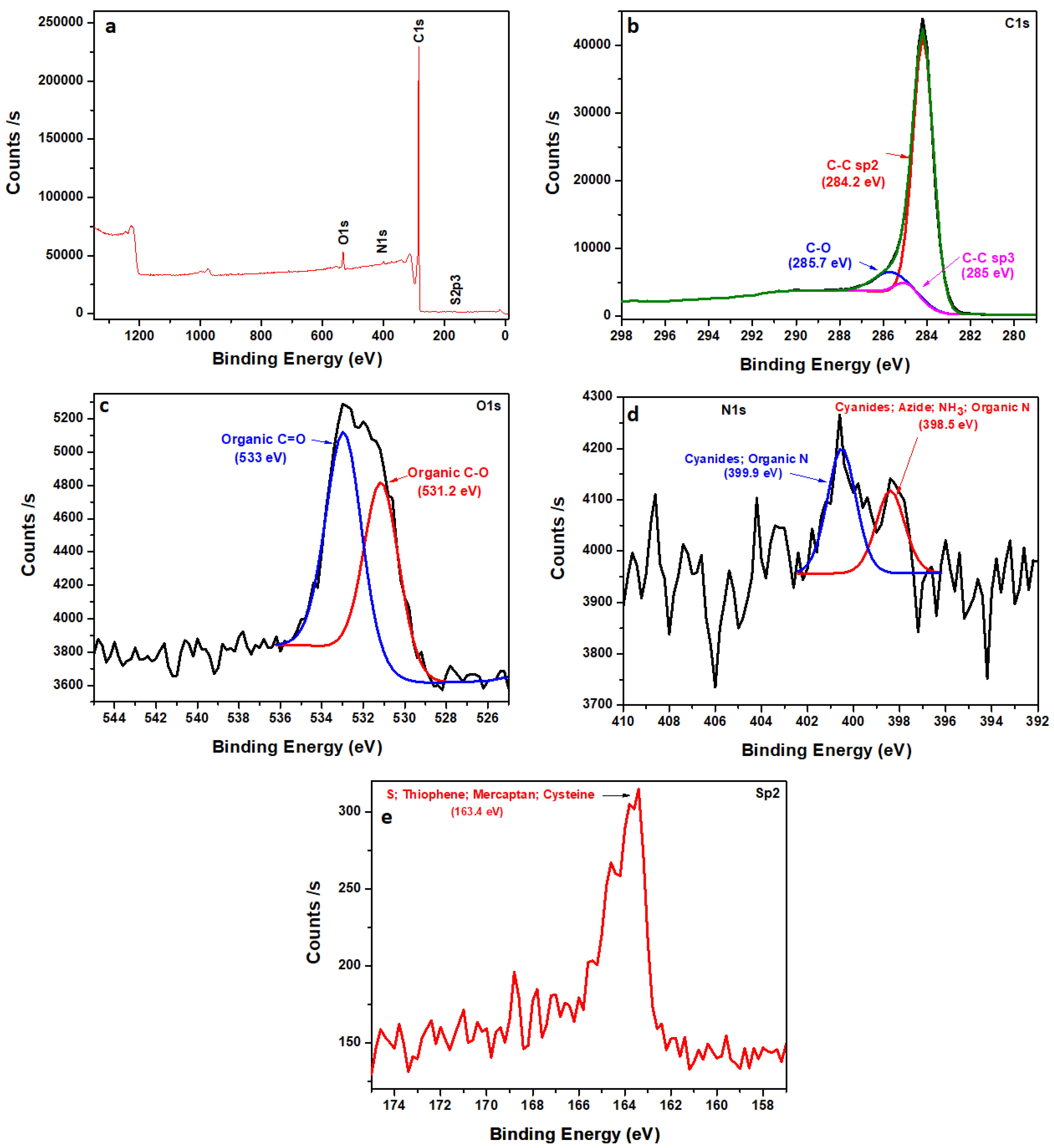
3.1. Morphological, Structural, and Compositional Analyses
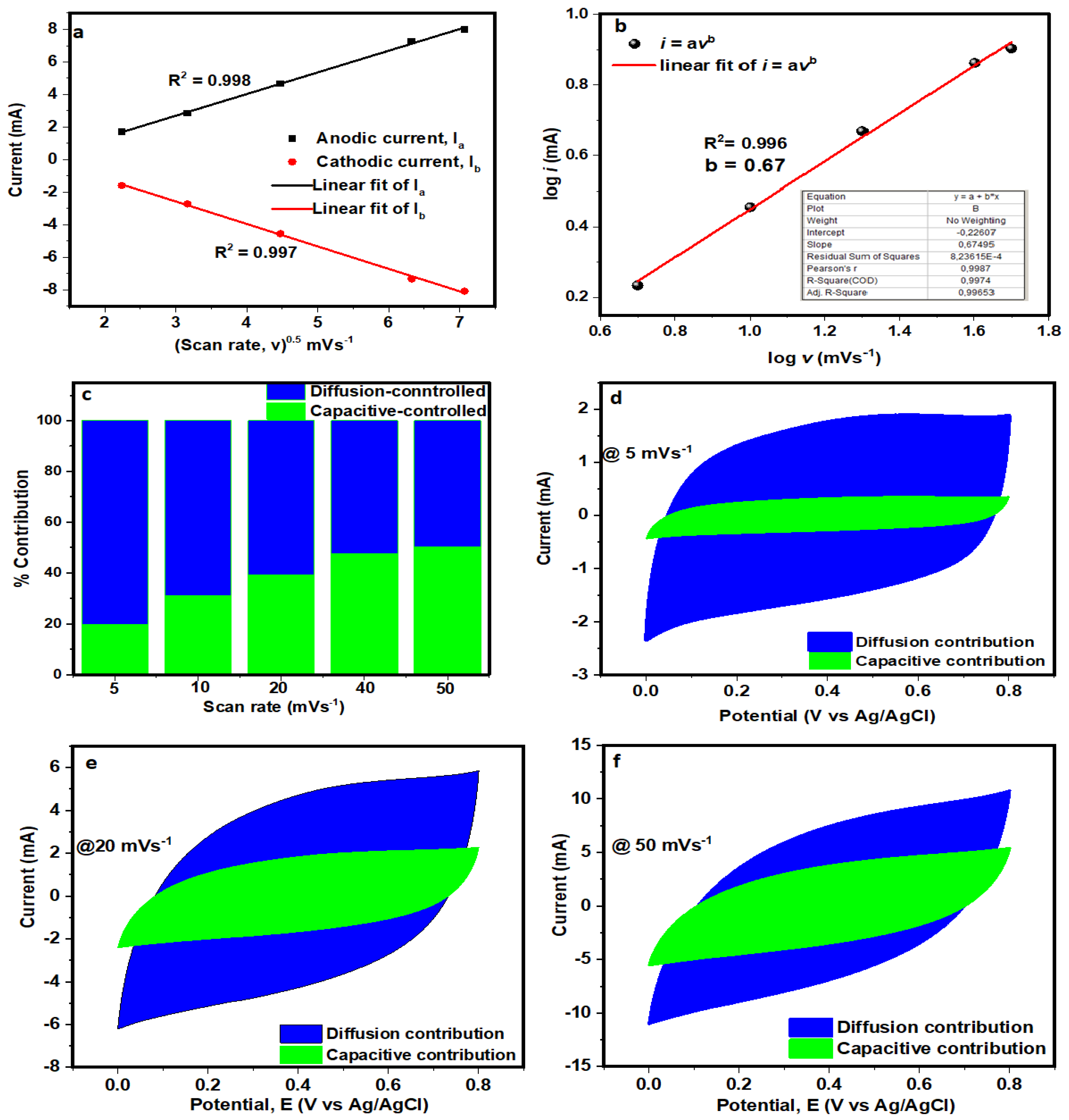
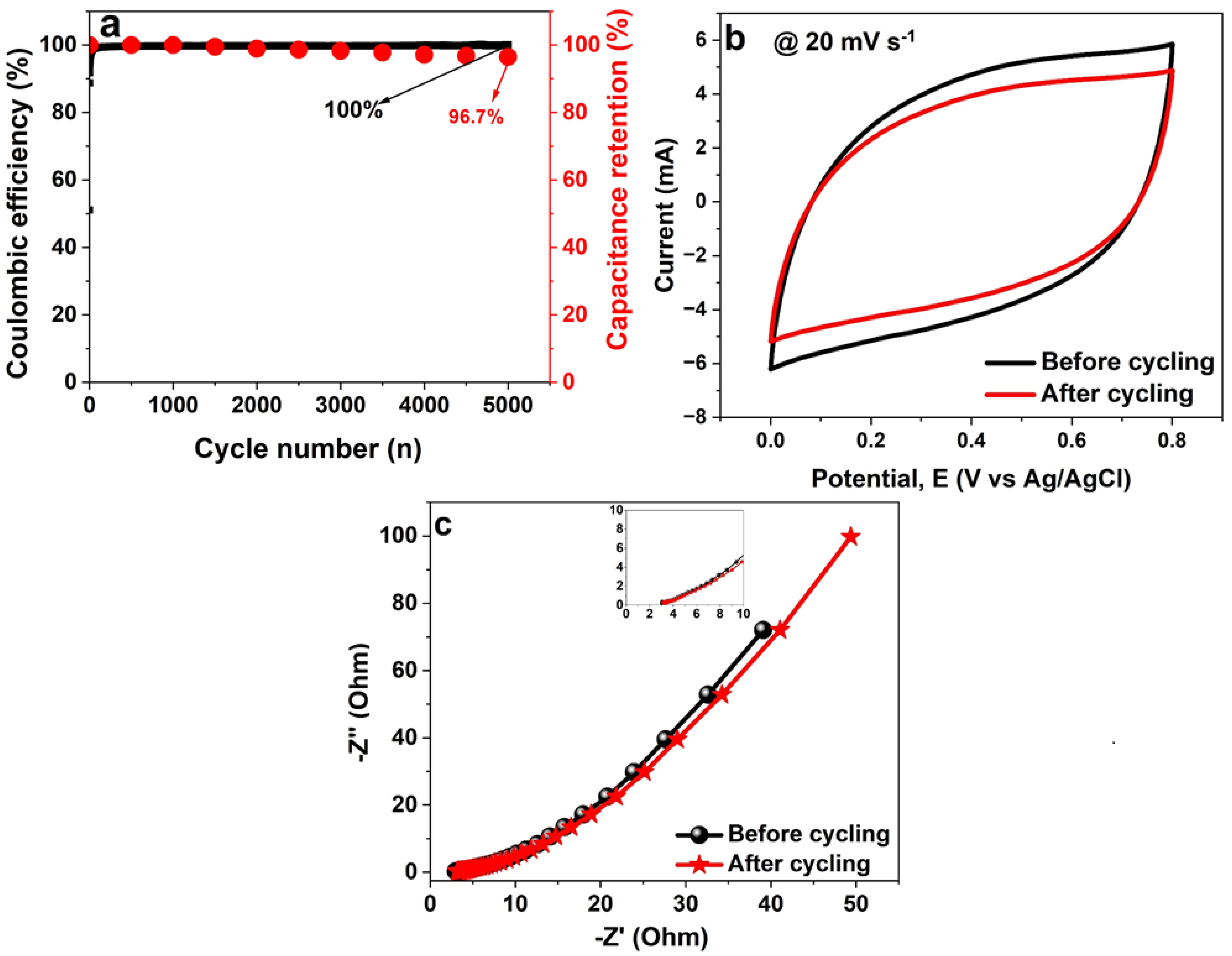
3.2. Electrochemical Characterisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 11, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Yan, D.; Wang, W.; Luo, X.; Chen, C.; Zeng, Y.; Zhu, Z. NiCo2O4 with oxygen vacancies as better performance electrode material for supercapacitor. Chem. Eng. J. 2018, 334, 864–872. [Google Scholar] [CrossRef]
- Xia, C.; Chen, W.; Wang, X.; Hedhili, M.N.; Wei, N.; Alshareef, H.N. Highly stable supercapacitors with conducting polymer core-shell electrodes for energy storage applications. Adv. Energy Mater. 2015, 5, 1401805. [Google Scholar] [CrossRef]
- Wang, W.D.; Zhang, P.P.; Gao, S.Q.; Wang, B.Q.; Wang, X.C.; Li, M.; Liu, F.; Cheng, J.P. Core-shell nanowires of NiCo2O4@ α-Co (OH)2 on Ni foam with enhanced performances for supercapacitors. J. Colloid Interface Sci. 2020, 579, 71–81. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Barzegar, F.; Mirghni, A.A.; Khaleed, A.A.; Masikhwa, T.M.; Manyala, N. Examination of High-Porosity Activated Carbon Obtained from Dehydration of White Sugar for Electrochemical Capacitor Applications. ACS Sustain. Chem. Eng. 2019, 7, 537–546. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Masikhwa, T.M.; Lindberg, S.; Matic, A.; Johansson, P.; Manyala, N. Comparison of ionic liquid electrolyte to aqueous electrolytes on carbon nanofibres supercapacitor electrode derived from oxygen-functionalized graphene. Chem. Eng. J. 2019, 375, 121906. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Madito, M.J.; Momodu, D.Y.; Mirghni, A.A.; Masikhwa, T.M.; Manyala, N. Synthesis of ternary NiCo-MnO2 nanocomposite and its application as a novel high energy supercapattery device. Chem. Eng. J. 2018, 335, 416–433. [Google Scholar] [CrossRef]
