A High Andean Hydrocolloid Extracted by Microatomization: Preliminary Optimization in Aqueous Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Suspension Conditioning
2.3. Suspension Stability Evaluation
2.3.1. Rheological Measurements
2.3.2. Turbidity Measurements
2.3.3. Particle Size and ζ Potential Measurements
2.3.4. Color Measurements
- −
- If the CI* is −40 to −20, colors range from blue-violet to deep green.
- −
- If the CI* is −20 to −2, colors range from deep green to yellowish green.
- −
- If the CI* is −2 to +2, this represents greenish yellow.
- −
- If the CI* is +2 to +20, colors range from pale yellow to deep orange.
- −
- If the CI* is +20 to +40, colors range from deep orange to deep red.
2.4. UV-Vis Sscanning
2.5. Chemometric Analysis by PCA
2.6. Suspension Stability Parameter Optimization
2.7. Statistical Analysis
3. Results and Discussion
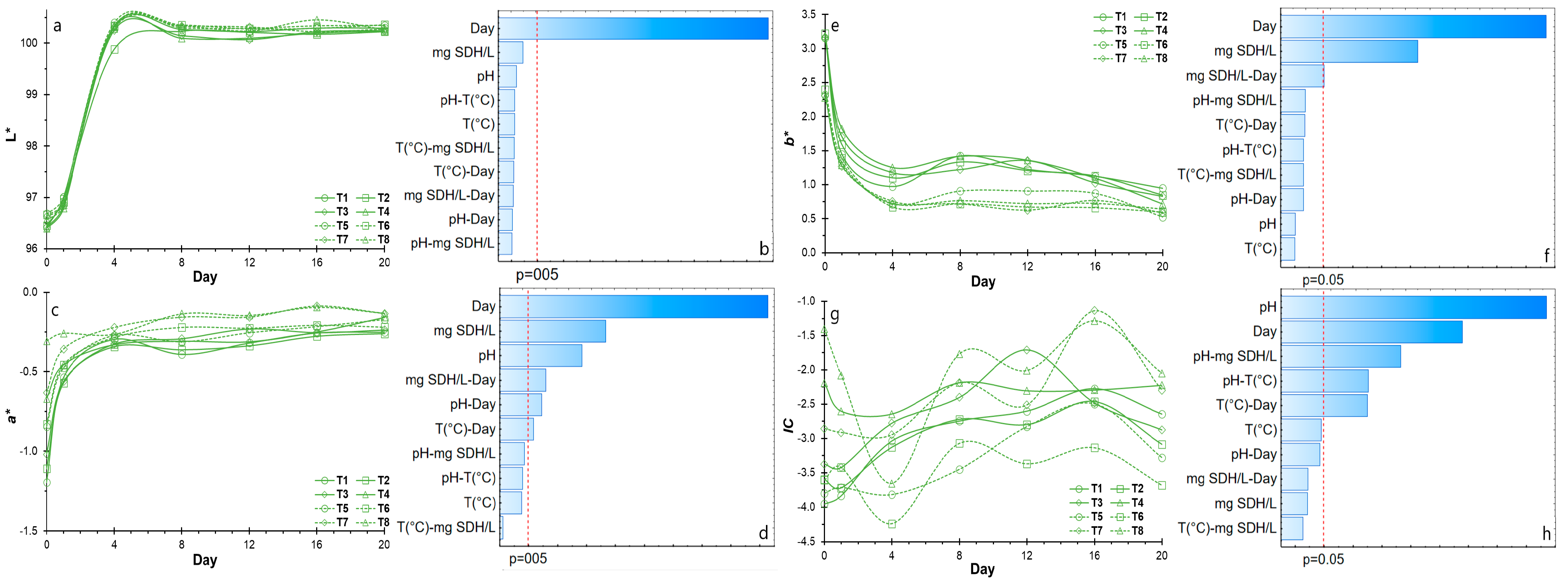
3.1. Suspended Solid Stability of NSH Suspensions
3.2. Color Stability of NSH Suspensions
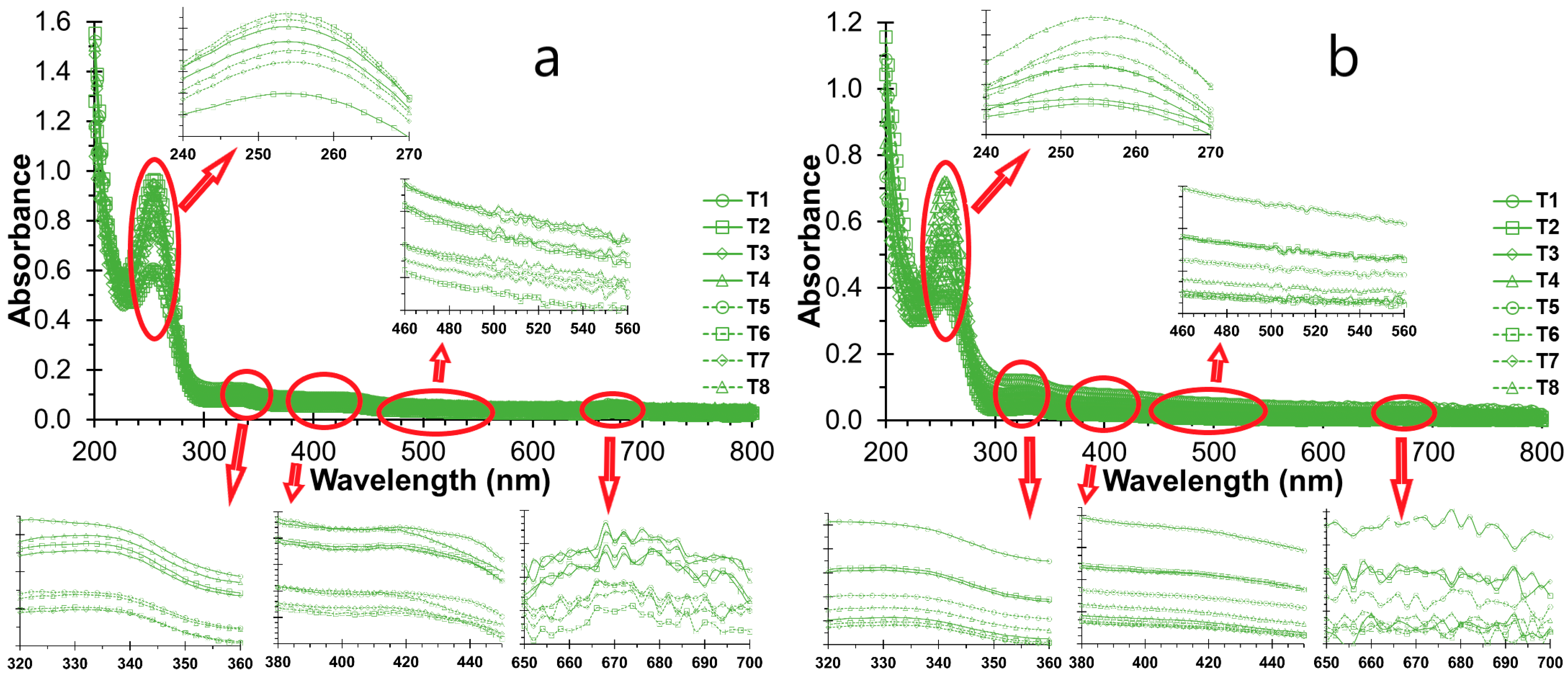
3.3. UV-Vis Scanning of NSH Suspensions
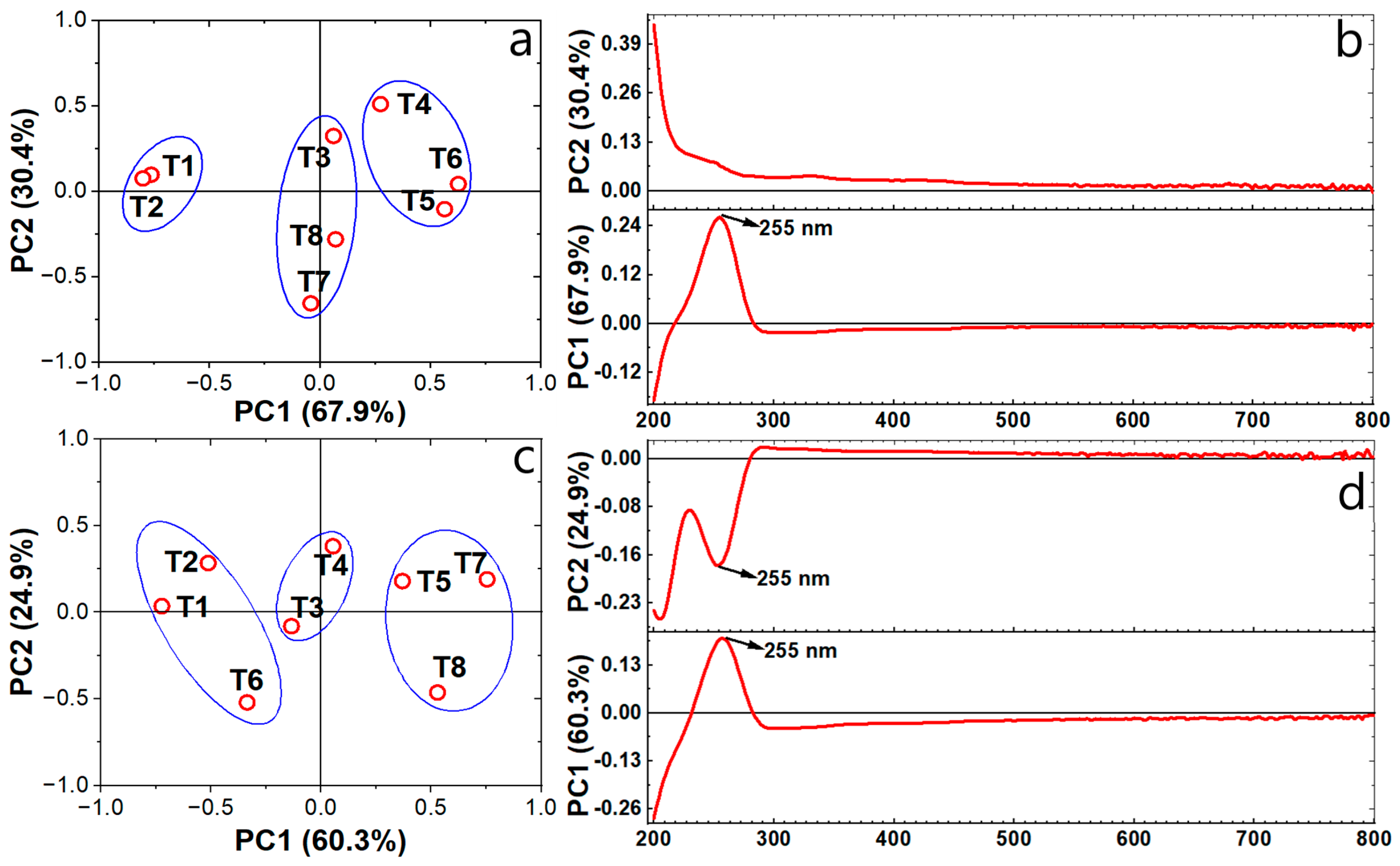
3.4. PCA Analysis of UV-Vis Spectra
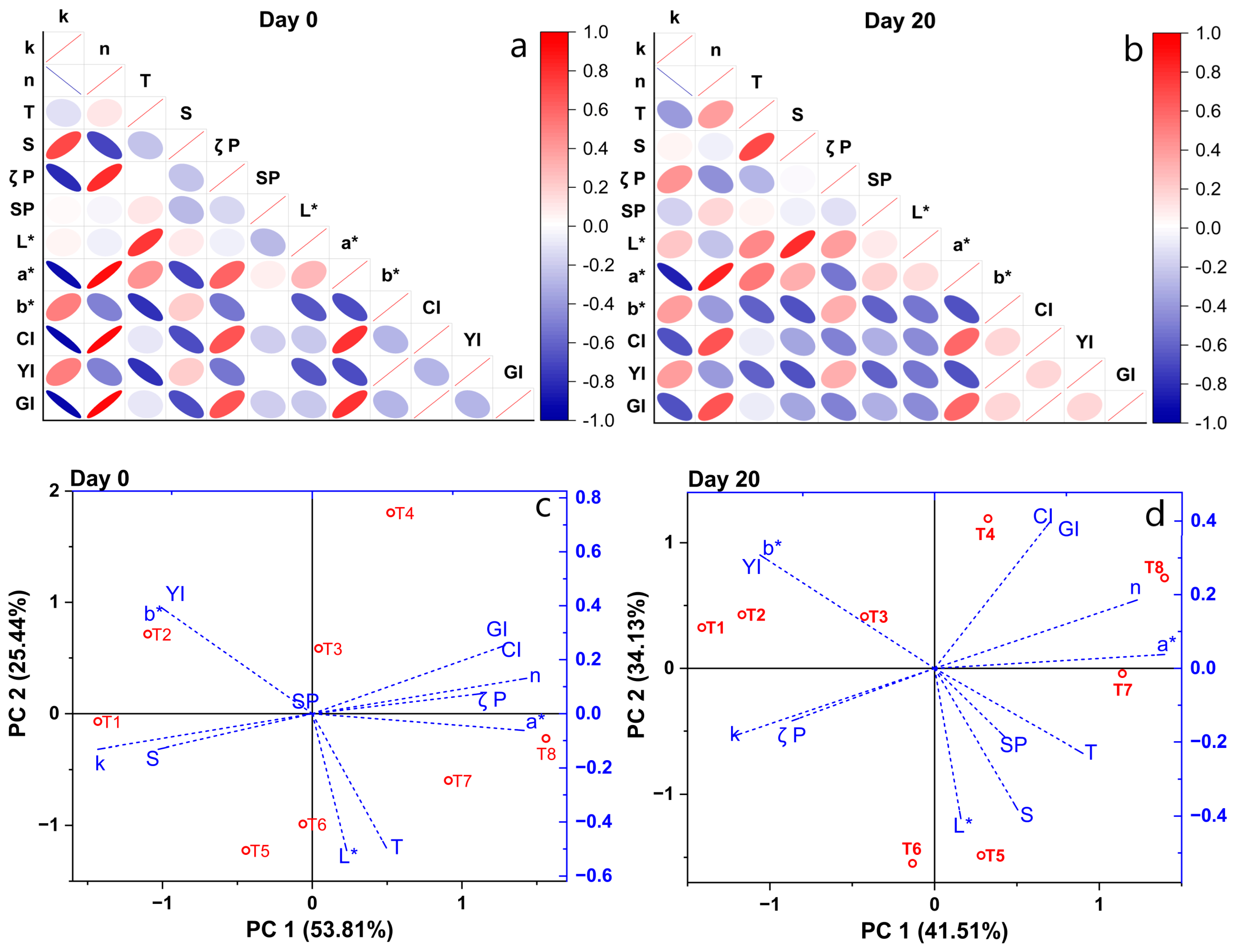
3.5. PCA Analysis of Suspension Properties
3.6. NSH Suspension Parameter Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shogren, R.; Wood, D.; Orts, W.; Glenn, G. Plant-based materials and transitioning to a circular economy. Sustain. Prod. Consum. 2019, 19, 194–215. [Google Scholar] [CrossRef]
- Ma, H.-W.; Shih, H.-C.; Liao, M.-I. Circular Economy and New Research Directions in Sustainability. In Pursuing Sustainability: OR/MS Applications in Sustainable Design, Manufacturing, Logistics, and Resource Management; Springer: Berlin/Heidelberg, Germany, 2021; pp. 141–168. [Google Scholar]
- Qin, Y. Production of seaweed-derived food hydrocolloids. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–69. [Google Scholar]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Aftab, K.; Hameed, S.; Umbreen, H.; Ali, S.; Rizwan, M.; Alkahtani, S.; Abdel-Daim, M.M. Physicochemical and functional potential of hydrocolloids extracted from some Solanaceae plants. J. Chem. 2020, 2020, 3563945. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Chang, C.-C.; Nagarajan, D.; Chen, C.-Y.; Chang, J.-S. Algae-derived hydrocolloids in foods: Applications and health-related issues. Bioengineered 2021, 12, 3787–3801. [Google Scholar] [CrossRef] [PubMed]
- Herbas-De la Cruz, R.K.; Choque-Quispe, Y.; Choque-Quispe, D.; Ligarda-Samanez, C.A.; Froehner, S.; Buleje-Campos, D.; Ramos-Pacheco, B.S.; Peralta-Guevara, D.E.; Pérez-Salcedo, R.; Yauris-Silvera, C.R. Flocculant capacity of hydrocolloid extracted from high Andean algae (Nostoc sphaericum) in the treatment of artificial wastewater: An approach. Case Stud. Chem. Environ. Eng. 2023, 8, 100515. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Mojo-Quisani, A.; Ligarda-Samanez, C.A.; Calla-Florez, M.; Ramos-Pacheco, B.S.; Zamalloa-Puma, L.M.; Peralta-Guevara, D.E.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Zamalloa-Puma, A. Preliminary characterization of a spray-dried hydrocolloid from a high Andean algae (Nostoc sphaericum). Foods 2022, 11, 1640. [Google Scholar] [CrossRef] [PubMed]
- Yashaswini, D.G.V.; Venkatesan, J.; Anil, S. Hydrocolloids from Marine Macroalgae: Isolation and Applications. In Algae for Food; CRC Press: Boca Raton, FL, USA, 2021; pp. 185–200. [Google Scholar]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid gel particles: Formation, characterization, and application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; de Morais, M.G. Microalgae polysaccharides: An overview of production, characterization, and potential applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Wang, W.; Li, Y. Advanced properties of gluten-free cookies, cakes, and crackers: A review. Trends Food Sci. Technol. 2020, 103, 200–213. [Google Scholar] [CrossRef]
