Research on the Performance and Modification Mechanism of Gutta-Percha-Modified Asphalt
Abstract
1. Introduction
2. Molecular Modeling and Verification
2.1. Molecular Modeling
2.1.1. BA Molecular Modeling
2.1.2. EUG Molecular Modeling
2.1.3. SEUG Molecular Modeling
2.2. Validation in Molecular Modeling
3. Determination of Test Materials, GPMA Preparation Process, and Design of Test Program
3.1. Test Materials
3.1.1. Base Asphalt
3.1.2. EUG
3.1.3. Additives
3.2. GPMA Preparation
3.2.1. SEUG Preparation Process
3.2.2. Preparation Process of GPMA
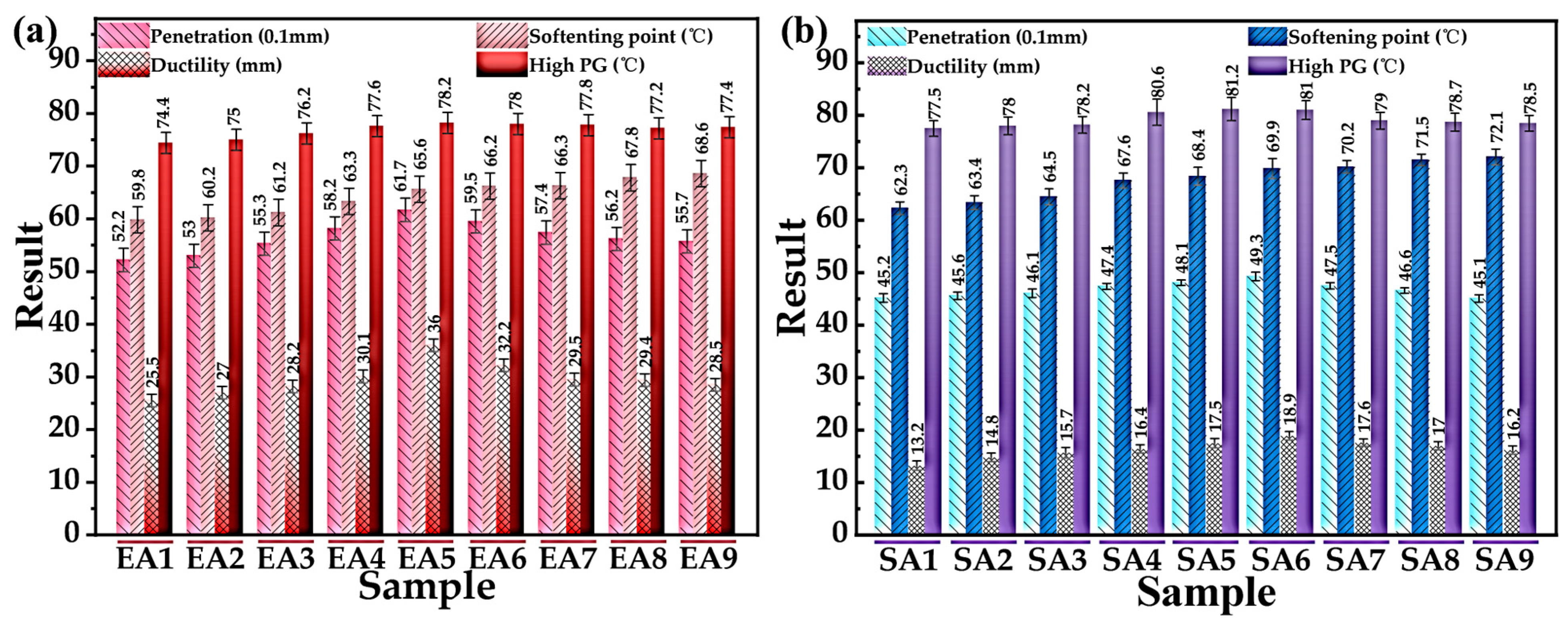
3.2.3. Determination of the Optimum Preparation Process for GPMA
3.3. Design of Experiments
3.3.1. Dynamic Shear Rheology (DSR) Test
3.3.2. Multiple Stress Creep Recovery (MSCR) Test
3.3.3. Low-Temperature Bending Beam Rheology (BBR) Test
3.3.4. Fourier Transform Infrared Spectroscopy (FTIR) Test
3.3.5. Fluorescence Microscopy (FM) Test
