Xanthan–Polyurethane Conjugates: An Efficient Approach for Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
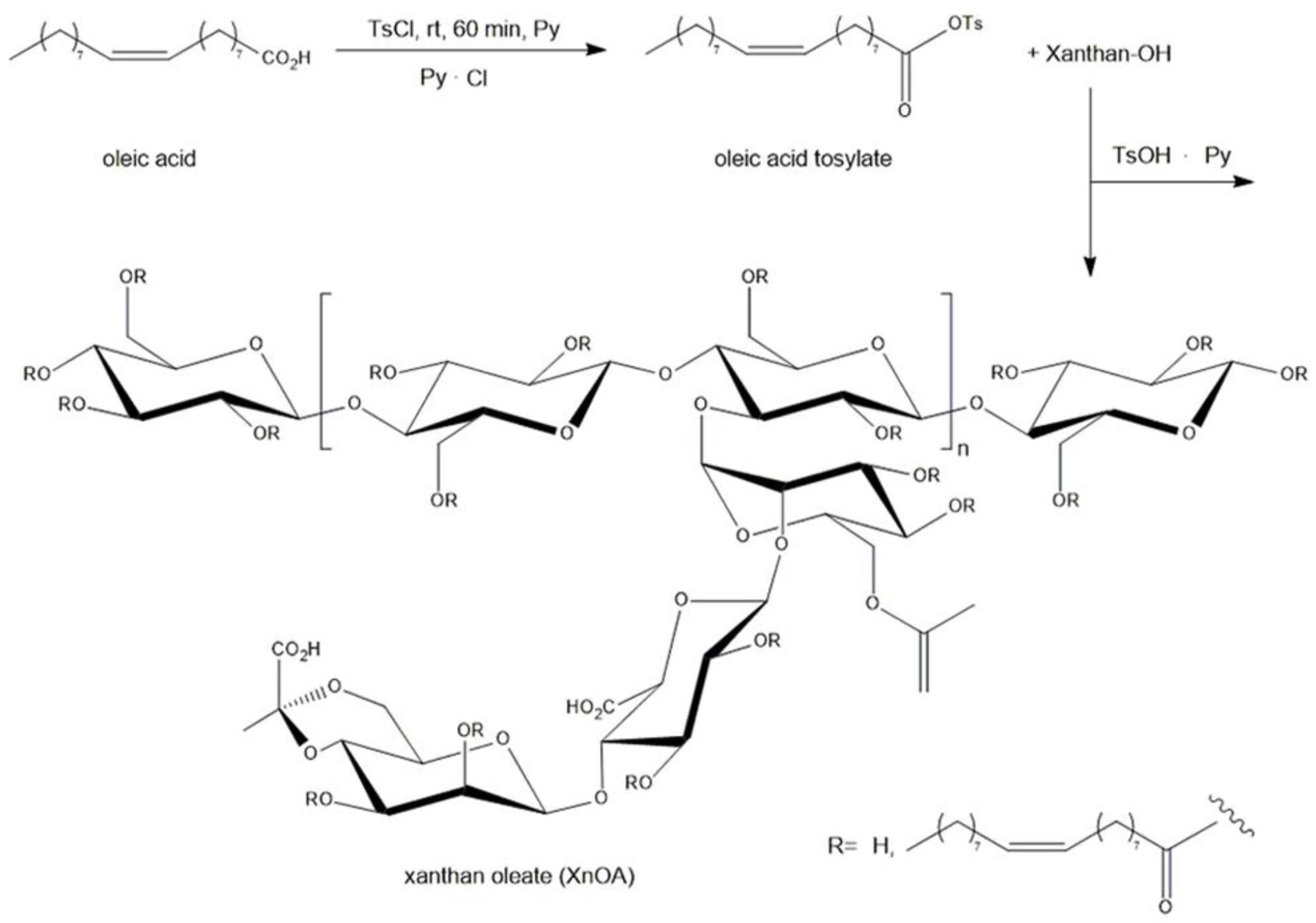
2.2. Synthesis of Xanthan Oleate
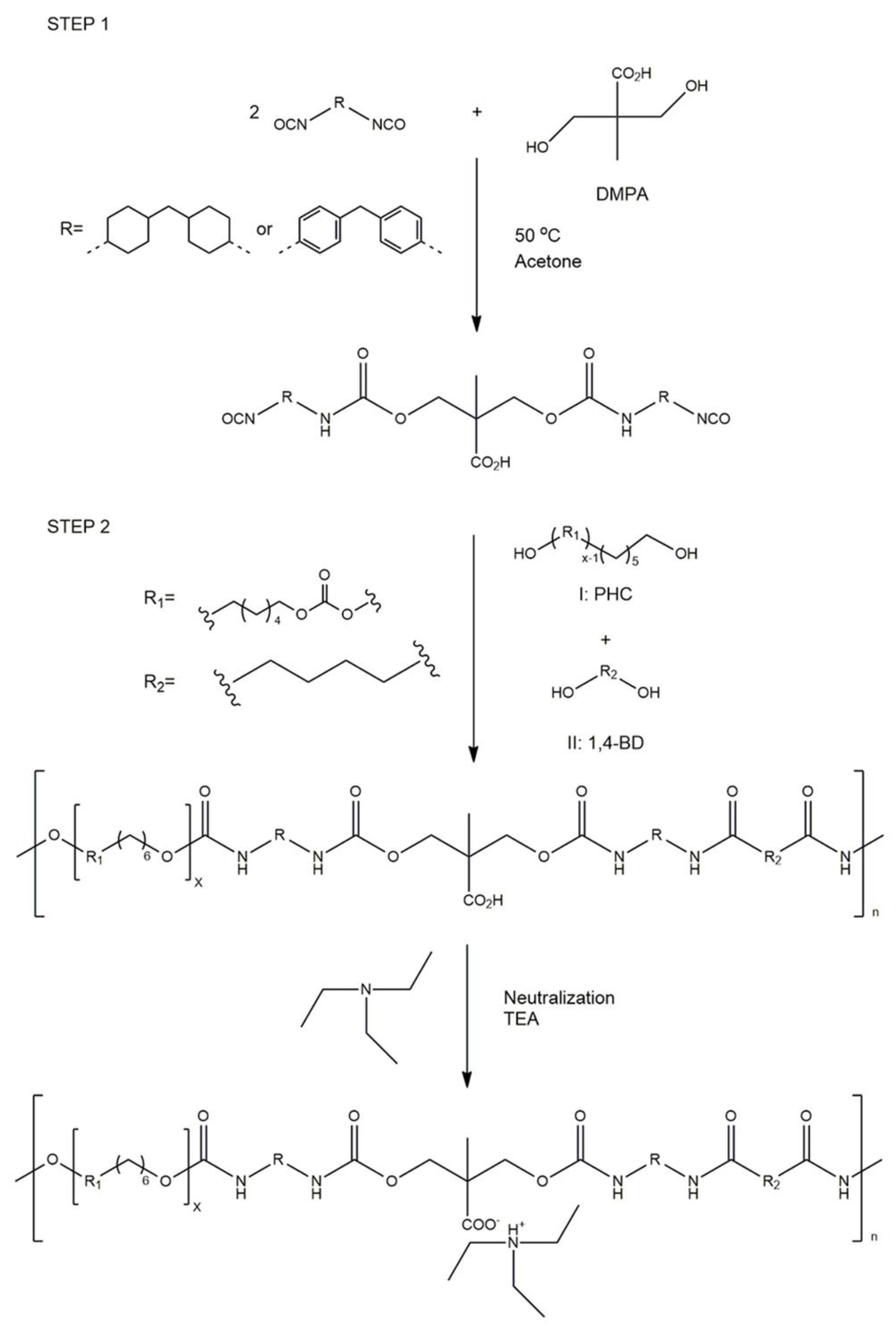
2.3. Synthesis of Polyurethane
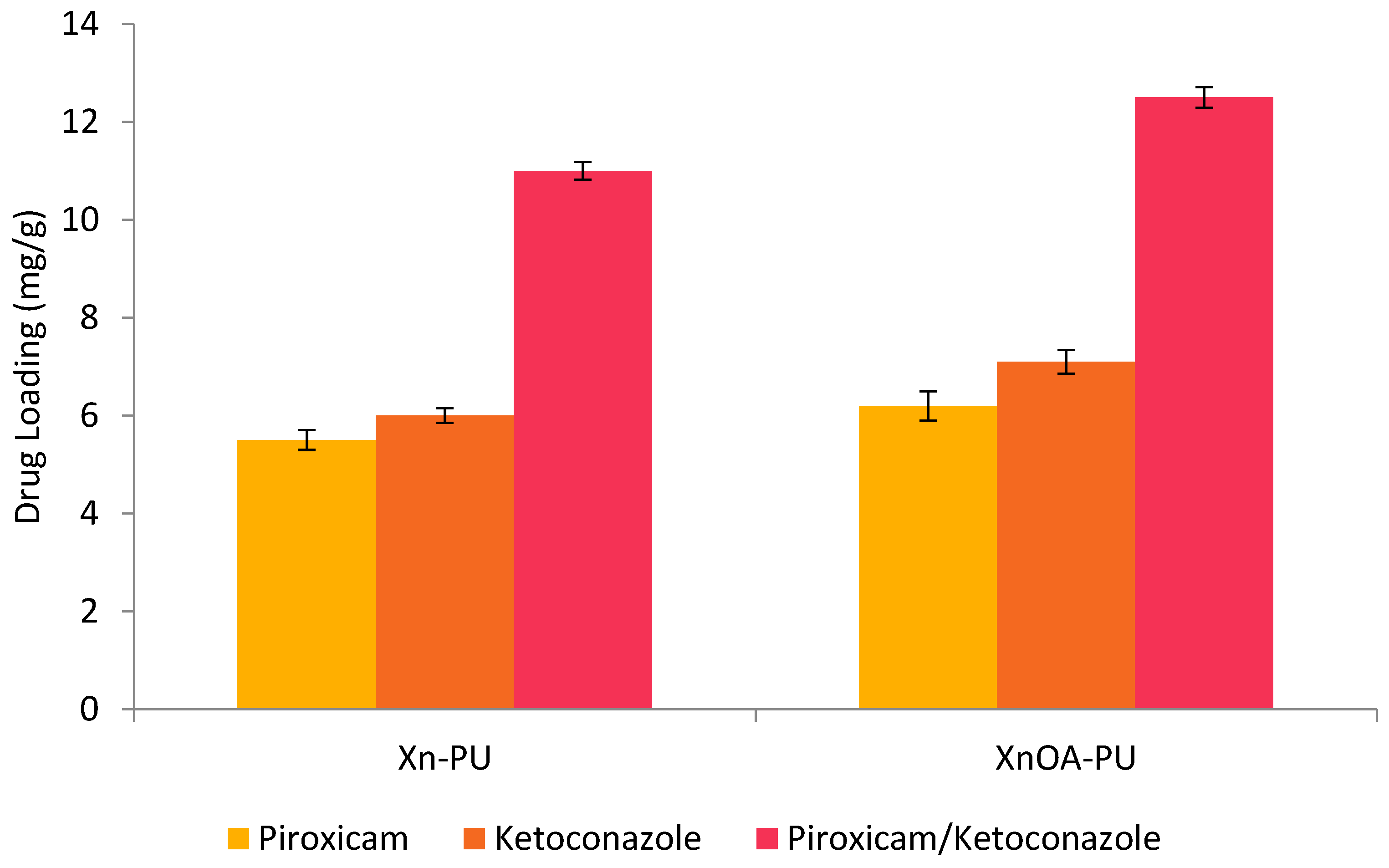
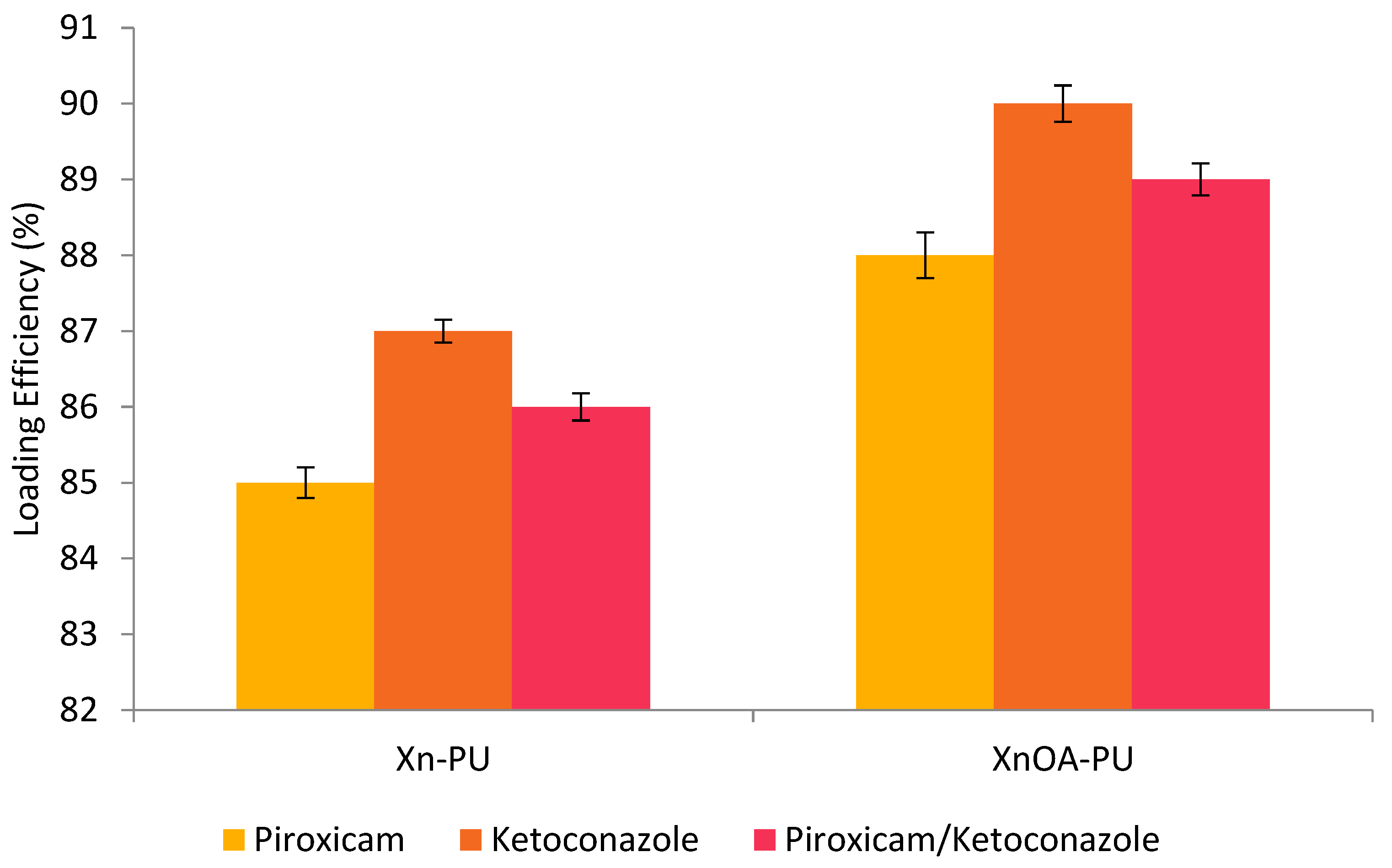
2.4. Preparation of Biomaterials
2.5. FTIR (Fourier Transform Infrared Spectroscopy) Analysis
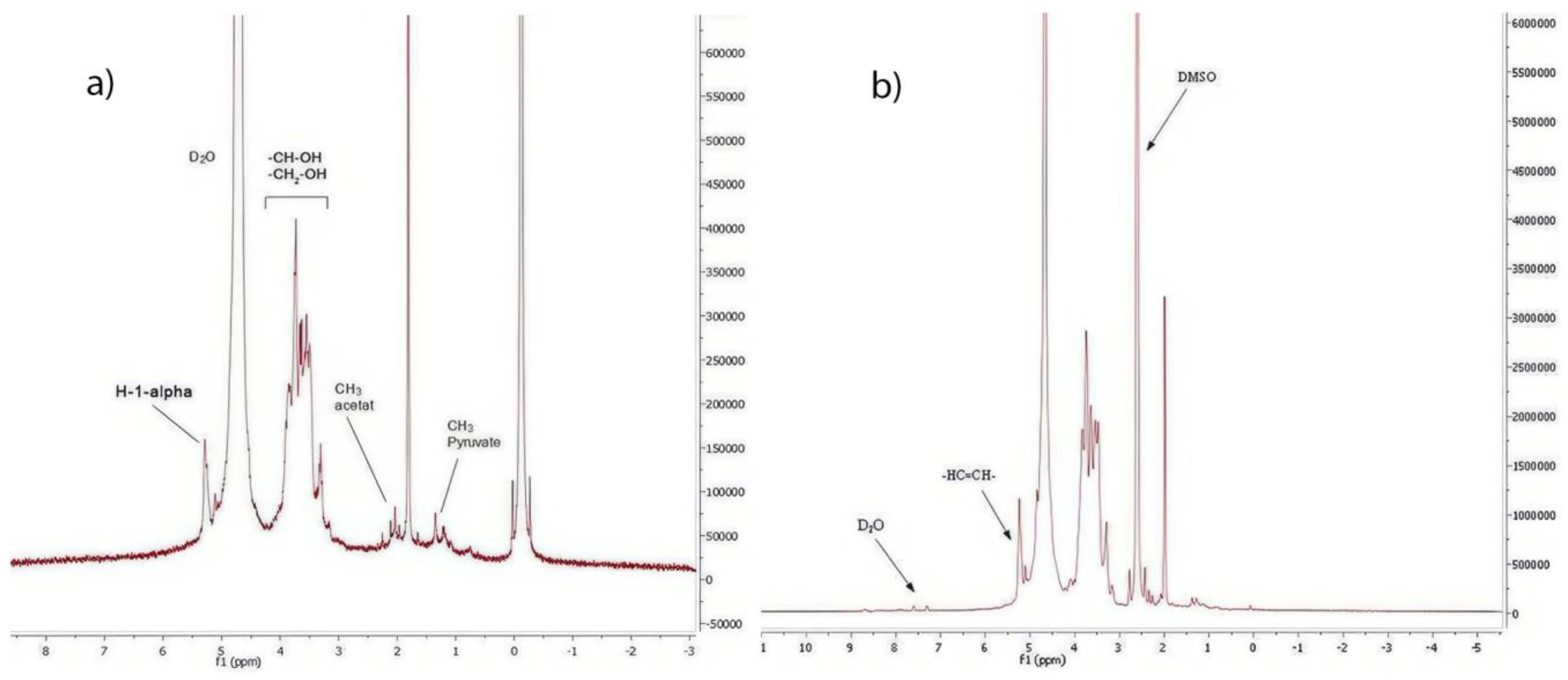
2.6. NMR (Proton Nuclear Magnetic Resonance) Analysis
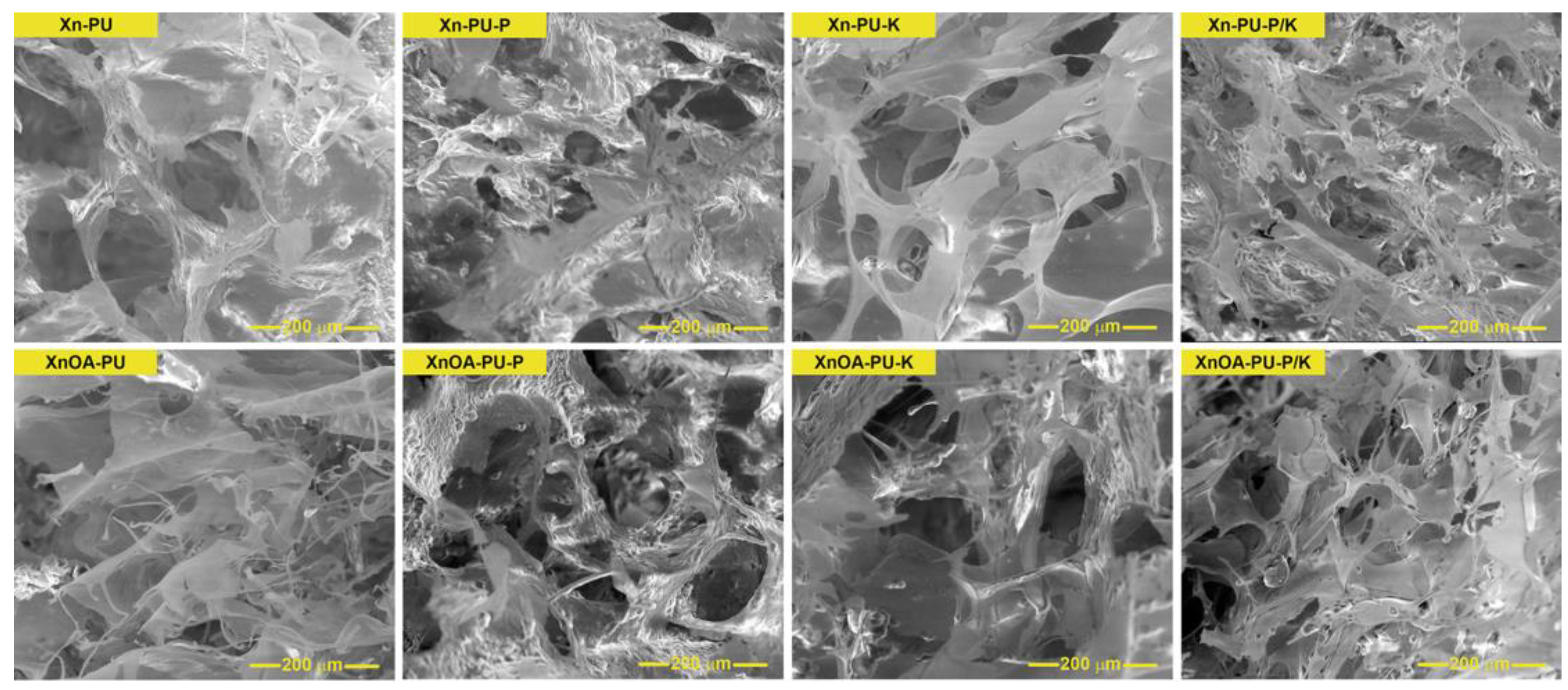
2.7. Scanning Electron Microscopy (SEM)
2.8. Mechanical Tests
2.9. Evaluation of Antimicrobial Activity
2.10. Evaluation of In Vitro Anti-Inflammatory Activity
2.11. In Vitro Drug Release
3. Results and Discussions
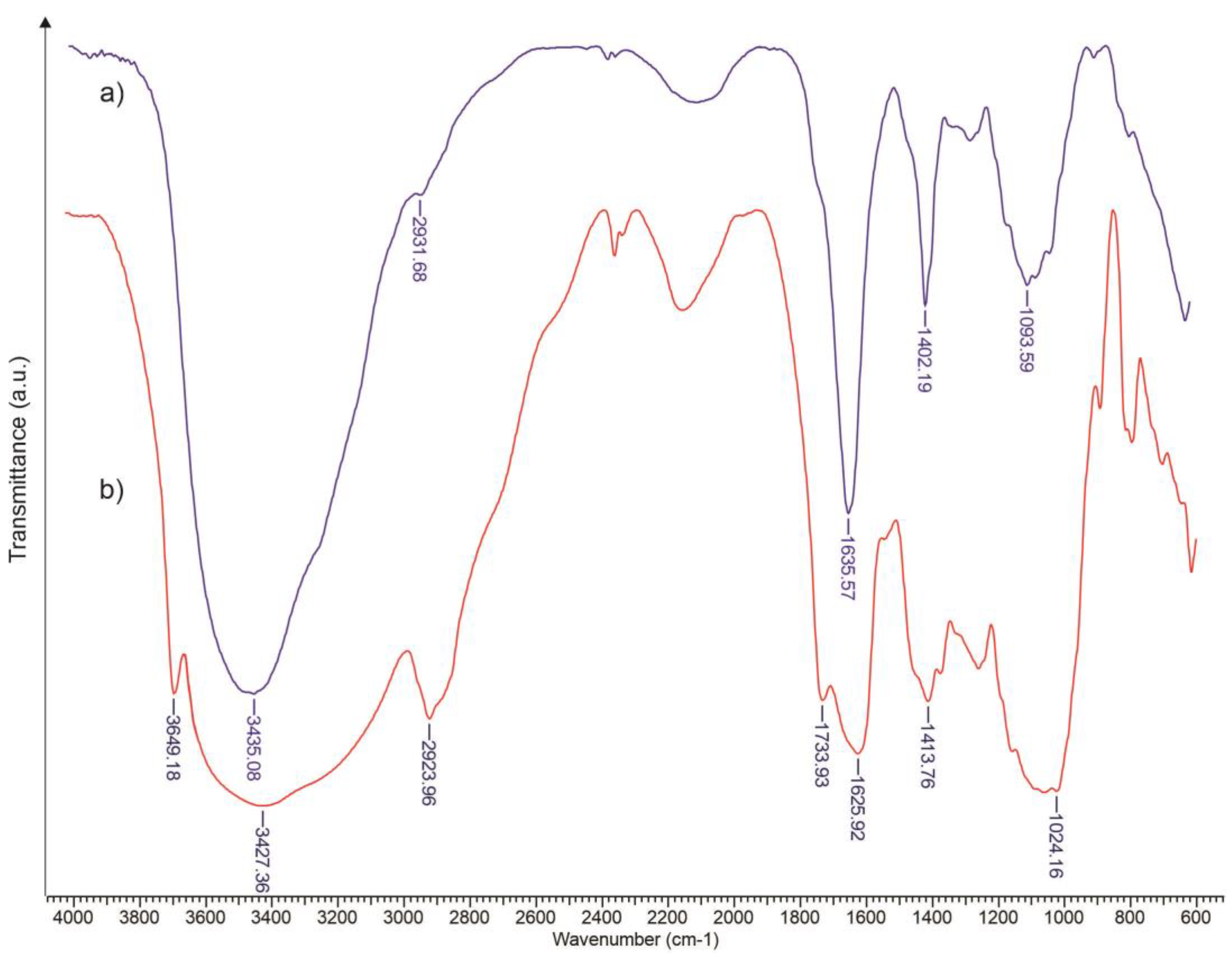
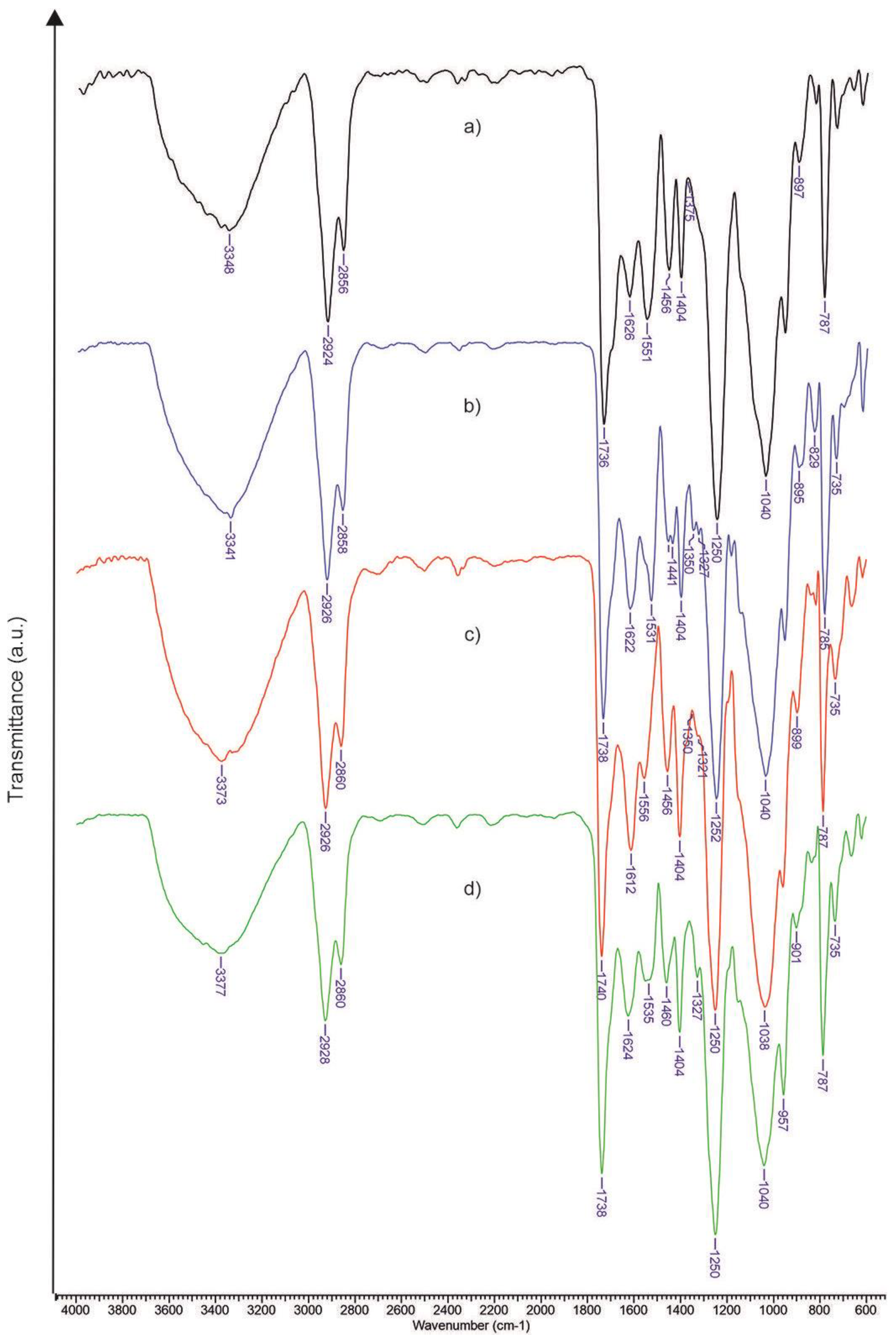
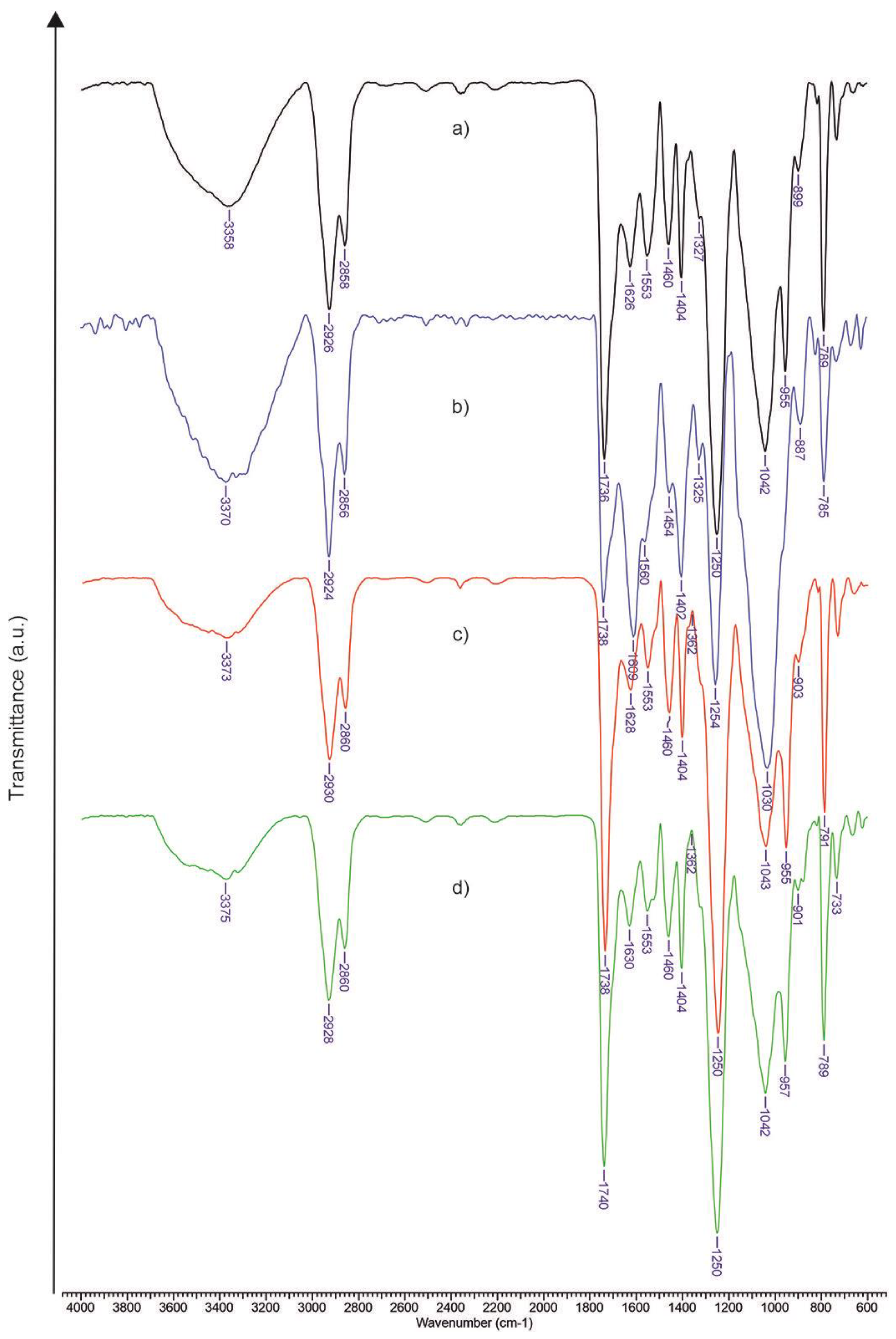
3.1. FTIR Assessment of Xanthan Modified with Oleic Acid
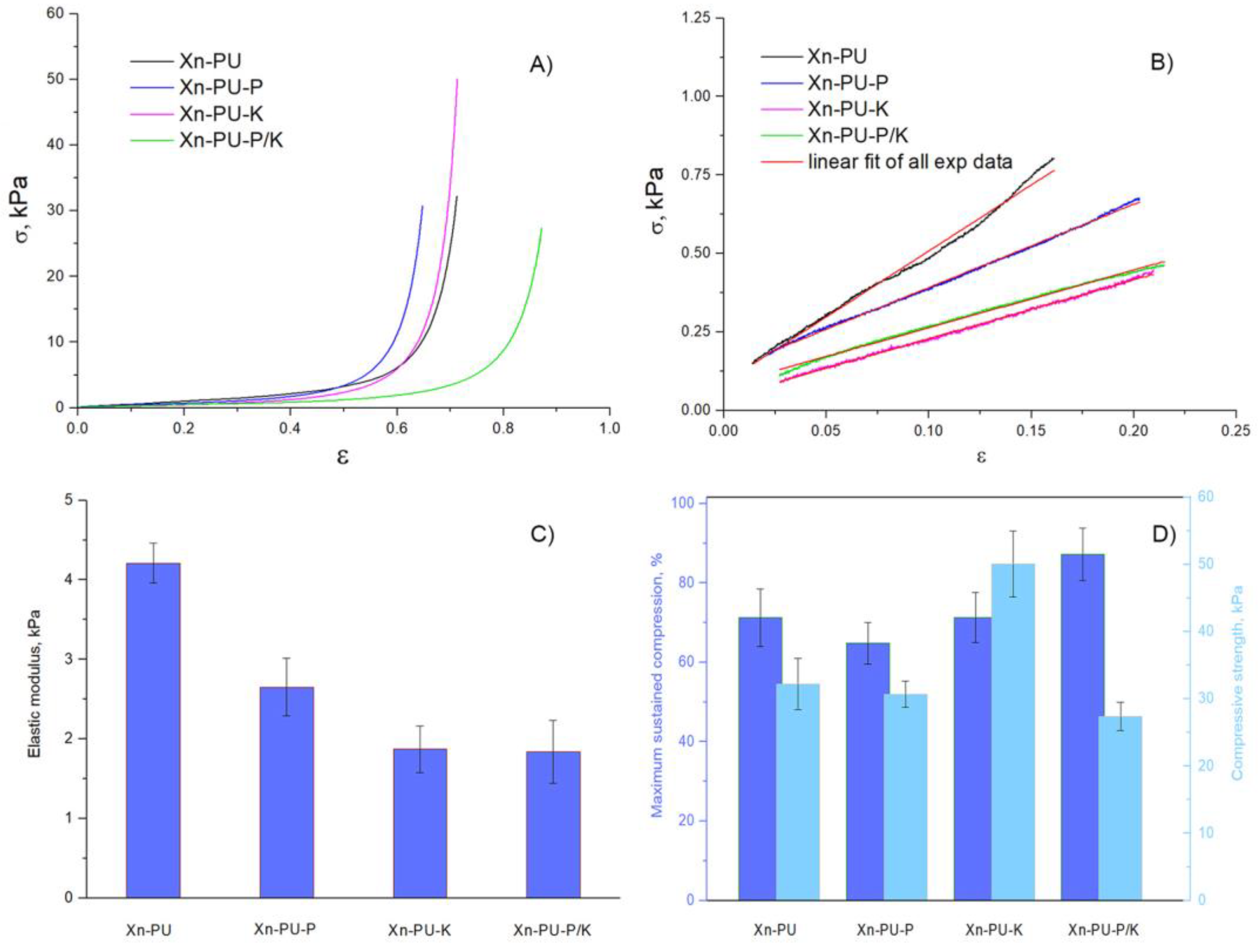
3.2. Mechanical Properties
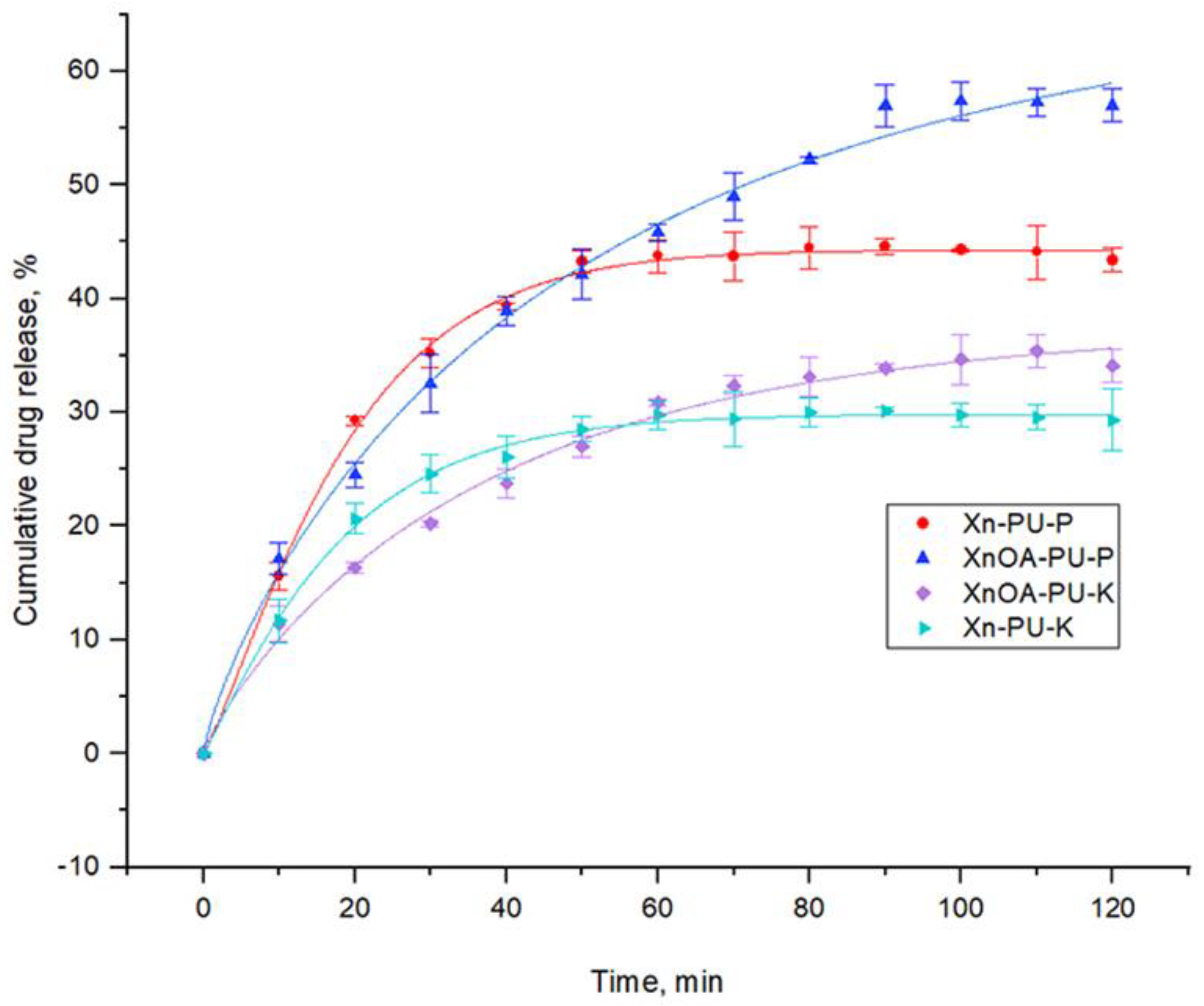
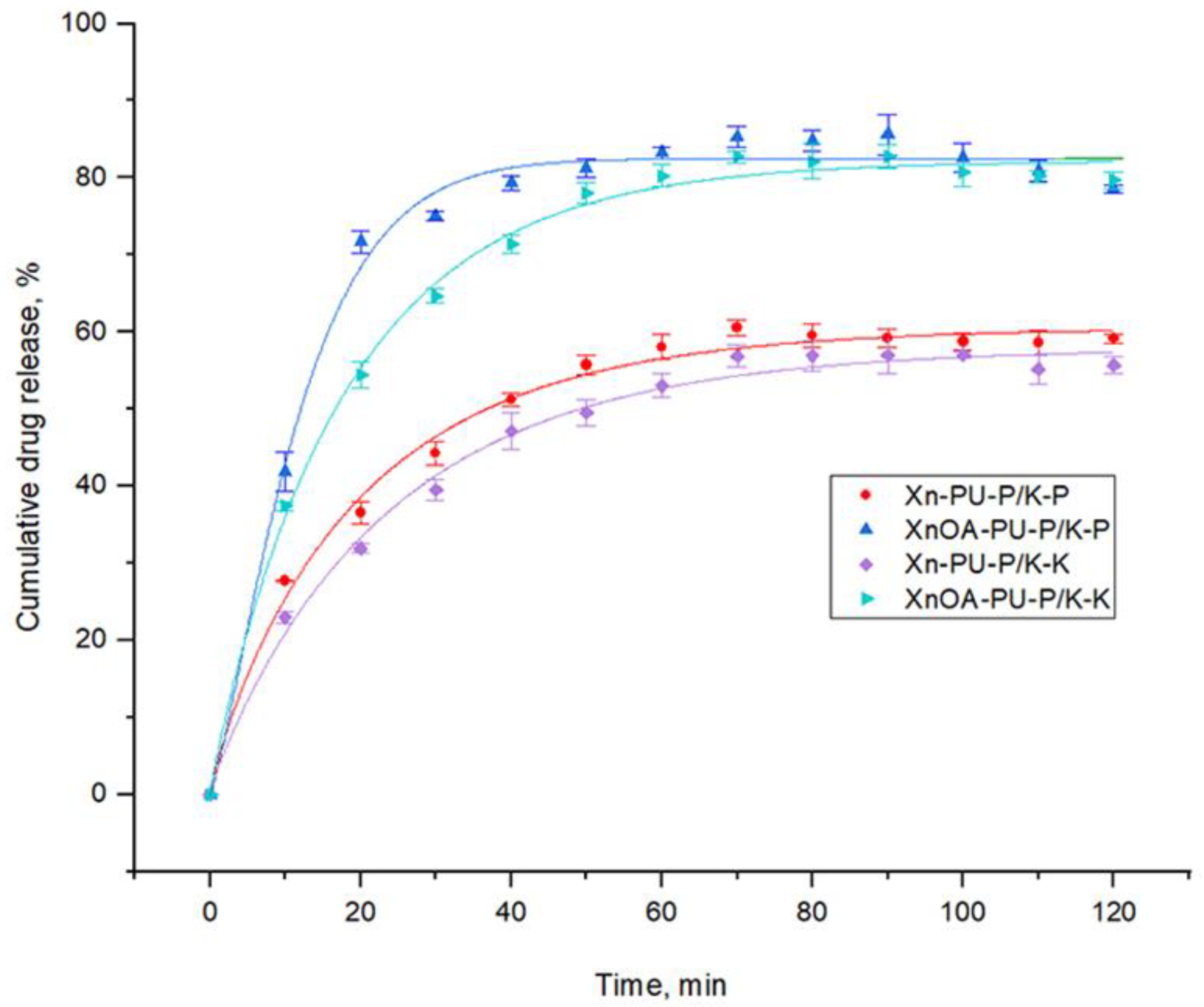
3.3. Drug Release Kinetics
3.4. Antimicrobial Activity
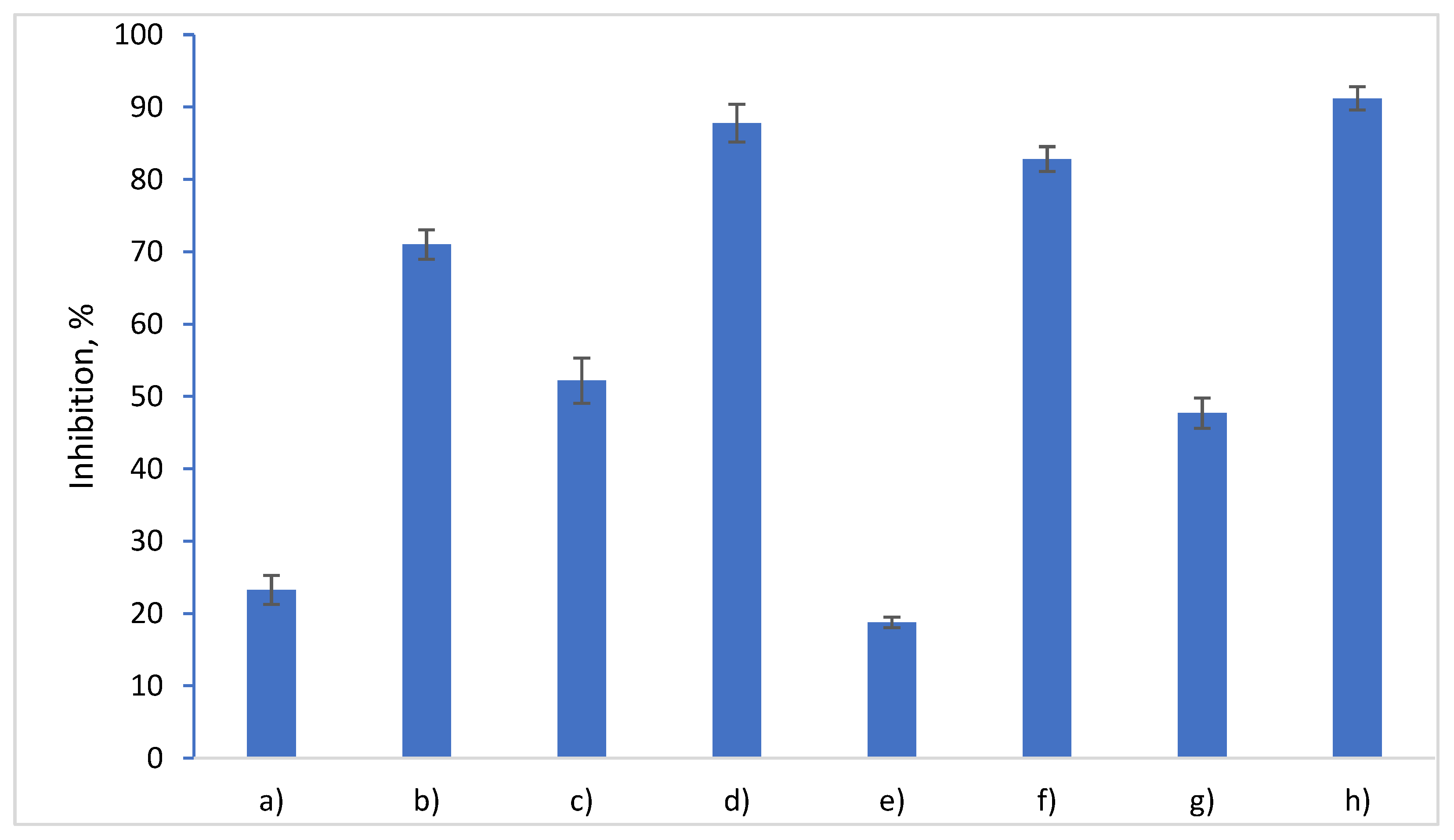
3.5. Evaluation of Anti-Inflammatory Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fardous, J.; Yamamoto, E.; Omoso, Y.; Nagao, S.; Inoue, Y.; Yoshida, K.; Ikegami, Y.; Zhang, Y.; Shirakigawa, N.; Ono, F.; et al. Development of a gel-in-oil emulsion as a transdermal drug delivery system for successful delivery of growth factors. J. Biosci. Bioeng. 2021, 132, 95–101. [Google Scholar] [CrossRef]
- Križman, K.; Novak, S.; Kristl, J.; Majdič, G.; Drnovšek, N. Long-acting silk fibroin xerogel delivery systems for controlled release of estradiol. J. Drug Deliv. Sci. Technol. 2021, 65, 102701. [Google Scholar] [CrossRef]
- Morad, H.; Jahanshahi, M.; Akbari, J.; Saeedi, M.; Gill, P.; Enayatifard, R. Novel topical and transdermal delivery of colchicine with chitosan based biocomposite nanofiberous system; formulation, optimization, characterization, ex vivo skin deposition/permeation, and anti-melanoma evaluation. Mater. Chem. Phys. 2021, 263, 124381. [Google Scholar] [CrossRef]
- Puri, A.; Frempong, D.; Mishra, D.; Dogra, P. Microneedle-mediated transdermal delivery of naloxone hydrochloride for treatment of opioid overdose. Int. J. Pharm. 2021, 604, 120739. [Google Scholar] [CrossRef]