- Rafe, A.; Razavi, S.M.A. The effect of p H and calcium ion on rheological behaviour of β-lactoglobulin-basil seed gum mixed gels. Int. J. Food Sci. Technol. 2013, 48, 1924–1931. [Google Scholar] [CrossRef]
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The role of hydrocolloids in gluten-free bread and pasta; rheology, characteristics, staling and glycemic index. Foods 2021, 10, 3121. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Pozo, L.M.F.; Ramos-Pacheco, B.S.; Palomino-Rincón, H.; Gutiérrez, R.J.G.; Peralta-Guevara, D.E. Effect of Inlet Air Temperature and Quinoa Starch/Gum Arabic Ratio on Nanoencapsulation of Bioactive Compounds from Andean Potato Cultivars by Spray-Drying. Molecules 2023, 28, 7875. [Google Scholar] [CrossRef] [PubMed]
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 2014, 155, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Paul, D. A review on Biological activities of common mallow (Malva sylvestris L.). Innovare J. Life Sci. 2016, 4, 1–5. [Google Scholar]
- Angeloni, C.; Pirola, L.; Vauzour, D.; Maraldi, T. Dietary polyphenols and their effects on cell biochemistry and pathophysiology. Oxidative Med. Cell. Longev. 2012, 2012, 583901. [Google Scholar] [CrossRef] [PubMed]
- Choque-Quispe, D.; Ligarda-Samanez, C.A.; Choque-Quispe, Y.; Solano-Reynoso, A.M.; Ramos-Pacheco, B.S.; Zamalloa-Puma, M.M.; Álvarez-López, G.J.; Zamalloa-Puma, A.; Choque-Quispe, K.; Alzamora-Flores, H. Multimetal removal in aqueous medium using a potato starch/nopal mucilage copolymer: A study of adsorption kinetics and isotherms. Results Eng. 2023, 18, 101164. [Google Scholar] [CrossRef]
- Puspita, M.; Setyawidati, N.A.R.; Pangestuti, R. Hydrocolloid production of Indonesian seaweeds. Encycl. Mar. Biotechnol. 2020, 1, 407–416. [Google Scholar]
- Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Pereira, L. Colloid producing seaweeds: Agarophytes, carrageenophytes and alginophytes biodiversity. Encycl. Mar. Biotechnol. 2020, 1, 161–326. [Google Scholar]
- Gomes Gradíssimo, D.; Pereira Xavier, L.; Valadares Santos, A. Cyanobacterial polyhydroxyalkanoates: A sustainable alternative in circular economy. Molecules 2020, 25, 4331. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Torres, F.G.; López, D. Preparation and characterization of polysaccharide films from the cyanobacteria Nostoc commune. Polym. Renew. Resour. 2017, 8, 133–150. [Google Scholar] [CrossRef]
- Jensen, S.; Petersen, B.O.; Omarsdottir, S.; Paulsen, B.S.; Duus, J.Ø.; Olafsdottir, E.S. Structural characterisation of a complex heteroglycan from the cyanobacterium Nostoc commune. Carbohydr. Polym. 2013, 91, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, X.; Chen, F.; Xiao, A. An ecofriendly agar extraction strategy using KOH for boiling extraction and MgCl2 for alkali neutralization. Sustain. Chem. Pharm. 2023, 31, 100909. [Google Scholar] [CrossRef]
- Gomes-Dias, J.S.; Teixeira-Guedes, C.I.; Teixeira, J.A.; Rocha, C.M.R. Red seaweed biorefinery: The influence of sequential extractions on the functional properties of extracted agars and porphyrans. Int. J. Biol. Macromol. 2023, 257, 128479. [Google Scholar] [CrossRef] [PubMed]