3.3.6. Scanning Electron Microscope (SEM) Test
3.3.7. Atomic Force Microscopy (AFM) Tests
4. Results and Discussion
4.1. Compatibility of GPMA with Asphalt
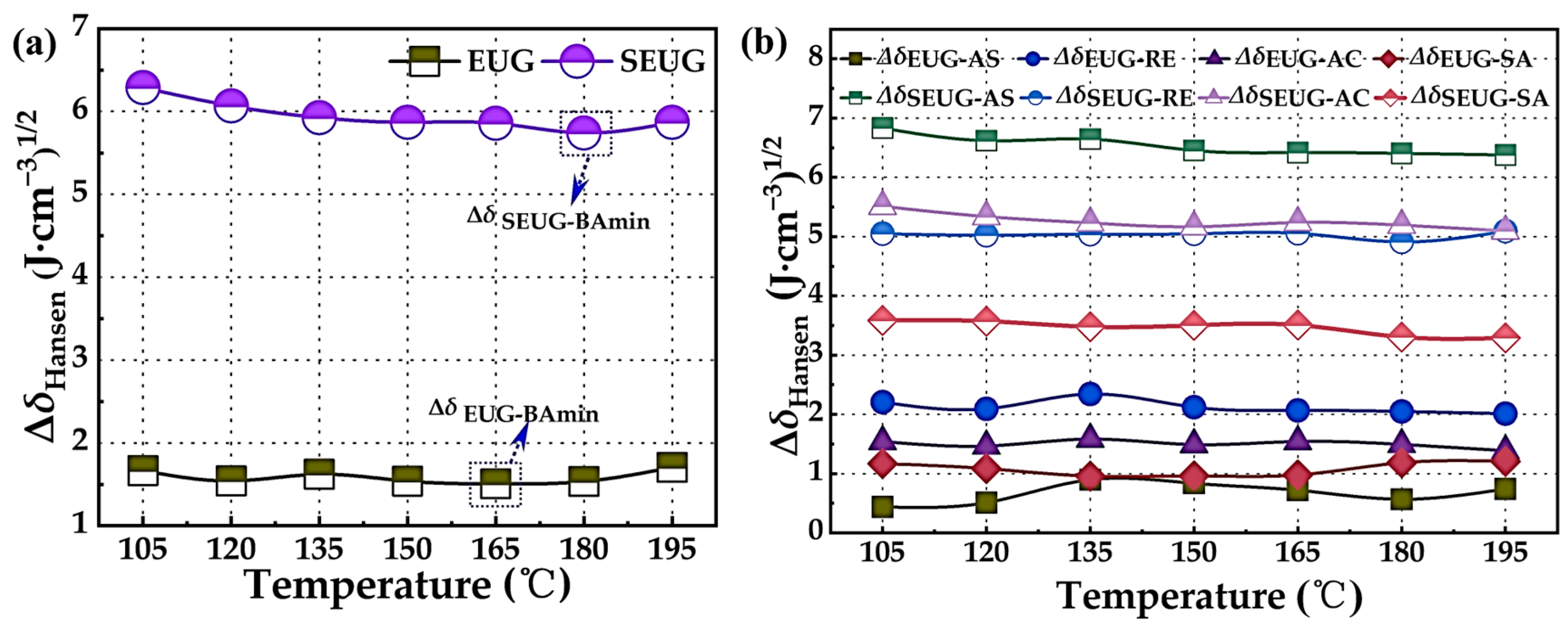
4.1.1. Hansen Solubility Parameter
4.1.2. Interaction Energies
4.2. Rheological Properties of GPMA
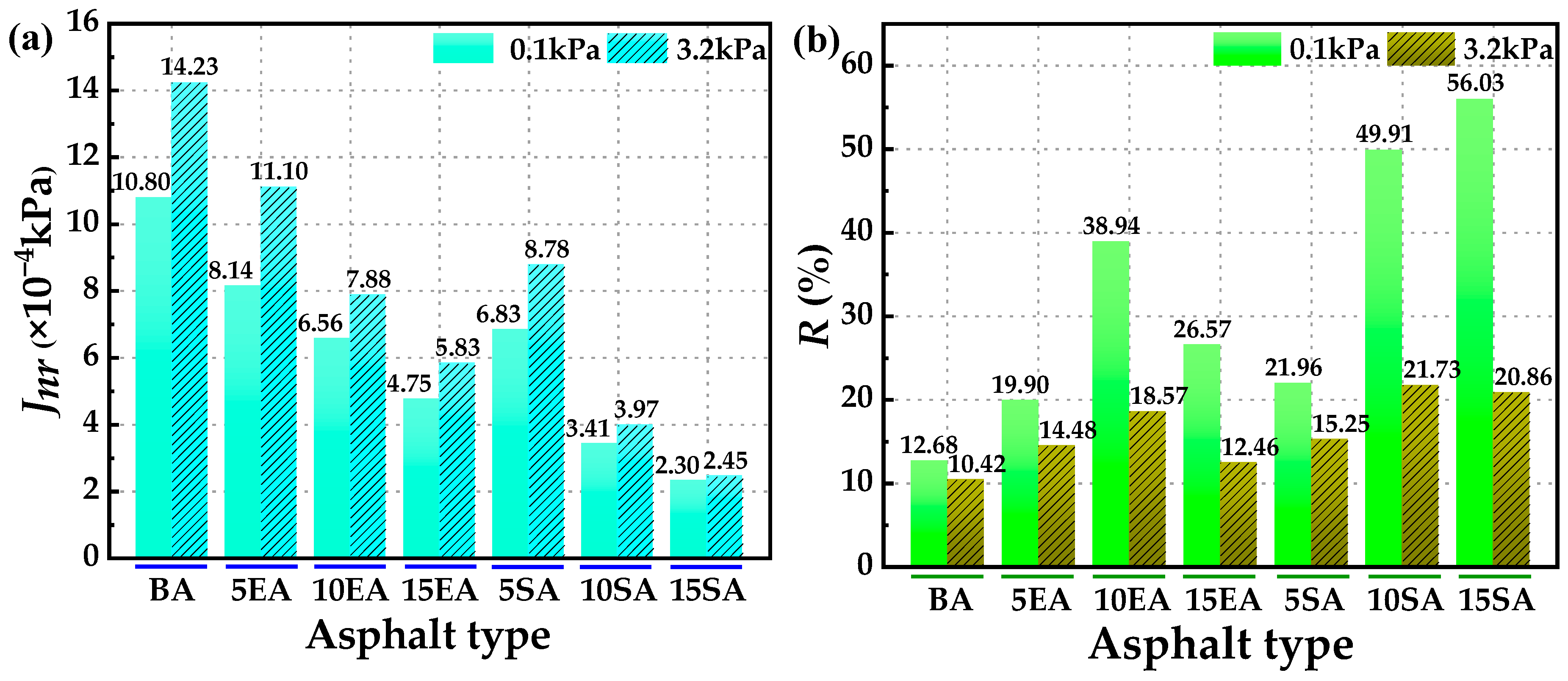
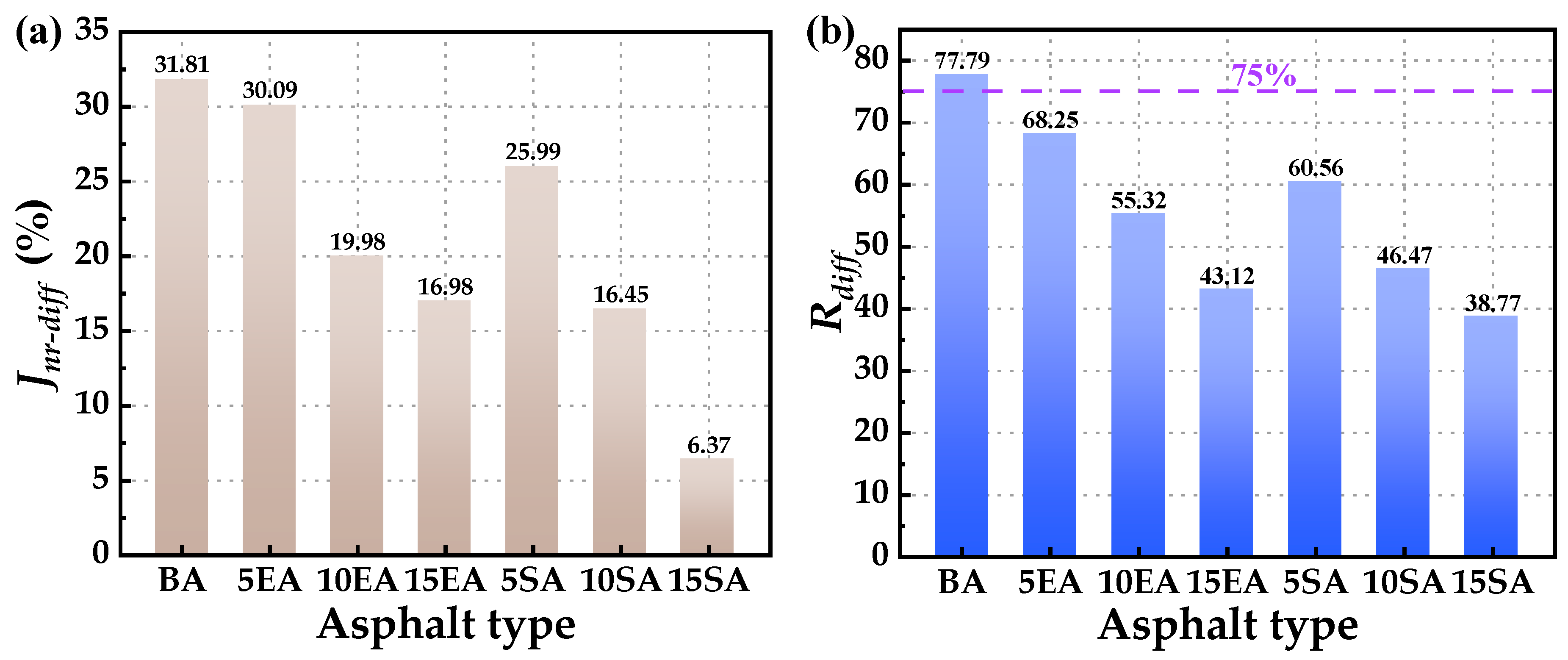
4.2.1. High-Temperature Rutting Resistance
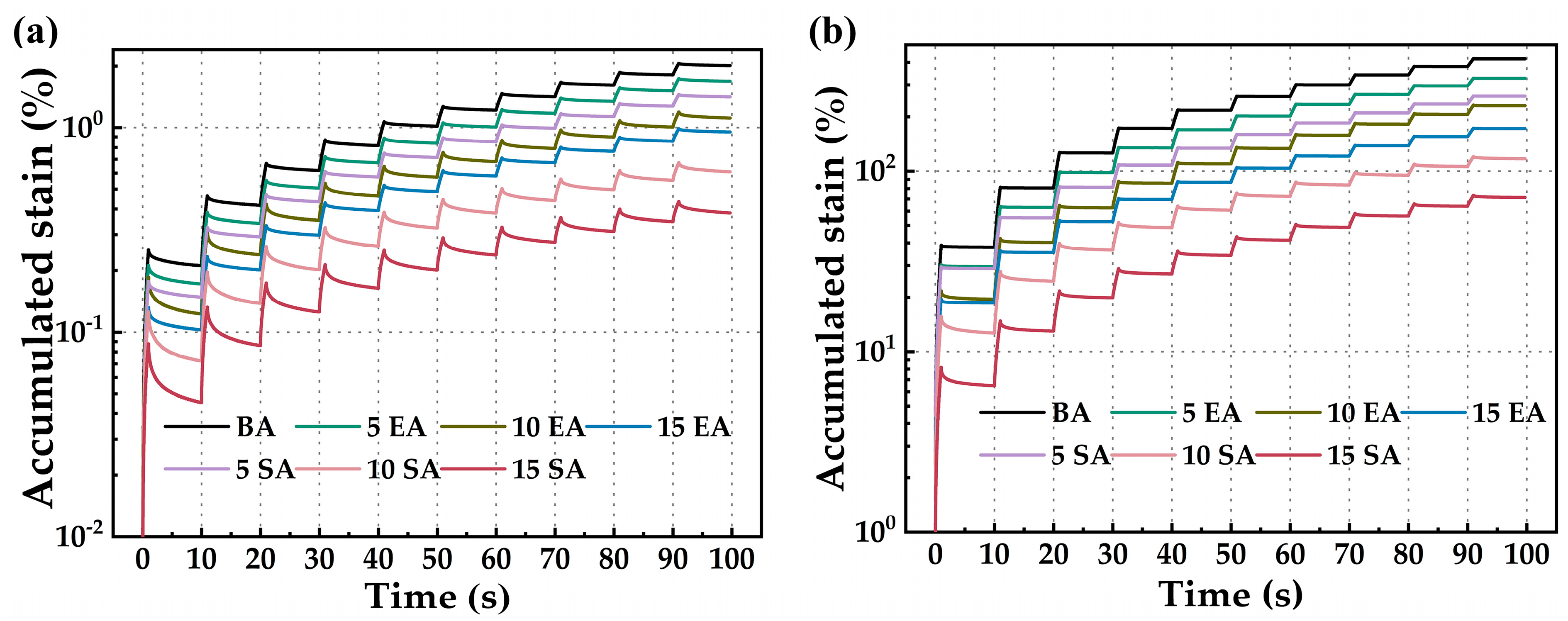
4.2.2. High-Temperature Creep Recovery Performance
4.2.3. Resistance to Cracking at Low Temperatures
4.3. Modified Mechanism of GPMA
4.3.1. FTIR Test
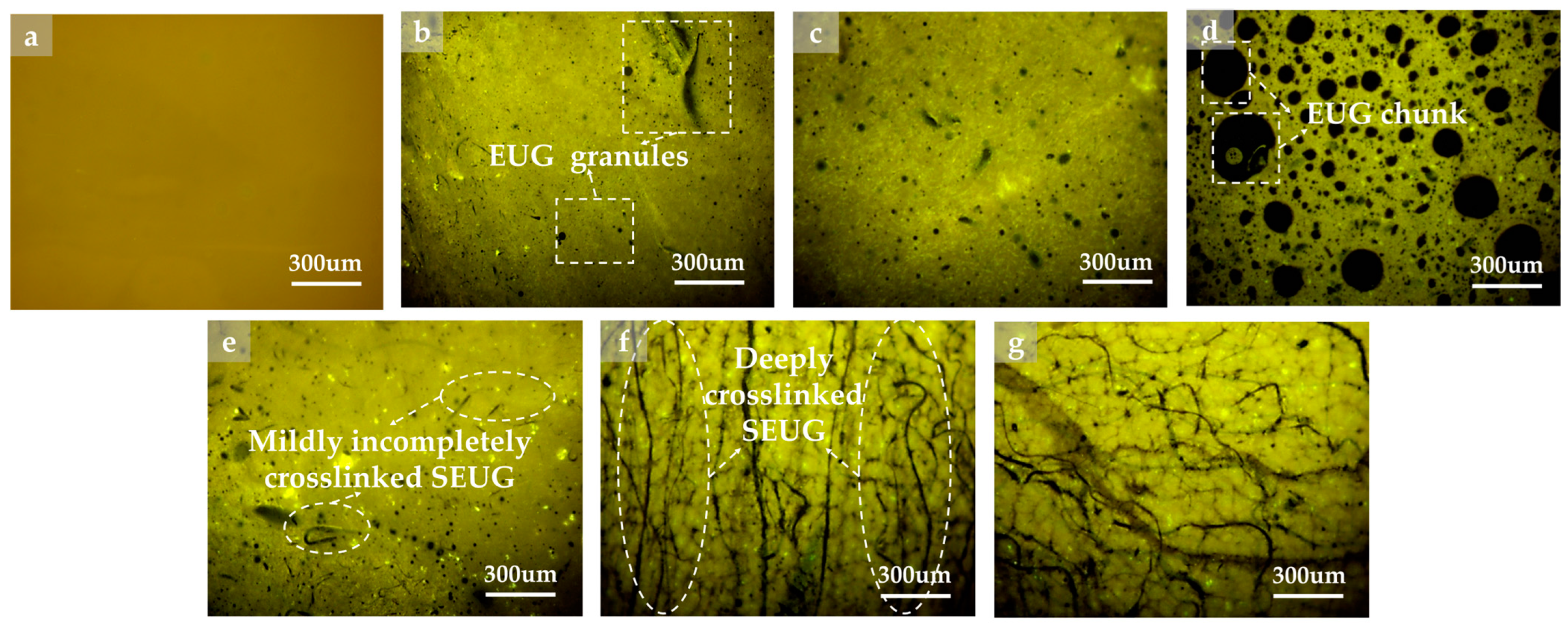
4.3.2. FM Test
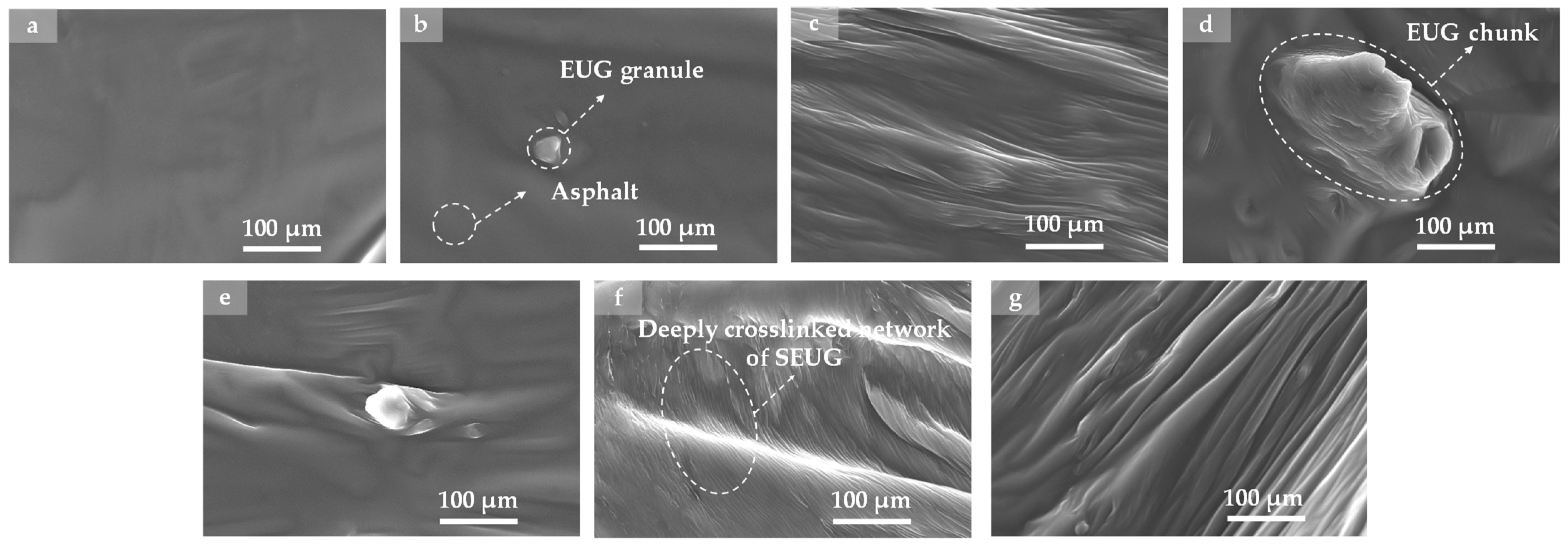
4.3.3. SEM Test
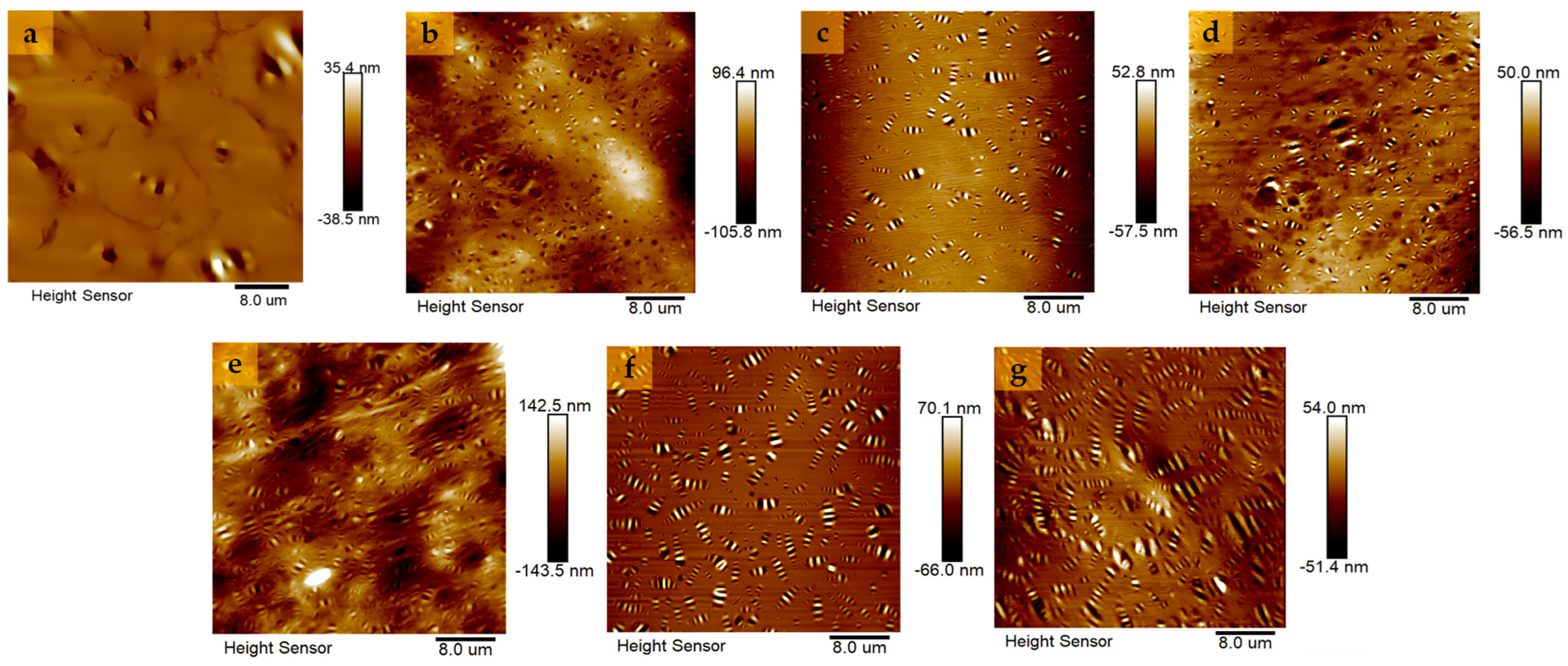
4.3.4. AFM Test
5. Conclusions
- (1)
- The MD results indicate that the compatibility between EUG and asphalt is superior, and the compatibility with BA achieves its optimum at a