- Solís, A.C.; Bento, D.; Nunes, S.; Valente, A.; Pais, A.; Vitorino, C. Rethinking transdermal drug delivery using PVA-NLC based films. Polymer 2021, 230, 124032. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L.; Wat, E.; Kan, C.W.; Leung, P.C.; Wang, W. Drug delivery system of dual-responsive PF127 hydrogel with polysaccharide-based nano-conjugate for textile-based transdermal therapy. Carbohydr. Polym. 2020, 236, 116074. [Google Scholar] [CrossRef]
- Karakurt, I.; Ozaltin, K.; Vargun, E.; Kucerova, L.; Suly, P.; Harea, E.; Minařík, A.; Štěpánková, K.; Lehocky, M.; Humpolícek, P.; et al. Controlled release of enrofloxacin by vanillin-crosslinked chitosan-polyvinyl alcohol blends. Mater. Sci. Eng. C 2021, 126, 112125. [Google Scholar] [CrossRef]
- Kim, H.S.; Yun, Y.H.; Shim, W.G.; Yoon, S.D. Preparation of atenolol imprinted polysaccharide based biomaterials for a transdermal drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 59, 101893. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Graft and crosslinked polymerization of polysaccharide gum to form hydrogel wound dressings for drug delivery applications. Carbohydr. Res. 2020, 489, 107949. [Google Scholar] [CrossRef]
- Cleymand, F.; Poerio, A.; Mamanov, A.; Elkhoury, K.; Ikhelf, L.; Jehl, J.P.; Kahn, C.J.F.; Ponçot, M.; Arab-Tehrany, E.; Mano, J.F. Development of novel chitosan/guar gum inks for extrusion-based 3D bioprinting: Process, printability and properties. Bioprinting 2021, 21, e00122. [Google Scholar] [CrossRef]
- Huaytragul, J.; Chalitangkoon, J.; Monvisade, P.; Chotsaeng, N. Enhancing chitosan solubility in alcohol: Water mixtures for film-forming systems releasing with turmeric extracts. J. Taiwan Inst. Chem. Eng. 2021, 123, 293–301. [Google Scholar] [CrossRef]
- Nornberg, A.B.; Martins, C.C.; Cervi, V.F.; Sari, M.H.M.; Cruz, L.; Luchese, C.; Wilhelm, E.A.; Fajardo, A.R. Transdermal release of methotrexate by cationic starch/poly(vinyl alcohol)-based films as an approach for rheumatoid arthritis treatment. Int. J. Pharm. 2022, 611, 121285. [Google Scholar] [CrossRef]
- Camargo, L.G.; de Freitas Rosa Remiro, P.; Rezende, G.S.; Di Carla Santos, S.; Franz-Montan, M.; Moraes, Â.M. Development of bioadhesive polysaccharide-based films for topical release of the immunomodulatory agent imiquimod on oral mucosa lesions. Eur. Polym. J. 2021, 151, 110422. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Lin, Q.; Xue, K.; Loh, X.J. Recent advances in supramolecular hydrogels for biomedical applications. Mater. Today Adv. 2019, 3, 100021. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Hosseinzadeh, H.; Pashaei, S. Fabrication of nanocellulose loaded poly(AA-co-HEMA) hydrogels for ceftriaxone controlled delivery and crystal violet adsorption. Polym. Compos. 2018, 40, E559–E569. [Google Scholar] [CrossRef]