- Miller, J.R. A brief history of supercapacitors. Battey Energy Technol. 2007, 61–78. [Google Scholar]
- Conway, B.E. Electrochemical Supercapacitors; Springer: Boston, MA, USA, 1999. [Google Scholar]
- McGill, C.A. A brief history of A Brief History. Pop. Sci. 2010, 235, 70–72. [Google Scholar]
- Oyedotun, K.O.; Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J.; Adegoke, K.A. Advances in Supercapacitor Development: Materials, Processes, and Applications. J. Electron. Mater. 2022, 52, 96–129. [Google Scholar] [CrossRef]
- Liang, Z.; Xia, H.; Liu, H.; Zhang, L.; Zhou, J.; Li, H.; Xie, W. Enhanced capacitance characteristic of microporous carbon spheres through surface modification by oxygen-containing groups. Results Phys. 2019, 15, 102586. [Google Scholar] [CrossRef]
- Kim, C. Electrochemical characterization of electrospun activated carbon nanofibres as an electrode in supercapacitors. J. Power Sources 2005, 142, 382–388. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S.; Moon, J.M.; Bae, D.J.; Lim, S.C.; Lee, Y.S.; Lee, Y.H. Electrochemical properties of high-power supercapacitors using single-walled carbon nanotube electrodes. Adv. Funct. Mater. 2001, 11, 387–392. [Google Scholar] [CrossRef]
- Hwang, S.-R.; Teng, H. Capacitance Enhancement of Carbon Fabric Electrodes in Electrochemical Capacitors through Electrodeposition with Copper. J. Electrochem. Soc. 2002, 149, A591. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Lai, C.C.; Ho, C.L.; Lo, C.T. Preparation of interconnected carbon nanofibers as electrodes for supercapacitors. Electrochim. Acta 2014, 127, 369–376. [Google Scholar] [CrossRef]
- Guler, G.S.; Yildiz, E.; Yazicioglu, N.; Sumnu, G.; Sahin, S. Reduction of lipid oxidation in olive oil using gelatin-based centrifugally spun fibers loaded with caffeic acid. Food Biosci. 2024, 59, 104009. [Google Scholar] [CrossRef]