- Corpus-Gomez, A.; Alcantara-Callata, M.; Celis-Teodoro, H.; Echevarria-Alarcón, B.; Paredes-Julca, J.; Paucar-Menacho, L.M. Cushuro (Nostoc sphaericum): Habitat, physicochemical characteristics, nutritional composition, forms of consumption and medicinal properties. Agroind. Sci 2021, 11, 231–238. [Google Scholar] [CrossRef]
- Torres-Maza, A.; Yupanqui-Bacilio, C.; Castro, V.; Aguirre, E.; Villanueva, E.; Rodríguez, G. Comparison of the hydrocolloids Nostoc commune and Nostoc sphaericum: Drying, spectroscopy, rheology and application in nectar. Sci. Agropecu. 2020, 11, 583–589. [Google Scholar] [CrossRef]
- Ponce, E. Nostoc: A different food and their presence in the precordillera of Arica. Idesia 2014, 32, 115–118. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ligarda-Samanez, C.A.; Choque-Quispe, Y.; Froehner, S.; Solano-Reynoso, A.M.; Moscoso-Moscoso, E.; Carhuarupay-Molleda, Y.F.; Peréz-Salcedo, R. Stability in Aqueous Solution of a New Spray-Dried Hydrocolloid of High Andean Algae Nostoc sphaericum. Polymers 2024, 16, 537. [Google Scholar] [CrossRef]
- Kamel, R.; Afifi, S.M.; Kassem, I.A.A.; Elkasabgy, N.A.; Farag, M.A. Arabinoxylan and rhamnogalacturonan mucilage: Outgoing and potential trends of pharmaceutical, environmental, and medicinal merits. Int. J. Biol. Macromol. 2020, 165, 2550–2564. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.; San Martin-González, M.F.; Garcia-Bravo, J.; Liceaga, A.M. Development of chia seed (Salvia hispanica) mucilage films plasticized with polyol mixtures: Mechanical and barrier properties. Int. J. Biol. Macromol. 2020, 163, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Deore, U.V.; Mahajan, H.S. Isolation and characterization of natural polysaccharide from Cassia Obtustifolia seed mucilage as film forming material for drug delivery. Int. J. Biol. Macromol. 2018, 115, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yin, J.; Nie, S.; Wan, Y.; Xie, M. Fractionation, physicochemical property and immunological activity of polysaccharides from Cassia obtusifolia. Int. J. Biol. Macromol. 2016, 91, 946–953. [Google Scholar] [CrossRef]
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem. 2023, 399, 133967. [Google Scholar] [CrossRef] [PubMed]
- Koko, M.Y.F.; Hassanin, H.A.M.; Qi, B.; Han, L.; Lu, K.; Rokayya, S.; Harimana, Y.; Zhang, S.; Li, Y. Hydrocolloids as promising additives for food formulation consolidation: A short review. Food Rev. Int. 2023, 39, 1433–1439. [Google Scholar] [CrossRef]
- Yemenicioğlu, A.; Farris, S.; Turkyilmaz, M.; Gulec, S. A review of current and future food applications of natural hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1389–1406. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Feng, X.; Ren, F.; Wang, J. Effect of hydrocolloids on gluten proteins, dough, and flour products: A review. Food Res. Int. 2023, 164, 112292. [Google Scholar] [CrossRef] [PubMed]
- Pak, A.M.; Nelyubina, Y.V.; Novikov, V.V. Natural hydrocolloids as biocompatible composite materials for food applications. Usp. Him. 2023, 92, RCR5102. [Google Scholar] [CrossRef]
- Brütsch, L.; Stringer, F.J.; Kuster, S.; Windhab, E.J.; Fischer, P. Chia seed mucilage–a vegan thickener: Isolation, tailoring viscoelasticity and rehydration. Food Funct. 2019, 10, 4854–4860. [Google Scholar] [CrossRef]
- Alpizar-Reyes, E.; Román-Guerrero, A.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Cruz-Olivares, J.; Pérez-Alonso, C. Rheological properties of tamarind (Tamarindus indica L.) seed mucilage obtained by spray-drying as a novel source of hydrocolloid. Int. J. Biol. Macromol. 2018, 107, 817–824. [Google Scholar] [CrossRef]