temperature of 165 °C. EUG forms a micellar system with asphaltenes and the saturates of asphaltene by polar interactions. SEUG is not compatible with asphalt; however, the compatibility between SEUG and asphalt is optimal at a temperature of 180 °C. SEUG forms π-π conjugation interactions with naphthene aromatics and polar aromatics present in asphaltene. The primary source of interaction energy between GP and asphalt molecules is predominantly derived from van der Waals forces.
- (2)
- The optimal preparation process of GPMA was discussed by using the gray correlation theory, and the results show that the optimal preparation process parameters of EUGMA were T1 = 145 °C, T2 = 165 °C; S1 =3000 r/min, S2 = 5000 r/min; and t1 = 60 min, t2 = 60 min. The optimal preparation process parameters of SEUGMA were T1 = 155 °C, T2 = 180 °C; S1 = 4000 r/min, S2 = 6000 r/min; and t1 = 90 min, t2 = 90 min. Since the T2 of EUGMA and SEUGMA are consistent with the results of MD simulation, the validity of MD simulation is proved.
- (3)
- The macro rheological test findings indicate that both EUG and SEUG have a considerable positive impact on the high-temperature stability and deformation resistance of asphalt. However, they have a negative effect on the low-temperature performance of asphalt. The 15wt% SEUGMA has superior high-temperature stability but poor resistance to low-temperature cracking. By conducting a microscopic performance test, it was determined that the preparation method of EUGMA involved physical blending, while the preparation method of SEUGMA primarily involved physical blending with a minor component of chemical mixing.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- 2023–2028 China Asphalt Industry Market Demand Forecast and Investment Strategic Planning Analysis Report; Prospective Industry Research Institute: Beijing, China, 2023.