- Doğan, N.; Doğan, C.; Eticha, A.; Gungor, M.; Control, Y.A.-F. Centrifugally spun micro-nanofibers based on lemon peel oil/gelatin as novel edible active food packaging: Fabrication, characterization, and application to prevent foodborne pathogens E. coli and S. aureus in cheese. Food Control 2022, 139, 109081. [Google Scholar]
- Aydın, H.; Kurtan, U.; Demir, M.; Karakuş, S. Synthesis and Application of a Self-Standing Zirconia-Based Carbon Nanofiber in a Supercapacitor. Energy Fuels 2022, 36, 2212–2219. [Google Scholar] [CrossRef]
- Levitt, A.S.; Alhabeb, M.; Hatter, C.B.; Sarycheva, A.; Dion, G.; Gogotsi, Y. Electrospun MXene/carbon nanofibers as supercapacitor electrodes. J. Mater. Chem. A 2018, 7, 269–277. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Ojha, G.P.; Park, J.; Kuk, Y.S.; Lee, E.J.; Kim, H.Y.; Park, S.J. Carbon nanofibers wrapped with zinc oxide nano-flakes as promising electrode material for supercapacitors. J. Colloid Interface Sci. 2018, 522, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Niu, H.; Wang, H.; Wang, W.; Jin, X.; Wang, H.; Zhou, H.; Lin, T. Micro-meso porous structured carbon nanofibers with ultra-high surface area and large supercapacitor electrode capacitance. J. Power Sources 2020, 482, 228986. [Google Scholar] [CrossRef]
- Dirican, M.; Yanilmaz, M.; Asiri, A.M.; Zhang, X. Polyaniline/MnO2/porous carbon nanofiber electrodes for supercapacitors. J. Electroanal. Chem. 2020, 861, 113995. [Google Scholar] [CrossRef]
- Rajapriya, A.; Keerthana, S.; Viswanathan, C.; Ponpandian, N. Direct growth of MoS2 hierarchical nanoflowers on electrospun carbon nanofibers as an electrode material for high-performance supercapacitors. J. Alloys Compd. 2021, 859, 157771. [Google Scholar] [CrossRef]
- Park, W.K.; Yoon, Y.; Song, Y.H.; Choi, S.Y.; Kim, S.; Do, Y.; Lee, J.; Park, H.; Yoon, D.H.; Yang, W.S. High-efficiency exfoliation of large-area mono-layer graphene oxide with controlled dimension. Sci. Rep. 2017, 7, 16414. [Google Scholar] [CrossRef]
