1. Introduction
Agriculture is one of the cornerstones of human society, feeding eight billion people on Earth. In agriculture, soil plays a crucial role [
1]. It serves as the substrate for crop growth, providing the necessary nutrients and moisture for plants to thrive [
2]. However, improper agricultural practices such as over-tillage, over-fertilization, and excessive use of chemicals can lead to soil degradation. Soil degradation results in decreased land productivity and serious issues like soil erosion, posing significant threats to the sustainability of human society [
3].
Soil aggregates are the basic units of soil structure [
1]. Enhancing the stability of soil aggregates is pivotal for improving the performance of degraded soils and restoring their functionality. The formation of soil aggregates in natural soils typically involves interactions among biological, physical, and chemical processes. Soil microorganisms decompose organic matter, yielding colloids and gelatinous substances that act as binding agents, facilitating the cohesion of soil particles into aggregates. However, in soils subjected to improper agriculture practices, these organic binding agents may not be effectively replenished, leading to a decline in aggregate stability and soil performance. Strategies for enhancing soil aggregate stability include the addition of organic matter and polymer amendments. Incorporating organic matter such as compost, manure, or crop residues can increase soil organic matter content, enhance soil microbial activity, and promote the formation and stabilization of aggregates [
4]. Nevertheless, the effectiveness of organic matter addition may require extended periods to manifest visibly, and there is a risk of introducing external pathogens or weed seeds. Another approach involves the application of polymer amendments, such as polyacrylamide (PAM) [
5,
6]; however, its efficacy is limited and it may suffer from a short duration of action [
7]. Consequently, there remains a lack of efficient, rapidly effective, and long-term effective soil stabilization methods.
In this study, we propose an efficient, rapid, and long-term stable method for enhancing soil aggregate stability and subsequently improving soil performance. We introduced polyvinyl alcohol (PVA) into the soil (at a concentration of 0.1% of the soil) by mixing aqueous PVA solution with the soil, followed by complete drying. The large surface area of clay and slit particles in the soil can irreversibly adsorb PVA onto the soil [
8,
9], and the drying treatments greatly enhance this interaction. Through this treatment, PVA acts as a soil binder, significantly enhancing the water stability of soil aggregates. Soil aggregates bonded with PVA do not disintegrate in wet sieving tests and remain stable even after 30 wet–dry cycles and a one-year field experiment without significant degradation. Compared to the original soil, the PVA-treated soil shows higher germination rates, highlighting its superiority over the original soil. Our method offers promising commercial prospects due to its low material cost, rapid effectiveness, low energy consumption, long duration, and yield-enhancing capabilities
2. Materials and Methods
Chemicals and soils: Polyvinyl alcohol (KURARAY Tokyo, Japan, POVAL™ 60–98, 98–99% mol% hydrolysis) was purchased from a commercial source. The first soil was collected from Chaozhou City, Guangdong Province. The second soil is yellow soil from Shanxi Province, and the third type is red soil from Fujian Province, and were both purchased from Taobao.com. All soils were dried and passed through a 0.05 mm sieve to remove large particles of sand. Tap water was used throughout the entire experiment.
Fabrication of PVA soil: Ten grams of PVA powder was added to 90 g of water and stirred in a 95 °C water bath until completely dissolved to obtain the PVA solution. Then, the PVA solution, dried soil, and water were mixed together in a composition such that the mass ratio of PVA to soil was 0.1% and the mass ratio of water to soil was 25%. The mixture was thoroughly mixed and then dried in an atmosphere.
Mechanical test of soil: Dry soil was mixed with water and different amounts of PVA solution, with the water content in the mixture set at 25%. The mixture was then transferred to a mold (20 cm in length, 3 cm in both width and thickness) and dried in the atmosphere. The flexural strength of the dried specimen was tested using a universal testing machine (XLD-20E, Jingkong Mechanical Testing Co., Ltd. Guangzhou, China) with a three-point bending setup. For the wet specimen, the soil sample was soaked in water for 1 h before the test.
Wet sieving test: Dried soil and PVA soil with particle sizes ranging from 0.5 to 2 mm were collected. These soils were placed on the sieve with a mesh size of 0.25 mm. Then, the sieve was immersed in a water bath and moved up and down with a displacement of 3 cm and a frequency of 30 times per minute for 10 min. After this process, the sample remaining on the sieve was collected and dried again. The change in mass before and after the sieving process was used to indicate the water stability of the soils.
Measurement of particle density: Ten grams of dried soil was placed in a 50 mL pycnometer and then 20 g of water was added into the bottle. The bottle was kept in a vacuum for 1 h to remove bubbles. Finally, the bottle was transferred to the atmosphere and filled with water. The mass of the bottle, soil, and water was weighed as
mbws. The same pycnometer filled only with water was weighed as
mbw. The particle density of soil,
, was determined as:
where
ms is the mass of PVA soil and
is the density of water at 25 °C (since the experiment is conducted at room temperature).