- Zhu, J.; Birgisson, B.; Kringos, N. Polymer modification of bitumen: Advances and challenges. Eur. Polym. J. 2014, 54, 18–38. [Google Scholar] [CrossRef]
- Ji, Y.H.; Guo, S.H.; Li, R. Mechanism of compatibility and stability of SBS modified asphalt. Acta Pet. Sin. (Pet. Process. Sect.) 2002, 3, 23–29. [Google Scholar]
- Lu, Y.Y.; Yu, L.M.; Fang, J.; Yun, Q.Q.; Lu, J.Y. Research development of polymer modified asphalt. New. Chem. Mater. 2020, 48, 222–225. [Google Scholar] [CrossRef]
- Fisher, H.L. Conversion of Rubber into Thermoplastic Products with Properties Similar to Gutta-Percha, Balata, and Shellac. Ind. Eng. Chem. 1927, 19, 1325–1333. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Dong, D.; Wang, Q. A review of the properties and extraction of Eucommia rubber. Guizhou Chem. Ind. 2006, 4, 4–8. [Google Scholar]
- Ma, J.; Lin, Y.; Liu, B.; Liu, R.; Wang, H. The Development Status and Prospect of Eucommia Ulmoides Gum in China. J. Anhui Agric. Sci. 2012, 40, 3396–3398. [Google Scholar] [CrossRef]
- Yan, R.F. An Age-Old and Young Natural Polymer—Gutta-Percha. Chin. Polym. Bull. 1989, 2, 39–40. [Google Scholar]
- Du, H.Y.; Xie, B.X.; Shao, S.M. Prospects and Research Progress of Gutta-percha. Cent. South Univ. (Engl. Ed.) 2003, 4, 95–99. [Google Scholar]
- Du, H.Y.; Zhao, G.; Lu, X.K. The Development Tendency of the Industrialization and Culture Techniques of Eucommia ulmoides in China. For. Res. 2000, 5, 554–561. [Google Scholar] [CrossRef]
- Liu, Y.J.; Du, H.Y.; Liu, L.W.; Leng, J.S. Shape memorypolymers and their composites in aerospace applications a review. Smart Mater. Struct. 2014, 23, 23001. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.H.; X, L.; Shen, M.; Wei, L.P.; Xin, Z.X. Fully Biobased Shape Memory Thermoplastic Vulcanizates from Poly (Lactic Acid) and Modified Natural Eucommia Ulmoides Gum with Co-Continuous Structure and Super Toughness. Polymers 2019, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Li, Z.G. Research on blending asphalt with eucommia ulmoides gum. J. PLA Univ. Sci. Technol. (Nat. Sci. Ed.) 2007, 2, 176–179. [Google Scholar]
- Li, Y. Engineering Characteristics of Eucommia Ulmoides Gum and Its Use in Asphalt Modifying. Highway 2008, 3, 147–150. [Google Scholar]
- Li, Z.G.; Deng, X.Y.; Shen, J.X. Test and Research on Effect of Eucommia Ulmoides Gum Blending with SBS Modified Asphalt. Highway 2008, 8, 217–220. [Google Scholar]
- Fang, J.H. Research on Vulcanized Eucommia Ulmoides Gum Blending with Asphalt Applied on West Zone of High-altitude and Chilliness. Highw. Eng. 2012, 4, 40–43. [Google Scholar]
- Chen, Z.Q.; Li, Z.G. Study on the vulcanization cross-linking degree of Eucommia gum used in asphalt modification. Highway 2013, 8, 267–270. [Google Scholar]
- Li, Z.G.; Li, C.; Cai, M.D. Modification mechanism and effect of grafted eucommia ulmoides gum on dry-process rubber bitumen mixture. Dongnan Daxue Xuebao Nat. Sci. 2014, 4, 845–848. [Google Scholar]
- Deng, X.Y.; Li, Z.G.; Huang, Y.X.; Luan, Y.B. Improving mechanism and effect analysis of sulfurated and grafted Eucommia Ulmoides Gum modified rubber asphalt. Constr. Build. Mater. 2017, 148, 715–722. [Google Scholar] [CrossRef]
- Li, Z.G.; Deng, S.Q.; Tan, F.M.; Xu, L. Analysis on temperature and time for mixing preparation of crumb rubber modified by eucommia ulmoides gum. Dongnan Daxue Xuebao Nat. Sci. 2020, 6, 1109–1114. [Google Scholar]
- Li, N.; Xu, J.J.; Xu, T. Preparation, properties and modification mechanism of vulcanized eucommia ulmoides gum modified asphalt. Constr. Build. Mater. 2021, 274, 121992. [Google Scholar] [CrossRef]
- Cui, S.C.; Guo, N.S.; Tan, Y.Q.; You, Z.P.; Chu, Z.Y.; Jin, X.; Guo, Z.X. Preparation and microstructural and thermal properties of a vulcanized Eucommia ulmoides gum modified asphalt. Constr. Build. Mater. 2023, 408, 133727. [Google Scholar] [CrossRef]
- Liu, Q.; Yue, H.; Jiang, H.; Chen, C. Molecular Dynamics and Dissipative Particle Dynamics Simulation of TPI/NR Blends. Mater. Rev. 2012, 26, 141–145. [Google Scholar]
- Li, D.D.; Greenfield, M.L. Viscosity, relaxation time, and dynamics within a model asphalt of larger molecules. J. Chem. Phys. 2014, 140, 34507. [Google Scholar] [CrossRef]
- Martín-Martínez, F.J.; Fini, E.H.; Buehler, M.J. Molecular asphaltene models based on Clar sextet theory. RSC Adv. 2015, 5, 753–759. [Google Scholar] [CrossRef]
- Ding, H.Y.; Wang, H.N.; Qu, X.; Aikaterini, V.; Gao, J.F.; You, Z.P. Towards an understanding of diffusion mechanism of bio-rejuvenators in aged asphalt binder through molecular dynamics simulation. J. Clean. Prod. 2021, 299, 126927. [Google Scholar] [CrossRef]
- Fardin, K.; Rajesh, K. Molecular simulations of asphalt rheology: Application of time–temperature superposition principle. J. Rheol. 2018, 62, 941–954. [Google Scholar] [CrossRef]
- Zhang, J.C.; Xue, Z.H. A comparative study on the properties of Eucommia ulmoides gum and synthetic trans-1,4-polyisoprene. Polym. Test. 2011, 30, 753–759. [Google Scholar] [CrossRef]
- Yan, S.M.; Guo, N.S.; Jin, X.; Chu, Z.Y.; Yan, S.T. The Study on Mathematical Simulation and Analysis of the Molecular Discrete System of the Sulfurated Eucommia Ulmoides Gum. Mathematics 2023, 11, 964. [Google Scholar] [CrossRef]
- Cui, S.Y.; Zhang, J.C.; Chen, Y.H.; Dong, M.J.; Liu, G.X.; Zhang, J.J.; Li, L.L.; Yue, H. Study on degrees of mesomorphic zone of polymer. III. Determination of the degree of crystallinity of Eucommia Ulmoides gum by VTFTIR and VTWAXD. Polym. Test. 2020, 89, 106605. [Google Scholar] [CrossRef]
- Sven, E.; Jan, M.; Reinhard, H. Computer simulation of thermal conductivity in vulcanized polyisoprene at variable strain and temperature. Phys. Rev. B 2017, 96, 54110. [Google Scholar] [CrossRef]
- Aleksandr, V.; Tommy, L.; Cornelia, B. Thermal Conductivities of Crosslinked Polyisoprene and Polybutadiene from Molecular Dynamics Simulations. Polymer 2021, 13, 315. [Google Scholar] [CrossRef]
- Johannes, K.; Carr, J.M.; Keal, T.W.; Thiel, W.; Wander, A.; Sherwood, P. DL-FIND: An open-source geometry optimizer for atomistic simulations. J. Phys. Chem. A 2009, 113, 11856–11865. [Google Scholar] [CrossRef]
- Yang, Y.L.; JiménezNegrón, O.A.; Kitchin, J.R. Machine-learning accelerated geometry optimization in molecular simulation. J. Chem. Phys. 2021, 154, 234704. [Google Scholar] [CrossRef]
- Zhang, W.N.; Yu, F.; Zhao, S.L.; Zhang, Z.Q.; He, Y.P. Progress in Molecular Dynamics and Hansen Solubility Parameters of Low Molecular Weight Gels. Chin. J. Appl. Chem. 2022, 39, 1803–1817. [Google Scholar] [CrossRef]
- Lv, Z.T.; Pan, L.; Zhang, J.M.; Lin, X.J. Molecular Dynamics Simulation of Adhesion Mechanism of Asphalt-aggregate Interface. J. Mater. Sci. Eng. 2022, 40, 809–815. [Google Scholar] [CrossRef]
- Tang, W.; Guo, Y.J.; LYU, Y.J.; Chen, H. Influence of Biological Rejuvenator on Molecular Agglomeration Behavior of Aged Asphalt. J. Chongqing Jiaotong Univ. Nat. Sci. 2022, 41, 92–97. [Google Scholar]
- Fang, Q.H. Development of Eucommia Ulmoides Gum Industry in China and Its Application in Tire. Tire Ind. 2020, 40, 387–393. [Google Scholar]
- He, M.J.; Zhang, D.H.; Chen, W.X. Polymer Physics, 3rd ed.; Fudan University Press: Shanghai, China, 2006. [Google Scholar]
- You, L.Y.; Theodora, S.C.; Dai, Q.L.; You, Z.P.; Khanal, A. Experimental and molecular dynamics simulation study on thermal, transport, and rheological properties of asphalt. Constr. Build. Mater. 2020, 265, 120358. [Google Scholar] [CrossRef]
- Li, G.N.; Tan, Y.Q.; Fu, Y.K.; Liu, P.F.; Fu, C.L.; Markus, O. Density, zero shear viscosity and microstructure analysis of asphalt binder using molecular dynamics simulation. Constr. Build. Mater. 2022, 345, 128332. [Google Scholar] [CrossRef]
- Menozzi, A.; Garcia, A.; Partl, M.N.; Tebaldi, G.; Schuetz, P. Induction healing of fatigue damage in asphalt test samples. Constr. Build. Mater. 2015, 74, 162–168. [Google Scholar] [CrossRef]
- JTG E20-2011; Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering. PRC Ministry of Transport (MOT): Beijing, China, 2011.