- Emiola, I.; Adem, R. Comparison of Minimization Methods for Rosenbrock Functions. In Proceedings of the 2021 29th Mediterranean Conference on Control and Automation (MED), Puglia, Italy, 22–25 June 2021; pp. 837–842. [Google Scholar]
- Choque-Quispe, D.; Froehner, S.; Ligarda-Samanez, C.A.; Ramos-Pacheco, B.S.; Palomino-Rincón, H.; Choque-Quispe, Y.; Solano-Reynoso, A.M.; Taipe-Pardo, F.; Zamalloa-Puma, L.M.; Calla-Florez, M. Preparation and chemical and physical characteristics of an edible film based on native potato starch and nopal mucilage. Polymers 2021, 13, 3719. [Google Scholar] [CrossRef]
- Hadimani, L.; Mittal, N. Development of a computer vision system to estimate the colour indices of Kinnow mandarins. J. Food Sci. Technol. 2019, 56, 2305–2311. [Google Scholar] [CrossRef]
- Nurani, L.H.; Edityaningrum, C.A.; Irnawati, I.; Putri, A.R.; Windarsih, A.; Guntarti, A.; Rohman, A. Chemometrics-Assisted UV-Vis Spectrophotometry for Quality Control of Pharmaceuticals: A Review. Indones. J. Chem. 2023, 23, 542–567. [Google Scholar] [CrossRef]
- Beattie, J.R.; Esmonde-White, F.W.L. Exploration of principal component analysis: Deriving principal component analysis visually using spectra. Appl. Spectrosc. 2021, 75, 361–375. [Google Scholar] [CrossRef]
- Andrade, C. Understanding Statistical Noise in Research: 3. Noise in Regression Analysis. Indian J. Psychol. Med. 2023, 45, 310–311. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, J. How much should we trust R2 and adjusted R2: Evidence from regressions in top economics journals and Monte Carlo simulations. J. Appl. Econ. 2023, 26, 2207326. [Google Scholar] [CrossRef]
- Camirand Lemyre, F.; Chalifoux, K.; Desharnais, B.; Mireault, P. Squaring things up with R2: What it is and what it can (and cannot) tell you. J. Anal. Toxicol. 2022, 46, 443–448. [Google Scholar] [CrossRef]
- Hapanowicz, J.; Szydlowska, A. Viscosity of plant meal suspension and the effectiveness of its calculation methods. Przem. Chem. 2018, 97, 985–990. [Google Scholar]
- Fidaleo, M.; Miele, N.A.; Armini, V.; Cavella, S. Design space of the formulation process of a food suspension by D-optimal mixture experiment and functional data analysis. Food Bioprod. Process. 2021, 127, 128–138. [Google Scholar] [CrossRef]
- Zhang, R.; Ghorbani, M.; Wong, S.; Frigaard, I.A. Vertical cementing displacement flows of shear-thinning fluids. Phys. Fluids 2023, 35, 113110. [Google Scholar] [CrossRef]
- Fujitani, Y. Effects of the preferential adsorption in a near-critical binary fluid mixture on dynamics of a droplet. Phys. Fluids 2022, 34, 092007. [Google Scholar] [CrossRef]
- Shan, L.; Tian, Y.; Jiang, J.; Zhang, X.; Meng, Y. Effects of pH on shear thinning and thickening behaviors of fumed silica suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2015, 464, 1–7. [Google Scholar] [CrossRef]
- Zhou, B.; Drusch, S.; Hogan, S.A. Rheological fingerprinting and tribological assessment of high internal phase emulsions stabilized by whey protein isolate: Effects of protein concentration and pH. Food Hydrocoll. 2022, 131, 107816. [Google Scholar] [CrossRef]
- Włodarska, K.; Pawlak-Lemańska, K.; Górecki, T.; Sikorska, E. Perception of apple juice: A comparison of physicochemical measurements, descriptive analysis and consumer responses. J. Food Qual. 2016, 39, 351–361. [Google Scholar] [CrossRef]
- Deng, J.K.; Liu, X.; Wu, X.Y.; Bi, J.F.; Jiao, Y.; Zhong, Y.G. Quality evaluation of apple (Substandard) juice from different cultivars based on analytic hierarchy process and grey interconnect degree analysis. J. Chin. Inst. Food Sci. Technol. 2017, 17, 197–208. [Google Scholar]