- Kolakovic, R.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.; Laaksonen, T. Nanofibrillar cellulose films for controlled drug delivery. Eur. J. Pharm. Biopharm. 2012, 82, 308–315. [Google Scholar] [CrossRef]
- Li, X.J.; Li, Y.; Meng, Y.; Pu, X.Q.; Qin, J.W.; Xie, R.; Wang, W.; Liu, Z.; Jiang, L.; Ju, X.J.; et al. Composite dissolvable microneedle patch for therapy of oral mucosal diseases. Biomater. Adv. 2022, 139, 213001. [Google Scholar] [CrossRef]
- Nematpour, N.; Farhadian, N.; Ebrahimi, K.S.; Arkan, E.; Seyedi, F.; Khaledian, S.; Shahlaei, M.; Moradi, S. Sustained release nanofibrous composite patch for transdermal antibiotic delivery. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124267. [Google Scholar] [CrossRef]
- Affes, S.; Aranaz, I.; Acosta, N.; Heras, Á.; Nasri, M.; Maalej, H. Chitosan derivatives-based films as pH-sensitive drug delivery systems with enhanced antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2021, 182, 730–742. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; Kura, A.; Hussein, M.Z.; Fakurazi, S. Preparation of chitosan nanoparticles as a drug delivery system for perindopril erbumine. Polym. Compos. 2016, 39, 544–552. [Google Scholar] [CrossRef]
- Potaś, J.; Szymańska, E.; Winnicka, K. Challenges in developing of chitosan—Based polyelectrolyte complexes as a platform for mucosal and skin drug delivery. Eur. Polym. J. 2020, 140, 110020. [Google Scholar] [CrossRef]
- Aguero, L.; Alpdagtas, S.; Ilhan, E.; Zaldivar-Silva, D.; Gunduz, O. Functional role of crosslinking in alginate scaffold for drug delivery and tissue engineering: A review. Eur. Polym. J. 2021, 160, 110807. [Google Scholar] [CrossRef]
- Karimi Khorrami, N.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and characterization of alginate-based films functionalized with nanostructured lipid carriers. Int. J. Biol. Macromol. 2021, 182, 373–384. [Google Scholar] [CrossRef]
- Dimofte, A.; Dinu, M.V.; Anghel, N.; Doroftei, F.; Spiridon, I. Xanthan and alginate-matrix used as transdermal delivery carrier for piroxicam and ketoconazole. Int. J. Biol. Macromol. 2022, 209, 2084–2096. [Google Scholar] [CrossRef]
- Martín-Illana, A.; Chinarro, E.; Cazorla-Luna, R.; Notario-Perez, F.; Veiga-Ochoa, M.D.; Rubio, J.; Tamayo, A. Optimized hydration dynamics in mucoadhesive xanthan-based trilayer vaginal films for the controlled release of tenofovir. Carbohydr. Polym. 2022, 278, 118958. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Veeramachineni, A.K.; Owh, C.; Loh, X.J. Biodegradable Polysaccharides for Controlled Drug Delivery. ChemPlusChem 2016, 81, 504–514. [Google Scholar] [CrossRef]