- Lei, Y.; Wei, Z.H.X.; Wang, H.N.; You, Z.P.; Yang, X.; Chen, Y. Effect of crumb rubber size on the performance of rubberized asphalt with bio-oil pretreatment. Constr. Build. Mater. 2021, 285, 122864. [Google Scholar] [CrossRef]
- Xiao, F.P.; Zong, Q.D.; Wang, J.G.; Chen, J.; Liu, J. Storage stability characterization and improvement of SBS and crumb rubber composite modified asphalt. Road. Mater. Pavement. 2022, 23, 509–526. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, L.; Xin, Z. Triple shape memory effect of foamed natural Eucommia ulmoides gum/high-density polyethylene composites. Polym. Advan. Technol. 2018, 29, 190–197. [Google Scholar] [CrossRef]
- Yao, X.; Li, C.; Xu, T. Multi-scale studies on interfacial system compatibility between asphalt and SBS modifier using molecular dynamics simulations and experimental methods. Constr. Build. Mater. 2022, 346, 128502. [Google Scholar] [CrossRef]
- Song, Z.F.; Wei, Z.Q.; Zheng, C.F.; Li, H.J.; Zhao, J.; Luo, H.S.; Jin, W.D.; Wang, F.Y. Microscopic mechanism and effect analysis of polymer modifiers on embrittlement and viscosity behaviour of asphalt. Results Eng. 2024, 22, 102204. [Google Scholar] [CrossRef]
- Sreeram, A.; Filonzi, A.; Komaragiri, S.; Lakshmi, R.K.; Masad, E.; Bhasin, A. Assessing impact of chemical compatibility of additives used in asphalt binders: A case study using plastics. Constr. Build. Mater. 2022, 359, 129349. [Google Scholar] [CrossRef]
- Press, D.T. The Three Dimensional Solubility Parameter and Solvent Diffusion Coeffocient. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, 1967. [Google Scholar]
- Zhang, H.L.; Chen, Z.H.; Xu, G.Q.; Shi, C.J. Evaluation of aging behaviors of asphalt binders through different rheological indices. Fuel 2018, 221, 78–88. [Google Scholar] [CrossRef]
- Xu, B.; Wang, K.; Zhou, W.C. Evaluation of High Temperature Performance of Foamed Asphalt Based on the Improved Rutting Factor. J. Mater. Sci. Eng. 2015, 33, 899–902. [Google Scholar] [CrossRef]
- Li, X.Z.; Sha, A.M.; Jiao, W.X.; Song, R.M.; Cao, Y.S.; Li, C.; Liu, Z.Z. Fractional derivative Burgers models describing dynamic viscoelastic properties of asphalt binders. Constr. Build. Mater. 2023, 408, 133552. [Google Scholar] [CrossRef]
- Lei, Z.; Chao, X.; Fei, G.; Tian, S.L.; Tan, Y.Q. Using DSR and MSCR Tests to Characterize High Temperature Performance of Different Rubber Modified Asphalt. Constr. Build. Mater. 2016, 127, 466–474. [Google Scholar] [CrossRef]
- Guo, Y.M.; Xu, L.; Wu, L.; Shen, X.Y. High-Temperature Performance Evaluation of Modified Asphalts Based on Multiple Stress Creep Recovery Test. J. Build. Mater. 2018, 21, 154–158. [Google Scholar]