Measurement of bulk density and porosity: The sieved PVA soil was added into a plastic collum with a volume of 1 L. Oscillation and percussion were applied to enhance package. Then, the PVA soil was weighed and the bulk density
calculated by dividing mass by volume. The porosity (
f) of PVA soil was determined as:
Measurement of hydraulic conductivity: The PVA-treated soil was placed in a cylindrical apparatus with a cross-sectional area of 30 cm2. Under constant head conditions and a steady vertical water flow, the volume of water passing through the specimen was measured over a specific time interval. Subsequently, the hydraulic conductivity of the specimen was calculated using Darcy’s law.
For untreated soil, hydraulic conductivity is measured by a falling head condition.
Pumpkin seedling cultivation: The pumpkin seeds were obtained from Daziran Agricultural Technology Co., Ltd. in Shouguang City, China. Before sowing, the seeds were soaked in water at 40 degrees Celsius for 1 h, then covered with a moist paper for 24 h. After that, the seeds were placed in wet soil and covered with 0.5–1 cm of soil. The seedlings were then placed in a bright environment, and the lid of the seedling tray was covered for the first four days. The temperature during the experiment ranged from 20 to 30 °C. Each of the 12-cell seedling trays had six cells filled with PVA–CZ soil and six cells filled with CZ soil. Each seedling tray counted as one trial. The number of germinated seeds on the seventh day was used to calculate the germination rate. On the second day, the germinated pumpkins were carefully removed from the soil, and the soil was washed off the roots with water and then the moisture was removed with paper towels. The roots were then trimmed and weighed. Six random pumpkin seedlings were selected for root mass measurement. On the seventh day, the pumpkins were separated from the soil, the roots were carefully trimmed off, and the weight of six pumpkin shoots was measured to assess the growth rate of pumpkins in different soils.
3. Results and Discussion
3.1. Design Principle
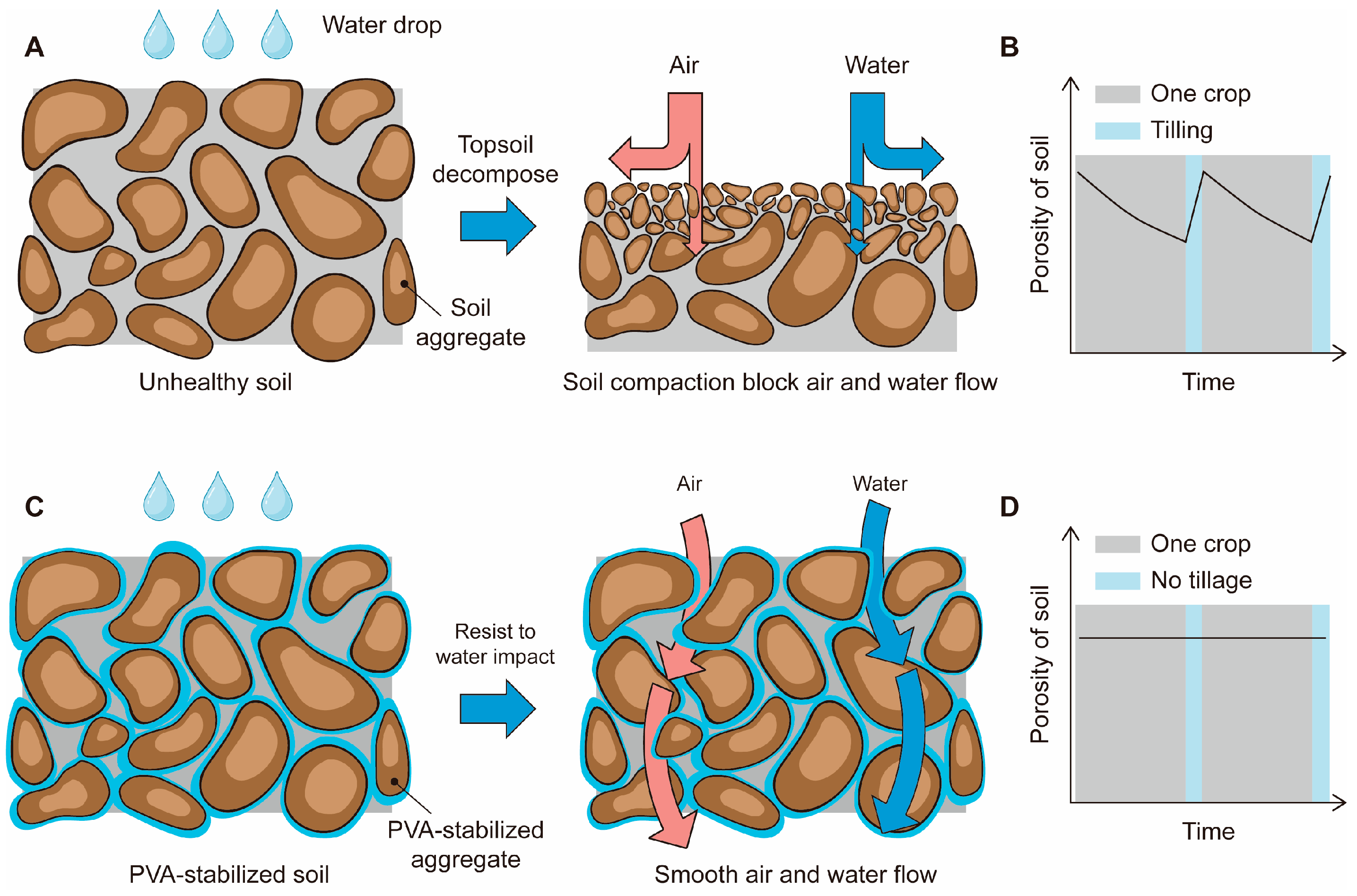
Soil aggregate stability is an important indicator of soil health [
10]. Unstable aggregates disintegrate under the impact of rainfall, forming smaller particles that can clog existing surface pores, leading to the formation of crusts. This, in turn, reduces soil permeability and aeration (
Figure 1A). At this point, operations such as plowing may seem necessary (
Figure 1B). However, plowing not only fails to solve the problem of crust formation permanently but also disturbs the soil further and could potentially make it less healthy. Additionally, lower aggregate stability can lead to surface soil erosion under rainfall [
11,
12,
13,
14].