- Raschip, I.E.; Dinu, M.V.; Fifere, N.; Darie-Nita, R.; Pamfil, D.; Popirda, A.; Logigan, C. Thermal, mechanical and water sorption properties of xanthan-based composite cryogels. Cell. Chem. Technol. 2020, 54, 915–924. [Google Scholar] [CrossRef]
- Spiridon, I.; Andrei, I.M.; Anghel, N.; Dinu, M.V.; Ciubotaru, B.I. Development and characterization of novel cellulose composites obtained in 1-ethyl-3-methylimidazolium chloride used as drug delivery systems. Polymers 2021, 13, 2176. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Lin, Q.; Liu, H.; Shen, C.; Nan, K.; Chen, H. In vitro and in vivo evaluation of xanthan gum-succinic anhydride hydrogels for the ionic strength-sensitive release of antibacterial agents. J. Mater. Chem. B 2016, 4, 1853–1861. [Google Scholar] [CrossRef]
- Anghel, N.; Dinu, V.M.; Verestiuc, L.; Spiridon, I.A. Transcutaneous Drug Delivery Systems Based on Collagen/Polyurethane Composites Reinforced with Cellulose. Polymers 2021, 13, 1845. [Google Scholar] [CrossRef]
- Bozyigit, I.; Javadi, A.; Altun, S. Strength properties of xanthan gum and guar gum treated kaolin at different water contents. J. Rock Mech. Geotech. Eng. 2021, 13, 1160–1172. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Razmjooei, M.; Safdarianghomsheh, R.; Shad, E.; Delvigne, F.; Khalesi, M. Semi-continuous production of xanthan in biofilm reactor using Xanthomonas campestris. J. Biotechnol. 2021, 328, 1–11. [Google Scholar] [CrossRef]
- Mohsin, A.; Akyliyaevna, K.A.; Zaman, W.Q.; Hussain, M.H.; Mohsin, M.Z.; Al-Rashed, S.; Tan, X.; Tian, X.; Aida, K.; Tariq, M.; et al. Kinetically modelled approach of xanthan production using different carbon sources: A study on molecular weight and rheological properties of xanthan. Int. J. Biol. Macromol. 2021, 193, 1226–1236. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Jiang, B.; Chen, J. A review of the enzymatic, physical, and chemical modification techniques of xanthan gum. Int. J. Biol. Macromol. 2021, 186, 472–489. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, C.; Yang, Y. Micellar thermodynamic behavior of branch-modified xanthan gum and aggregating structures in aqueous and saline solutions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124317. [Google Scholar] [CrossRef]