- Du, Z.Y.; Jiang, C.S.; Yuan, J.; Xiao, F.P.; Wang, J.G. Low temperature performance characteristics of polyethylene modified asphalts—A review. Constr. Build. Mater. 2020, 264, 120704. [Google Scholar] [CrossRef]
- Jin, X. Study on Rheological Properties and Inherent Mechanism of TPU Modified Asphalt and Mastic. Ph.D. Thesis, Dalian Maritime University, Dalian, China, 2021. [Google Scholar]
- Meegoda, J.N.; Gao, S.Y. Roughness Progression Model for Asphalt Pavements Using Long-Term Pavement Performance Data. Transp. Eng. J. ASCE 2014, 140, 4014037. [Google Scholar] [CrossRef]
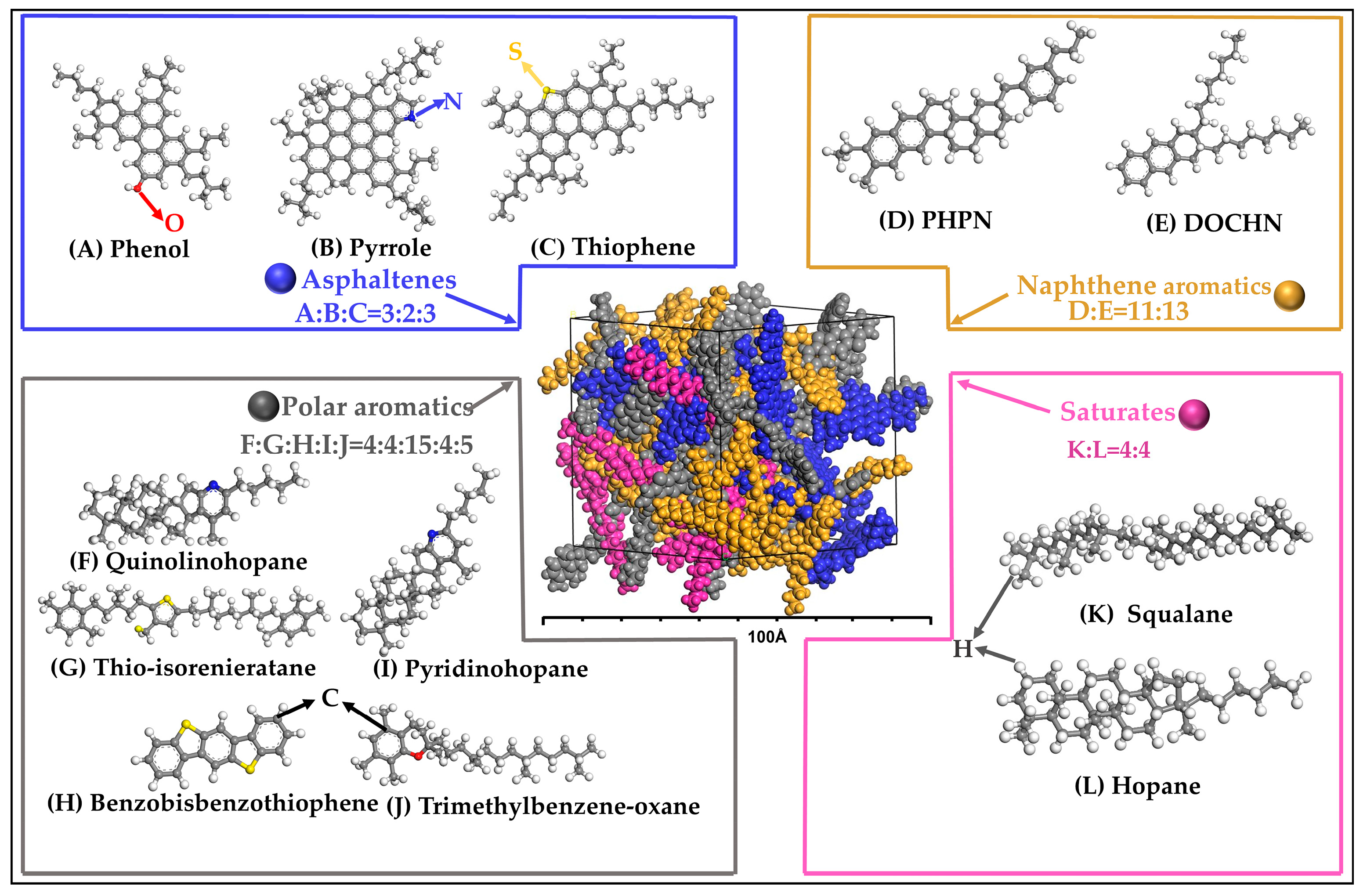

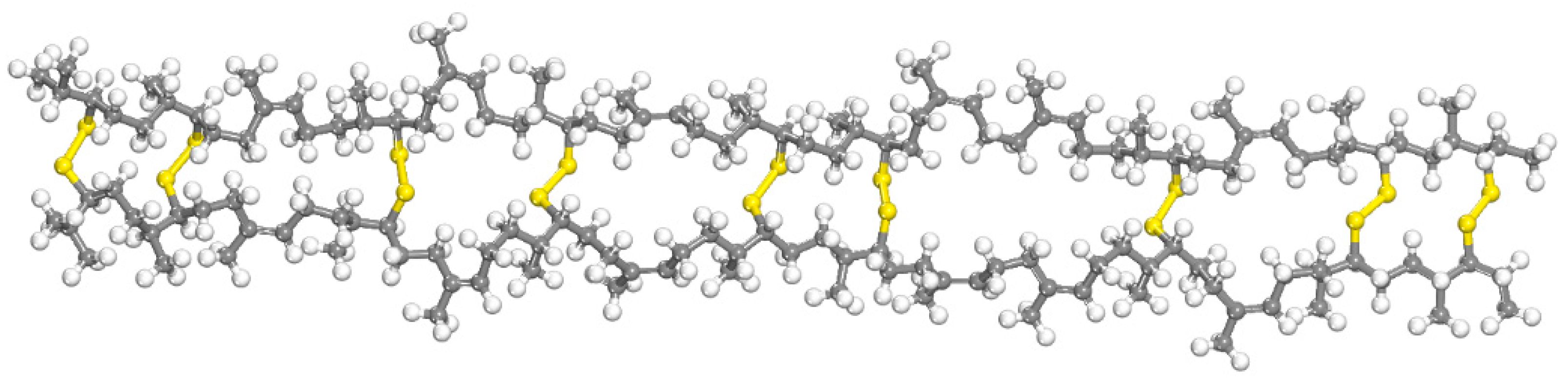
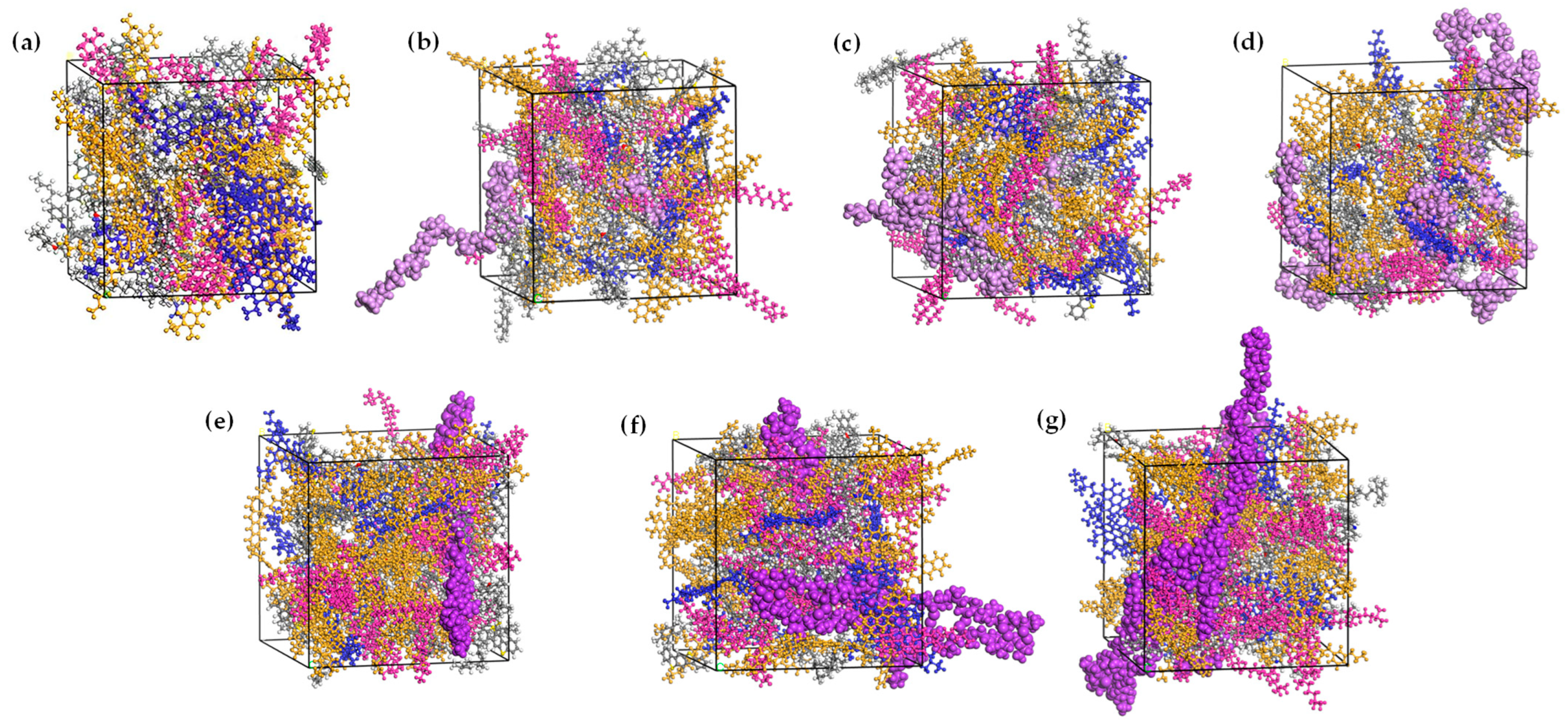

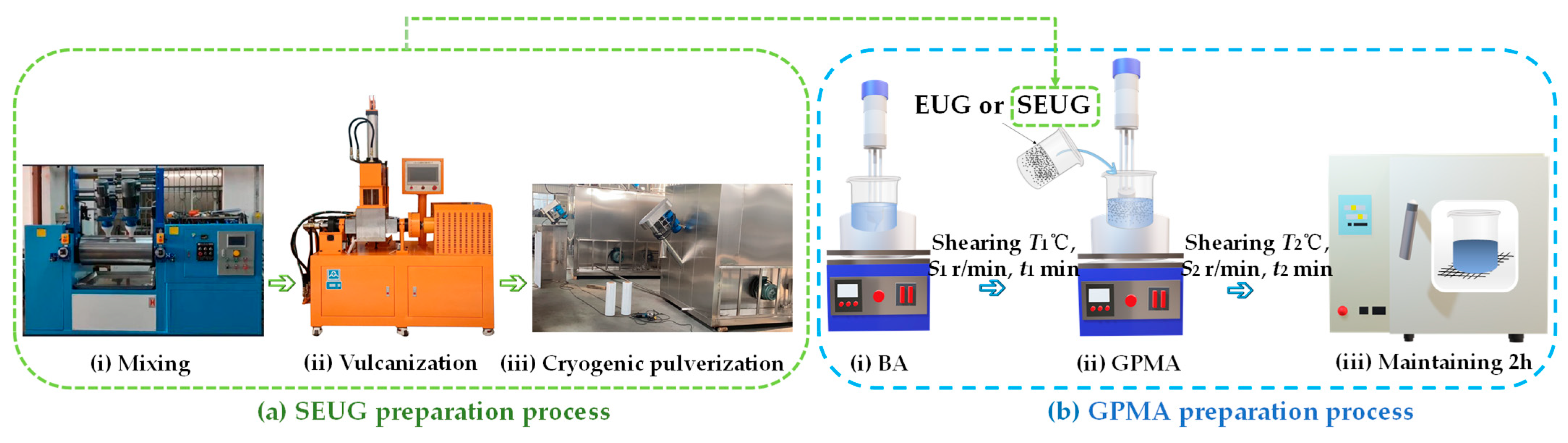





| Molecular Models | Criteria | MD Results | EL | Errors (%) |
|---|---|---|---|---|
| EUG | ρ (g·cm−3) | 0.882 | 0.91~0.98 [38] | 3.3 |
| δHansen (g·cm−3)1/2 | 16.53 | 16.2~17.0 [23,39] | 2~2.8 | |
| SEUG | ρ (g·cm−3) | 0.984 | 1.009 | 2.5 |
| δHansen (g·cm−3)1/2 | 11.041 | - | - | |
| BA | ρ (g·cm−3) | 0.998 | 0.968~1.034 [40,41] | 4.1~4.9 |
| δHansen (g·cm−3)1/2 | 17.802 | 13.30~22.50 [42] | 1.0 | |
| 5wt% EUGMA | ρ (g·cm−3) | 1.004 | 1.037 | 3.2 |
| 10wt% EUGMA | ρ (g·cm−3) | 1.006 | 1.042 | 3.5 |
| 15wt% EUGMA | ρ (g·cm−3) | 1.007 | 1.049 | 4.0 |
| 5wt% SEUGMA | ρ (g·cm−3) | 1.024 | 1.057 | 3.1 |
| 10wt% SEUGMA | ρ (g·cm−3) | 1.028 | 1.062 | 3.2 |
| 15wt% SEUGMA | ρ (g·cm−3) | 1.032 | 1.067 | 3.3 |
| Property | Result | Specification Limits | Testing Method [43] | |
|---|---|---|---|---|
| Penetration (25 °C, 0.1 mm) | 90.5 | 80~100 | T0604—2011 | |
| Softening point (R&B, °C) | 47.7 | ≥40 | T0606—2011 | |
| Ductility (5 °C, cm) | 9.5 | - | T0605—2011 | |
| RTFOT Residuum | Mass loss rate (%) | 0.05 | ≤±0.8 | T0610—2011 |
| Penetration ratio (25 °C, %) | 61.1 | ≥57 | T0610—2011 | |
| Ductility (5 °C, cm) | 8.2 | ≥8 | T0610—2011 | |
| Asphalt Type | Sample Number | Shear Temperature (°C) | Shear Rate (r/min) | Shear Time (min) |
|---|---|---|---|---|
| EUGMA | EA1 | T1: 130; T2:150 | S1: 3000; S2: 3000 | t1: 40; t2: 40 |
| EA2 | T1: 130; T2:150 | S1: 3000; S2: 5000 | t1: 40; t2: 60 | |
| EA3 | T1: 130; T2:150 | S1: 5000; S2: 5000 | t1: 60; t2: 60 | |
| EA4 | T1: 145; T2:165 | S1: 3000; S2: 3000 | t1: 40; t2: 60 | |
| EA5 | T1: 145; T2:165 | S1: 3000; S2: 5000 | t1: 60; t2: 60 | |
| EA6 | T1: 145; T2:165 | S1: 5000; S2: 5000 | t1: 40; t2: 40 | |
| EA7 | T1: 160; T2:180 | S1: 3000; S2: 3000 | t1: 60; t2: 60 | |
| EA8 | T1: 160; T2:180 | S1: 3000; S2: 5000 | t1: 40; t2: 40 | |
| EA9 | T1: 160; T2:180 | S1: 5000; S2: 5000 | t1: 40; t2: 60 | |