The goal of this study is to create a soil composed of water-stable aggregates, thereby fundamentally preventing the occurrence of crust formation and compaction (
Figure 1C) [
15]. By improving the water stability of the aggregates, they are better able to withstand the impact of rainfall while maintaining good permeability. This stable soil, with its stable porosity, can also decrease the frequency of tillage (
Figure 1D).
Based on considerations of safety [
16,
17], biodegradability [
18,
19,
20], and adhesion [
8,
9], we selected polyvinyl alcohol (PVA) as the soil adhesive. We enhanced the bonding effect through a process of mixing and drying. In this study, we investigated three types of soils: soil from the author’s hometown of Chaozhou, referred to as CZ soil; soil from the Loess Plateau (from Shanxi province), referred to as yellow soil; and soil from South China (from Fujian province), referred to as red soil. The latter two are widely distributed soils in China and are generally considered to have poor performance, requiring improvement. The samples treated with PVA are referred to as PVA–CZ soil, PVA–yellow soil, and PVA–red soil, respectively.
3.2. Mechanical Performance
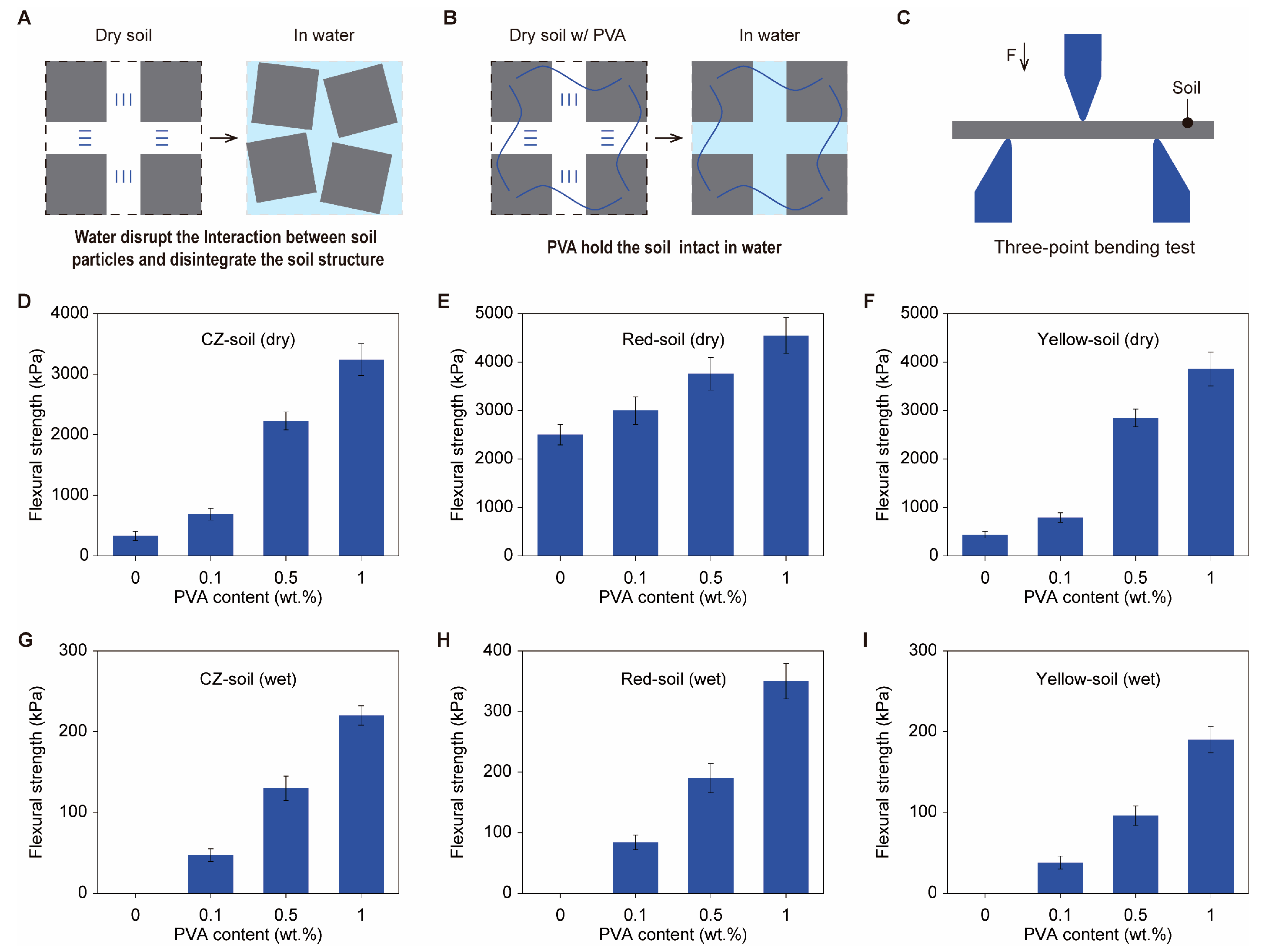
The mechanical properties of soil are closely related to the amount of binder it contains. As shown in
Figure 2A, when dry soil that lacks an organic binder is immersed in water, the interactions between soil particles are disrupted by the water, leading to soil disintegration. However, when PVA is added, the irreversible adhesion of PVA to soil particles can maintain the shape of the soil in water (
Figure 2B). In this regard, a higher content of PVA will impart greater mechanical strength to the soil, especially under wet conditions.
We conducted three-point bending tests (
Figure 2C) to assess the mechanical properties of soils with different PVA concentrations. Taking CZ soil as an example, the original soil strength was ≈330 kPa. After adding 0.1% PVA, the strength increased to ≈1000 kPa (
Figure 2D). When the PVA content was increased to 1%, the strength further increased to ≈3500 kPa (
Figure 2D). The same trend was observed for dried red soil (
Figure 2E) and yellow soil (
Figure 2F).
The addition of PVA imparts superior mechanical properties to soils under wet conditions. In the absence of PVA, CZ soil, yellow soil, and red soil all disintegrate in water, as shown in
Figure S1. We thus regard their flexural strengths as zero. However, with the addition of PVA, these three types of soils can maintain their initial shapes in water, as shown in the photos in