- Byram, P.K.; Sunka, K.C.; Barik, A.; Kaushal, M.; Dhara, S.; Chakravorty, N. Biomimetic silk fibroin and xanthan gum blended hydrogels for connective tissue regeneration. Int. J. Biol. Macromol. 2020, 165, 874–882. [Google Scholar] [CrossRef]
- Westin, C.B.; Nagahara, M.H.T.; Decarli, M.C.; Kelly, D.J.; Moraes, Â.M. Development and characterization of carbohydrate-based thermosensitive hydrogels for cartilage tissue engineering. Eur. Polym. J. 2020, 129, 109637. [Google Scholar] [CrossRef]
- Demir, G.C.; Erdemli, Ö.; Keskin, D.; Tezcaner, A. Xanthan-gelatin and xanthan-gelatin-keratin wound dressings for local delivery of Vitamin C. Int. J. Pharm. 2022, 614, 121436. [Google Scholar] [CrossRef]
- Saadatlou, G.A.; Pircheraghi, G. Concentrated regimes of xanthan-based hydrogels crosslinked with multifunctional crosslinkers. Carbohydr. Polym. Technol. Appl. 2021, 2, 100047. [Google Scholar] [CrossRef]
- Gil, M.C.; Park, S.J.; Lee, B.S.; Park, C.; Lee, B.J. Dual thermal stabilizing effects of xanthan gums via glycosylation and hydrogen bonding and in vivo human bioavailability of desmopressin in orodispersible film. Int. J. Pharm. 2023, 637, 122879. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Aamir, M.N.; Haseeb, M.T.; Abbas Bukhari, S.N.; Farid ul Haq, M.; Akhtar, N. Design, physico-chemical assessment and pharmacokinetics of a non-toxic orodispersible film for potential application in musculo-skeletal disorder. J. Drug Deliv. Sci. Technol. 2021, 65, 102726. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Dhaliwal, A.S. Controlled release of amoxicillin and antioxidant potential of gold nanoparticles-xanthan gum/poly (Acrylic acid) biodegradable nanocomposite. J. Drug Deliv. Sci. Technol. 2020, 55, 101384. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, F.; Ling, J.; Ouyang, X.K.; Wang, Y.G. Delivery of curcumin using a zein-xanthan gum nanocomplex: Fabrication, characterization, and in vitro release properties. Colloids Surf. B Biointerfaces 2021, 204, 111827. [Google Scholar] [CrossRef]
- Kamaci, M. Polyurethane-based hydrogels for controlled drug delivery applications. Eur. Polym. J. 2020, 123, 109444. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Abrami, M.; Grassi, M.; Zoso, A.; Chiono, V.; Ciardelli, G. Dual stimuli-responsive polyurethane-based hydrogels as smart drug delivery carriers for the advanced treatment of chronic skin wounds. Bioact. Mater. 2021, 6, 3013–3024. [Google Scholar] [CrossRef]
- P, S.; Reshmi, C.R.; Sundaran, S.P.; Binoy, A.; Mishra, N.; A, S. β-Cyclodextrin functionalized polyurethane nano fibrous membranes for drug delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102759. [Google Scholar] [CrossRef]