- Kannappan, S.; Kaliyappan, K.; Manian, R.K.; Pandian, A.S.; Yang, H.; Lee, Y.S.; Jang, J.H.; Lu, W. Graphene based Supercapacitors with Improved Specific Capacitance and Fast Charging Time at High Current Density. arXiv 2013, arXiv:1311.1548. [Google Scholar]
- Madito, M.J. Correlation of the graphene fermi-level shift and the enhanced electrochemical performance of graphene-manganese phosphate for hybrid supercapacitors: Raman spectroscopy analysis. ACS Appl. Mater. Interfaces 2021, 13, 37014–37026. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, F.; Momodu, D.Y.; Fashedemi, O.O.; Bello, A.; Dangbegnon, J.K.; Manyala, N. Investigation of different aqueous electrolytes on the electrochemical performance of activated carbon-based supercapacitors. RSC Adv. 2015, 5, 107482–107487. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, Y.Q.; Xia, X.H.; Shi, S.J.; Lu, Y.; Wang, X.L.; Gu, C.D.; Tu, J.P. Self-assembled porous NiCo2O4 hetero-structure array for electrochemical capacitor. J. Power Sources 2013, 239, 157–163. [Google Scholar] [CrossRef]
- Das, S.K.; Pradhan, L.; Jena, B.K.; Basu, S. Polymer derived honeycomb-like carbon nanostructures for high capacitive supercapacitor application. Carbon 2022, 201, 49–59. [Google Scholar] [CrossRef]
- Pokharel, J.; Gurung, A.; Baniya, A.; He, W.; Chen, K.; Pathak, R.; Lamsal, B.S.; Ghimire, N.; Zhou, Y. MOF-derived hierarchical carbon network as an extremely-high-performance supercapacitor electrode. Electrochim. Acta 2021, 394, 139058. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Momodu, D.Y.; Naguib, M.; Mirghni, A.A.; Masikhwa, T.M.; Khaleed, A.A.; Kebede, M.; Manyala, N. Electrochemical performance of two-dimensional Ti3C2-Mn3O4 nanocomposites and carbonized iron cations for hybrid supercapacitor electrodes. Electrochim. Acta 2019, 301, 487–499. [Google Scholar] [CrossRef]
- Wang, J.; Quan, Y.; Wang, G.; Wang, D.; Xiao, J.; Gao, S.; Xu, H.; Liu, S.; Cui, L. 3D hollow cage copper cobalt sulfide derived from metal-organic frameworks for high-performance asymmetric supercapacitors. Cryst. Eng. Comm. 2021, 23, 7385–7396. [Google Scholar] [CrossRef]