Figure S2. In three-point bending tests, with a PVA content of 0.1%, the flexural strength of wet PVA–CZ soil is approximately 50 kPa, and this strength increases to above 200 kPa when the PVA content is further increased to 1% (
Figure 2G). Similar improvements in flexural strength can be observed for samples of red soil and yellow soil after being moistened (
Figure 2H,I). These results reveal that the addition of 0.1% PVA endows the soil with measurable flexural strength in a wet state, indicating a fundamental change in soil stability that further leads to profound changes in other physical properties as well.
The water stability of PVA-treated soils is attributed to the interaction between PVA and the soil particles. PVA is a hydrophilic and neutral polymer, possessing numerous side-chain -OH groups that can act as both hydrogen bond donors and acceptors. In this work, the textures of the three soils are as follows: CZ soil has 13% clay, 29% silt, and 58% sand; yellow soil has 1% clay, 71% silt, and 28% sand; red soil has 4% clay, 76% silt, and 20% sand. Although their textures are quite different, we observe a similarity: these soils contain -OH groups, as evidenced by the stretching peak ranging from 3500 to 3700 cm
−1 in the FTIR-ATR spectra (
Figure S3) [
21]. In this sense, when the PVA solution is mixed with soil, PVA chains are distributed among the soil particles. Due to the large surface area of clay and silt particles, PVA is more likely to be absorbed by these particles through hydrogen bonding. Then, when the mixture is dried, the water molecules between the PVA and soil particles are removed, significantly increasing the contact between PVA and clay and silt particles, which implies the formation of a large number of hydrogen bonds. The formation of multiple hydrogen bonds between PVA and soil particles ensures a strong interaction. Moreover, the dense hydrogen bonding also prevents disruption by water when the PVA soil is wetted again. In brief, the large number of hydrogen bonds between PVA and soil particles contributes to the high water stability of the PVA soil.
3.3. Stability of Soil in Water
Water stability is significantly enhanced with increasing concentrations of PVA in soil. However, considerations of cost-effectiveness dictate that the lowest effective concentration of PVA should be used. Our experiments have shown that a PVA concentration of 0.05% does not achieve the necessary level of water stability in the soil. Therefore, we have chosen to proceed with a PVA concentration of 0.1%, as this concentration strikes a balance between the desired stability and economic viability.