- Xu, J.; Hao, T.; Liu, C.; Bi, J.; Sun, J.; Wen, Z.; Hou, Z.; Wei, J. pH-Responsive and degradable polyurethane film with good tensile properties for drug delivery in vitro. Mater. Today Commun. 2021, 29, 102969. [Google Scholar] [CrossRef]
- Hassan, A.S.; Sangeetha, A.B.; Shobana, C.S.; Mythili, A.; Suresh, S.; Abirami, B.; Alharbi, R.A.; Aloyuni, S.A.; Abdel-Hadi, A.; Awad, M.F.; et al. In-vitro assessment of first-line antifungal drugs against Aspergillus spp. caused human keratomycoses. J. Infect. Public Health 2020, 13, 1907–1911. [Google Scholar] [CrossRef]
- Hedayati, N.; Montazer, M.; Mahmoudirad, M.; Toliyat, T. Ketoconazole and Ketoconazole/β-cyclodextrin performance on cotton wound dressing as fungal skin treatment. Carbohydr. Polym. 2020, 240, 116267. [Google Scholar] [CrossRef]
- Khona, D.K.; Roy, S.; Ghatak, S.; Huang, K.; Jagdale, G.; Baker, L.A.; Sen, C.K. Ketoconazole resistant Candida albicans is sensitive to a wireless electroceutical wound care dressing. Bioelectrochemistry 2021, 142, 107921. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Li, R.; Pang, L.; Zhu, S.; Ma, J.; Du, L.; Jin, Y. Electroporation-enhanced transdermal drug delivery: Effects of logP, pKa, solubility and penetration time. Eur. J. Pharm. Sci. 2020, 151, 105410. [Google Scholar] [CrossRef]
- Pénzes, T.; Blazsó, G.; Aigner, Z.; Falkay, G.; Eros, I. Topical absorption of piroxicam from organogels—In vitro and in vivo correlations. Int. J. Pharm. 2005, 298, 47–54. [Google Scholar] [CrossRef]
- Salma, H.; Melha, Y.M.; Sonia, L.; Hamza, H.; Salim, N. Efficient Prediction of In Vitro Piroxicam Release and Diffusion from Topical Films Based on Biopolymers Using Deep Learning Models and Generative Adversarial Networks. J. Pharm. Sci. 2021, 110, 2531–2543. [Google Scholar] [CrossRef]
- Jiang, H.; Duan, L.; Ren, X.; Gao, G. Hydrophobic association hydrogels with excellent mechanical and self-healing properties. Eur. Polym. J. 2019, 112, 660–669. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef]
- Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Lefnaoui, S.; Hadjsadok, A.; Amrane, A.; Kebir, M.; Zhang, J.; Assadi, A.A.; Mouni, L. Evaluation of Physicochemical and Amphiphilic Properties of New Xanthan Gum Hydrophobically Functionalized Derivatives. Sustainability 2023, 15, 6345. [Google Scholar] [CrossRef]
- Dinu, M.V.; Gradinaru, A.C.; Lazar, M.M.; Dinu, I.A.; Raschip, I.E.; Ciocarlan, N.; Aprotosoaie, A.C. Physically cross-linked chitosan/dextrin cryogels entrapping Thymus vulgaris essential oil with enhanced mechanical, antioxidant and antifungal properties. Int. J. Biol. Macromol. 2021, 184, 898–908. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef]