- Mohammadi-Jam, S.; Waters, K.E.; Greenwood, R.W. A review of zeta potential measurements using electroacoustics. Adv. Colloid Interface Sci. 2022, 309, 102778. [Google Scholar] [CrossRef]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting zeta potential, the key feature of interfacial phenomena, with applications and recent advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Drechsler, A.; Caspari, A.; Synytska, A. Influence of roughness and capillary size on the zeta potential values obtained by streaming potential measurements. Surf. Interface Anal. 2020, 52, 991–995. [Google Scholar] [CrossRef]
- Manilo, M.V.; Lebovka, N.I.; Barany, S. Effects of sort and concentration of salts on the electrosurface properties of aqueous suspensions containing hydrophobic and hydrophilic particles: Validity of the Hofmeister series. J. Mol. Liq. 2019, 276, 875–884. [Google Scholar] [CrossRef]
- Wei, B.; Hu, X.; Li, H.; Wu, C.; Xu, X.; Jin, Z.; Tian, Y. Effect of pHs on dispersity of maize starch nanocrystals in aqueous medium. Food Hydrocoll. 2014, 36, 369–373. [Google Scholar] [CrossRef]
- Ohshima, H. Electrophoretic mobility of a cylindrical colloidal particle with a slip surface. Colloid Polym. Sci. 2020, 298, 151–156. [Google Scholar] [CrossRef]
- Zhang, Z. A new method for estimating zeta potential of carboxylic acids’ functionalised particles. Mol. Phys. 2023, 122, e2260014. [Google Scholar] [CrossRef]
- Molina, A.K.; Corrêa, R.C.G.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules 2023, 28, 1200. [Google Scholar] [CrossRef]
- Podsędek, A. Food Colorants. In Chemical and Functional Properties of Food Components; CRC Press: Boca Raton, FL, USA, 2023; pp. 285–302. [Google Scholar]
- Šubert, J.; Kolář, J.; Čižmárik, J. Theory and practice of pharmacopoeial control of the quality of drugs and excipients VII. The colour reference solutions of the European pharmacopoeia (Ph. Eur.). Ceska A Slov. Farm. Cas. Ceske Farm. Spol. A Slov. Farm. Spol. 2018, 67, 30–31. [Google Scholar]
- Weiss, V.; Okun, Z.; Shpigelman, A. Utilization of hydrocolloids for the stabilization of pigments from natural sources. Curr. Opin. Colloid Interface Sci. 2023, 68, 101756. [Google Scholar] [CrossRef]
- Mastanjević, K.; Krstanović, V.; Lukinac, J.; Jukić, M.; Vulin, Z.; Mastanjević, K. Beer–the importance of colloidal stability (non-biological haze). Fermentation 2018, 4, 91. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Mitharwal, S.; Rani, P.; Shanker, M.A.; Kumar, A.; Aslam, R.; Barut, Y.T.; Kothakota, A.; Rustagi, S.; Bhati, D. The influence of non-thermal technologies on color pigments of food materials: An updatedreview. Curr. Res. Food Sci. 2023, 6, 100529. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- Singh, T.; Pandey, V.K.; Dash, K.K.; Zanwar, S.; Singh, R. Natural bio-colorant and pigments: Sources and applications in food processing. J. Agric. Food Res. 2023, 12, 100628. [Google Scholar] [CrossRef]
- Imchen, T.; Singh, K.S. Marine algae colorants: Antioxidant, anti-diabetic properties and applications in food industry. Algal Res. 2023, 69, 102898. [Google Scholar] [CrossRef]
- Murata, M. Browning and pigmentation in food through the Maillard reaction. Glycoconj. J. 2021, 38, 283–292. [Google Scholar] [CrossRef]
- Selvarajan, E.; Veena, R.; Manoj Kumar, N. Polyphenol oxidase, beyond enzyme browning. In Microbial Bioprospecting for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2018; pp. 203–222. [Google Scholar]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic browning of fruit and vegetables: A review. In Enzymes in Food Technology: Improvements and Innovations; Springer: Berlin/Heidelberg, Germany, 2018; pp. 63–78. [Google Scholar]