| SEUGMA | SA1 | T1: 140; T2:165 | S1: 4000; S2: 4000 | t1: 60; t2: 90 |
| SA2 | T1: 140; T2:165 | S1: 4000; S2: 6000 | t1: 90; t2: 60 | |
| SA3 | T1: 140; T2:165 | S1: 6000; S2: 6000 | t1: 90; t2: 90 | |
| SA4 | T1: 155; T2:180 | S1: 4000; S2: 4000 | t1: 90; t2: 60 | |
| SA5 | T1: 155; T2:180 | S1: 4000; S2: 6000 | t1: 90; t2: 90 | |
| SA6 | T1: 155; T2:180 | S1: 6000; S2: 6000 | t1: 60; t2: 90 | |
| SA7 | T1: 170; T2:195 | S1: 4000; S2: 4000 | t1: 90; t2: 90 | |
| SA8 | T1: 170; T2:195 | S1: 4000; S2: 6000 | t1: 60; t2: 90 | |
| SA9 | T1: 170; T2:195 | S1: 6000; S2: 6000 | t1: 90; t2: 60 |
| Sample Number | |||||
|---|---|---|---|---|---|
| Penetration (0.1 mm) | Softening Point (°C) | Ductility (cm) | High-Temperature Grading Temperature (°C) | ||
| EA 1 | 0.356 | 0.374 | 0.333 | 0.580 | 0.411 |
| EA 2 | 0.376 | 0.385 | 0.368 | 0.621 | 0.438 |
| EA 3 | 0.451 | 0.415 | 0.402 | 0.724 | 0.498 |
| EA 4 | 0.600 | 0.498 | 0.471 | 0.897 | 0.616 |
| EA 5 | 1.000 | 0.636 | 1.000 | 1.000 | 0.909 |
| EA 6 | 0.705 | 0.686 | 0.580 | 0.963 | 0.734 |
| EA 7 | 0.550 | 0.695 | 0.447 | 0.929 | 0.655 |
| EA 8 | 0.488 | 0.868 | 0.443 | 0.840 | 0.660 |
| EA 9 | 0.467 | 1.000 | 0.412 | 0.868 | 0.687 |
| SA 1 | 0.544 | 0.333 | 0.462 | 0.570 | 0.477 |
| SA 2 | 0.570 | 0.360 | 0.544 | 0.605 | 0.520 |
| SA 3 | 0.605 | 0.392 | 0.605 | 0.620 | 0.556 |
| SA 4 | 0.721 | 0.521 | 0.662 | 0.891 | 0.699 |
| SA 5 | 0.803 | 0.570 | 0.778 | 1.000 | 0.788 |
| SA 6 | 1.000 | 0.695 | 1.000 | 0.961 | 0.914 |
| SA 7 | 0.731 | 0.721 | 0.790 | 0.690 | 0.733 |
| SA 8 | 0.645 | 0.891 | 0.721 | 0.662 | 0.730 |
| SA 9 | 0.538 | 1.000 | 0.645 | 0.645 | 0.707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Cui, S.; Guo, N.; Chu, Z.; Zhang, J.; Yan, S.; Jin, X. Research on the Performance and Modification Mechanism of Gutta-Percha-Modified Asphalt. Polymers 2024, 16, 1860. https://doi.org/10.3390/polym16131860
Yan S, Cui S, Guo N, Chu Z, Zhang J, Yan S, Jin X. Research on the Performance and Modification Mechanism of Gutta-Percha-Modified Asphalt. Polymers. 2024; 16(13):1860. https://doi.org/10.3390/polym16131860
Chicago/Turabian StyleYan, Simeng, Shichao Cui, Naisheng Guo, Zhaoyang Chu, Jun Zhang, Sitong Yan, and Xin Jin. 2024. "Research on the Performance and Modification Mechanism of Gutta-Percha-Modified Asphalt" Polymers 16, no. 13: 1860. https://doi.org/10.3390/polym16131860
APA StyleYan, S., Cui, S., Guo, N., Chu, Z., Zhang, J., Yan, S., & Jin, X. (2024). Research on the Performance and Modification Mechanism of Gutta-Percha-Modified Asphalt. Polymers, 16(13), 1860. https://doi.org/10.3390/polym16131860