- Pan, H.; Yang, J.; Wang, S.; Xiong, Z.; Cai, W.; Liu, J. Facile fabrication of porous carbon nanofibers by electrospun PAN/dimethyl sulfone for capacitive deionization. J. Mater. Chem. A 2015, 3, 13827–13834. [Google Scholar] [CrossRef]
- Nan, W.; Zhao, Y.; Ding, Y.; Shende, A.R.; Fong, H.; Shende, R.V. Mechanically flexible electrospun carbon nanofiber mats derived from biochar and polyacrylonitrile. Mater. Lett. 2017, 205, 206–210. [Google Scholar] [CrossRef]
- Yan, X.; You, H.; Liu, W.; Wang, X.; Wu, D. Free-standing and heteroatoms-doped carbon nanofiber networks as a binder-free flexible electrode for high-performance supercapacitors. Nanomaterials 2019, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, Y.; Shi, J.; Song, Y.; Liu, L. High-performance supercapacitor electrodes based on porous flexible carbon nanofiber paper treated by surface chemical etching. Chem. Eng. J. 2014, 249, 216–225. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Y.; Chi, H.; Zhao, H.; Hu, J.; Xiao, G. Boosted electrochemical properties of polyimide-based carbon nanofibers containing micro/mesopore for high-performance supercapacitors by thermal rearrangement. J. Energy Storage 2022, 47, 103672. [Google Scholar] [CrossRef]
- Yu, S.; Myung, N.V. Minimizing the diameter of electrospun polyacrylonitrile (PAN) nanofibers by design of experiments for electrochemical application. Electroanalysis 2018, 30, 2330–2338. [Google Scholar] [CrossRef]
- Fan, Q.; Ma, C.; Wu, L.; Wei, C.; Wang, H.; Song, Y.; Shi, J. Preparation of cellulose acetate derived carbon nanofibers by ZnCl2 activation as a supercapacitor electrode. RSC Adv. 2019, 9, 6419–6428. [Google Scholar] [CrossRef]



| Material | SSA (m2 g−1) | Specific Capacitance (F g−1) | Stability | Electrolyte | Reference |
|---|---|---|---|---|---|
| PBI/DMAc | 1220 | 202 @ 1 mA cm2 | N/A | 1 M H2SO4 | [18] |