- Power, A.C.; Chapman, J.; Chandra, S.; Cozzolino, D. Ultraviolet-visible spectroscopy for food quality analysis. In Evaluation Technologies for Food Quality; Springer: Berlin/Heidelberg, Germany, 2019; pp. 91–104. [Google Scholar]
- Mignani, A.G.; Ciaccheri, L.; Mencaglia, A.A.; Cimato, A. Optical absorption spectroscopy for quality assessment of extra virgin olive oil. In Olive Oil-Constituents, Quality, Health Properties and Bioconversions; IntechOpen: London, UK, 2012; pp. 47–62. [Google Scholar]
- Alpatova, A.L.; Shan, W.; Babica, P.; Upham, B.L.; Rogensues, A.R.; Masten, S.J.; Drown, E.; Mohanty, A.K.; Alocilja, E.C.; Tarabara, V.V. Single-walled carbon nanotubes dispersed in aqueous media via non-covalent functionalization: Effect of dispersant on the stability, cytotoxicity, and epigenetic toxicity of nanotube suspensions. Water Res. 2010, 44, 505–520. [Google Scholar] [CrossRef]
- Kim, J.-S. Study of flavonoid/hydroxypropyl-β-cyclodextrin inclusion complexes by UV-Vis, FT-IR, DSC, and X-ray diffraction analysis. Prev. Nutr. Food Sci. 2020, 25, 449. [Google Scholar] [CrossRef]
- Urbano, M.; De Castro, M.D.L.; Pérez, P.M.; García-Olmo, J.; Gomez-Nieto, M.A. Ultraviolet–visible spectroscopy and pattern recognition methods for differentiation and classification of wines. Food Chem. 2006, 97, 166–175. [Google Scholar] [CrossRef]
- Bilancia, M.T.; Caponio, F.; Sikorska, E.; Pasqualone, A.; Summo, C. Correlation of triacylglycerol oligopolymers and oxidised triacylglycerols to quality parameters in extra virgin olive oil during storage. Food Res. Int. 2007, 40, 855–861. [Google Scholar] [CrossRef]
- Thiruchenduran, S.; Maheswari, K.U.; Prasad, T.; Rajeswari, B.; Suneetha, W.J. UV-Vis scanning coupled with PCA as an alternative method for phytochemical screening of natural products –Costus Igneus leaf metabolites. J. Pharmacogn. Phytochem. 2017, 6, 411–416. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Sergio, T.-G.; Sabina, W.; Beatriz, M.-R. Nanoencapsulation of Aloe vera in Synthetic and Naturally Occurring Polymers by Electrohydrodynamic Processing of Interest in Food Technology and Bioactive Packaging. J. Agric. Food Chem. 2017, 65, 4439–4448. [Google Scholar]
- Lazzari, E.; Schena, T.; Marcelo, M.C.A.; Primaz, C.T.; Silva, A.N.; Ferrao, M.F.; Bjerk, T.; Caramao, E.B. Classification of biomass through their pyrolytic bio-oil composition using FTIR and PCA analysis. Ind. Crops Prod. 2018, 111, 856–864. [Google Scholar] [CrossRef]
- Dankowska, A.; Domagała, A.; Kowalewski, W. Quantification of Coffea arabica and Coffea canephora var. robusta concentration in blends by means of synchronous fluorescence and UV-Vis spectroscopies. Talanta 2017, 172, 215–220. [Google Scholar] [CrossRef]
| Treatment | pH | Dose NSH (g/L) | Temperature (°C) |
|---|---|---|---|
| T1 | 6.5 | 0.10 | 60 |
| T2 | 6.5 | 0.10 | 80 |
| T3 | 4.5 | 0.10 | 60 |
| T4 | 4.5 | 0.10 | 80 |
| T5 | 6.5 | 0.07 | 60 |
| T6 | 6.5 | 0.07 | 80 |
| T7 | 4.5 | 0.07 | 60 |
| T8 | 4.5 | 0.07 | 80 |
| k *** | n *** | Turbidity *** (% Transmittance) | Sedimentation *** (% Transmittance) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 20 | ** | Day 0 | Day 20 | ** | |||||||||||
| ±s | * | ±s | * | ±s | * | ±s | * | |||||||||
| T1 | 2.5 | 1.38 | 98.71 | 0.01 | a | 97.57 | 0.52 | c | < | 77.49 | 0.22 | b | 72.61 | 0.5 | a | < |
| T2 | 1.79 | 1.41 | 98.47 | 0.02 | b | 97.60 | 0.06 | c | < | 77.1 | 0.03 | b | 71.57 | 0.5 | b | < |
| T3 | 1.12 | 1.52 | 98.64 | 0.06 | c | 97.90 | 0.1 | c | < | 74.81 | 0.16 | c | 63.17 | 0.29 | c | < |