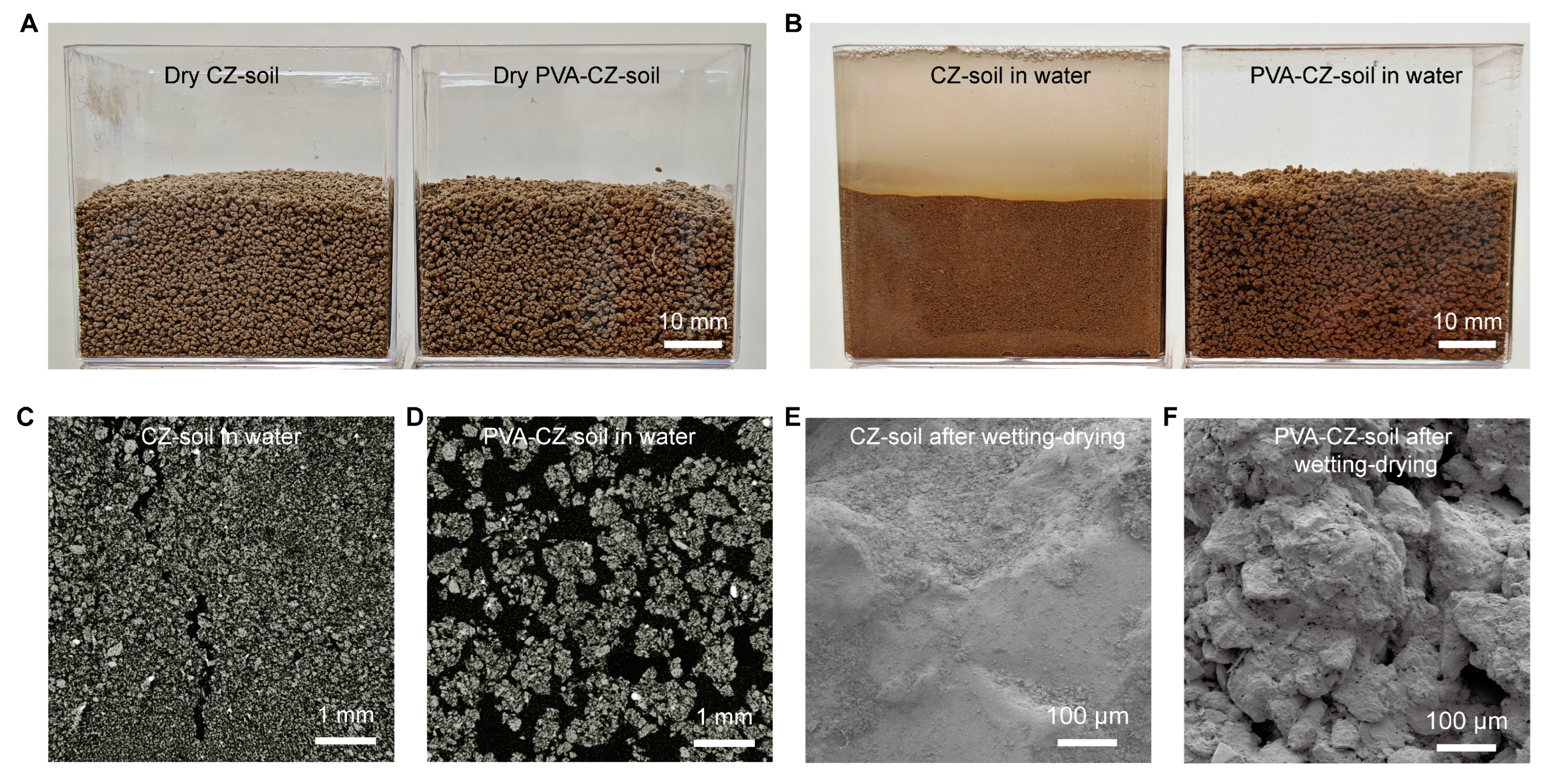
We investigated the changes in soil due to PVA during wet–dry cycles. We dried samples of CZ soil containing 0.1% PVA and those without PVA and sieved them to obtain particles in the size range of 0.5–2 mm for study. During drying, there was no significant difference between the two types of soils (
Figure 3A). However, upon adding water, the aggregates of PVA-free CZ soil disintegrated, whereas the PVA–CZ soil maintained the same appearance as when it was dry (
Figure 3B). This phenomenon was also observed in yellow soil and red soil (
Figure S4).
We used computed tomography (CT) to analyze the structure of CZ soil in water. The PVA-free CZ soil had a large number of aggregates estimated to be less than 0.1 mm in size (
Figure 3C), which is much smaller than their initial size range of 0.5–2 mm. Meanwhile, PVA–CZ soil retained large aggregates in water, with no signs of aggregate breakdown (
Figure 3D). The PVA–CZ soil had more voids, which are beneficial for maintaining soil moisture and high permeability. After wet–dry cycles, the surface of PVA-free soil became denser due to the tendency of small aggregates to compact densely (
Figure 3E). On the other hand, the sample with PVA remained rough and porous (
Figure 3F).