- Paiva, D.; Goncalves, C.; Vale, I.; Bastos, M.; Magalhaes, F.D. Oxidized Xanthan Gum and Chitosan as Natural Adhesives for Cork. Polymers 2016, 8, 259. [Google Scholar] [CrossRef]
- Ledea Lozano, O.E.; Diaz Gomez, M.F.; Molerio, J. 1H-NMR spectroscopy study of oleic acid and methyl oleate ozonation in different reaction conditions. Rev. CENIC Cienc. Quim. 2003, 34, 3–8. [Google Scholar]
- National Library of Medicine, National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 11 April 2024).
- Van Den Mooter, G.; Wuyts, M.; Blaton, N.; Busson, R.; Grobet, P.; Augustijns, P.; Kinget, R. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur. J. Pharm. Sci. 2001, 12, 261–269. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Ueda, H.; Takata, Y.; Minamihata, K.; Wakabayashi, R.; Kamiya, N.; Goto, M. Co-amorphous formation of piroxicam-citric acid to generate supersaturation and improve skin permeation. Eur. J. Pharm. Sci. 2021, 158, 105667. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F. Crystallinity changes in lyocell and viscose-type fibres by caustic treatment. Eur. Polym. J. 2002, 38, 2225–2230. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A. Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps. Gels 2022, 8, 240. [Google Scholar] [CrossRef]
- Atiyah, N.A.; Albayati, T.M.; Atiya, M.A. Interaction behavior of curcumin encapsulated onto functionalized SBA-15 as an efficient carrier and release in drug delivery. J. Mol. Struct. 2022, 1260, 132879. [Google Scholar] [CrossRef]
- Permanadewi, I.; Kumoro, A.C.; Wardhani, D.H.; Aryanti, N. Modelling of controlled drug release in gastrointestinal tract simulation. J. Phys. Conf. Ser. 2019, 1295, 012063. [Google Scholar] [CrossRef]
- Lu, T.; ten Hagen, T.L.M. A novel kinetic model to describe the ultra-fast triggered release of thermosensitive liposomal drug delivery systems. J. Control. Release 2020, 324, 669–678. [Google Scholar] [CrossRef]
- Ignacio, M.; Chubynsky, M.V.; Slater, G.W. Interpreting the Weibull fitting parameters for diffusion-controlled release data. Phys. A Stat. Mech. Its Appl. 2017, 486, 486–496. [Google Scholar] [CrossRef]
- Cutsem, J.V.; Gerven, F.V.; Cauwenbergh, G.; Odds, F.; Janssen, P.A.J. The antiinflammatory effects of ketoconazole. J. Am. Acad. Dermatol. 1991, 25, 257–261. [Google Scholar] [CrossRef]

| Physical Property | Ketoconazole | Piroxicam | |
|---|---|---|---|