| T4 | 0.79 | 1.57 | 98.38 | 0.03 | d | 97.48 | 0.06 | c | < | 73.95 | 0.81 | d | 66.28 | 0.14 | d | < |
| T5 | 1.37 | 1.45 | 99.42 | 0.01 | e | 99.06 | 0.49 | b | < | 82.85 | 0.40 | a | 77.09 | 0.22 | e | < |
| T6 | 1.13 | 1.51 | 99.89 | 0.01 | f | 99.67 | 0.1 | a | < | 74.18 | 0.45 | c,d | 78.59 | 0.36 | f | < |
| T7 | 0.97 | 1.55 | 99.63 | 0.03 | g | 99.33 | 0.11 | a,b | < | 72.95 | 0.62 | e | 73.73 | 0.22 | g | < |
| T8 | 0.64 | 1.62 | 99.51 | 0.05 | h | 99.77 | 0.06 | a | < | 74.67 | 0.04 | c,d | 74.52 | 0.1 | h | < |
| ζ Potential (mV) | Particle Size (nm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 20 | ** | Day 0 | Day 20 | ** | |||||||||
| ±s | * | ±s | * | ±s | * | ±s | * | |||||||
| T1 | −27.35 | 1.87 | b,c | −24.27 | 0.26 | a | > | 941.00 | 19.16 | a | 316.33 | 11.07 | b | < |
| T2 | −29.26 | 1.47 | c | −25.22 | 1.45 | a | < | 626.17 | 33.04 | d | 342.17 | 27.94 | b | < |
| T3 | −23.18 | 1.29 | a | −24.77 | 1.29 | a | > | 630.67 | 19.11 | d | 464.00 | 45.42 | a | < |
| T4 | −24.39 | 1.51 | a,b | −24.98 | 0.79 | a | > | 884.03 | 53.28 | a,b | 363.03 | 34.15 | b | < |
| T5 | −25.87 | 1.62 | a,b,c | −25.19 | 1.09 | a | > | 708.37 | 66.71 | c,d | 473.13 | 23.06 | a | < |
| T6 | −26.76 | 0.88 | a,b,c | −23.62 | 0.31 | a | < | 725.67 | 88.15 | c,d | 344.37 | 26.33 | b | < |
| T7 | −26.08 | 0.72 | a,b,c | −26.41 | 0.69 | a | > | 776.33 | 50.80 | b,c | 453.33 | 44.21 | a | < |
| T8 | −22.95 | 1.38 | a | −26.20 | 1.28 | a | > | 688.97 | 32.12 | c,d | 309.97 | 8.07 | b | < |
| L* | a* | b* | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 20 | ** | Day 0 | Day 20 | ** | Day 0 | Day 20 | ** | |||||||||||||
| ±s | * | ±s | * | ±s | * | ±s | * | ±s | * | ±s | * | ||||||||||
| T1 | 95.92 | 0.01 | f | 99.79 | 0.01 | b | < | −1.20 | 0.01 | a | −0.25 | 0.01 | d,e | < | 3.16 | 0.01 | c | 0.95 | 0.01 | a | < |
| T2 | 95.95 | 0.01 | e | 99.73 | 0.02 | c | < | −1.11 | 0.01 | b | −0.26 | 0.01 | e | < | 3.22 | 0.01 | a | 0.85 | 0.01 | b | < |
| T3 | 95.93 | 0.01 | f | 99.74 | 0.01 | c | < | −1.02 | 0.01 | c | −0.24 | 0.01 | c,d | < | 3.15 | 0.01 | c | 0.83 | 0.02 | b | < |
| T4 | 95.90 | 0.01 | g | 99.74 | 0.02 | c | < | −0.67 | 0.01 | d | −0.16 | 0.01 | b | < | 3.19 | 0.01 | b | 0.71 | 0.02 | c | < |
| T5 | 96.18 | 0.01 | a | 99.85 | 0.02 | a | < | −0.85 | 0.01 | e | −0.17 | 0.01 | b | < | 2.32 | 0.01 | e | 0.52 | 0.01 | f | < |
| T6 | 96.17 | 0.01 | b | 99.86 | 0.07 | a | < | −0.82 | 0.01 | f | −0.22 | 0.01 | c | < | 2.39 | 0.01 | d | 0.60 | 0.02 | e | < |
| T7 | 96.14 | 0.01 | c | 99.77 | 0.02 | b,c | < | −0.63 | 0.01 | g | −0.13 | 0.01 | a | < | 2.31 | 0.01 | e | 0.58 | 0.02 | e | < |
| T8 | 96.07 | 0.01 | d | 99.76 | 0.01 | b,c | < | −0.31 | 0.01 | h | −0.13 | 0.00 | a | < | 2.28 | 0.01 | f | 0.64 | 0.01 | d | < |
| CI* | YI | GI | |||||||||||||||||||
| T1 | −3.95 | 0.01 | g | −2.65 | 0.13 | b | < | 431.9 | 0.71 | c | 129.57 | 0.71 | a | < | −0.35 | 0.00 | g | −0.26 | 0.01 | b | < |
| T2 | −3.60 | 0.03 | e | −3.09 | 0.14 | c,d | < | 440.1 | 0.71 | a | 115.65 | 1.44 | b | < | −0.33 | 0.00 | e | −0.29 | 0.01 | c,d | < |
| T3 | −3.38 | 0.02 | d | −2.88 | 0.06 | b,c | < | 431.4 | 0.71 | c | 112.92 | 2.84 | b | < | −0.31 | 0.00 | d | −0.28 | 0.01 | b,c | < |
| T4 | −2.18 | 0.03 | b | −2.22 | 0.16 | a | > | 436.0 | 0.71 | b | 97.63 | 3.31 | c | < | −0.20 | 0.00 | b | −0.22 | 0.02 | a | > |
| T5 | −3.80 | 0.03 | f | −3.28 | 0.22 | d | < | 317.0 | 0.71 | e | 71.63 | 1.66 | f | < | −0.34 | 0.00 | f | −0.31 | 0.02 | d | < |
| T6 | −3.58 | 0.04 | e | −3.62 | 0.23 | e | > | 327.5 | 0.71 | d | 81.44 | 2.84 | e | < | −0.33 | 0.00 | e | −0.34 | 0.03 | e | > |