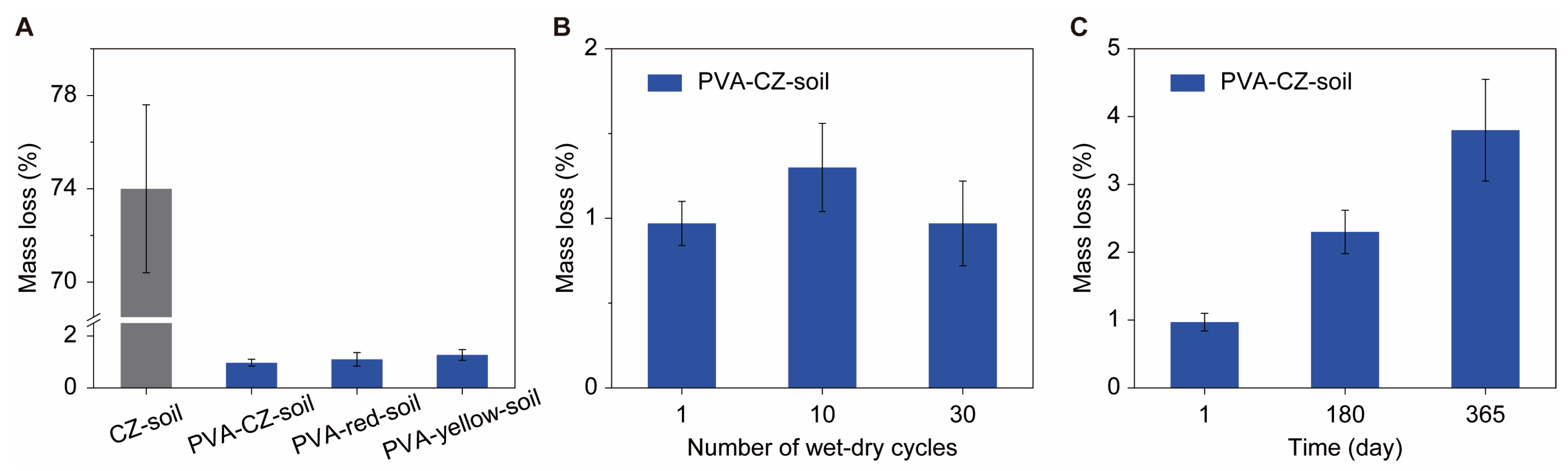
We evaluated the water stability of soil improved by PVA using the wet sieving method [
10]. Soil samples with and without 0.1% PVA, with particle sizes ranging from 0.5 to 1 mm, were subjected to wet sieving using a 0.25 mm sieve, and the changes in mass after wet sieving were recorded. As shown in
Figure 4A, the mass reduction of CZ soil without PVA after one wet sieving exceeded 70%, while the one with only 0.1% PVA showed a mass change of about 1%. After multiple wet–dry cycles, the stability of soil in wet sieving did not show a significant decrease. For instance, the soil after 30 wet–dry cycles only shows a reduction of 1% in the wet sieving test (
Figure 4B). After one year of cultivation in the field, subject to precipitation, we repeated the wet sieving process on the soil and found that the mass reduction was again less than 5% (
Figure 4C). This demonstrates the exceptional stability of the PVA-treated soil even under conditions of significant precipitation. The high water stability of PVA-improved soil is mainly attributed to its strong binding with mineral particles in the soil, while the low degradation rate of PVA in the soil ensures its long-term stability. Such long-term stability makes PVA a much better soil binder than the classical PAM, whose efficiency decays in a few months.