| Hydrogen bond | Donor | 0 | 6 |
| Acceptor | 6 | 6 | |
| LogP, octanol/water partition coefficient | 4.35 | 3.06 | |
| Topological polar surface area, Å | 69.1 | 108 | |
| Sample | TCI, (A1376/A2902) | HBI, (A3336/A1336) | LOI, (A1437/A899) |
|---|---|---|---|
| Xn-PU | 0.77 | 0.171 | 1.608 |
| Xn-PU-P | 0.73 | 0.893 | 1.503 |
| Xn-PU-K | 1.175 | 0.229 | 3.52 |
| Xn-PU-P/K | 1.075 | 0.821 | 2.01 |
| XnOA-PU | 1.124 | 0.89 | 2.024 |
| XnOA-PU-P | 0.815 | 1.184 | 0.406 |
| XnOA-PU-K | 1.137 | 1.122 | 5.371 |
| XnOA-PU-P/K | 0.789 | 1.071 | 1.733 |
| Sample’s Code | Average Pore Size, µm | Average Pore Wall Thickness, µm |
|---|---|---|
| Xn-PU | 110.03 ± 18.55 | 42.47 ± 4.79 |
| Xn-PU-P | 51.17 ± 17.77 | 14.22 ± 2.12 |
| Xn-PU-K | 58.43 ± 12.03 | 6.54 ± 1.69 |
| Xn-PU-P/K | 39.44 ± 13.48 | 23.84 ± 4.03 |
| XnAO-PU | 50.35 ± 8.23 | 5.82 ± 1.31 |
| XnAO-PU-P | 72.04 ± 17.54 | 27.37 ± 4.46 |
| XnAO-PU-K | 74.71 ± 20.74 | 22.83 ± 5.31 |
| XnAO-PU-P/K | 50.19 ± 13.02 | 19.62 ± 2.86 |
| Sample | M∞, % | β | k, min−1 | Correlation Coefficient, R2 |
|---|---|---|---|---|
| Xn-PU-P | 44.290 ± 0.323 | 1.207 ± 0.218 | 0.051 ± 0.009 | 0.99813 |
| XnOA-PU-P | 67.322 ± 4.798 | 0.823 ± 0.074 | 0.020 ± 0.003 | 0.99557 |
| Xn-PU-K | 29.837 ± 0.250 | 1.109 ± 0.137 | 0.054 ± 0.006 | 0.9973 |
| XnOA-PU-K | 37.707 ± 1.822 | 0.902 ± 0.094 | 0.027 ± 0.003 | 0.99323 |
| Xn-PU-P/K-K | 57.920 ± 1.332 | 0.942 ± 0.094 | 0.042 ± 0.002 | 0.99253 |
| XnOA-PU-P/K-K | 82.216 ± 1.003 | 0.958 ± 0.074 | 0.055 ± 0.002 | 0.9956 |
| Xn-PU-P/K-P | 60.567 ± 1.237 | 0.897 ± 0.093 | 0.050 ± 0.003 | 0.99216 |
| XnOA-PU-P/K-P | 82.498 ± 1.030 | 1.267 ± 1.175 | 0.078 ± 0.090 | 0.99032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghel, N.; Spiridon, I.; Dinu, M.-V.; Vlad, S.; Pertea, M. Xanthan–Polyurethane Conjugates: An Efficient Approach for Drug Delivery. Polymers 2024, 16, 1734. https://doi.org/10.3390/polym16121734
Anghel N, Spiridon I, Dinu M-V, Vlad S, Pertea M. Xanthan–Polyurethane Conjugates: An Efficient Approach for Drug Delivery. Polymers. 2024; 16(12):1734. https://doi.org/10.3390/polym16121734
Chicago/Turabian StyleAnghel, Narcis, Iuliana Spiridon, Maria-Valentina Dinu, Stelian Vlad, and Mihaela Pertea. 2024. "Xanthan–Polyurethane Conjugates: An Efficient Approach for Drug Delivery" Polymers 16, no. 12: 1734. https://doi.org/10.3390/polym16121734
APA StyleAnghel, N., Spiridon, I., Dinu, M.-V., Vlad, S., & Pertea, M. (2024). Xanthan–Polyurethane Conjugates: An Efficient Approach for Drug Delivery. Polymers, 16(12), 1734. https://doi.org/10.3390/polym16121734