| PAN-co-butadiene based CNF | N/A | 172.0 @ 1 A g−1 | 100% after 2000 cycles | 2 M KOH | [22] |
| PAN and MXene in DFM | N/A | 205.0 mF cm−2 @ 50.0 mV/s | N/A | 1 M H2SO4 | [26] |
| PAN–DMSO/DMF | 212 | 43 @ 2 mV s−1 | N/A | 1 M NaCl | [40] |
| PAN + biochar + DMF | 30.12 | 37.60 @ 500.0 m (A g−1) | N/A | 1 M NaOH | [41] |
| PAA–PDPP/DMF | 130.6 | 182 @ 1 A g−1 | 88% after 10,000 cycles | 6 M KOH | [42] |
| Porous CNFs | N/A | 362.0 @ 0.2 A g−1 | N/A | 6 M KOH | [43] |
| hydroxyl-containing polyimide (HPI) nanofibres | 1107.6 | 263.9 @ 0.5 A g−1 | 99.8% after 10,000 cycles | 6 M KOH | [44] |
| PAN and 0.1 wt.% in N, N-dimethylformamide | 684 | 10.0 @ 10.0 mV s−1 | N/A | 1 M KCl | [45] |
| CA–DMAc/acetone | 1188 | 202 @ 0.1 A g−1, | 92% after 5000 cycles | 6 M KOH | [46] |
| PBI_NCFs | 49 | 78 @ 0.5 A g−1 | 96.7% after 5000 cycles | 2.5 M KNO3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyedotun, K.O.; Makgopa, K.; Nkambule, T.T.; Mathe, M.K.; Otun, K.O.; Mamba, B.B. Nanostructured Carbon Fibres (NCF): Fabrication and Application in Supercapacitor Electrode. Polymers 2024, 16, 1859. https://doi.org/10.3390/polym16131859
Oyedotun KO, Makgopa K, Nkambule TT, Mathe MK, Otun KO, Mamba BB. Nanostructured Carbon Fibres (NCF): Fabrication and Application in Supercapacitor Electrode. Polymers. 2024; 16(13):1859. https://doi.org/10.3390/polym16131859
Chicago/Turabian StyleOyedotun, Kabir O., Katlego Makgopa, Thabo T. Nkambule, Mkhulu K. Mathe, Kabir O. Otun, and Bhekie B. Mamba. 2024. "Nanostructured Carbon Fibres (NCF): Fabrication and Application in Supercapacitor Electrode" Polymers 16, no. 13: 1859. https://doi.org/10.3390/polym16131859
APA StyleOyedotun, K. O., Makgopa, K., Nkambule, T. T., Mathe, M. K., Otun, K. O., & Mamba, B. B. (2024). Nanostructured Carbon Fibres (NCF): Fabrication and Application in Supercapacitor Electrode. Polymers, 16(13), 1859. https://doi.org/10.3390/polym16131859