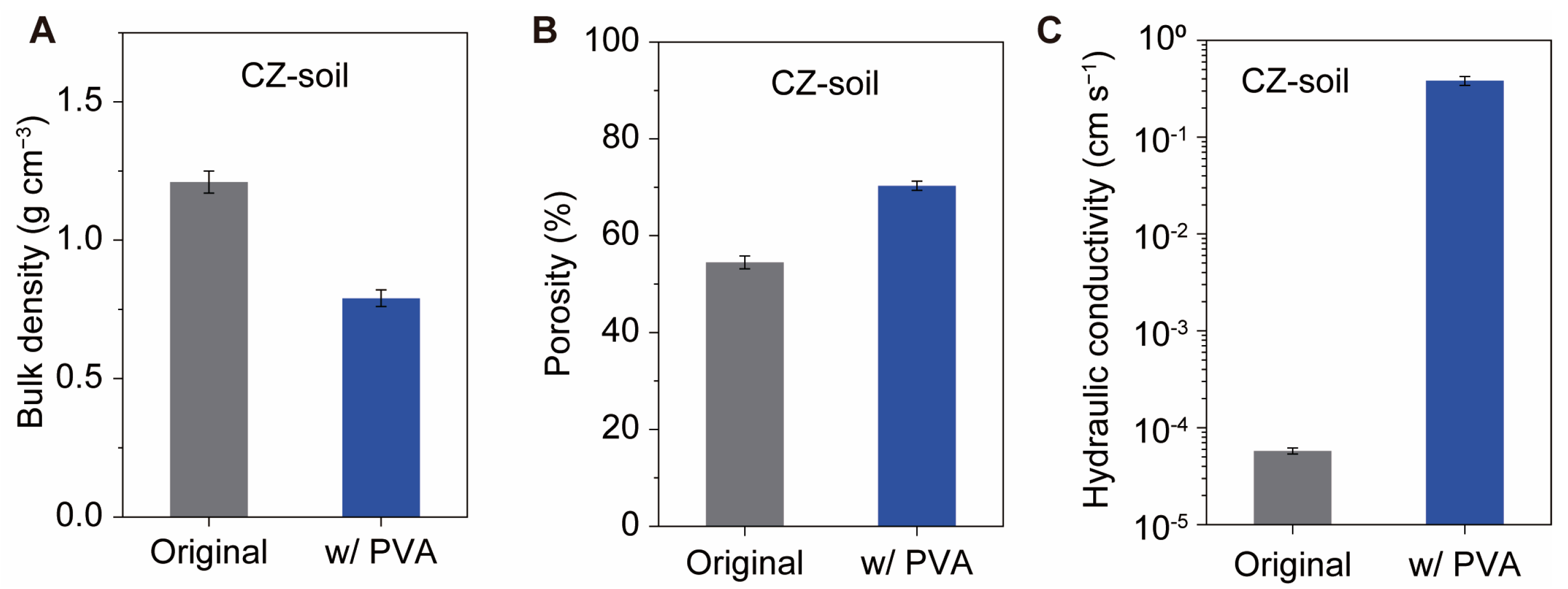
3.4. Physical Properties
The significant improvement in water stability greatly enhanced the performance of the soil. We compared the physical properties of the three types of soils with and without 0.1% PVA. The bulk density of the original CZ soil was 1.2 g cm
−3 (
Figure 5A), with corresponding porosities of 54% (
Figure 5B). After PVA amendment, the bulk density of the soil was 0.79 g cm
−3 (
Figure 5A), resulting in a calculated porosity of 70% (
Figure 5B), which is 16% higher than that of the original samples. This reduction in bulk density and increase in porosity is also observed in the case of red soil and yellow soil (
Figure S5A–D).
PVA treatment significantly improves soil permeability. The saturated hydraulic conductivity of soil without PVA was 6 × 10
−5 cm s
−1. After PVA amendment, the PVA–CZ soil exhibits a high hydraulic conductivity of 0.4 cm s
−1, which is four orders of magnitude higher than that of the original soil (
Figure 5C). The high hydraulic conductivity of CZ soil is due to its small particle size and low porosity, while PVA–CZ soil maintains larger pores even when submerged in water (
Figure 3C), greatly facilitating water permeation. The hydraulic conductivity of both red soil and yellow soil can also be enhanced by this PVA treatment (
Figure S5E,F). The low hydraulic conductivity of the original soil can lead to surface water accumulation, which not only hinders air diffusion into the soil but also causes runoff and soil erosion. This is one of the reasons for soil erosion in the Loess Plateau. The addition of PVA significantly increases soil permeability, preventing surface water runoff and erosion. Moreover, its high water stability also helps to prevent soil erosion. The method of using PVA-stabilized soil has significant implications for soil and water conservation in regions such as the Loess Plateau.
3.5. Application in Seedling Cultivation
The treatment with PVA significantly enhances the soil’s performance during cultivation. We compared the performance of PVA–CZ soil with CZ soil in seedling cultivation, with pumpkin being the study object. As shown in
Figure 6A, pumpkin seeds were planted in a 12-cell seedling tray, with half using PVA–CZ soil and the other half using CZ soil. In a typical scenario, pumpkin seeds emerged from PVA–CZ soil after two days (
Figure 6A). Pumpkin seedlings grew well in PVA–CZ soil after seven days, whereas in this typical test, no pumpkin seed succeeded in germinating from the CZ soil (
Figure 6B). We counted the germination rate over six experiments and the results showed that 60% more pumpkins germinated from the PVA–CZ soil compared to CZ soil (
Figure 6C). We removed the soil from the roots of the pumpkin seeds on day 2 and photographed them as shown in
Figure 6D. Pumpkin roots in CZ soil had only one main root and the root system was short and sparse, while those grown in PVA–CZ soil had five long roots with many secondary roots. The mass of the root system in the ones cultivated in PVA–CZ soil was about twice that in CZ soil (
Figure 6E), indicating a preferred condition was provided by the PVA–CZ soil. The strong root system supported strong growth. On day seven, the mass of the shoot of the pumpkins in PVA–CZ soil was higher than in CZ soil (
Figure 6E), further demonstrating the beneficial effects of PVA–CZ soil on plant growth.