| T7 | −2.85 | 0.03 | c | −2.30 | 0.16 | a | < | 315.8 | 1.35 | e | 79.84 | 3.08 | e | < | −0.26 | 0.00 | c | −0.22 | 0.02 | a | < |
| T8 | −1.40 | 0.02 | a | −2.05 | 0.07 | a | < | 311.7 | 1.82 | f | 88.05 | 1.12 | d | < | −0.13 | 0.00 | a | −0.2 | 0.01 | a | < |
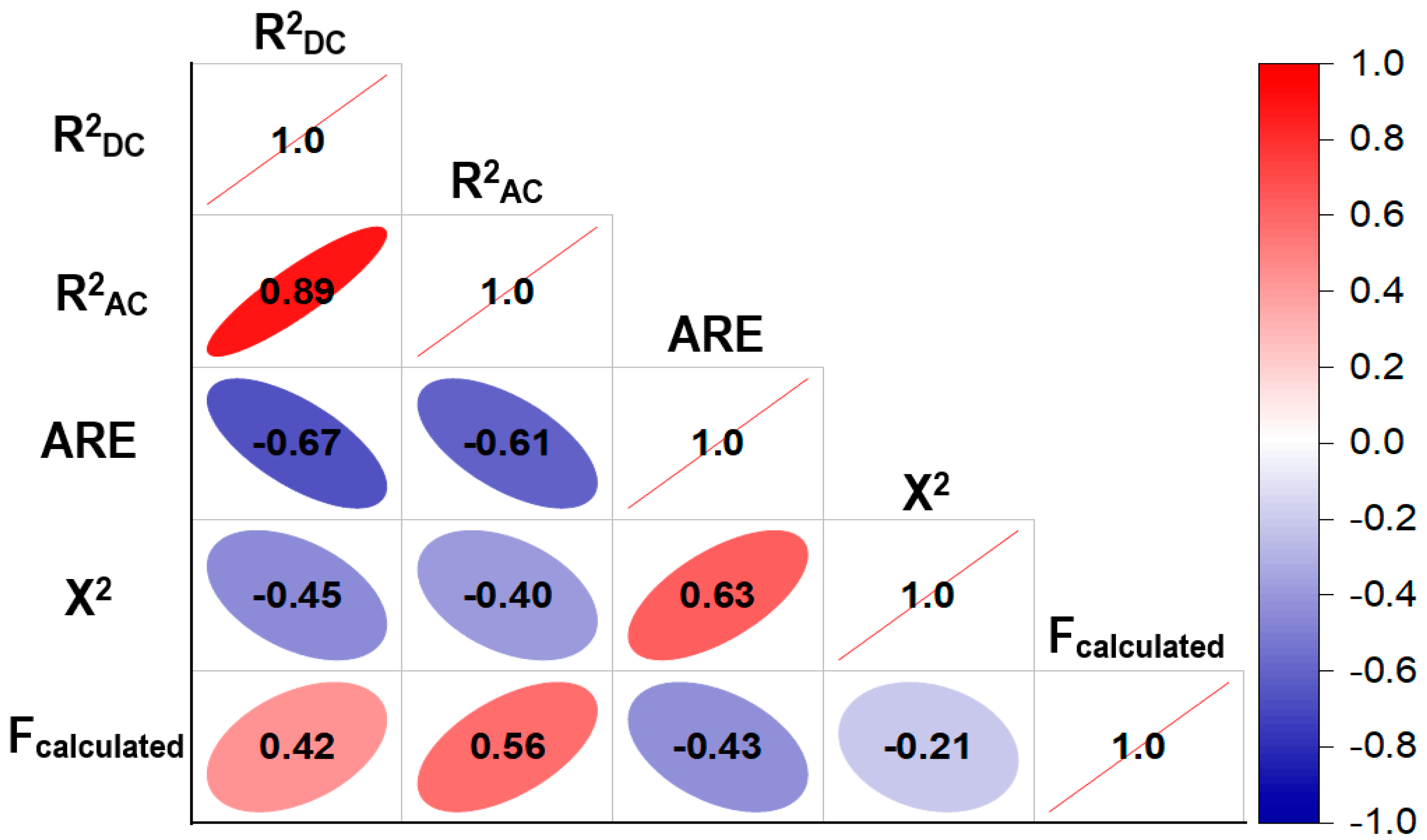
| NSH Suspension Property | Model | R2DC | R2AC | ARE | X2 | Fcalculated | Fcritical |
|---|---|---|---|---|---|---|---|
| Sedimentation (%T) | L | 0.9416 | 0.8978 | 1.3666 | 0.1580 | 21.5011 | 0.0063 |
| LITF | 0.9844 | 0.8907 | 0.8467 | 0.0412 | 10.5095 | 0.2318 | |
| Turbidity (%T) | L | 0.9545 | 0.9204 | 0.1818 | 0.0032 | 27.9894 | 0.0038 |
| LITF | 0.9986 | 0.9902 | 0.0355 | 0.0001 | 119.0552 | 0.0700 | |
| Z potential (mV) | L | 0.4559 | 0.0479 | 2.1507 | 0.1302 | 1.1173 | 0.4409 |
| LITF | 0.9078 | 0.3545 | 1.0444 | 0.0219 | 1.6408 | 0.5353 | |
| Particle size (nm) | L | 0.5551 | 0.2215 | 9.3394 | 35.8759 | 1.6638 | 0.3104 |
| LITF | 0.9409 | 0.5864 | 4.0750 | 4.9762 | 2.6539 | 0.4381 | |
| Color index | L | 0.5969 | 0.2947 | 9.8341 | 0.3408 | 1.9748 | 0.2599 |
| LITF | 0.9889 | 0.9225 | 2.0976 | 0.0094 | 14.8829 | 0.1959 |
| Coefficient | T | S | ζP | PS | CI |
|---|---|---|---|---|---|
| Intercept | 100.04 | 85.37 | −55.30 | 1570.49 | 0.28 |
| pH | −0.73 | 0.62 | 2.47 | 20.17 | −0.29 |
| T | 0.07 | 0.31 | 0.17 | −26.50 | 0.09 |
| C | 15.90 | −573.74 | 351.28 | −2035.28 | −108.54 |
| pHxT | 0.01 | −0.04 | 0.01 | 1.64 | −0.02 |
| pHxC | 1.31 | 60.69 | −29.44 | −1772.78 | 16.54 |
| TxC | −1.19 | −0.18 | −2.44 | 155.83 | 0.31 |
| Condition Criteria | Parameters | Minimum * | Maximum * | Optimum | Case Application for Day 20 |
|---|---|---|---|---|---|
| Restriction | pH | 4.00 | 6.50 | 4.50 | 4.50 |
| T (°C) | 40.00 | 90.00 | 84.55 | 85.00 | |
| C (g/L) | 0.05 | 0.15 | 0.08 | 0.08 | |
| Objective function (maximize) | Sedimentation (%T) | --- | --- | 72.34 | 74.33 ± 0.35 |
| Subject to | Turbidity (%T) | 90.00 | 99.00 | 99.00 | 99.27 ± 0.83 |
| ζ potential (mV) | --- | --- | −25.64 | −26.55 | |
| Particle size (nm) | 300.00 | 400.00 | 300.00 | 311.21 ± 21.54 | |
| Color index | −2.00 | 2.00 | −2.00 | −2.14 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choque-Quispe, Y.; Choque-Quispe, D.; Ligarda-Samanez, C.A.; Solano-Reynoso, A.M.; Froehner, S.; Ramos-Pacheco, B.S.; Carhuarupay-Molleda, Y.F.; Sumarriva-Bustinza, L.A. A High Andean Hydrocolloid Extracted by Microatomization: Preliminary Optimization in Aqueous Stability. Polymers 2024, 16, 1777. https://doi.org/10.3390/polym16131777
Choque-Quispe Y, Choque-Quispe D, Ligarda-Samanez CA, Solano-Reynoso AM, Froehner S, Ramos-Pacheco BS, Carhuarupay-Molleda YF, Sumarriva-Bustinza LA. A High Andean Hydrocolloid Extracted by Microatomization: Preliminary Optimization in Aqueous Stability. Polymers. 2024; 16(13):1777. https://doi.org/10.3390/polym16131777
Chicago/Turabian StyleChoque-Quispe, Yudith, David Choque-Quispe, Carlos A. Ligarda-Samanez, Aydeé M. Solano-Reynoso, Sandro Froehner, Betsy S. Ramos-Pacheco, Yakov Felipe Carhuarupay-Molleda, and Liliana Asunción Sumarriva-Bustinza. 2024. "A High Andean Hydrocolloid Extracted by Microatomization: Preliminary Optimization in Aqueous Stability" Polymers 16, no. 13: 1777. https://doi.org/10.3390/polym16131777
APA StyleChoque-Quispe, Y., Choque-Quispe, D., Ligarda-Samanez, C. A., Solano-Reynoso, A. M., Froehner, S., Ramos-Pacheco, B. S., Carhuarupay-Molleda, Y. F., & Sumarriva-Bustinza, L. A. (2024). A High Andean Hydrocolloid Extracted by Microatomization: Preliminary Optimization in Aqueous Stability. Polymers, 16(13), 1777. https://doi.org/10.3390/polym16131777