In addition to pumpkin seedling cultivation, we explored the use of PVA–CZ soil, PVA–red soil, and PVA–yellow soil using various qualitative tests. This includes the cultivation of corn (
Figure S6) and mini-cyclamen (
Figure S7). We also experimented with growing a multitude of species in a glass tank using PVA–red soil, an endeavor that would have been unattainable with native soil (
Figure S8). We believe that these PVA soils hold great promise not only in agriculture but also excel as superior substrates for potted plants in horticulture.
3.6. Comparison with Existing Methods
We performed a comparative analysis of our PVA-based soil enhancement method against other soil amelioration techniques that utilize compost, biochar, or PAM. These three categories of materials are the popular choices for soil modification. Our evaluation primarily concentrates on the material costs, as well as the time and energy investments associated with each method.
Composting is a process that requires significant time investment, typically taking several months to mature [
22]. Once prepared, it then needs an additional extended period to exert a beneficial effect on the soil, as its potency relies on microbial decomposition [
23]. In this regard, composting is a time-consuming process. In contrast, our method begins to exert its effects as soon as the soil–PVA mixture is dried. Under sunny conditions or in dry weather, the time required for our method is minimal, usually amounting to just one week, and often less than that—perhaps two or three days. Furthermore, PVA solutions are commercially available, which further enhances the time efficiency of our approach.
Biochar is another type of soil amendment, but its production often requires high-temperature conditions, which can be energy-intensive [
24,
25]. In contrast, our PVA solution is easily obtainable from commercial sources and requires minimal energy for mixing with soil. Additionally, the drying process is naturally aided by sunlight or wind. Furthermore, biochar shares similar issues with compost, in that its effectiveness is cumulative and it also needs a long time to reach its full potential.
PAM is the most extensively researched polymer soil conditioner, offering a unique advantage in terms of lower material costs. In comparison, compost or biochar typically require application in much larger quantities, often tons per hectare, while PAM is applied in kilogram amounts. However, PAM is limited by its relatively low efficiency and rapid decline in effectiveness over time. The material cost of our PVA method may be comparable to PAM’s if we opt to focus on modifying only the topsoil; the materials usage is about ten kilograms per hectare.
Our PVA-based soil stabilization method offers significant benefits in terms of time, energy, and material savings. Additionally, it provides the added advantages of simplicity, long-term effectiveness, and tolerance to various additives, thereby expanding its potential applications. The mixing and drying process is straightforward and can be further simplified by adopting a spray-drying method, particularly when focusing on topsoil improvement. With respect to stability over time, our experimental results have shown that the PVA-treated soil remains stable with no observable decline after one year, demonstrating its exceptional long-term stability. This stability is a result of the strong interaction between PVA and the soil, as well as the absence of PVA-degrading microorganisms in the soil. This long-term stability reduces the overall input of PVA into the soil over a longer period. Our PVA method holds a high tolerance to other additives. PVA functions by binding the soil particles in the soil, and the addition of materials such as compost, biochar, and sawdust does not interfere with PVA’s efficacy. Our method is compatible with other soil improvement techniques, opening the door to a wider range of soil modification possibilities.
3.7. Potentials and Limitations
To focus on the efficacy of PVA, the prior discussion has concentrated solely on its interaction with soil, excluding other substances or additives to prevent overcomplication. Thus, the discussion serves as a proof of concept, leaving significant room for future enhancements and optimizations for actual application.
The PVA–soil mixture can be further refined by integrating organic materials such as straw or sawdust, as well as inorganic materials like vermiculite and perlite. The inclusion of light organic matter can reduce bulk density (
Figure S9A), while the porous inorganic material can enhance porosity (
Figure S9B), both of which are instrumental in improving soil properties.
The mixing and drying process can be optimized by a spraying-drying process. Field experiments have demonstrated that spraying the plowed soil with a PVA solution, at a rate of 10 g of PVA per square meter, is adequate to stabilize the topsoil.
This PVA-based soil improvement method holds promise for horticultural applications, where natural peat is commonly used, often leading to wetland destruction. Replacing peat with PVA soil in potting has been shown to be effective (
Figures S7 and S8).
Our method can be employed to mitigate soil erosion caused by water. Enhancing soil stability in water and increasing hydraulic conductivity minimizes soil detachment and runoff, protecting the soil from erosion due to rainfall.
PVA-stabilized soil may be more suitable for sponge city initiatives, signified by the tunable and high hydraulic conductivity. The larger the size of the aggregate, the greater the hydraulic conductivity (
Figure S10). The highly permeable PVA soils are thus the preferable sponge, having the potential to alleviate the negative impacts of heavy rain in urban areas.
Finally, it is imperative to acknowledge that the long-term environmental impact of PVA in soil requires thorough investigation. While PVA is recognized as a safe material for humans by the FDA, and it is plausible to infer that it poses minimal harm to the environment when used in soil, the precise effects of this polymer on soil microorganisms and other living organisms have not been well-documented. Comprehensive studies are essential before the widespread application of PVA in soil can be confidently recommended.